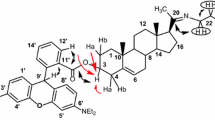
Abstract
A series of new aromatic esters based on 4,16-pregnadiene-6,20-dione skeleton, namely 3β-benzoyloxy-4,16-pregnadiene-6,20-dione and 3β-furoyloxy-4,16-pregnadiene- 6,20-dione, which may be good inhibitors for the 5α-reductase enzyme and show high antiandrogenic activity, were synthesized starting from diosgenin. The structures of the steroids were characterized by elemental analysis, 1H NMR, 13C NMR, IR and mass spectrum. Single crystal X-ray diffraction measurement on one of the new compounds, 3β-(p-methoxybenzoyloxy)-4,16-pregnadiene-6,20-dione revealed that the A, B, C, and D ring adopted half chair, distorted chair, distorted chair, and distorted envelope conformation, respectively. The molecules in the crystal were packed face-to-face at the normal van der Waals distances.

Similar content being viewed by others
Introduction
Studies on steroids stimulated renewed interest because apart from their estrogenic and anabolic/androgenic activity [1–3] steroids were found to show various biological activities including cytotoxicity [4], antiproliferative activity [5], antimicrobial activity [6], anti-glioma activity [7], inhibition of cholesterol α-glucosyltransferase [8], neuromuscular blocking activity [9], differential activities on prostate cancer cells [10], anti-osteoporosis activity [11], and anti-aging activity [12]. Up until now steroids have showed potential applications as 5α-reductase inhibitors [13], as modulators of inflammation and immunity [14], as inhibitors of 17α-hydroxylase/C17,20-lyase [15], as predictor of tamoxifen response in premenopausal breast cancer [16], as progesterone receptor antagonist [17], as glucocorticoid receptor imaging agents [18], as inhibitors of protein tyrosine phosphatase 1B [19], as reversal agents of multidrug resistance in cancer cells [20], as inhibitors of 17β-hydroxysteroid dehydrogenase [21], and as ligands for drug vectors [22]. Some steroidal compounds were used for photodynamic therapy [23], for cancer chemotherapy [24], and for DNA delivery [25]. Moreover, cholesteric liquid crystal [26] and steroid-based organogelator [27] were also studied. Recently Cabeza and collegues synthesized a series of 3β-benzoyloxy-4,16-pregnadiene-6,20-dione (1a-e, Figure 1) and 3β- cycloalkylcarbonyloxy-4,16-pregnadiene-6,20-dione (2a-d, Figure 1) compounds starting from 16-dehydropregnenolone acetate (16-DPA) and evaluated their biological activities. Of these steroids 1a is a good inhibitor for the 5α-reductase enzyme [13] while 1b-e and 2a-d showed high antiandrogenic activity [28, 29]. In continuation of our study on steroids [30, 31] and to explore an improved way for synthesis of new steroidal compounds from the cheap starting material diosgenin, herein we report the synthesis and structural characterization of a series of new aromatic esters based on 4,16-pregnadiene-6,20-dione skeleton.
Experimental
All chemical reagents were purchased from commercial sources and used as received unless stated otherwise. Melting points were determined on a XT-4 melting apparatus and the quoted temperatures were uncorrected. Elemental analysis was carried out on an Elmentar Vario EL III system. 1H NMR and 13C NMR spectra were recorded on a Bruker AM 400 spectrometer. CDCl3 was used as solvent and chemical shifts recorded were internally referenced to Me4Si (0 ppm). IR spectra were obtained on a Thermo Electron Corporation Nicolet 380 FT-IR spectrophotometer. Mass spectra were recorded on a Shimadzu QP-2010 instrument using electron impact ionization (EI) at 70 eV. LC-MS spectra were obtained on an Agilent 6000 LC-MS instrument equipped with a SunFire C18 column (4.6 × 50 mm, 3.5 μm) under the following conditions: mobile phase: H2O (0.05% trifluoroacetic acid (TFA)) (A)/acetonitrile (0.05% TFA) (B); elution program: gradient from 5 to 95% of B in 1.6 min at 2.2 ml/min; temperature: 50°; detection: UV (214 nm) and MS (ESI, pos mode, 70 to 1000 amu). All of the measured samples were dissolved in methanol. X-Ray crystal structure was measured on a Bruker Smart CCD diffractometer by using Mo Kα radiation at 293 K.
Pseudodiosgenin diacetate (4) [32]
To a solution of diosgenin 3 (16.0 g, 38.6 mmol) and ammonium chloride (2.5 g, 46.7 mmol) in acetic anhydride (82 ml) was added pyridine (1.5 ml) and the mixture was heated at 140°C for 8-9 h. The resulting solution was cooled to room temperature and poured into ice water. The precipitate was filtered and recrystallized from methanol to give the product pseudodiosgenin diacetate (4) as yellow solid (16.6 g, 86%). M.p. 70-72°C.
3β-Acetoxy-5,16-pregnadiene-20-one (5)
A solution of pseudodiosgenin diacetate (4) (15.4 g, 30.8 mmol) in acetic anhydride (76 ml) was diluted with water (4.8 ml) and acetic acid (50 ml). The mixture was cooled to 0°C then a solution of chromium(VI) oxide (8.9 g, 89.0 mmol) in acetic acid (25 ml) was added dropwise in 1 h. After the addition the solution was allowed to warm to 10-16°C and the stirring was continued for 5 h at this temperature. Sodium bisulphite (9.3 g, 89.4 mmol) in water (30 ml) was added then the mixture was refluxed for 3 h, cooled and poured into water to give sticky solids. The crude product was crystallized from methanol then purified by means of column chromatography on silica gel with petroleum ether (b.p. 60-90°C) and ethyl acetate (4:1, v/v) as eluent to afford product 5 (4.5 g, 41%) as yellow needles. M.p. 170-172°C (literature [32] value: 171-172°C). Rf 0.58 (petroleum ether/ethyl acetate, 4:1, v/v).
Compound 6 was synthesized as described in literature [33] in 90% yield and as described in literature [13] in 95% yield. M.p. 170-172°C (literature [13] value: 170-172°C). Rf 0.57 (petroleum ether/ethyl acetate, 4:1, v/v).
Compound 7 was synthesized as described in literature [13] in 84% yield. M.p. 243-245°C (literature [13] value: 244-245°C). Rf 0.56 (petroleum ether/ethyl acetate, 4:1, v/v).
Compound 8 was synthesized as described in literature [13] in 73% yield. M.p. 192-194°C (literature [13] value: 193-195°C). Rf 0.58 (petroleum ether/ethyl acetate, 4:1, v/v).
3β-Hydroxypregna-4,16-diene-6,20-dione (9)
A solution of steroid 8 (0.3 g, 0.8 mmol) in methanol (45 ml) and 2% aqueous sodium hydroxide (3 ml) was stirred for 30 min at room temperature. The resulting solution was poured into ice water (45 ml) then extracted with ethyl acetate (3 × 30 ml). The organic phase was dried over anhydrous magnesium sulfate and the solvent was removed in vacuum. The residue was purified by column chromatography on silica gel with petroleum ether and ethyl acetate (1:1, v/v) as eluent to afford product 9 (0.25 g, 74%) as pale yellow needles. M.p. 168-170°C (literature [13] value: 168-170°C). Rf 0.52 (petroleum ether/ethyl acetate, 4:1, v/v).
3β-(p-Methoxybenzoyloxy)pregna-4,16-diene-6,20-dione (10a)
A solution containing steroid 9 (0.10 g, 0.3 mmol), p-methoxybenzoic acid (0.13 g, 0.7 mmol), 1,3-dicyclohexylcarbodiimide (DCC, 0.10 g, 0.5 mmol) and 4-dimethylaminopyridine (DMAP, 0.06 g, 0.5 mmol) in methylene dichloride (6 ml) was stirred for 2 h at about 10°C. Ice water was added and the reaction mixture was extracted with chloroform (3 × 20 ml). The organic phase was dried over anhydrous magnesium sulfate and the solvent was removed in vacuum. The crude product was dissolved in ethyl acetate and filtered through a column containing silica gel. The organic solvent was removed in vacuum to give a white crystalline solid 10a (0.14 g, 71%). M.p. 244-246°C. Rf 0.50 (petroleum ether/ethyl acetate, 4:1, v/v). 1H NMR (CDCl3): 0.94 (s, 3 H, H-C(18)); 1.10 (s, 3 H, H-C(19)); 2.28 (s, 3 H, H-C(21)); 3.87 (s, 3 H, OMe); 5.56 (m, 1 H, H-C(3)); 6.23 (m, 1 H, H-C(4)); 6.71 (t, 1 H, J = 1.3 Hz, H-C(16)); 6.93 (m, 2 H, H-Ph); 8.02 (m, 2 H, H-Ph). 13C NMR (CDCl3): 15.8 (C(18)); 19.7 (C(19)); 27.1 (C(21)); 69.5 (C(3)); 131.8 (C(4)); 143.8 (C(16)); 147.9 (C(5)); 154.9 (C(17)); 165.9 (ester C = O); 196.6 (C(20)); 201.9 (C(6)). IR (KBr): 3075, 2958, 1706, 1690, 1656, 1629. EI-MS: 312 (12, M+ + 1 - p-MeO-C6H4COO), 175 (31), 157 (38), 121 (30), 105 (41), 93 (33), 91 (66), 79 (51), 77 (46), 43 (100). LC-MS: 485.4 (M+ + Na, retention time 2.20 min). Anal. Calcd. for C29H34O5: C, 75.30; H, 7.41; Found: C, 75.12; H, 7.37.
Other steroidal compounds 10b-j were prepared in a similar procedure and the physical data of the new steroids were as follows.
3β-(o-Methoxybenzoyloxy)pregna-4,16-diene-6,20-dione (10b)
White powder. Yield 45%. M.p. 240-242°C. Rf 0.42 (petroleum ether/ethyl acetate, 4:1, v/v). 1H NMR (CDCl3): 0.93 (s, 3 H, H-C(18)); 1.07 (s, 3 H, H-C(19)); 2.27 (s, 3 H, H-C(21)); 3.89 (s, 3 H, OMe); 5.56 (m, 1 H, H-C(3)); 6.23 (m, 1 H, H-C(4)); 6.73 (t, J = 1.6 Hz, H-C(16)); 6.96 (m, 2 H, H-Ph); 7.46 (m, 1 H, H-Ph); 7.80 (m, 1 H, H-Ph). 13C NMR (CDCl3): 17.5 (C(18)); 22.0 (C(19)); 26.5 (C(21)); 71.8 (C(3)); 136.0 (C(4) and C(16)); 146.1 (C(5)); 149.9 (C(17)); 167.0 (ester C = O); 198.9 (C(20)); 203.8 (C(6)). IR (KBr): 3040, 2946, 1688. LC-MS: 485.4 (M+ + Na, retention time 2.13 min). Anal. Calcd. for C29H34O5: C, 75.30; H, 7.41; Found: C, 75.04; H, 7.46.
3β-(o-Methylbenzoyloxy)pregna-4,16-diene-6,20-dione (10c)
White powder. Yield 62%. M.p. 182-184°C. Rf 0.39 (petroleum ether/ethyl acetate, 3:1, v/v). 1H NMR (CDCl3): 0.94 (s, 3 H, H-C(18)); 1.08 (s, 3 H, H-C(19)); 2.28 (s, 3 H, H-C(21)); 2.60 (s, 3 H, Me-Ph); 5.56 (m, 1 H, H-C(3)); 6.24 (m, 1 H, H-C(4)); 6.72 (t, 1 H, J = 1.8 Hz, H-C(16)); 7.23 (m, 2 H, H-Ph); 7.40 (m, 1 H, H-Ph); 7.93 (m, 1 H, H-Ph). 13C NMR (CDCl3): 15.8 (C(18)); 19.7 (C(19)); 27.1 (C(21)); 69.6 (C(3)); 132.1 (C(4)); 143.9 (C(16)); 148.0 (C(5)); 154.9 (C(17)); 168.4 (ester C = O); 196.7 (C(20)); 201.8 (C(6)). IR (KBr): 3070, 2946, 1688. LC-MS: 469.4 (M+ + Na, retention time 2.28 min). Anal. Calcd. for C29H34O4: C, 78.00; H, 7.67; Found: C, 77.81; H, 7.63.
3β-(m-Methylbenzoyloxy)pregna-4,16-diene-6,20-dione (10d)
White powder. Yield 69%. M.p. 138-140°C. Rf 0.42 (petroleum ether/ethyl acetate, 3:1, v/v). 1H NMR (CDCl3): 0.95 (s, 3 H, H-C(18)); 1.11 (s, 3 H, H-C(19)); 2.27 (s, 3 H, H-C(21)); 5.60 (m, 1 H, H-C(3)); 6.24 (m, 1 H, H-C(4)); 6.71 (t, 1 H, J = 1.8 Hz, H-C(16)); 7.32 (m, 2 H, H-Ph); 7.44 (m, 1 H, H-Ph); 7.84 (m, 1 H, H-Ph). 13C NMR (CDCl3): 15.8 (C(18)); 19.7 (C(19)); 27.1 (C(21)); 69.8 (C(3)); 138.0 (C(4)); 143.9 (C(16)); 148.0 (C(5)); 154.9 (C(17)); 166.4 (ester C = O); 196.6 (C(20)); 201.9 (C(6)). IR (KBr): 3060, 2945, 1688, 1589, 1457. LC-MS: 469.4 (M+ + Na, retention time 2.29 min). Anal. Calcd. for C29H34O4: C, 78.00; H, 7.67; Found: C, 78.18; H, 7.71.
3β-(p-Nitrobenzoyloxy)pregna-4,16-diene-6,20-dione (10e)
Pale yellow powder. Yield 46%. M.p. 174-176°C. Rf 0.36 (petroleum ether/ethyl acetate, 3:1, v/v). 1H NMR (CDCl3): 0.95 (s, 3 H, H-C(18)); 1.12 (s, 3 H, H-C(19)); 2.28 (s, 3 H, H-C(21)); 5.62 (m, 1 H, H-C(3)); 6.21 (m, 1 H, H-C(4)); 6.72 (t, 1 H, J = 1.8 Hz, H-C(16)); 8.23 (m, 2 H, H-Ph); 8.28 (m, 2 H, H-Ph). 13C NMR (CDCl3): 15.8 (C(18)); 19.6 (C(19)); 27.1 (C(21)); 71.0 (C(3)); 130.8 (C(4)); 135.4 (C(16)); 148.6 (C(5)); 154.9 (C(17)); 164.3 (ester C = O); 196.6 (C(20)); 201.8 (C(6)). IR (KBr): 3062, 2946, 1688, 1528, 1456. LC-MS: 500.3 (M+ + Na, retention time 2.17 min). Anal. Calcd. for C28H31NO6: C, 70.42; H, 6.54; N, 2.93; Found: C, 70.57; H, 6.52; N, 2.87.
3β-(o-Nitrobenzoyloxy)pregna-4,16-diene-6,20-dione (10f)
Pale yellow powder. Yield 55%. M.p. 158-161°C. Rf 0.29 (petroleum ether/ethyl acetate, 3:1, v/v). 1H NMR (CDCl3): 0.92 (s, 3 H, H-C(18)); 1.05 (s, 3 H, H-C(19)); 2.27 (s, 3 H, H-C(21)); 5.60 (m, 1 H, H-C(3)); 6.17 (m, 1 H, H-C(4)); 6.70 (t, 1 H, J = 1.8 Hz, H-C(16)); 7.63-7.68 (m, 2 H, H-Ph); 7.72 (m, 1 H, H-Ph); 7.95 (m, 1 H, H-Ph). 13C NMR (CDCl3): 15.8 (C(18)); 19.6 (C(19)); 27.1 (C(21)); 71.5 (C(3)); 133.0 (C(4)); 143.8 (C(16)); 148.6 (C(5)); 154.9 (C(17)); 165.1 (ester C = O); 196.6 (C(20)); 201.6 (C(6)). IR (KBr): 3033, 2945, 1732, 1687, 1656, 1628, 1587, 1540, 1456. LC-MS: 500.3 (M+ + Na, retention time 2.11 min). Anal. Calcd. for C28H31NO6: C, 70.42; H, 6.54; N, 2.93; Found: C, 70.25; H, 6.49; N, 2.90.
3β-(m-Nitrobenzoyloxy)pregna-4,16-diene-6,20-dione (10g)
Pale yellow powder. Yield 47%. M.p. 176-178°C. Rf 0.38 (petroleum ether/ethyl acetate, 3:1, v/v). 1H NMR (CDCl3): 0.94 (s, 3 H, H-C(18)); 1.12 (s, 3 H, H-C(19)); 2.27 (s, 3 H, H-C(21)); 5.63 (m, 1 H, H-C(3)); 6.20 (m, 1 H, H-C(4)); 6.71 (t, J = 1.2 Hz, H-C(16)); 7.66 (m, 1 H, H-Ph); 8.36-8.43 (m, 2 H, H-Ph); 8.86 (m, 1 H, H-Ph). 13C NMR (CDCl3): 15.8 (C(18)); 19.6 (C(19)); 27.1 (C(21)); 71.0 (C(3)); 135.4 (C(4)); 143.8 (C(16)); 148.6 (C(5)); 154.9 (C(17)); 164.1 (ester C = O); 196.6 (C(20)); 201.7 (C(6)). IR (KBr): 3040, 2945, 1689, 1526, 1456. LC-MS: 500.3 (M+ + Na, retention time 2.17 min). Anal. Calcd. for C28H31NO6: C, 70.42; H, 6.54; N, 2.93; Found: C, 70.27; H, 6.58; N, 2.88.
3β-(3,5-Dinitrobenzoyloxy)pregna-4,16-diene-6,20-dione (10h)
Pale yellow powder. Yield 45%. M.p. 168-170°C. Rf 0.59 (petroleum ether/ethyl acetate, 2:1, v/v). 1H NMR (CDCl3): 0.95 (s, 3 H, H-C(18)); 1.15 (s, 3 H, H-C(19)); 2.29 (s, 3 H, H-C(21)); 5.70 (m, 1 H, H-C(3)); 6.20 (m, 1 H, H-C(4)); 6.72 (t, 1 H, J = 1.4 Hz, H-C(16)); 9.16 (m, 2 H, H-Ph); 9.24 (m, 1 H, H-Ph). 13C NMR (CDCl3): 15.8 (C(18)); 19.5 (C(19)); 27.1 (C(21)); 72.2 (C(3)); 133.8 (C(4)); 143.8 (C(16)); 148.6 (C(5)); 154.8 (C(17)); 162.2 (ester C = O); 196.6 (C(20)); 201.6 (C(6)). IR (KBr): 3037, 2946, 1689, 1543, 1457. LC-MS: 500.3 (M+ + Na, retention time 3.14 min). Anal. Calcd. for C28H30N2O8: C, 64.36; H, 5.79; N, 5.36; Found: C, 64.22; H, 5.75; N, 5.19.
3β-(o-Chlorobenzoyloxy)pregna-4,16-diene-6,20-dione (10i)
White powder. Yield 64%. M.p. 192-194°C. Rf 0.32 (petroleum ether/ethyl acetate, 3:1, v/v). 1H NMR (CDCl3): 0.95 (s, 3 H, H-C(18)); 1.11 (s, 3 H, H-C(19)); 2.27 (s, 3 H, H-C(21)); 5.59 (m, 1 H, H-C(3)); 6.24 (m, 1 H, H-C(4)); 6.71 (t, 1 H, J = 1.8 Hz, H-C(16)); 7.32 (m, 1 H, H-Ph); 7.36 (m, 2 H, H-Ph); 7.87 (m, 1 H, H-Ph). 13C NMR (CDCl3): 15.8 (C(18)); 19.6 (C(19)); 27.1 (C(21)); 70.5 (C(3)); 133.9 (C(4)); 143.8 (C(16)); 148.2 (C(5)); 154.9 (C(17)); 165.2 (ester C = O); 196.6 (C(20)); 201.7 (C(6)). IR (KBr): 3040, 2946, 1688, 1522, 1456. LC-MS: 490.2 (M+ + Na, retention time 2.23 min). Anal. Calcd. for C28H31ClO4: C, 72.01; H, 6.69; Found: C, 71.83; H, 6.65.
3β-(2-Furoyloxy)pregna-4,16-diene-6,20-dione (10j)
White powder. Yield 77%. M.p. 180-182°C. Rf 0.40 (petroleum ether/ethyl acetate, 2:1, v/v). 1H NMR (CDCl3): 0.94 (s, 3 H, H-C(18)); 1.09 (s, 3 H, H-C(19)); 2.28 (s, 3 H, H-C(21)); 5.57 (m, 1 H, H-C(3)); 6.20 (m, 1 H, H-C(4)); 6.52 (t, 1 H, J = 1.8 Hz, H-C(16)); 6.71 (m, 1 H, H-Ar); 7.20 (m, 1 H, H-Ar); 7.59 (m, 1 H, H-Ar). 13C NMR (CDCl3): 15.8 (C(18)); 19.6 (C(19)); 27.1 (C(21)); 69.9 (C(3)); 128.7 (C(4)); 143.8 (C(16)); 148.1 (C(5)); 154.8 (C(17)); 158.3 (ester C = O); 196.6 (C(20)); 201.8 (C(6)). IR (KBr): 3042, 2946, 1720, 1689, 1657, 1580, 1523, 1457. LC-MS: 445.2 (M+ + Na, retention time 2.06 min). Anal. Calcd. for C26H30O5: C, 73.91; H, 7.16; Found: C, 73.73; H, 7.21.
Results and Discussion
The synthetic route of the target steroids 3β-benzoyloxypregna-4,16-diene-6,20-dione (10a-i) and 3β-furoyloxypregna-4,16-diene-6,20-dione (10j) was showed in Figure 2. At first the starting material diosgenin was transformed by acetylation and oxidation to 3β-acetoxy-5,16-pregnadiene-20-one (16-DPA, 5). The transformation could be achieved stepwise or in a one-pot reaction [32]. We adopted the former in consideration that the purification of the product was easier to yield 16-DPA of higher purity. Epoxidation of 16-DPA to form steroid 6 was conducted by using 30% H2O2/HCO2H [33] or m-chloroperoxybenzoic acid (m-CPBA) [13] oxidation system in 90% or 95% yield.
Conversion of compound 6 to 7 was carried out in a similar way as reported in literature [13] and the yield of steroid 7 was improved from 81% to 84%. Dehydration of 7 with thionyl chloride in pyridine resulted compound 8. We found that the quality of the solvent pyridine had an effect on the yield of the steroid 8. When dried pyridine (refluxed in the presence of potassium hydroxide for 2 h before distillation) was used instead of pyridine of analytically pure grade without treatment, the yield of 8 was improved to 73% from the literature [13] value 66%. Compound 8 was hydrolyzed with aqueous sodium hydroxide in methanol to afford 3β-hydroxypregna-4,16-diene-6,20-dione (9). The procedure for workup was improved by using extraction (with ethyl acetate) and concentration replacing filtration and subsequent recrystallization and pure product 9 was obtained in 74% yield, significantly higher than the literature [13] value 60%.
The target steroids 3β-benzoyloxypregna-4,16-diene-6,20-dione (10a-i) and 3β- furoyloxypregna-4,16-diene-6,20-dione (10j) were synthesized through Steglich reaction from 9, substituted benzoic acid or furoic acid, and DCC catalyzed by DMAP in fair to good yields (45%-77%), depending upon the structure of the aryl acids. It seems that the steric hindrance from the aryl acid structure leads to decrease in the yield. Therefore the highest yield of the Steglich reaction came from furoic acid (77%) while the reactions with dinitrobenzoic acid or nitrobenzoic acid gave the lowest yield.
The chemical structures of steroids 10a-j were fully characterized by elemental analysis, 1H NMR, 13C NMR, IR and mass spectrum and melting point measurements. Characteristic chemical shifts in 1H NMR spectra of steroids 10a-j were summarized in Table 1. All the chemical shifts of protons in methyl groups, OCH moieties, C = CH moieties, and aryl rings were in the reasonable range and the spectra data were in good accordance with the structures.
Single crystals of 10a suitable for X-ray crystal diffraction measurement were obtained by slow evaporation of a solution of 10a in 1:1 petroleum ether and ethyl acetate. X-Ray data for crystals of 10a were collected by graphite-monochromatized Mo Kα radiation at 293 K and the crystal data and experimental details for compound 10a were summarized in Table 2. The structure was solved by direct methods and refined by full-matrix least-squares with anisotropic temperature factors for the non-hydrogen atoms. The hydrogen atoms bonded to the carbon atoms were assigned based on the expected bonding geometry. The hydrogen atoms were refined isotropically in the final least-squares cycles.
The crystal structure and molecular packing of steroid 10a were shown in Figure 3 and Figure 4 respectively. It can be seen that the molecule consists of three six-membered rings (A, B, and C ring) and one five-membered ring (D ring), all trans fused. The six-membered rings A, B, and C occur in a half chair, distorted chair, and distorted chair conformation, respectively. Ring D adopts a distorted envelope conformation. The molecules in the crystal were packed face-to-face at the normal van der Waals distances, similar to the crystal structure of 3β-(p-fluorobenzoyloxy)-4,16-pregnadiene-6,20-dione [34]. CCDC-787721 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/data_request/cif
Conclusions
In summary, ten new aromatic esters based on 4,16-pregnadiene-6,20-dione skeleton, namely 3β-benzoyloxy-4,16-pregnadiene-6,20-dione and 3β-furoyloxy-4,16-pregnadiene-6,20- dione were synthesized starting from diosgenin. The structures of the steroids were characterized by elemental analysis, 1H NMR, 13C NMR, IR and mass spectrum. Single crystal X-ray diffraction measurement on one of the new compounds, 3β-(p-methoxybenzoyloxy)-4,16-pregnadiene-6,20-dione revealed that the A, B, C, and D ring adopted half chair, distorted chair, distorted chair, and distorted envelope conformation, respectively. The molecules in the crystal were packed face-to-face at the normal van der Waals distances. These new steroids may show high antiandrogenic activity and serve as good inhibitors for the 5α-reductase enzyme. Investigation of the inhibitory activity of the new steroids for the 5α-reductase enzyme will be conducted in the near future.
References
Yarrow JF, McCoy SC, Borst SE: Tissue selectivity and potential clinical applications of trenbolone (17β-hydroxyestra-4,9,11-trien-3-one): a potent anabolic steroid with reduced androgenic and estrogenic activity. Steroids. 2010, 75: 377-389. 10.1016/j.steroids.2010.01.019.
Morioka M, Kamizono A, Takikawa H, Mori A, Ueno H, Kadowaki S, Nakao Y, Kato K, Umezawa K: Design, synthesis, and biological evaluation of novel estradiol-bisphosphonate conjugates as bone-specific estrogens. Bioorg Med Chem. 2010, 18: 1143-1148. 10.1016/j.bmc.2009.12.041.
Reyes-Moreno M, Ruiz-García JA, Ibarra-Reyes Y, Fuente-Hernández A, Vélez- Castro H, Hernández-Balmaseda I, Martínez-Hormaza I, Rodeiro-Guerra I, Ramírez JS, Reyes SM, Montiel-Smith S: Synthesis and anabolic/androgenic evaluation of novel 9α-fluorosteroids. Eur J Med Chem. 2009, 44: 4567-4571. 10.1016/j.ejmech.2009.06.025.
Bunyathaworn P, Boonananwong S, Kongkathip B, Kongkathip N: Further study on synthesis and evaluation of 3,16,20-polyoxygenated steroids of marine origin and their analogs as potent cytotoxic agents. Steroids. 2010, 75: 432-444. 10.1016/j.steroids.2010.02.011.
Kim T-K, Chen J, Li W, Zjawiony J, Miller D, Janjetovic Z, Tuckey RC, Slominski A: A new steroidal 5,7-diene derivative, 3β-hydroxyandrosta-5,7-diene-17β- carboxylic acid, shows potent anti-proliferative activity. Steroids. 2010, 75: 230-239. 10.1016/j.steroids.2009.12.004.
Rasras AJM, Al-Tel TH, Al-Aboudi AF, Al-Qawasmeh RA: Synthesis and antimicrobial activity of cholic acid hydrazone analogues. Eur J Med Chem. 2010, 45: 2307-2313. 10.1016/j.ejmech.2010.02.006.
Leng TD, Zhang JX, Xie J, Zhou SJ, Huang YJ, Zhou YH, Zhu WB, Yan GM: Synthesis and anti-glioma activity of 25(R)-spirostan-3β,5α,6β,19-tetrol. Steroids. 2010, 75: 224-229. 10.1016/j.steroids.2009.12.005.
Gunasekara S, Vrielink A, Stubbs KA: Preliminary studies into the inhibition of the cholesterol α-glucosyltransferase from Helicobacter pylori using azasugars. Carbohyd Res. 2010, 345: 960-964. 10.1016/j.carres.2010.03.003.
Dubey S, Sharma AK, Jindal DP, Harvey A, Singh R, Bodhankar SL: Synthesis and neuromuscular blocking activity of 16-(2- and 3-pyridylmethylene) dehydroepiandrosterone derivatives. Steroids. 2010, 75: 323-329. 10.1016/j.steroids.2010.01.009.
Bastien D, Leblanc V, Asselin É, Bérubé G: First synthesis of separable isomeric testosterone dimers showing differential activities on prostate cancer cells. Bioorg Med Chem Lett. 2010, 20: 2078-2081. 10.1016/j.bmcl.2010.02.077.
Liu J, Zhang X, Zhao M, Peng S: Synthesis, evaluation and 3D QSAR analysis of novel estradiol-RGD octapeptide conjugates with oral anti-osteoporosis activity. Eur J Med Chem. 2009, 44: 1689-1704. 10.1016/j.ejmech.2008.09.036.
Weng Y, Xiang L, Matsuura A, Zhang Y, Huang Q, Qi J: Ganodermasides A and B, two novel anti-aging ergosterols from spores of a medicinal mushroom Ganoderma lucidum on yeast via UTH1 gene. Bioorg Med Chem. 2010, 18: 999-1002. 10.1016/j.bmc.2009.12.070.
Pérez-Ornelas V, Cabeza M, Bratoeff E, Heuze I, Sánchez M, Ramírez E, Naranjo-Rodríguez E: New 5α-reductase inhibitors: in vitro and in vivo effects. Steroids. 2005, 70: 217-224.
Chapman KE, Odermatt A: Steroids: modulators of inflammation and immunity. J Steroid Biochem Mol Biol. 2010, 120: 67-68. 10.1016/j.jsbmb.2010.04.022.
Iványi Z, Wölfling J, Görbe T, Szécsi M, Wittmann T, Schneider G: Synthesis of regioisomeric 17β-N-phenylpyrazolyl steroid derivatives and their inhibitory effect on 17α-hydroxylase/C17,20-lyase. Steroids. 2010, 75: 450-456.
Källström A-C, Salme R, Rydén L, Nordenskjöld B, Jönsson P-E, Stål O: 17β-Hydroxysteroid dehydrogenase type 1 as predictor of tamoxifen response in premenopausal breast cancer. Eur J Cancer. 2010, 46: 892-900.
Cabeza M, García-Lorenzana M, Garcés M, Heuze I, Teran N, Bratoeff E: New-D- homoandrost-4,6-diene derivatives as potent progesterone receptor antagonist. Steroids. 2010, 75: 101-108. 10.1016/j.steroids.2009.11.001.
Wuest F, Carlson KE, Katzenellenbogen JA: Expeditious synthesis of steroids containing a 2-methylsulfanyl-acetyl side chain as potential glucocorticoid receptor imaging agents. Steroids. 2008, 73: 69-76. 10.1016/j.steroids.2007.08.013.
Xu J-Q, Shen Q, Li J, Hu L-H: Dammarans from Gynostemma pentaphyllum and synthesis of their derivatives as inhibitors of protein tyrosine phosphatase 1B. Bioorg Med Chem. 2010, 18: 3934-3939. 10.1016/j.bmc.2010.04.073.
Xiong J, Taniguchi M, Kashiwada Y, Sekiya M, Yamagishi T, Takaishi Y: Papyriferic acid derivatives as reversal agents of multidrug resistance in cancer cells. Bioorg Med Chem. 2010, 18: 2964-2975. 10.1016/j.bmc.2010.02.046.
Bydal P, Luu-The V, Labrie F, Poirier D: Steroidal lactones as inhibitors of 17β-hydroxysteroid dehydrogenase type 5: chemical synthesis, enzyme inhibitory activity, and assessment of estrogenic and androgenic activities. Eur J Med Chem. 2009, 44: 632-644. 10.1016/j.ejmech.2008.03.020.
Yaya AR, Touaibia M, Massarweh G, Rochon FD, Breau L: Synthesis of 17α- substituted ethynylestradiols: potential ligands for drug vectors. Steroids. 2010, 75: 489-498. 10.1016/j.steroids.2010.03.004.
Novakova V, Zimcik P, Miletin M, Kopecky K, Ivincová J: A phthalocyanine- mestranol conjugate for photodynamic therapy prepared via click chemistry. Tetrahedron Lett. 2010, 51: 1016-1018. 10.1016/j.tetlet.2009.12.075.
Oaksmith JM, Ganem B: Synthesis of a COMC-estradiol conjugate for targeted, tissue-selective cancer chemotherapy. Tetrahedron Lett. 2009, 50: 3497-3498. 10.1016/j.tetlet.2009.03.083.
Radchatawedchakoon W, Watanapokasin R, Krajarng A, Yingyongnarongkul B: Solid phase synthesis of novel asymmetric hydrophilic head cholesterol-based cationic lipids with potential DNA delivery. Bioorg Med Chem. 2010, 18: 330-342. 10.1016/j.bmc.2009.10.057.
Köysal O: Conductivity and dielectric properties of cholesteric liquid crystal doped with single wall carbon nanotube. Synth Met. 2010, 160: 1097-1100.
Edelsztein VC, Burton G, Di Chenna PH: Self-assembly of a silylated steroid-based organogelator and its use as template for the in situ sol-gel polymerization of tetraethyl orthosilicate. Tetrahedron. 2010, 66: 2162-2167. 10.1016/j.tet.2010.01.065.
Cabeza M, Heuze I, Sánchez M, Bratoeff E, Ramírez E, Rojas A, Orozco A, Mungía A, Agustín G, Cuatepotzo L, Gonzalez C, Palma S, Padilla D, Perez V, Jimenez G: Relative binding affinity of novel steroids to androgen receptors in hamster prostate. J Enzyme Inhib Med Chem. 2005, 20: 357-364. 10.1080/14756360500148924.
Cabeza M, Bratoeff E, Ramírez E, Heuze I, Recillas S, Berrios H, Cruz A, Cabrera O, Perez V: Biological activity of novel progesterone derivatives having a bulky ester side chains at C-3. Steroids. 2008, 73: 838-843. 10.1016/j.steroids.2008.03.006.
Li H, Song Y, Peng X: Improved synthesis of mestranol and ethinyl estradiol (EE) related degradation products as authentic references. Steroids. 2008, 73: 488-494. 10.1016/j.steroids.2007.12.024.
Li H, Song Y, Ge F: 17α-Ethynyl-3-methoxyestra-1,3,5(10),9(11)-tetraen-17-ol. Acta Crystallogr E. 2008, 64: o783-10.1107/S1600536808005254.
Bandhoria P, Gupta VK, Gupta DK, Jain SM, Varghese B: Crystal structure of 3β-acetoxy-pregna-5,16-dien-20-one (16 DPA). J Chem Crystallogr. 2006, 36: 161-166. 10.1007/s10870-005-9000-5.
Sun F, Lu H, Ge W: Synthesis of epoxy-progesterone derivatives and spectroscopic analysis. J Mol Sci (in Chinese). 2007, 23: 209-212.
Soriano-García M, Valencia N, Flores E, Bratoeff E, Ramírez E, Cabeza M: Crystal structure and synthesis of β-(p-fluorobenzoyloxy)-4,16-pregnadiene-6,20-dione. Anal Sci. 2003, 19: x79-x80.
Acknowledgements
The authors acknowledge the financial support of the project by Shanghai Natural Science Foundation (No. 06ZR14001).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
JL carried out the synthetic experiments and drafted the manuscript. HL completed the molecular design, the arrangement of the work and modification of the manuscript. YL participated in the separation and purification of compounds, acquisition of data, and collection of literature and helped to draft the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Li, J., Li, H. & Li, Y. Synthesis and characterization of new aromatic esters based on 4,16-pregnadiene-6,20-dione skeleton. Chemistry Central Journal 4, 18 (2010). https://doi.org/10.1186/1752-153X-4-18
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1752-153X-4-18