Abstract
Background
The hepatocyte growth factor (HGF) stimulates mitogenesis, motogenesis, and morphogenesis in a wide range of tissues, including epithelial cells, on binding to the receptor tyrosine kinase c-Met. Abnormal c-Met signalling contributes to tumour genesis, in particular to the development of invasive and metastatic phenotypes. The human microbial pathogen Helicobacter pylori can induce chronic gastritis, peptic ulceration and more rarely, gastric adenocarcinoma. The H. pylori effector protein cytotoxin associated gene A (CagA), which is translocated via a type IV secretion system (T4SS) into epithelial cells, intracellularly modulates the c-Met receptor and promotes cellular processes leading to cell scattering, which could contribute to the invasiveness of tumour cells. Using a logical modelling framework, the presented work aims at analysing the c-Met signal transduction network and how it is interfered by H. pylori infection, which might be of importance for tumour development.
Results
A logical model of HGF and H. pylori induced c-Met signal transduction is presented in this work. The formalism of logical interaction hypergraphs (LIH) was used to construct the network model. The molecular interactions included in the model were all assembled manually based on a careful meta-analysis of published experimental results. Our model reveals the differences and commonalities of the response of the network upon HGF and H. pylori induced c-Met signalling. As another important result, using the formalism of minimal intervention sets, phospholipase Cγ1 (PLCγ1) was identified as knockout target for repressing the activation of the extracellular signal regulated kinase 1/2 (ERK1/2), a signalling molecule directly linked to cell scattering in H. pylori infected cells. The model predicted only an effect on ERK1/2 for the H. pylori stimulus, but not for HGF treatment. This result could be confirmed experimentally in MDCK cells using a specific pharmacological inhibitor against PLCγ1. The in silico predictions for the knockout of two other network components were also verified experimentally.
Conclusion
This work represents one of the first approaches in the direction of host-pathogen systems biology aiming at deciphering signalling changes brought about by pathogenic bacteria. The suitability of our network model is demonstrated by an in silico prediction of a relevant target against pathogen infection.
Similar content being viewed by others
Background
H. pylori is a highly successful micro-aerophilic spiral-shaped bacterium that has colonized the gastric epithelium of half of the human population [1, 2]. H. pylori is a major risk factor for peptic ulcer disease, gastric cancer and gastric mucosa-associated lymphoid tissue (MALT) lymphoma [3]. It was the first bacterial pathogen to be classified as a class I carcinogen by the WHO. Gastric cancer remains the second deadliest cancer worldwide, which makes H. pylori infection, also in light of growing bacterial resistances to antibiotics, a significant global health problem [4].
H. pylori has evolved elaborate mechanisms to manipulate host cells during infection. Following colonization of the gastric epithelial apical surface and adhesion, various H. pylori virulence factors interfere with signalling pathways in gastric epithelial cells. The presence of a pathogenicity island (cag PAI) in H. pylori is strongly associated with the development of gastric diseases. The cag PAI encodes a T4SS that mediates translocation of bacterial virulence factors into the host cell [5]. The three major H. pylori virulence factors involved in bacterial-epithelial interactions that are associated with an increased risk of severe gastritis, gastric atrophy and/or gastric cancer, are the cag pathogenicity island (cag PAI), the vacuolating cytotoxin A (VacA), and the blood group antigen-binding adhesionA2 (BabA2), which binds Lewis B on gastric epithelial cells [3]. CagA, one of the main virulence factors of H. pylori, also encoded in the PAI, is translocated via the T4SS into the host cell cytoplasm, where it modulates cellular functions. Attachment of CagA-positive H. pylori induces cell scattering in human gastric epithelial cells [6]. Cell scattering comprises cell spreading and elongation, and the cells become motile. Therefore, cell scattering is one readout for the motogenic response of H. pylori infected cells. Recent studies have shown that CagA intracellularly modulates the receptor tyrosine kinase c-Met [6]. Binding of the natural ligand HGF to c-Met stimulates mitogenesis, motogenesis, and morphogenesis in epithelial cells [7]. Abnormal c-Met signalling has been strongly related to tumour genesis, in particular to the development of invasive and metastatic phenotypes [8]. Numerous experiments indicate a particular role of HGF and the proto-oncogene c-Met in tumour invasive growth [6]. It has been shown that c-Met signalling induced by H. pylori leads to the activation of ERK1/2 in AGS cells [6]. ERK1/2 activity promotes cell scattering in a transcription independent manner. It has also been shown that activation of ERK1/2 is critical for the induction of cell scattering in H. pylori-infected epithelial cells [6], which could contribute to the invasiveness of tumour cells. Therefore, blocking the activation of ERK1/2 represents a promising intervention goal to prevent H. pylori induced signalling changes, which could play a role for cancer metastasis.
The induction of cell scattering by H. pylori in epithelial cells, is an example how human microbial pathogens modulate signal transduction in the cell by translocated bacterial proteins. The presented work aims at translating these complex interactions into a logical network model.
Signalling networks have not yet been modelled at a scale comparable to metabolic and regulatory networks. More than 500 members of the protein kinase superfamily of enzymes alone are encoded in the human genome, which allows for an enormous complexity of signalling. The wealth of data generated, describing signalling networks in molecular detail at a rapidly increasing rate makes the reconstruction of such large networks a difficult task [9, 10]. The most often used formalism to model cellular networks is kinetic analysis, which has been applied to signalling networks of smaller size [11] or for modelling single pathways [12]. A large scale reconstruction of signalling networks relying on kinetic data has not yet appeared due to the lack of available kinetic data for the interactions in the network. The data obtained by recent Genomics and Proteomics high throughput technologies are often only qualitative or semi-quantitative. Therefore, qualitative (i.e. parameter-free) modelling seems to be the only feasible approach at the moment to represent and analyse large-scale signalling networks in a computer. A functional analysis of the network structure already enables to address important issues, such as detection of network-wide interdependencies, identification of intervention strategies and qualitative predictions on the effect of perturbations.
In our view it would be invalid to try to construct a complete network model of H. pylori infection due to the limited number of detailed information about the cellular processes triggered by this pathogen. Thus, in contrast to a H. pylori infection model, we explicitly only considered the signal transduction events that directly arise from c-Met-receptor-mediated signalling, which becomes modulated by the H. pylori virulence factor CagA.
In order to construct a qualitative network model of c-Met activation by HGF and H. pylori we used here a methodology introduced previously [13, 14] relying on logical interaction hypergraphs (LIH). Our model reveals the differences and commonalities of the response of the network upon HGF and H. pylori induced c-Met signalling. Another goal of this study was to use the logical model to generate in silico predictions and to verify these experimentally. As one case study demonstrating the predictive capabilities of our model, we determine suitable interventions that prevent an activation of ERK1/2, because of the above mentioned decisive role of ERK1/2 for cell scattering and tumour invasive growth.
Results and Discussion
Logical modelling of signal transduction networks
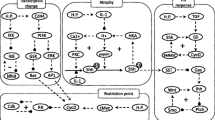
For the reconstruction and qualitative analysis of the signal flow network we employ a logical modelling framework (Boolean networks represented as logical interaction hypergraphs) as introduced previously [13, 14]. Boolean network modelling for biological systems has so far mainly been applied to the analysis of medium-scale regulatory networks [15–18]. In contrast to regulatory networks (whose behaviour is mainly determined by their regulatory feedback loops), signalling networks are much stronger structured in input, intermediate and output layers and the input signals usually govern the response of the network. For this characteristic network topology we introduced logical interaction hypergraphs (LIHs) [13] as a special representation of Boolean networks, which is well suited to formalize, visualize and analyse logical models of signal transduction networks. As in all Boolean networks, nodes in the network represent species (e.g. kinases, adaptor molecules or transcription factors) each having an associated logical state (in the binary case as used herein only "on" (1) or "off" (0)) determining whether the species is active (or present) or not. Signalling events are encoded as Boolean operations on the network nodes. For example, protein kinase C alpha (PKCα) can be activated (gets "on", i.e. value 1) if the level of calcium AND of diacylglycerol (DAG) is high, i.e. calcium and DAG must be "on" to activate PKCα (see connection 46 in Figure 1). Usually, a node can be activated by more than one signalling event, which are then OR-connected, e.g. the MAPKKK Raf1 becomes active if either Ras OR PKCα is active (Figure 1).
In general, in LIHs we make only use of the Boolean operators AND (·), OR (+), and NOT (!), which are sufficient to represent any logical relationship. A signalling event in a LIH is an AND connection of nodes (negation of node values using the NOT operator are allowed) describing one opportunity how the target species of this connection can be activated. Hence, for the first example described above we would write
DAG AND Ca2+ → PKCα or shorter DAG ·Ca2+ → PKCα
In a graphical representation of the network (see PKCα node in Figure 1), such an AND connection is displayed as a hyperarc. In contrast to arcs in graphs, a hyperarc (in hypergraphs) may have several start or end nodes. Clearly, in some cases, only one species is involved in activating another as in the case
Ras → Raf1.
In these special cases, the hyperarc is a simple arc as occurring in graphs; we will nevertheless refer to it as a hyperarc. As already pointed out, a species may be activated via several distinct signalling events (hyperarcs), i.e. all these signalling events are OR-connected. This is illustrated by Raf1, which can be activated via
Ras → Raf1 OR PKCα → Raf1.
Accordingly, all the hyperarcs pointing into a species are OR connected. In this way we can easily interpret Figure 1, which displays graphically the interactions given in Table 1.
Once we have constructed an LIH, we may start to analyse it. A typical scenario is that we apply a pattern of inputs to the network and we would like to know how the nodes in the network will respond to this stimulation. As explained in [13], by propagating input signals along the logical (hyperarc) connections (which is equivalent to compute the logical steady state resulting from the input stimuli) we obtain the qualitative response of the network. It depends on the functionality of positive or negative feedback loops in the network whether we can resolve a complete and unique logical response of all nodes for a given set of input stimuli (for example, negative feedback loops may prevent the existence of a logical steady state [13]). Feedback loops are usually present in signalling networks, however, one can often identify at least one connection in each loop that becomes active at a later time-scale and does not play a significant role for the early signalling events. Setting these late-event connections inactive, one obtains an acyclic network for which always a unique network response for a given set of inputs can be computed. Using this technique, one can easily perform in silico experiments, for example check how knockouts (or knockins) alter the network response.
With the idea of minimal intervention sets (MISs, [13, 14]) one may even directly search for those interventions that enforce a desired response (e.g. inactivation of a transcription factor). As described by Klamt et al. [14], MISs can be computed by testing systematically which combinations of knockouts (KOs) and knockins (KIs) fulfil a specified intervention goal. One usually starts with the single KOs and KIs: one clamps the logical value (0 or 1, respectively) of the respective node, computes the resulting logical steady state (as explained above) and verifies whether the intervention goal is achieved. All those KOs and KIs, that were not successful are then combined in pairs which may lead to some MISs of size two. Then all combinations with 3 interventions (that are not a superset of the MISs of size one or two) are tested and so forth. Obviously, especially computing the MISs of higher cardinality becomes a highly combinatorial problem and one usually restricts oneself to the low cardinality MISs. There are also some heuristics that can be used to accelerate the computation [14].
Another advantage of LIHs is, that we can easily derive the (signed and directed) interaction graph underlying the logical model: we only have to split hyperarcs with two or more start nodes (i.e. the AND connections) into simple arcs. Interaction graphs cannot be used to give on/off predictions, however, they provide an appropriate formalism to search for signalling paths and feedback loops. Another useful feature that can be extracted from interaction graphs is the dependency matrix as introduced in [13, 14] which displays network-wide interdependencies between all pairs of species. For example, a species A is an activator (inhibitor) of another species B, if at least one path leads from A to B and if all those paths are positive (negative). This kind of information can be very useful for predicting effects of perturbations.
The model studied in this work was implemented in and analysed with CellNetAnalyzer, a comprehensive toolbox for functional analysis of cellular networks [14].
Logical network of HGF and H. pylori triggered c-Met signal transduction
The logical network of HGF and H. pylori induced c-Met signal transduction was constructed as a bottom up approach. We restricted our network model explicitly to the signal transduction events that arise from stimulation or modulation of the c-Met receptor. Other virulence factors of H. pylori than CagA that target different receptors of the host epithelial cell are therefore not included in the model. The modeling objective was to construct a logical network model that is capable to predict qualitatively how CagA modulates the c-Met receptor, and to compare HGF versus CagA induced c-Met signal transduction. Only accurate and well-defined interactions were included in the model. The molecular interactions were all assembled manually by extensive use of literature search engines and databases (see Methods). Only data obtained from epithelial cell lines were considered for the model. The data were subjected to a careful meta-analysis; only data that were consistent with the current knowledge and did not interfere with recent publications were taken.
Activation of the c-Met receptor by its endogenous ligand HGF leads to autophosphorylation of specific tyrosine residues in its cytoplasmic domain within the intracellular activation loop (Y1234 and Y1235) resulting in an activation of the intrinsic kinase activity. Subsequently, a multifunctional signal transducer docking site is formed by phosphorylation of Y1349 and Y1356 [19]. This docking site recruits intracellular adapters via Src homology-2 domains (SH2), phosphotyrosine binding domains (PTB) and Met binding domains (MBD).
Recent studies have shown that infection of gastric epithelial cells by the bacterial pathogen H. pylori targets the c-Met receptor and provokes some c-Met-related cellular responses [6]. This includes especially the enhancement of cell scattering, which is mainly mediated by the bacterial virulence factor CagA, translocated during infection via a T4SS into gastric epithelial cells.
In the following, the biochemical steps included in the logical model will be described briefly. The numbering corresponds to the hyperarcs in Figure 1 and Table 1.
1. HGF is the natural ligand of c-Met. Upon ligand binding, c-Met undergoes autophosphorylation of specific tyrosine residues within the intracellular region. Phosphorylation of Y1230, Y1234 and Y1235 located within the activation loop of the tyrosine kinase domain activates the intrinsic kinase activity of c-Met [19].
2. Gab1 is subsequently recruited to activated c-Met by direct binding to the tyrosine phosphate residues Y1349 and Y1356 of the receptor [20].
3.-7. The c-Met/Gab1 complex forms a multivalent binding site for a number of downstream molecules, including SHP2, PI3K, Grb2.
3. Fusion of Gab1 with c-Met induces tyrosine phosphorylation and interaction with SHP2 [21].
4. Activated c-Met also interacts with SHP2 independently of Gab1 [22].
5. The adaptor protein Grb2 binds to the Y1356 docking site of c-Met [23].
6. c-Met associates withPI3K via Gab1 [23, 24].
7. c-Cbl is a substrate of the activated tyrosine kinase receptor c-Met [25].
8. PI3K can also be activated by c-Cbl [26].
9. Gab1 interacts with Crk and CrkL, two proteins with SH2 and SH3 protein interaction domains. This interaction is mediated via the SH2 domains [27].
10. The c-Met receptor associates with the Shc1 adaptor, via the SH2 domain [28].
11. Grb2 binds to Shc via its SH2-domain [29].
12. Phosphorylated c-Cbl interacts with the SH2 domain of Crk/CrkL [25].
13. Activated Crk/CrkL recruits DOCK180 through its SH3 domain [30].
14. DOCK180 binds and activates Rac1 [31].
15. Rac1 directly binds and phosphorylates the transcription factor STAT3 [32].
16. Activated c-Met directly phosphorylates STAT3 [33].
17. PI3K activates ILK [34].
18. PI3K converts the plasma membranelipid PIP2 to PIP3 [35]. The phosphatase PTEN selectively removes the 3-phosphate of PIP3 to regenerate PIP2, counteracting PI3K activity [36].
19. PDK1 is a key PIP3-binding protein [37].
20. Akt1 is subsequently activated by PDK1 and ILK [34, 38].
21. Akt1 phosphorylates a single serine residue of Rac1 [39].
22. Akt1 activates PAK1 [40].
23. Activation of Rac1 leads to the activation of PAK1 [39, 41].
24. Rac1 interacts with IQGAP-1, thereby crosslinking actin filaments. Under these conditions IQGAP-1 does not bind to β-catenin and cannot dissociate α-catenin from the cadherin-catenin complex, leading to strong adhesion. When Rac1 is not active, activated Calmodulin allows IQGAP-1 to interact with β-catenin to dissociate α-catenin from the cadherin-catenin complex [42].
25.,26. PAK1 stimulates JNK activity through a MAP kinase regulatory cascade. PAK1 regulates the activity of an unknown MAP kinase kinase kinase, which controls activity of MKK4. We used a dotted hyperarc between PAK1 and MKK4 to indicate an unknown compound that links PAK1 to MKK4 [43].
27. JNK phophorylates the transcription factor c-Jun [44].
28. JNK phosphorylates the transcription factor Elk1 [45].
29. JNK phosphorylates ATF2 on Thr-69 and Thr-71 [46].
30.-32. HGF activates NF-κB through an Akt1 -> PAK1 pathway [40]. PAK1 is required for the activation of NF-κB by activating the IKK complex through an unknown kinase. The hyperarc connecting PAK1 and IKK is therefore also shown as unknown link. IκBα is phophorylated by the IKK complex and undergoes ubiquitin-mediated degradation, allowing nuclear translocation of NF-κB [40].
33. Gab1 interacts with RasGAP SH2 domains, only under conditions when SHP2 is not activated. SHP2 downregulates RasGAP by dephosphorylating RasGAP binding sites on Gab1 [47, 48].
34. Grb2 forms a complex with SOS1 and recruits it to the plasma membrane [49].
35. SOS1 is an exchange factor and activator of Ras, which is downregulated by RasGAP [50].
36.-38. Activated Ras activates the Raf1 kinase, which in turn activates the MAP kinase MEK. MEK activation leads to ERK1/2 activation [51, 52]. Ras proteins activate at least three families of downstream effector signalling pathways, involving Raf kinases, phosphatidylinositol 3 (PI 3)-kinase, and Ral-specific guanine nucleotide exchange factors (Ral-GEFs) [53]. PI3K is also a downstream component of the adaptor Gab1, as such it is included in our model. We did not include the Ras/PI3K/PIP3 and the Ras/Ral-GEF/Ral pathways, because they are not described in the context of c-Met signaling. For our model, we therefore only considered the Ras-Raf-MEK-ERK pathway.
39. ERK1/2 phosphorylates Elk1 on its C-terminal activation domain [54].
40. ERK1/2 phosphorylates and stabilizes c-Myc [55].
41. ERK1/2 phosphorylates ETS1 [56].
42. Phosphorylated Gab1 recruits PLCγ1 [57].
43.,44. PLCγ1 hydrolyses PIP2 to produce IP3 and DAG [58].
45. IP3 raises the Calcium level by opening Ca2+-channels [59].
46. The activation of PKCα is Ca2+-dependent. Provided a high Ca2+-level, DAG binds to and activates PKCα [59, 60].
47. PKCα promotes activation of Raf1 by direct phosphorylation [61, 62].
48. Calmodulin is a Ca2+-receptor protein and is regulated by the Ca2+-level [63].
49. IQGAP-1 is a regulator of E-cadherin mediated cell-cell adhesion. When Rac1 is not active, activated Calmodulin allows IQGAP-1 to interact with β-catenin to dissociate α-catenin from the cadherin-catenin complex. This leads to a reduction of cell-cell adhesions [42, 64, 65].
50. Attached H. pylori translocates CagA via T4SS [66]. Upon membrane localization translocated CagA undergoes subsequent tyrosine phosphorylation by c-Src and Fyn [67]. Thus the node CagA in the logical network corresponds to phosphorylated CagA.
51. Whereas resting host cells are characterized by almost inactive Src kinases due to phosphorylation of their regulatory loops by C-terminal Src kinase (CSK), it is known that H. pylori can transiently activate src family kinases by an unknown mechanism, which is figuratively shown as an inactivation of CSK [68].
52.-54. Nascently phosphorylated CagA activates CSK and thereby leads to a subsequent inactivation of c-Src and Fyn [69]. As the activation of CSK via CagA occurs significantly later than the activation of other downstream events in the network, we set the time scale parameter of connection 52 on 2. Experimental data presented by Tsutsumi et al. in 2003 [69] provide the basis for setting up two time scale scenarios. In the early events the translocated CagA protein undergoes tyrosine phophorylation by Src family kinases (see step 51). In the later events tyrosine phophorylated CagA binds and activates CSK, which in turn phosphorylates and inactivates Src family kinases. The phosphorylation of CagA has to occur at an earlier timepoint, because CagA-CSK interaction involves the SH2-domain of CSK and is strictly dependent on CagA tyrosine phophorylation [69]. CSK then works as a negative regulator of CagA-Shp2 signalling, because the inactivated Src family kinases lead to a down-regulation of the levels of CagA phophorylation and subsequent diminished CagA-Shp2 complex formation.
In 2002 Mimuro et al. published data, that suggested that CagA could interact with Grb2 and thereby activate Ras-Raf-Mek-ERK [70]. This observation was clearly contradicted later by other groups. The groups of Hatakeyama [71] and Naumann [6] were not able to reproduce the published results of Mimuro et al.. In light of these results and after careful evaluation of the available data we decided not to include the direct interaction between CagA and Grb2 in our network model.
55. Phosphorylated CagA directly binds to the adaptor proteins Crk and CrkL [72].
56. Phosphorylated CagA binds and activates SHP2 [73].
57. SHP2 inactivates FAK by dephosphorylation and thereby induces/enhances cell scattering [74].
58. FAK phosphorylates PIPKIγ661 [75].
59. Tyrosine phosphorylated PIPKIγ661 associates with Talin [75].
60. FAK physically associates with Calpain2 and spatially couples it to its upstream regulator ERK1/2 [76].
61. CagA intracellularly interacts with phosphorylated c-Met [6].
62. In contrast to HGF activated c-Met, CagA replaces Gab1 and/or Grb2 and leads to PLCγ1 activation [6].
The model was built on the current understanding of the biochemical processes involved in HGF and H. pylori induced c-Met signal transduction and iteratively improved by validation with published experimental results. If the proposed model failed to reproduce the experimental results, it was modified accordingly.
In total, the network model contains 54 species and 62 hyperarcs (plus 15 input and output arcs). The set of nodes includes 5 input elements (HGF and H. pylori as the events starting the signalling cascade, PTEN as externally regulated signal and PIP2 and c-Met as externally provided constituents that are always present in the cell) and 10 elements in the output layer (among these the seven transcription factors STAT3, ATF2, Elk1, c-Jun, c-Myc, ETS1, NF-κB).
Network analysis: induction of the signalling cascades by HGF and by H. pylori
We first analysed the interaction graph underlying the logical network. Two (negative) feedback loops can be found in the network regulating CagA phosphorylation by src family kinases. These loops, however, are not active during the early events because both feedback loops involve connection 52 (CagA → CSK) having a time-scale parameter of 2. The computation of signalling paths revealed that 86 paths connect the input node HGF with one of the output nodes, whereas H. pylori may influence the ouput nodes only via 63 signalling paths (but every output node can still be reached). Below we will discuss the reason for this difference in the number of signalling paths and show what the consequences of this reduced flexibility is.
The dependency matrix of the interaction graph (computed for time scale 1, Figure 2) displays all functional dependencies between each pair of species. In the following we discuss some of these dependencies. HGF is an activator for the transcription factors STAT3, ATF2, c-Jun and NF-κB, i.e. there are only positive paths from HGF to these nodes which can thus only mediate activating effects. In contrast, HGF is an ambivalent factor for ERK1/2 and its downstream effectors Elk1, c-Myc and ETS1, i.e. there is at least one inhibiting and one activating path emanating from HGF to these species. The reason is that HGF has a positive effect on ERK1/2 via Grb2-Sos1-Ras-Raf1-MEK but does also signal through RasGAP, which has an inhibitory effect on Ras and therefore on the MAP kinase cascade. However, we notice that the activity of RasGAP on the other hand is downregulated by SHP2 so that the above pathway running over Ras is functional. In contrast to HGF, H. pylori is an activator for all seven transcription factors because ERK1/2 cannot be activated via Grb2-Sos1-Ras-Raf1-MEK- and therefore only through the positive pathway PKCα-Raf1-MEK-ERK1/2 (the latter is also functional with HGF). Another example for coexisting positive and negative effects is the α-catenin/β-catenin/E-cadherin-complex, for which both H. pylori and HGF are ambivalent factors, because they signal through Rac1, which has an inhibitory effect on IQGAP-1, and via calmodulin with a positive effect.
We then used the logical model to compute the response of the network (i) when the network is triggered with HGF (Figure 3; H. pylori stimulation is off) and (ii) when c-Met is stimulated by H. pylori (Figure 4; HGF = 0), both for the early events (connection 52 is off).
HGF triggered signalling network (screenshot of CellNetAnalyzer). Each species and each hyperarc has an associated text box displaying a logical value. Blue boxes indicate clamped (fixed) values prior computing the logical steady state (i.e. the network response). Green boxes ("on") indicate an active species or activating signal flow, respectively. Red boxes ("off") indicate inactive (or absent) species or, in the case of hyperarcs, connections along which no activation of the end species of the hyperarc takes place.
Interestingly, the resulting on/off states for the output nodes are identical for both triggers, but the signalling pathways that lead to these differ significantly (Figures 3 and 4). The main cause for these differences lies in the fact that H. pylori modulates the c-Met receptor by translocating CagA, which replaces adaptor molecules like Gab1 and Grb2 at the c-Met receptor and leads to PLCγ1 activation [6]. Therefore, signalling events, including the whole PI3K pathway, that originate from the c-Met/Gab1 binding site in the HGF stimulated network are thus not included in the H. pylori triggered network. That is also the reason why the number of paths connecting H. pylori with the output layer is lower compared to the HGF case.
Target identification: deactivation of ERK1/2
To evaluate the usefulness and robustness of our model for the generation of in silico predictions, deactivation of ERK1/2 in the H. pylori triggered network was defined as an intervention goal having biomedical relevance. In silico identified targets would provide clues for anti-cancer therapies, as prevention of ERK1/2 activation would have an impact on cell scattering and thus invasiveness of tumour cells [6].
A strong feature of CellNetAnalyzer (CNA) is the possibility to compute minimal intervention sets (MISs). This function can be used to predict targets for knockouts (or possibly knockins) that lead to a desired response defined by an intervention goal. Accordingly, ERK1/2 repression (i.e. a logical level of 0) was defined as intervention goal and H. pylori input was set to 1 and HGF input set to 0. When searching only for single interventions (i.e. the cardinality of the intervention sets was restricted to 1), CNA calculated 15 minimal intervention sets each proposing one knockout target (H. pylori, c-Met, CagA/c-Met, CagA, c-Src, Fyn, PLCγ1, PIP2, IP3, Ca2+, DAG, PKCα, ERK1/2, MEK, Raf1). When HGF was the stimulus (H. pylori input was set to 0 and HGF input set to 1), CNA finds only 6 knockout targets for ERK1/2 repression (HGF, c-Met, HGF/c-Met, ERK1/2, MEK, Raf1).
By comparing the MISs for the two scenarios ((i) stimulation with H. pylori and (ii) stimulation with HGF) we can identify targets, that imply a deactivation of ERK1/2 for scenario (i) but not for (ii). Among those we chose PLCγ1 for further evaluation.
Figures 5 and 6 show the computed network response for the in silico knockouts of PLCγ1 for H. pylori and HGF stimulation, respectively. In agreement with the computed MISs, ERK1/2 is deactivated in the case of H. pylori stimulation (Figure 6), but is still activated in the case of HGF activation (Figure 5), demonstrating the different response of the network for the two stimuli. Our model shows that the knockout of PLCγ1 can be bypassed via Grb2 – SOS1 – Ras – Raf1 – MEK in the case of HGF stimulation. Next we wanted to verify this prediction experimentally. Apart from PLCγ1, we also selected PI3K (no qualitative effect on ERK1/2 in both scenarios) and MEK (which is, for both cases, a MIS for deactivating ERK1/2) as two further knockout candidates to be tested in experiments.
Experimental validation of the predicted results from the model
MDCK cells were used as a model system for the epithelial cells of the human gastric mucosa. The cells were stimulated with HGF or infected with H. pylori and the effect on ERK1/2 phosphorylation was evaluated via Western Blot analysis after cell lysis using anti-ERK1/2 and phospho-specific ERK1/2 antibodies. As shown in Figure 7a, ERK1/2 was activated after stimulation of the cells with HGF, as well as after infection with H. pylori, as demonstrated by the Western Blot analysis.
Differential regulation of ERK1/2 activity in response to stimulation with HGF and H. pylori infection. a) MDCK cells were infected with H. pylori or stimulated with HGF as indicated, or left untreated. Total cell lysates were subjected to SDS-PAGE and immunoblotted. The basal level of ERK was used as load control. b) MDCK epithelial cells were pretreated with pharmacological inhibitors of PI3K (LY294002), MEK (PD98059) or PLCγ1 (U73122) and infected with H. pylori or activated by HGF. The basal level of ERK was used as load control. Western blot analysis shows that inhibition of PI3K has no effect on ERK1/2 activation. Treatment of the cells with MEK inhibitor blocks ERK1/2 activation by H. pylori and HGF. Inhibition of PLCγ1 blocks ERK1/2 activation in H. pylori transfected cells, but not in HGF activated cells.
We then repeated the above experiments with different specific inhibitors to inhibit MEK, PI3K and PLCγ1. When treating the cells with the pharmacological inhibitor PD98059, which specifically inhibits MEK, our experimental results clearly indicated that ERK1/2 phophorylation was effectively reduced in the case of HGF stimulation as well as after infection with H. pylori (Figure 7b). This corresponds to what we expect since MEK is the only upstream effector of ERK1/2 in our model.
Inhibition of PI3K using the specific inhibitor LY294002 had no effect on ERK1/2 phosphorylation for both stimuli, again in agreement with the model prediction.
For the inhibition of PLCγ1 we used the specific inhibitor U73122. The Western Blot analysis could show that in the case of HGF stimulation, ERK1/2 activation was similar strong as in the untreated cells, but in the case of H. pylori infection ERK1/2 phophorylation was strongly reduced (Figure 7b). This result confirms the prediction of the in silico knockout of PLCγ1 in the logical model, which predicted no effect in the case of HGF simulation and a suppressive effect in the case of H. pylori infection (Figures 5 and 6).
Conclusion
A logical model of HGF- and H. pylori induced c-Met signal transduction was presented. We used the formalism of logical interaction hypergraphs for network representation and analysis and it turned out that this qualitative approach is suitable for analysing a number of important aspects in this signalling network.
In a case study we could demonstrate the capability of the model to identify targets for ERK1/2 deactivation using the formalism of minimal intervention sets. Three network species (PI3K, MEK and PLCγ1) were chosen for in silico knockout. The effects on ERK1/2 activity predicted from the model for both HGF and H. pylori stimulus could all be confirmed by experimental results in MDCK cell culture, demonstrating the suitability of our logical model to generate in silico predictions for the effect of certain knockouts of species. This approach can also be used for the in silico identification of new targets against pathogen infection, as demonstrated here by the identification of PLCγ1 as a target for ERK1/2 deactivation. Inhibition of PLCγ1 prevents H. pylori induced cell scattering, providing a possible intervention strategy against invasive gastric cancer.
As a major finding, our work shows that PLCγ1 represents a key factor in H. pylori-induced ERK activation. Further, the identification of PLCγ1 as a target for inactivation of ERK could also have therapeutic implications. Inhibition of ERK1/2, which is critically important for the induction of cell scattering in H. pylori-infected epithelial cells, could represent a target for the treatment of invasive stomach cancers caused by H. pylori infection.
In the light of our observation, that the activation of ERK by growth factor induced c-Met signalling is not influenced by a PLCγ1 inhibition, the use of specific pharmaceutical inhibitors for PLCγ1 might thus represent a promising therapeutic strategy to prevent the pathogenic effects in H. pylori infection. Furthermore, H. pylori eradication therapy however is complicated by increasing global antibiotic resistance in the pathogen. Understanding of important epithelial signal transduction pathways (e.g. c-Met) modulated by H. pylori could delineate potential chemopreventative agents to target oncogenic pathways. Therefore, our study discovered PLCγ1 as a potential novel therapeutic target that has the advantage that the microorganism can hardly develop resistances.
This work represents one of the first approaches in the direction of host-pathogen systems biology and is a first step to decipher signalling changes brought about by pathogenic bacteria. The need for the identification of new targets in treating pathogen infection in light of the growing emergence of resistance against antibiotics is a formidable task for the discovery of novel anti-infectives. In this context host-pathogen systems biology can provide highly relevant clues, not only for the elucidation how the pathogen captures the host signalling mechanisms, but also for the identification of novel targets for therapeutic interventions.
In our future work, the presented logical network will be systematically extended. This includes in particular the incorporation of relevant processes related to the cytoskeleton, tight junctions and adherence junctions. The three output nodes α-catenin/β-catenin/E-cadherin, Talin and Calpain2 represent starting points for this future work.
Methods
Model generation, data collection and meta-analysis
Literature mining for the assembling of the biochemical data for the construction of the network model was done using databases for protein-protein interaction data and by manually searching the published literature in the relevant fields through Pubmed. We used two comprehensive, manually literature-curated databases on human protein-protein interaction, the Human Protein Reference Database (HPRD.org) [77] and IntAct [78]. For a query usually the protein name or the gene name respectively was used and the obtained hits were all evaluated manually by checking the primary literature.
Published data were evaluated carefully and only approved results, which are not contradicted by recently published data, were incorporated into the model. Only results obtained from epithelial cell cultures were considered for the model. This meta-analysis of the utilized experimental data included evaluation of all the primary data and published figures. Only data that were consistent and in agreement with the current knowledge were taken.
Building and analysing the network model using CellNetAnalyzer
The logical network model (Table 1 and Figure 1) was implemented and analyzed with CellNetAnalyzer [13, 14, 79]. The network diagram was drawn using the CellDesigner Software (Version 3.5, The Systems Biology Institute, Tokyo, Japan) and then exported as an image file to CellNetAnalyzer.
Experimental part: materials and methods
Cell stimulation, and H. pylori infection
MDCK (Madin-Darby Canine Kidney) cells were grown in RPMI 1640 medium containing 4 mM glutamin (Invitrogen), 100 U ml-1 penicillin, 100 μg ml-1 streptomycin, and 10% FCS (Invitrogen) in a humidified 5% CO2 atmosphere at 37°C. The cells were seeded in tissue culture plates for 48 h before infection. 16 h before infection, the medium was replaced by fresh RPMI 1640 without serum.
H. pylori wild-type strain P1 [6] was cultured on agar plates containing 10% horse serum under microaerophilic conditions at 37°C for 48 h. For the infection, bacteria were harvested in PBS, pH 7.4, and added to the host cells at a multiplicity of infection of 100.
The cells were infected with H. pylori, or were treated with 50 ng/ml HGF (Calbiochem). Pharmacological inhibitors for the inhibition of MEK (PD98059, 50 μM, Calbiochem), PLCγ1 (U73122, 5 μM, Calbiochem), and PI3K (LY294002, 25 μM, Calbiochem) were added to the cells 30 min before stimulation with HGF, or infection with H. pylori.
Cell lysis and Western Blots
For Western Blot analysis, MDCK cells were harvested at different time points after infection, and stimulation with HGF respectively, in lysis buffer (50 mM Tris-HCl, pH 7.5, 5 mM EDTA, 100 mM NaCl, 1% Triton X-100, and 10% glycerol) containing 2 mM Na3VO4, 1 mM PMSF, 1 mg/ml aprotinin, and 1 mg/ml pepstatin. Total cell lysates were subjected to SDS-PAGE and immunoblotting. Immunoblots were developed using enhanced chemiluminescence (ECL, Amersham Biosciences). Antibodies used in this work were anti-ERK 2 (K-23) (Santa Cruz Biotechnology), and phospho-p44/p42 MAPK (Thr202/Tyr204) antibody (Cell Signaling). The secondary antibody used was HRP conjugated anti-rabbit (Dianova).
Abbreviations
- AKT1:
-
Protein kinase B
- ATF2:
-
activating transcription factor 2
- Ca2+:
-
Calcium-Ions
- CagA:
-
cytotoxine-associated gene A
- c-Cbl Cas-Br-M:
-
(murine) ecotropic retroviral transforming sequence
- c-JUN v-jun:
-
sarcoma virus 17 oncogene homolog
- c-Met:
-
met proto-oncogene (hepatocyte growth factor receptor)
- c-Met/HGF:
-
Complex
- c-Myc:
-
avian myelocytomatosis virus oncogene cellular homolog
- CNA:
-
CellNetAnalyzer
- Crk/CrkL v-crk:
-
sarcoma virus CT10 oncogene homolog
- CSK:
-
c-src tyrosine kinase
- c-SRC:
-
v-src sarcoma (Schmidt-Ruppin A-2) viral oncogene homolog
- DAG:
-
Diacylglycerol
- DOCK180:
-
dedicator of cytokinesis 1
- ELK1:
-
member of ETS oncogene family
- ERK1/2:
-
extracellular signal-regulated kinase 1/2
- ETS E26-AMV:
-
virus oncogene cellular homolog
- FAK:
-
focal adhesion kinase
- FYN FYN oncogene related to SRC:
-
FGR, YES
- Gab1 GRB2:
-
-associated binding protein 1
- Grb2:
-
growth factor receptor-bound protein 2
- H. pylori:
-
Helicobacter pylori
- HGF:
-
Hepatocyte growth factor
- IκBα:
-
Inhibitor of κBα
- IKK IκB:
-
kinase
- ILK:
-
Integrin-linked kinase
- IP3 Inositol-1:
-
4,5-triphosphat
- IQGAP-1 IQ:
-
motif containing GTPase activating protein 1
- JNK:
-
c-Jun kinase
- MEK:
-
mitogen-activated protein kinase kinase 1
- MKK4:
-
Mitogen-activated protein kinase kinase 4
- NF-κB:
-
Nuclear factor κB
- PAK1 p21:
-
-activated kinase 1
- PDK1:
-
3-Phosphoinositide-dependent protein kinase-1
- PI3K phosphoinositide-3-kinase:
-
regulatory subunit 1 (p85 alpha)
- PIP2 Phosphatidylinositol-4:
-
5-bisphosphat
- PIP3 Phosphatidylinositol (3:
-
4,5) trisphosphate
- PIPKIγ661:
-
type I phosphatidylinositol phosphate kinase isoform-γ661
- PKCalpha:
-
protein kinase C alpha
- PLCγ1:
-
phospholipase C gamma 1
- PTEN:
-
Phosphatase and tensin homolog
- RAC1:
-
ras-related C3 botulinum toxin substrate
- Raf1 v-raf-1:
-
murine leukemia viral oncogene homolog 1
- RasGAP:
-
Ras GTPase activating protein
- Shc1 SHC:
-
(Src homology 2 domain containing) transforming protein 1
- SHP2 SH2:
-
containing protein tyrosine phosphatase 2
- SOS1:
-
son of sevenless homolog 1
- STAT3:
-
signal transducer and activator of transcription 3
References
Hu LT, Foxall PA, Russell R, Mobley HL: Purification of recombinant Helicobacter pylori urease apoenzyme encoded by ureA and ureB. Infect Immun. 1992, 60: 2657-2666.
Covacci A, Telford JL, Giudice GD, Parsonnet J, Rappuoli R: Helicobacter pylori Virulence and Genetic Geography. Science. 1999, 284: 1328-1333. 10.1126/science.284.5418.1328
Crabtree JE, Naumann M: Epithelial Cell Signaling in Helicobacter pylori Infection. Current Signal Transduction Therapy. 2006, 1: 53-65.
Beswick EJ, Suarez G, Reyes VE: H pylori and host interactions that influence pathogenesis. World Journal of Gastroenterology. 2006, 12: 5599-5605.
Naumann M: Pathogenicity island-dependent effects of Helicobacter pylori on intracellular signal transduction in epithelial cells. Int J Med Microbiol. 2005, 5: 335-41. 10.1016/j.ijmm.2005.06.007.
Churin Y, Al-Ghoul L, Kepp O, Meyer TF, Birchmeier W, Naumann M: Helicobacter pylori CagA protein targets the c-Met receptor and enhances the motogenic response. J Cell Biol. 2003, 161: 249-255. 10.1083/jcb.200208039
Peruzzi B, Bottaro DP: Targeting the c-Met Signaling Pathway in Cancer. Clin Cancer Res. 2006, 12: 3657-3660. 10.1158/1078-0432.CCR-06-0818
Furge KA, Zhang YW, Vande Woude GF: Met receptor tyrosine kinase: enhanced signaling through adapter proteins. Oncogene. 2000, 19: 5582-5589. 10.1038/sj.onc.1203859
Papin JA, Palsson BO: Topological analysis of mass-balanced signaling networks: a framework to obtain network properties including crosstalk. Journal of Theoretical Biology. 2004, 227: 283-297. 10.1016/j.jtbi.2003.11.016
Papin JA, Hunter T, Palsson BO, Subramaniam S: Reconstruction of cellular signaling networks and analysis of their properties. Nat Rev Mol Cell Biol. 2005, 6: 99-111. 10.1038/nrm1570
Alon U, Surette MG, Barkai N, Leibler S: Robustness in bacterial chemotaxis. Nature. 1999, 397: 168-171. 10.1038/16483
Schoeberl B, Eichler-Jonsson C, Gilles ED, Muller G: Computational modeling of the dynamics of the MAP kinase cascade activated by surface and internalized EGF receptors. Nat Biotech. 2002, 20: 370-375. 10.1038/nbt0402-370.
Klamt S, Saez-Rodriguez J, Lindquist J, Simeoni L, Gilles E: A methodology for the structural and functional analysis of signaling and regulatory networks. BMC Bioinformatics. 2006, 7: 56- 10.1186/1471-2105-7-56
Klamt S, Saez-Rodriguez J, Gilles E: Structural and functional analysis of cellular networks with CellNetAnalyzer. BMC Systems Biology. 2007, 1: 2- 10.1186/1752-0509-1-2
Mendoza L, Thieffry D, Alvarez-Buylla ER: Genetic control of flower morphogenesis in Arabidopsis thaliana: a logical analysis. Bioinformatics. 1999, 15: 593-606. 10.1093/bioinformatics/15.7.593
Thomas R, D'Ari R: Biological feedback. 1990, Boca Raton: CRC Press
Albert R, Othmer HG: The topology of the regulatory interactions predicts the expression pattern of the Drosophila segment polarity genes. J Theor Biology. 2003, 223: 1-18. 10.1016/S0022-5193(03)00035-3.
Chaves M, Albert R, Sontag ED: Robustness and fragility of Boolean models for genetic regulatory networks. J Theor Biol. 2005, 235: 431-49. 10.1016/j.jtbi.2005.01.023
Ferracini R, Longati P, Naldini L, Vigna E, Comoglio PM: Identification of the major autophosphorylation site of the Met/hepatocyte growth factor receptor tyrosine kinase. J Biol Chem. 1991, 266: 19558-19564.
Weidner KM, Di Cesare S, Sachs M, Brinkmann V, Behrens J, Birchmeier W: Interaction between Gab1 and the c-Met receptor tyrosine kinase is responsible for epithelial morphogenesis. Nature. 1996, 384: 173-176. 10.1038/384173a0
Schaeper U, Gehring NH, Fuchs KP, Sachs M, Kempkes B, Birchmeier W: Coupling of Gab1 to c-Met, Grb2, and Shp2 Mediates Biological Responses. J Cell Biol. 2000, 149: 1419-1732. 10.1083/jcb.149.7.1419
Fixman ED, Fournier TM, Kamikura DM, Naujokas MA, Park M: Pathways downstream of Shc and Grb2 are required for cell transformation by the tpr-Met oncoprotein. J Biol Chem. 1996, 271: 13116-13122. 10.1074/jbc.271.22.13116
Ponzetto C, Bardelli A, Zhen Z, Maina F, la Zonca P, Giordano S, Graziani A, Panayotou G, Comoglio PM: A multifunctional docking site mediates signaling and transformation by the hepatocyte growth factor/scatter factor receptor family. Cell. 1994, 77: 261-271. 10.1016/0092-8674(94)90318-2
Graziani A, Gramaglia D, Cantley LC, Comoglio PM: The tyrosine-phosphorylated hepatocyte growth factor/scatter factor receptor associates with phosphatidylinositol 3-kinase. J Biol Chem. 1991, 33: 22087-22090.
Garcia-Guzman M, Larsen E, Vuori K: The proto-oncogene c-Cbl is a positive regulator of Met-induced MAP kinase activation: a role for the adaptor protein Crk. Oncogene. 2000, 19: 4058-4065. 10.1038/sj.onc.1203750
Fournier TM, Lamorte L, Maroun CR, Lupher M, Band H, Langdon W, Park M: Cbl-transforming variants trigger a cascade of molecular alterations that lead to epithelial mesenchymal conversion. Mol Biol Cell. 2000, 11: 3397-3410F.
Garcia-Guzman M, Dolfi F, Zeh K, Vuori K: Met-induced JNK activation is mediated by the adapter protein Crk and correlates with the Gab1 – Crk signaling complex formation. Oncogene. 1999, 18: 7775-7786. 10.1038/sj.onc.1203198
Pelicci G, Giordano S, Zhen Z, Salcini AE, Lanfrancone L, Bardelli A, Panayotou G, Waterfield MD, Ponzetto C, Pelicci PG, Comoglio PM: The motogenic and mitogenic responses to HGF are amplified by the Shc adaptor protein. Oncogene. 1995, 10: 1631-1638.
Ravichandran KS: Signaling via Shc family adapter proteins. Oncogene. 2001, 20: 6322-6330. 10.1038/sj.onc.1204776
Hasegawa H, Kiyokawa E, Tanaka S, Nagashima K, Gotoh N, Shibuya M, Kurata T, Matsuda M: DOCK180, a major CRK-binding protein, alters cell morphology upon translocation to the cell membrane. Mol Cell Biol. 1996, 16: 1770-1776.
Kiyokawa E, Hashimoto Y, Kobayashi S, Sugimura H, Kurata T, Matsuda M: Activation of Rac1 by a Crk SH3-binding protein, DOCK180. Genes Dev. 1998, 12: 3331-3336.
Simon AR, Vikis HG, Stewart S, Fanburg BL, Cochran BH, Guan KL: Regulation of STAT3 by direct binding to the Rac1 GTPase. Science. 2000, 290: 144-147. 10.1126/science.290.5489.144
Boccaccio C, Ando M, Tamagnone L, Bardelli A, Michieli P, Battistini C, Comoglio PM: Induction of epithelial tubules by growth factor HGF depends on the STAT pathway. Nature. 1998, 391: 285-288. 10.1038/34657
Delcommenne M, Tan C, Gray V, Rue L, Woodgett J, Dedhar S: Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc Natl Acad Sci USA. 1998, 95: 11211-11216. 10.1073/pnas.95.19.11211
Czech MP: PIP2 and PIP3: complex roles at the cell surface. Cell. 1998, 100: 603-606. 10.1016/S0092-8674(00)80696-0.
Downes CP, Leslie NR, Batty IH, van der Kaay J: Metabolic switching of PI3K-dependent lipid signals. Biochem Soc Trans. 2007, 35: 188-192. 10.1042/BST0350188
Tian X, Rusanescu G, Hou W, Schaffhausen B, Feig LA: PDK 1 mediates growth factor-induced Ral-GEF activation by a kinase-independent mechanism. The EMBO Journal. 2002, 21: 1327-1338. 10.1093/emboj/21.6.1327
Wymann MP, Pirola L: Structure and function of phosphoinositide 3-kinases. Biochim Biophys Acta. 1998, 1436: 127-150.
Royal I, Lamarche-Vane N, Lamorte L, Kaibuchi K, Park M: Activation of cdc42, rac, PAK, and rho-kinase in response to hepatocyte growth factor differentially regulates epithelial cell colony spreading and dissociation. Mol Biol Cell. 2000, 11: 1709-1725.
Fan S, Gao M, Meng Q, Laterra JJ, Symons MH, Coniglio S, Pestell RG, Goldberg ID, Rosen EM: Role of NF-κB signaling in hepatocyte growth factor/scatter factor-mediated cell protection. Oncogene. 2005, 24: 1749-1766. 10.1038/sj.onc.1208327
Churin Y, Kardalinou E, Meyer TF, Naumann M: Pathogenicity island-dependent activation of Rho GTPases Rac1 and Cdc42 in Helicobacter pylori infection. Mol Microbiol. 2001, 40: 815-823. 10.1046/j.1365-2958.2001.02443.x
Noritake J, Watanabe T, Sato K, Wang S, Kaibuchi K: IQGAP1 a key regulator of adhesion and migration. J Cell Sci. 2005, 118: 2085-2092. 10.1242/jcs.02379
Zhang S, Han J, Mary A, Chernoff&cjs0952 J, Knaus UG, Ulevitch RJ, Bokoch GM: Rho Family GTPases Regulate p38 Mitogen-activated Protein Kinase through the Downstream Mediator Pak1. J Biol Chem. 1995, 270: 23934-23936. 10.1074/jbc.270.41.23934
Gupta S, Barrett T, Whitmarsh A J, Cavanagh J, Sluss HK: Selective interaction of JNK protein kinase isoforms with transcription factors. EMBO J. 1996, 15: 2760-2770.
Zinck R, Cahill MA, Kracht M, Sachsenmaier C, Hipskind RA: Protein synthesis inhibitors reveal differential regulation of mitogen-activated protein kinase and stress-activated protein kinase pathways that converge on Elk-1. Mol Cell Biol. 1995, 15: 4930-4938.
Gupta S, Campbell D, Dérijard B, Davis RJ: Transcription factor ATF2 regulation by the JNK signal transduction pathway. Science. 1995, 267: 389-393. 10.1126/science.7824938
Neel BG, Gu H, Pao L: The 'Shp'ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem Sci. 2003, 28: 284-293. 10.1016/S0968-0004(03)00091-4
Montagner A, Yart A, Dance M, Perret B, Salles JP, Raynal P: A novel role for Gab1 and SHP2 in epidermal growth factor-induced Ras activation. J Biol Chem. 2005, 280: 5350-5360. 10.1074/jbc.M410012200
Li N, Batzer A, Daly R, Yajnik V, Skolnik E, Chardin P, Bar-Sagi D, Margolis B, Schlessinger J: Guanine-nucleotide-releasing factor Sos1 binds to Grb2 and links receptor tyrosine kinases to Ras signaling. Nature. 1993, 363: 85-88. 10.1038/363085a0
Clark GJ, Der CJJ: Aberrant function of the Ras signal transduction pathway in human breast cancer. Breast Cancer Res Treat. 1995, 35: 133-44. 10.1007/BF00694753
Howe LR, Leevers SJ, Gomez N, Nakielny S, Cohen P, Marshall CJ: Activation of the MAP kinase pathway by the protein kinase raf. Cell. 1992, 71: 335-342. 10.1016/0092-8674(92)90361-F
Jeffers M, Fiscella M, Webb CP, Anver M, Koochekpour S, Vande Woude GF: The mutationally activated Met receptor mediates motility and metastasis. Proc Natl Acad Sci USA. 1998, 95: 14417-14422. 10.1073/pnas.95.24.14417
Rusanescu G, Gotoh T, Tian X, Feig LA: Regulation of Ras signaling specificity by protein kinase C. Mol Cell Biol. 2001, 21: 2650-2658. 10.1128/MCB.21.8.2650-2658.2001
Marais R, Wynne J, Treisman R: The SRF accessory protein Elk-1 contains a growth factor-regulated transcriptional activation domain. Cell. 1993, 73: 381-393. 10.1016/0092-8674(93)90237-K
Grill C, Gheyas F, Dayananth P, Jin W, Ding W, Qiu P, Wang L, Doll RJ, English JM: Analysis of the ERK1, 2 transcriptome in mammary epithelial cells. Biochem J. 2004, 381: 635-644. 10.1042/BJ20031688
Paumelle R, Tulasne D, Kherrouche Z, Plaza S, Leroy C, Reveneau S, Vandenbunder B, Fafeur V: Hepatocyte growth factor/scatter factor activates the ETS1 transcription factor by a RAS-RAF-MEK-ERK signaling pathway. Oncogene. 2002, 21: 2309-2319. 10.1038/sj.onc.1205297
Gual P, Giordano S, Williams TA, Rocchi S, Van Obberghen E, Comoglio PM: Sustained recruitment of phospholipase C-gamma to Gab1 is required for HGF-induced branching tubulogenesis. Oncogene. 2000, 19: 1509-1518. 10.1038/sj.onc.1203514
Kim MJ, Kim E, Ryu SH, Suh PG: The mechanism of phospholipase C-gamma1 regulation. Exp Mol Med. 2000, 32: 101-109.
Berridge MJ, Irvine RF: Inositol phosphates and cell signaling. Nature. 1989, 341: 197-205. 10.1038/341197a0
Nishizuka Y: Studies and perspectives of protein kinase C. Science. 1986, 233: 305-312. 10.1126/science.3014651
Morrison DK, Cutler RE: The complexity of Raf-1 regulation. Curr Opin Cell Biol. 1997, 9: 174-179. 10.1016/S0955-0674(97)80060-9
Kolch W, Heidecker G, Kochs G, Hummel R, Vahidi H, Mischak H, Finkenzeller G, Marine D, Rapp UR: Protein kinase Cα activates Raf-1 by direct phosphorylation. Nature. 1993, 364: 249-252. 10.1038/364249a0
Soderling TR: The Ca-calmodulin-dependent protein kinase cascade. Trends Biochem Sci. 1999, 24: 232-236. 10.1016/S0968-0004(99)01383-3
Mateer SC, Morris LE, Cromer DA, Bensenor LB, Bloom GS: Actin filament binding by a monomeric IQGAP 1 fragment with a single calponin homology domain. Cell Motility and the Cytoskeleton. 2004, 58: 231-241. 10.1002/cm.20013.
Mateer SC, McDaniel AE, Nicolas V, Habermacher GM, Lin MJ, Cromer DA, King ME, Bloom GS: The mechanism for regulation of the F-actin binding activity of IQGAP1 by calcium/calmodulin. J Biol Chem. 2002, 277: 12324-12333. 10.1074/jbc.M109535200
Backert S, Ziska E, Brinkmann V, Zimny-Arndt U, Fauconnier A, Jungblut PR, Naumann M, Meyer TF: Translocation of the Helicobacter pylori CagA protein in gastric epithelial cells by a type IV secretion apparatus. Cellular Microbiology. 2000, 2: 155-164. 10.1046/j.1462-5822.2000.00043.x
Stein M, Bagnoli F, Halenbeck R, Rappuoli R, Fantl WJ, Covacci A: c-Src/Lyn kinases activate Helicobacter pylori CagA through tyrosine phosphorylation of the EPIYA bf motfis. Mol Microbiol. 2002, 43: 971-980. 10.1046/j.1365-2958.2002.02781.x
Okada M, Nada S, Yamanashi Y, Yamamoto T, Nakagawa H: CSK: a protein-tyrosine kinase involved in regulation of src family kinases. J Biol Chem. 1989, 266: 24249-24252.
Tsutsumi R, Higashi H, Higuchi M, Okada M, Hatakeyama M: Attenuation of Helicobacter pylori CagA x SHP-2 signaling by interaction between CagA and C-terminal Src. J Biol Chem. 2003, 278: 3664-3670. 10.1074/jbc.M208155200
Mimuro H, Suzuki T, Tanaka J, Asahi M, Haas R, Sasakawa C: Grb2 is a key mediator of helicobacter pylori CagA protein activities. Mol Cell. 2002, 10: 745-755. 10.1016/S1097-2765(02)00681-0
Higashi H, Nakaya A, Tsutsumi R, Yokoyama K, Fujii Y, Ishikawa S, Higuchi M, Takahashi A, Kurashima Y, Teishikata Y, Tanaka S, Azuma T, Hatakeyama M: Helicobacter pylori CagA induces Ras-independent morphogenetic response through SHP-2 recruitment and activation. J Biol Chem. 2004, 279: 17205-17216. 10.1074/jbc.M309964200
Suzuki M, Mimuro H, Suzuki T, Park M, Yamamoto T, Sasakawa C: Interaction of CagA with Crk plays an important role in Helicobacter pylori-induced loss of gastric epithelial cell adhesion. J Exp Med. 2005, 202: 1235-1247. 10.1084/jem.20051027
Higashi H, Tsutsumi R, Muto S, Sugiyama T, Azuma T, Asaka M, Hatakeyama M: SHP-2 tyrosine phosphatase as an intracellular target of Helicobacter pylori CagA protein. Science. 2002, 295: 683-686. 10.1126/science.1067147
Tsutsumi R, Takahashi A, Azuma T, Higashi H, Hatakeyama M: Focal adhesion kinase is a substrate and downstream effector of SHP-2 complexed with Helicobacter pylori CagA. Mol Cell Biol. 2006, 26: 261-276. 10.1128/MCB.26.1.261-276.2006
Ling K, Doughman RL, Firestone AJ, Bunce MW, Anderson RA: Type I gamma phosphatidylinositol phosphate kinase targets and regulates focal adhesions. Nature. 2002, 420: 89-93. 10.1038/nature01082
Carragher NO, Westhoff MA, Fincham VJ, Schaller MD, Frame MC: A novel role for FAK as a protease-targeting adaptor protein: regulation by p42 ERK and Src. Curr Biol. 2003, 13: 1442-1450. 10.1016/S0960-9822(03)00544-X
Mishra GR, Suresh M, Kumaran K, Kannabiran N, Suresh S, Bala P, Shivakumar K, Anuradha N, Reddy R, Raghavan TM, Menon S, Hanumanthu G, Gupta M, Upendran S, Gupta S, Mahesh M, Jacob B, Mathew P, Chatterjee P, Arun KS, Sharma S, Chandrika KN, Deshpande N, Palvankar K, Raghavnath R, Krishnakanth R, Karathia H, Rekha B, Nayak R, Vishnupriya G, Kumar HG, Nagini M, Kumar GS, Jose R, Deepthi P, Mohan SS, Gandhi TK, Harsha HC, Deshpande KS, Sarker M, Prasad TS, Pandey A: Human protein reference database-2006 update. Nucleic Acids Res. 2006, 34: D411-D414. 10.1093/nar/gkj141
Kerrien S, Alam-Faruque Y, Aranda B, Bancarz I, Bridge A, Derow C, Dimmer E, Feuermann M, Friedrichsen A, Huntley R, Kohler C, Khadake J, Leroy C, Liban A, Lieftink C, Montecchi-Palazzi L, Orchard S, Risse J, Robbe K, Roechert B, Thorneycroft D, Zhang Y, Apweiler R, Hermjakob H: IntAct-open source resource for molecular interaction data. Nucleic Acids Res. 2007, 35: D561-D565. 10.1093/nar/gkl958
For academic purposes, CellNetAnalyzer including its manual can be obtained for free via the web-site., http://www.mpi-magdeburg.mpg.de/projects/cna/cna.html
Acknowledgements
The authors thank for the support of the Federal Ministry of Education and Research "Forschungseinheiten der Systembiologie" (FORSYS, BMBF-0313922) and HepatoSys, and the Ministry of Education of Saxony-Anhalt (Research Centre Dynamical Systems, XD3639HP/0306).
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors' contributions
RF constructed and analysed the network model and was in charge of writing the manuscript. MM and TK constructed the network model, collected the data and contributed to the manuscript. NW carried out the experimental contributions. SK helped in the construction and the analysis of the logical model and wrote theoretical parts of the manuscript. EDG contributed with suggestions in model analysis. MN conceived and coordinated the study, participated in its design, and supervised the writing process of the manuscript. All authors have read and approved the final version of the manuscript.
Raimo Franke, Melanie Müller contributed equally to this work.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Franke, R., Müller, M., Wundrack, N. et al. Host-pathogen systems biology: logical modelling of hepatocyte growth factor and Helicobacter pylori induced c-Met signal transduction. BMC Syst Biol 2, 4 (2008). https://doi.org/10.1186/1752-0509-2-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1752-0509-2-4