Abstract
Background
Naturally occurring apoptosis is a developmental process that shapes the retina by eliminating overproduced neurons. In the absence of the proapoptotic Bcl-2 family member BAX, developmental apoptosis in the retina is disrupted and extra neurons survive. It is unknown how BAX is activated or if this regulation varies between neuronal types and subtypes. Since the Bcl-2 family members BIM, BID, and BBC3 (PUMA) are powerful direct activators of BAX, we used mice deficient for each of these genes to investigate their importance in developmental apoptosis.
Results
Bax deficient mice have an increase in retinal ganglion cells (RGCs), bipolar cells and dopaminergic amacrine cells, but not photoreceptors, horizontal cells or cholinergic amacrine cells. The retinas of adult Bim and Bid deficient mice appeared to have no increase in any retinal cell type. Bbc3 deficient mice, either homozygous or heterozygous for a null allele of Bbc3, had an increase in the same cell types as Bax deficient mice. An analogous result may occur in the brain where, similar to Bax deficient mice, Bbc3 deficient mice have a larger gross brain weight compared to wild type mice. In contrast to its developmental role, BBC3 did not appear to be a primary factor in BAX-dependent axonal injury induced neurodegeneration in adult RGCs.
Conclusion
The regulation of BAX activation in the retina appears to be complex, dependent on the developmental stage of the animal, the nature of the insult and even the type of neuron.
Similar content being viewed by others
Introduction
Apoptosis in the retina has a major role in neuronal development and neurodegeneration. Retinal development is a complex process involving the cell fate commitment and differentiation of seven cell types and numerous cell-subtypes. Cell death is an important component of determining the final composition of the retina as most cell types are overproduced during development [1, 2]. Numerous mechanisms have been suggested to play a role in determining the ultimate number of retinal neurons [3, 4]. For instance, competition for neurotrophic support was suggested to be important in determining retinal ganglion cell (RGC) number. However, even though extensive manipulation of the neurotrophic deprivation pathway affected the rate of cell death, it did not alter the final number of RGCs [4–6]. To date it is unclear what molecular pathways are critical for determining final cell number in the retina or other parts of the central nervous system (CNS).
It is known that BAX is an important proapoptotic molecule in retinal development, after RGC axonal injury, and in glaucoma [1, 7, 8]. Bax is required in the developmental cell death of retinal ganglion cells (RGCs), photoreceptor cells, and at least some retinal interneurons [1, 9]. BAX is a member of the Bcl-2 family of apoptotic regulators, and BAX activation is a final step in triggering apoptosis. Numerous other Bcl-2 family members carry out either prosurvival or prodeath functions by regulating BAX activity. This regulation carries significance in the retina where BAX overexpression alone did not alter RGC number [10]. Cell signaling pathways alter individual Bcl-2 family member activity, which ultimately determine whether BAX (or BAK1, another prodeath Bcl-2 family member that is capable of triggering apoptosis) is activated. During retinal development, it is unclear what Bcl-2 family members regulate BAX activation. To understand cell death during retinal development, it is important to define the critical steps to activating BAX.
There are at least 7 BH3-only proteins, prodeath Bcl-2 family members. Three of these, BIM, BBC3 and BID are powerful direct activators of BAX[11]. Doonan and colleagues [12] showed that BIM did not play a significant role in determining the final number of retinal neurons, though it might have a minor role in determining the rate of RGC death during development. BID is expressed in the developing retina [13], but it is not known whether it is activated (truncated) or has any role in regulating developmental cell death. BBC3 (also known as PUMA) is expressed in the developing retina [13]. Interestingly, BBC3 is known to be critical in some cells after insults that have been implicated in developmental neuronal death. For instance, BBC3 is known to play a role in certain cell types after cytokine withdrawal [14, 15] and following suppression of neuronal activity [16]. Recently, BBC3 has also been shown to be critical for the death of newly generated neurons in the adult brain [17, 18]. In addition BBC3 is a powerful mediator of neuronal apoptosis after various insults in the adult [19–21].
Here we examine the role of the direct activators of BAX that have not been assessed, BID and BBC3, to determine if they are important for BAX activation in retinal development. There were no obvious changes in retinal cell number in adult Bid deficient mice. Bbc3 deficiency significantly increases several different retinal cell types, including RGCs. Interestingly, RGC developmental death and death after axonal injury is BAX dependent [1, 7, 8, 22], but Bbc3 deficiency only protects RGCs from developmental death. These data suggest that BAX dependent apoptotic signaling in RGCs is differentially regulated even at the level of BAX activation. Furthermore, these data define a role for BBC3 in neuronal development.
Results
Biddeficiency does not alter retinal cell numbers
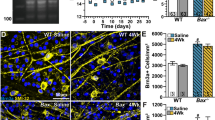
Neurons in the adult retina reside in three layers of cell bodies: the ganglion cell layer (GCL), inner nuclear layer (INL), and outer nuclear layer (ONL), which are connected by two synaptic layers: the inner plexiform layer (IPL) and the outer plexiform layer (OPL). Developmental death plays a role in the organization and final number of neurons in each layer. Both BIM and BID expression have previously been shown in the developing retina throughout the time when cell number is being refined [13]. Bim deficiency delayed developmental cell death throughout the retina, but did not appear to affect final cell number [12]. Retinal cross sections of adult Bim-/- mice were normal (Figure 1). Similarly, deficiency in Bid did not appear to alter the number of surviving cells in the adult retina (Figure 1). Counts of total neurons in the ganglion cell layer (GCL; the inner layer of the retina) of Nissl stained retinal flat mounts confirmed that neither BIM nor BID affects cell number in the GCL (given as % wild type, WT 100 ± 8%; Bid-/- , 102 ± 4%; Bim-/- , 106 ± 4%; n = 4 for each genotype, P > 0.5). In some instances, these BH3-only proteins may act in consort with other BH3-only proteins [11, 23]. Therefore we investigated whether this was true with BIM and BID. The Bim-/- Bid-/- double mutant retinas had normal morphology (Figure 1) and did not have extra retinal neurons in the GCL as judged by cell counts of retinal flat mounts (105 ± 6% of, n = 4; P > 0.5).
Deficiency in Bim or Bid do not alter adult retinal morphology. Retinal structure for both Bim and Bid deficient animals (aged 6 weeks or more) appeared to be grossly normal with no obvious increase in neuronal number in any retinal layer. Furthermore, no extra cells were obvious in adult mice that were deficient in both Bim and Bid, Bim-/- Bid-/-. At least 3 retinas for each genotype were assessed. ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer; scale bar, 50 μm.
Bbc3deficiency increases neuronal number in the retina
BBC3 expression has been reported during the postnatal wave of developmental death in the retina [13]; therefore, we examined the Bbc3 null retina to see whether its presence is required for normal retinal development. Compared to the wild type retina, cross-section thickness progressively increases in Bbc3 heterozygote and Bbc3 null retinas corresponding to the loss of one or both alleles of Bbc3 (Figure 2A). This increase in size is due to increased cellularity in the INL and GCL and a thicker IPL (the synaptic layer between the GCL and INL) in the Bbc3 mutant retinas. In contrast, the ONL, which contains photoreceptor cell bodies, appears unaffected. The architecture of the Bbc3 null retina is consistent with the phenotype observed in the Bax null retina and a disruption of developmental apoptosis (Figure 2A). However, the phenotype in the Bbc3 heterozygote was unexpected, as there does not appear to be an increase in retinal neurons in Bax heterozygote mice.
Deficiency in Bbc3 increases neuron number in the adult retina. (A) Histological analysis of the retinas of Bbc3-/- and Bbc3+/- mice (aged 6 weeks or more) reveal an increase in thickness of inner nuclear layer (INL), suggesting an increase in INL neurons. Similarly, there appears to be an increase in ganglion cell layer (GCL) neurons. The retinas of Bax-/- mice also appeared to have an increase in INL and GCL cells. However, no obvious increase in cell number was preset in Bax heterozygous mice. No increase in the number of photoreceptors, which make up the outer nuclear layer (ONL) was observed in either Bbc3 or Bax deficient retinas. (B) To confirm the observation of an increase in GCL neurons in Bbc3 and Bax deficient retinas, GCL neurons were stained in flat mount using a modified Nissl stain. (C) Cell counts confirmed an increase in GCL neurons in Bbc3-/-, Bbc3+/-, and Bax-/- neurons. At least 5 retinas were counted per genotype. Note, for cell counts, endothelial cells were excluded based on their elongated morphology. *, P < 0.05; scale bar, 100 μm in A, 50 μm in C.
In order to assess the extent of protection in the GCL, where Bax is known to be important in mediating developmental cell death [1, 7], a modified Nissl stain was performed on retinal flatmounts (Figure 2B). The total number of GCL neurons was significantly increased in the Bbc3+/- and Bbc3-/- mice (Figure 2C, P < 0.01). Quantification of the Bax+/- retinas yielded no significant difference, while in Bax-/- retinas GCL neurons were significantly increased (Figure 2C; P < 0.01). These results are consistent with the retinal cross-sections and also indicate no significant difference between the Bbc3 and Bax knockouts in terms of the number of GCL neurons.
BAX contributes to final neuronal number due to its role in postnatal death [1, 24]. To confirm that BBC3 plays a similar role in postnatal cell death, dying cells were counted in postnatal day 4 retinas, a time when cells are dying in both the GCL and the developing outer retina (NBL, which consists of neuronal precursors and differentiated neuronal cells) [2, 24]. CASP3 activation (cleaved caspase) was significantly reduced in both layers of Bbc3-/- retinas compared to wild type (Figure 3; P < 0.001). Thus, BBC3 plays a critical role in the apoptotic death of retinal neurons in the postnatal retina. It is important to note that there were still occasional dying cells in both layers, suggesting another caspase-dependent cell death pathway eliminates a small number of retinal cells in the absence of BBC3.
BBC3 is important for cell death in postnatal retinal neurons. (A) At postnatal day 4, Bbc3-/- retinas have far fewer cleaved CASP3+ (dying) cells compared to wild type retinas (arrows indicate examples of activated CASP3+ cells). (B) The number of CASP3+ cells was significantly different in both the retinal ganglion cell layer and the developing outer retina (P = 0.001; n = 3 for each genotype). Scale bar, 100 μm.
Inner retinal cell types that require BAX for developmental apoptosis share a BBC3-dependent cell death pathway
The different types of retinal neurons are overproduced and eliminated in different amounts [25]. However, whether similar or different, the cell signaling pathways responsible for developmental death have not been identified in any retinal cell type. We sought to determine if differences between the Bax null and Bbc3 null phenotype could identify key cell-type specific differences in the regulation of apoptotic proteins by assessing whether all types or a subset of retinal neurons are affected in each mutant. We quantified which cell types increased as a result of Bbc3 or Bax deficiency in the GCL and INL. The ganglion cell layer contains two types of neurons, RGCs and displaced amacrine cells. POU4F1 is a specific marker for RGCs and is expressed in 80% of adult RGCs [26]. Bbc3+/- and Bbc3-/- mice had significantly more RGCs than wild type mice (Figure 4 andTable 1). The increase was similar to that seen in Bax+/- and Bax-/- mice. Both Bbc3-/- and Bax-/- had approximately twice the number of POU4F1+ cells. In addition, the percent of RGCs in the GCL in the mutant mice does not appreciably differ from controls (e.g., % DAPI+ GCL cells, Bbc3+/+ 36%; Bbc3-/- 41%). This result indicates that the naturally occurring cell death of displaced amacrine cells is also disrupted in the Bbc3 mutants. However, all displaced amacrine cells do not appear to undergo BBC3 or BAX-dependent cell death. Deficiency in either Bbc3 or Bax did not effect the final number of cholinergic displaced amacrine cells (CHAT+; Figure 4 andTable 1), which make up approximately 20% of displaced amacrine cells in the GCL in wild type mice [27].
Bbc3 increases the number of ganglion cell layer neurons in the adult retina. There is an increase in POU4F1 retinal ganglion cells (red) in both Bbc3 and Bax deficient retinas. The number of cholinergic amacrine cells (green) is not increased in either Bbc3 of Bax deficient retinas. Note, CHAT+ cell numbers where similar to controls in both the inner nuclear layer (INL) and in the GCL (displaced amacrine cells). Quantification of CHAT and POU4F1 positive cells are given in table 1. Scale bar, 50 μm.
There are three broad types of retinal neuron in the inner nuclear layer: amacrine cells, bipolar cells, and horizontal cells. As in the GCL, developmental cell death of cholinergic (CHAT+) amacrine cells in the INL did not appear to require BBC3 or BAX (Figure 5 andTable 1). However, the number of dopaminergic (TH+) amacrine cells was increased in Bbc3-/- and Bax-/- retinas by over three fold. Interestingly, there was a significant increase in dopaminergic amacrine cells in Bbc3+/- but not Bax+/- mice. Similarly, there was a significant increase in bipolar cells (VSX2+) in Bbc3+/-, Bbc3-/- and Bax-/- but not Bax+/- mice. Horizontal cells (CALB1+) are not known to undergo significant amount of Bcl-2 family dependent death during development [25]. As expected horizontal cell numbers were similar in retinas of all genotypes. Overall, these results confirm the varying amounts to which different types of retinal neurons are overproduced and eliminated. Despite these differences BBC3 appears to be the primary activator of BAX in the developing retina, with the complete loss of BBC3 or BAX resulting in similar phenotypes.
BBC3 is important for the death of several retinal interneurons. Bbc3 and Bax deficiency increased the number of bipolar cells (VSX2+ cells) but not the number of horizontal cells (CALB1+ cells) in the inner nuclear layer (INL). The number of dopaminergic amacrine cells (TH), which reside in the INL were assessed in flat mount since there are so few in the retina. Deficiency in either Bbc3 or Bax greatly increased the number of these cells surviving in the adult retina. Quantification of VSX2, CALB1 and TH positive cells are given in table 1. Scale bar, 50 μm.
BBC3 contributes to developmental apoptosis in other regions of the CNS
Developmental apoptosis is an essential component in the proper development of the entire CNS [22]. Similar to the retina, a large amount of this naturally occurring cell death coincides with synapse formation [28]. The importance of BAX-dependent cell death pathways in the developing brain has been observed regionally and by increased gross brain weight in Bax-/- mice compared to wild type mice and varies with sex [29]. Similarly, in Bbc3-/- mice there was approximately a 15% increase in gross brain weight compared to wild type mice in both males and females (Table 2; P < 0.005 for each sex).
Bbc3deficiency does not prevent RGC death after axonal insult
Bbc3 appears to be as critical as Bax in the developmental death of RGCs. Since Bax is required for RGC death in glaucoma [8] and after optic nerve injury (Figure 6 and [7]), Bbc3's role in the death of adult RGCs from axonal insult was tested. Counts of total GCL neurons showed that significant loss of RGCs occurred in Bbc3-/- mice at 7 and 14 days after CONC compared to the mice undergoing a sham procedure (Figure 6). This result differs from Bax-/- mice, where no significant loss of RGCs was reported 14 days [7] and 21 days [8] after CONC. In order to assess whether the extent of RGC loss is diminished following axonal injury in Bbc3-/- mice, RGC layer neurons were counted 60 days post CONC, which is well after the normal period of cell death. 60 days after axonal injury, wild type retinas lost 44% of RGC layer neurons (Figure 6). Note, approximately 55% of RGC layer neurons are displaced amacrine cells [27] and only RGCs die after direct axonal injury [30], so the observed loss of 44% of RGC layer neurons equals complete RGC loss. Bax deficiency continued to provide complete protection at 60 days, but the percentage of RGC loss that occurred with Bbc3 deficiency was similar to wild type (Figure 6). Unlike BAX, BBC3 only has a role in developmental death and is not a factor in long-term RGC survival after an acute axonal injury.
BBC3 is not required for the death of adult retinal ganglion cells (RGCs) after axonal injury. Nissl stained flat mounts of ganglion cell layer (GCL) neurons (left) in Bbc3 deficient mice shows that RGCs are still lost at 7, 14 or 60 days after CONC. Note, only RGCs die after CONC. Approximately 55% of RGC layer neurons are amacrine cells [27], so a loss of 45% of RGC layer neurons equals complete RGC loss. In contrast to Bbc3 deficiency, Bax deficiency completely protects RGCs from apoptosis after CONC even out to 60 days. N > 5 for each genotype and time point.
Counting total number of GCL neurons is a good way to determine if there is long-term protection, but the method may not be sensitive enough to detect a delay in RGC death. To determine if Bbc3 deficiency delayed RGC death, the number of CASP3+ cells in Bbc3-/- and control retinas were counted at 3 (just as RGCs begin to die) and 5 days after CONC. At 3 days after CONC, the first day that significant numbers of dying RGCs are observed, there were approximately an equal number of CASP3+ cells in Bbc3+/+ and Bbc3-/- retinas (Bbc3+/+ 100 ± 8.1; Bbc3-/- 89.1 ± 13.5; P = 0.5; N = 9 for each genotype). Given that there are more RGCs in Bbc3-/- retinas it is possible that BBC3 has a small role in RGC death after CONC; a caveat is that the nerve is bigger which may provide some cushion from the mechanical trauma of the injury. By 5 days there were significantly more in the Bbc3-/- retinas compared to control, but this increase appears to be in proportion to the increase in the RGCs in these retinas (given as percent control ± SEM; Bbc3+/+ 100 ± 7.6; Bbc3-/- 140.5 ± 12.3; P = 0.023; N = 5 for each genotype). Thus, BBC3 does not appear to have a major role in RGC death after axonal injury.
Discussion
In the central nervous system Bcl-2 family members are involved in developmental cell death as well as in most cell death that is the result of trauma and disease [31]. BAX is a powerful prodeath member of the Bcl-2 family. In fact BAX activation is often a critical step in triggering apoptosis. BAX activation is tightly controlled by the interaction of other Bcl-2 family members. Prosurvival family members antagonize BAX activation while BH3 only proteins, another type of Bcl-2 family prodeath proteins, facilitate BAX activation [32]. In the retina, BAX has been reported to be critical for the developmental cell death of many retinal cell types [1, 9]. Furthermore, BAX activation is an important step for the death of adult retina neurons after an insult, including for axonally injured RGCs [7, 8]. Currently, the complete set of Bcl-2 prosurvival and prodeath proteins that control BAX activation in developing retinal cells or in axonally injured RGCs is unknown.
Most retinal neurons are overproduced during development and BAX is involved in the programmed cell death that prunes the extra neurons [22]. The gross morphology of Bax-/- retinas has been described [1, 24]. In Bax-/- mice there are approximately twice as many RGCs compared to wild type retinas [1, 22]. Also, the inner nuclear layer of Bax-/- retinas is much thicker than controls suggesting that there are more retinal interneurons (bipolar, amacrine and/or horizontal cells). We confirmed the involvement of BAX in RGC death. Furthermore, cell counts showed that BAX is required for the death of bipolar cells but not horizontal, which may not be overproduced in large numbers [2, 25]. Interestingly, subtypes of amacrine cells differed in their requirement for BAX. BAX appears to be required for the death of dopaminergic but not cholinergic cells even though cholinergic cells are overproduced and die during postnatal development [25]. Overexpression of Bcl-2, a powerful prosurvival member of the Bcl-2 family that can antagonize all of the prodeath BH3-only proteins results in a similar phenotype as Bax deficiency [25] with the exception that Bcl2 overexpression produced far more dopaminergic amacrine cells than Bax deficiency. Thus, the Bcl-2 family appears to be important in developmental cell death of numerous retinal cell types and the importance of specific family members can vary even amongst the subtypes of a specific neuron.
BAX activation likely requires BH3-only proteins. Mice lacking the BH3-only protein BIM had delayed cell death in all retinal layers during development [12]. However, adult retinas in Bim-/- mice appeared to have normal numbers of retinal cells [12] (and see Figure 1). Doonan et al., [12] hypothesize that other BH3-only proteins ultimately are recruited to kill developing retinal neurons. This study left unresolved whether in the normal retina a BIM-dependent pathway was responsible for killing developing retinal neurons. To determine if other BH3-only proteins had a role in retinal developmental death we focused on BID and BBC3, which like BIM antagonize all the prosurvival family members [33] and are capable of directly activating BAX, BID and BBC3 [11]. Bid deficiency did not affect the final number of retinal neurons. Surprisingly, since it has not been implicated in neuronal developmental cell death, BBC3 was required for death in all of retinal cell types that have BAX dependent developmental death. It appears that BAX activation during development is entirely BBC3 dependent. Thus, regulation of BBC3 appears to be a dominant factor determining a cell's commitment to die in the developing retina and the brain.
It is possible that the levels of prosurvival Bcl-2 family members relative to BBC3 (and other prodeath Bcl-2 family members) are key to determining which neurons survive naturally occurring developmental cell death in the postnatal retina. In fact, this model has been suggested to be important in RGC death after axon injury [34]. In developing retinal neurons BCL2 appears to be ubiquitously expressed [35]. BCL2 is required for the survival of a subset of RGCs after the naturally occurring window of developmental death [36], supporting the idea that neuronal survival is normally achieved by tipping the balance in favor of the prosurvival family members. However, the absence of BCL2 does not increase RGC death during the major wave of developmental cell death [36]. This result may be due to a high level of other prosurvival Bcl-2 family members in RGCs. BCL2L1 (BCL-XL) is expressed in the developing central nervous system and is required for survival of immature neurons [37, 38]. Assessing the function of BCL2L1 and other prosurvival family members in the developing retina would critically test this possibility. In addition, it is notable that despite the threshold set by prosurvival family members and the presence and importance of BIM (retinal developmental death is delayed in its absence [12]), BBC3 is specifically required for death to occur. Therefore, further study of the regulation and/or specificity of BBC3 is needed to understand the processes governing naturally occurring cell death in the retina.
It is unclear how BBC3 expression is regulated in the developing retina. The effect that Bbc3 heterozygosity has in increasing neuronal number indicates that its transcriptional control is important. Bbc3 expression is known to be rapidly induced in response to various insults, including neurotrophic deprivation, by multiple transcription factors [39]. The death of newly generated neurons in the adult brain is similar to the death of developing retinal neurons in that it requires BBC3 [18, 21]. However, Bbc3 expression in the newly generated adult neurons is dependent on a major regulator of Bbc3 expression, TRP53 [18]. Surprisingly, TRP53 is not the major regulator of Bbc3 expression because retinal cell death in development appears to be Trp53 independent [40]. Bbc3 expression is also known to be regulated by other transcription factors, including, TRP73, E2Fs, FOXO3A, JUN and MYC [39]. Knockout of many of these transcription factors E2fs, Foxo3a (unpublished data), and Jun (unpublished data) has no lasting effect on retinal development, at least in terms of the gross number of retinal neurons. These observations limit the number of known Bbc3 regulatory pathways that could be important in determining adult neuronal number.
Many physiological processes influence cell death in the developing retina and are potentially upstream of BBC3, including neurotrophin signaling, electrical activity, and refinement of spatial patterning [3, 4, 41]. However, manipulations of these processes differ from Bbc3 deficiency in that they only affect the rate of cell death and do not alter adult neuronal number (e.g. [3–6]). Interestingly, electrical activity has been suggested to be important in maintaining RGC survival in development [42] and synaptic activity can suppress Bbc3 expression, in a Trp53 independent manner, in developing neurons [16]. Though, it is unclear how electrical activity regulates Bbc3 activity, perhaps understanding this link may lead to the identification of molecules important in determining neuronal number in the CNS.
Bax deficiency also prevents apoptosis after axonal injury from a mechanical trauma [7, 8] or ocular hypertension (glaucoma) [8]. Unlike during developmental apoptosis, Bbc3 deficiency did not change the time course of RGC death after axonal injury. This is perhaps not surprising since Bim deficiency [43] completely inhibited cell death in the RGC layer for 4 days after axotomy in explant culture. It is unclear if BAX activation in RGCs is completely dependent on BIM because longer time points were not examined. In hippocampal neurons both BIM and BBC3 can trigger cell death in response to the same insult [17, 44]. It will be interesting to determine if Bim deficiency provides long-term protection to RGCs after axonal injury. If it does not, it is possible that BBC3 ultimately is capable of killing RGCs after axonal injury.
Conclusion
BBC3-dependent BAX activation is required for developmental cell death of many retinal cell types. Since BBC3 appears to be critical for the developmental cell death of a number of different types of neurons, understanding how it is regulated will help to identify the molecular mechanisms that control neuronal number in the mammalian CNS. Unlike in developmental apoptosis, axonal injury-induced death of RGCs, BAX activation did not require BBC3. Thus, in vivo and in an insult-dependent manner, different BH3-only proteins control BAX activation even in the same neuronal cell type.
Methods
Animals
Null alleles of Bbc3tm1Gpz[45] (a generous gift from Gerard Zambetti), Bimtm1.1Ast (the Jackson Laboratory stock number 004524) and Bidtm1Sjk [46], were separately backcrossed at least 10 times into C57BL/6J prior to our receiving them. All colonies were maintained by intercrossing. Mice were maintained in a 12-hour light dark cycle and were fed chow and water ad libitum. All experiments were conducted in accordance with the Association for Research in Vision and Ophthalmology's statement on the use of animals in ophthalmic research and were approved by the University of Rochester's University Committee on Animal Resources.
Tissue Collection, Plastic Sections and Flat mounts
Plastic sections were obtained as previously described [8, 47]. Briefly, eyes were fixed by immersion in 2.5% gluteraldehyde and 2% paraformaldehyde at 4°C for 24 hours. Then eyes were dehydrated and embedded in technovit, 2.5 μm sections were cut, and sections that included the optic nerve were stained with cresyl violet. Retinal flat mounts were performed as previously described [8, 47]. For RGC layer counts 2 40× fields from each retinal quadrant approximately 1 40× field from the margin of the retina were used for each retina. All cells within the field were counted except endothelial cells, which have an obvious elongated, non-neuronal morphology. For the total count for each retina, all eight fields were added together. To determine brain weights mice were weighed, anesthetized, and transcardially perfused with 4% paraformaldehyde. The entire brain was then removed and weighed.
Immunohistochemistry and Cell Counts
Eyes were fixed in 4% paraformaldehyde in PBS at room temperature. The anterior segment was removed and the resultant eye cup was either processed for cryosectioning or whole mount staining. For immunohistochemistry on retinal sections, cryosections were blocked by incubating in 10% horse serum in 0.3% Triton X-100 in PBS, pH 7.3, for one hour at room temperature. Primary antibodies diluted in 0.1% Triton X-100 in PBS (PBST) containing 5% horse serum were then applied overnight at 4°C. The following day the sections were washed and treated for two hours with Alexafluor-conjugated secondary antibodies (Invitrogen) diluted in PBST. Each section was also counterstained with DAPI. GCL neuronal nuclei (DAPI), retinal ganglion cells (anti-POU4F1, Santa-Cruz, 1:200), cholinergic amacrine cells (anti-CHAT; Millipore, 1:200), and bipolar cells (anti-VSX2 (CHX10), Exalpha Biologicals, 1:200) were counted in sections. Only central sections located at or within 500 um near the optic nerve head (ONH) were used for counting. Within a section, each marker was counted in a 440 μm length of retina on each side of the ONH. This method was repeated on four sections per retina. For an individual retina the score was the average of the eight counts. Due to the fewer number of horizontal cells in the retina, calbindin D (CALB1, Sigma Aldrich, 1:1000) positive cells were counted over entire sections. Counts were performed at equivalent retinal eccentricities near the optic nerve head on four sections per retina. Dying cells (activated caspase 3 positive; R&D Systems, 1:500) in the developing retina on postnatal day 4 were also counted over the entire retinal section, similarly to the adult horizontal cell counts. Dopaminergic amacrine cells (anti-TH, Millipore, 1:500) and activated caspase 3 positive cells in adult retinas were counted in retinal flat mounts, since there are so few of these cells in the retina. For flat mounts, retinas were dissected free of the eye cup and blocked in PBST+10% horse serum for 4 hours. Retinas were incubated in primary antibodies diluted in PBST+5% horse serum for three overnights at 4°C. Counts were performed on two 20× fields from each quadrant approximately 220 μm from the peripheral edge of the retina (one half of a 20× field). Adult retinas were all between 6 weeks and 12 weeks of age. Four or more adult retinas of each genotype were quantified.
Optic nerve injury
Controlled optic nerve crush (CONC) was performed on adult mice as previously described [8]. The optic nerve was crushed approximately 3-6 mm behind the eye for 5 seconds using self closing forceps (Roboz RS-5027). Retinas were harvested at various time points after the procedure. RGC layer counts after CONC were performed on nissl stained retinas as described above. Unmanipulated contralateral eyes or contralateral eyes that had a sham surgery performed (no crush of the optic nerve) were used as control eyes.
Statistical Analysis
At least 3 animals were assessed for each genotype for all experimental conditions. For cell experiments involving quantification of results the experimenter was blinded to genotype and/or experimental group. ANOVA was used to compare between all genotypes. Upon finding statistical significant, multiple comparison tests were performed using the Tukey-Kramer method. P < 0.05 was considered significant.
References
Mosinger Ogilvie J, Deckwerth TL, Knudson CM, Korsmeyer SJ: Suppression of developmental retinal cell death but not of photoreceptor degeneration in Bax-deficient mice. Invest Ophthalmol Vis Sci. 1998, 39: 1713-1720.
Young RW: Cell death during differentiation of the retina in the mouse. J Comp Neurol. 1984, 229: 362-373. 10.1002/cne.902290307.
Valenciano AI, Boya P, de la Rosa EJ: Early neural cell death: numbers and cues from the developing neuroretina. Int J Dev Biol. 2009, 53: 1515-1528. 10.1387/ijdb.072446av.
Isenmann S, Kretz A, Cellerino A: Molecular determinants of retinal ganglion cell development, survival, and regeneration. Prog Retin Eye Res. 2003, 22: 483-543. 10.1016/S1350-9462(03)00027-2.
Pollock GS, Robichon R, Boyd KA, Kerkel KA, Kramer M, Lyles J, Ambalavanar R, Khan A, Kaplan DR, Williams RW, Frost DO: TrkB receptor signaling regulates developmental death dynamics, but not final number, of retinal ganglion cells. J Neurosci. 2003, 23: 10137-10145.
Harada C, Harada T, Nakamura K, Sakai Y, Tanaka K, Parada LF: Effect of p75NTR on the regulation of naturally occurring cell death and retinal ganglion cell number in the mouse eye. Dev Biol. 2006, 290: 57-65. 10.1016/j.ydbio.2005.08.051.
Li Y, Schlamp CL, Poulsen KP, Nickells RW: Bax-dependent and independent pathways of retinal ganglion cell death induced by different damaging stimuli. Exp Eye Res. 2000, 71: 209-213. 10.1006/exer.2000.0873.
Libby RT, Li Y, Savinova OV, Barter J, Smith RS, Nickells RW, John SW: Susceptibility to neurodegeneration in a glaucoma is modified by Bax gene dosage. PLoS Genet. 2005, 1: 17-26. 10.1371/journal.pgen.0010017.
Hahn P, Lindsten T, Ying GS, Bennett J, Milam AH, Thompson CB, Dunaief JL: Proapoptotic bcl-2 family members, Bax and Bak, are essential for developmental photoreceptor apoptosis. Invest Ophthalmol Vis Sci. 2003, 44: 3598-3605. 10.1167/iovs.02-1113.
Bernard R, Dieni S, Rees S, Bernard O: Physiological and induced neuronal death are not affected in NSE-bax transgenic mice. J Neurosci Res. 1998, 52: 247-259. 10.1002/(SICI)1097-4547(19980501)52:3<247::AID-JNR1>3.0.CO;2-D.
Ren D, Tu HC, Kim H, Wang GX, Bean GR, Takeuchi O, Jeffers JR, Zambetti GP, Hsieh JJ, Cheng EH: BID, BIM, and PUMA are essential for activation of the BAX- and BAK-dependent cell death program. Science. 2010, 330: 1390-1393. 10.1126/science.1190217.
Doonan F, Donovan M, Gomez-Vicente V, Bouillet P, Cotter TG: Bim expression indicates the pathway to retinal cell death in development and degeneration. J Neurosci. 2007, 27: 10887-10894. 10.1523/JNEUROSCI.0903-07.2007.
Donovan M, Doonan F, Cotter TG: Decreased expression of pro-apoptotic Bcl-2 family members during retinal development and differential sensitivity to cell death. Dev Biol. 2006, 291: 154-169. 10.1016/j.ydbio.2005.12.026.
Han J, Flemington C, Houghton AB, Gu Z, Zambetti GP, Lutz RJ, Zhu L, Chittenden T: Expression of bbc3, a pro-apoptotic BH3-only gene, is regulated by diverse cell death and survival signals. Proc Natl Acad Sci USA. 2001, 98: 11318-11323. 10.1073/pnas.201208798.
You H, Pellegrini M, Tsuchihara K, Yamamoto K, Hacker G, Erlacher M, Villunger A, Mak TW: FOXO3a-dependent regulation of Puma in response to cytokine/growth factor withdrawal. J Exp Med. 2006, 203: 1657-1663. 10.1084/jem.20060353.
Leveille F, Papadia S, Fricker M, Bell KF, Soriano FX, Martel MA, Puddifoot C, Habel M, Wyllie DJ, Ikonomidou C, et al: Suppression of the intrinsic apoptosis pathway by synaptic activity. J Neurosci. 2010, 30: 2623-2635. 10.1523/JNEUROSCI.5115-09.2010.
Bunk EC, König HG, Bernas T, Engel T, Henshall DC, Kirby BP, Prehn JHM: BH3-only proteins BIM and PUMA in the regulation of survival and neuronal differentiation of newly generated cells in the adult mouse hippocampus. Cell Death and Disease. 2010, 1: e15-10.1038/cddis.2009.13.
Liu D, Ou L, Clemenson GD, Chao C, Lutske ME, Zambetti GP, Gage FH, Xu Y: Puma is required for p53-induced depletion of adult stem cells. Nat Cell Biol. 2010, 12: 993-998. 10.1038/ncb2100.
Kieran D, Woods I, Villunger A, Strasser A, Prehn JH: Deletion of the BH3-only protein puma protects motoneurons from ER stress-induced apoptosis and delays motoneuron loss in ALS mice. Proc Natl Acad Sci USA. 2007, 104: 20606-20611. 10.1073/pnas.0707906105.
Steckley D, Karajgikar M, Dale LB, Fuerth B, Swan P, Drummond-Main C, Poulter MO, Ferguson SS, Strasser A, Cregan SP: Puma is a dominant regulator of oxidative stress induced Bax activation and neuronal apoptosis. J Neurosci. 2007, 27: 12989-12999. 10.1523/JNEUROSCI.3400-07.2007.
Biswas SC, Ryu E, Park C, Malagelada C, Greene LA: Puma and p53 play required roles in death evoked in a cellular model of Parkinson disease. Neurochem Res. 2005, 30: 839-845. 10.1007/s11064-005-6877-5.
White FA, Keller-Peck CR, Knudson CM, Korsmeyer SJ, Snider WD: Widespread elimination of naturally occurring neuronal death in Bax-deficient mice. J Neurosci. 1998, 18: 1428-1439.
Schneider-Jakob S, Corazza N, Badmann A, Sidler D, Stuber-Roos R, Keogh A, Frese S, Tschan M, Brunner T: Synergistic induction of cell death in liver tumor cells by TRAIL and chemotherapeutic drugs via the BH3-only proteins Bim and Bid. Cell Death Dis. 2010, 1: e86-10.1038/cddis.2010.66.
Pequignot MO, Provost AC, Salle S, Taupin P, Sainton KM, Marchant D, Martinou JC, Ameisen JC, Jais JP, Abitbol M: Major role of BAX in apoptosis during retinal development and in establishment of a functional postnatal retina. Dev Dyn. 2003, 228: 231-238. 10.1002/dvdy.10376.
Strettoi E, Volpini M: Retinal organization in the bcl-2-overexpressing transgenic mouse. J Comp Neurol. 2002, 446: 1-10. 10.1002/cne.10177.
Xiang M, Zhou L, Macke JP, Yoshioka T, Hendry SH, Eddy RL, Shows TB, Nathans J: The Brn-3 family of POU-domain factors: primary structure, binding specificity, and expression in subsets of retinal ganglion cells and somatosensory neurons. J Neurosci. 1995, 15: 4762-4785.
Jeon CJ, Strettoi E, Masland RH: The major cell populations of the mouse retina. J Neurosci. 1998, 18: 8936-8946.
Oppenheim RW: Cell death during development of the nervous system. Annu Rev Neurosci. 1991, 14: 453-501. 10.1146/annurev.ne.14.030191.002321.
Holmes MM, McCutcheon J, Forger NG: Sex differences in NeuN- and androgen receptor-positive cells in the bed nucleus of the stria terminalis are due to Bax-dependent cell death. Neuroscience. 2009, 158: 1251-1256. 10.1016/j.neuroscience.2008.11.020.
Kielczewski JL, Pease ME, Quigley HA: The effect of experimental glaucoma and optic nerve transection on amacrine cells in the rat retina. Invest Ophthalmol Vis Sci. 2005, 46: 3188-3196. 10.1167/iovs.05-0321.
Shacka JJ, Roth KA: Regulation of neuronal cell death and neurodegeneration by members of the Bcl-2 family: therapeutic implications. Curr Drug Targets CNS Neurol Disord. 2005, 4: 25-39. 10.2174/1568007053005127.
Brenner D, Mak TW: Mitochondrial cell death effectors. Curr Opin Cell Biol. 2009, 21: 871-877. 10.1016/j.ceb.2009.09.004.
Certo M, Del Gaizo Moore V, Nishino M, Wei G, Korsmeyer S, Armstrong SA, Letai A: Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006, 9: 351-365. 10.1016/j.ccr.2006.03.027.
Nickells RW: Variations in the rheostat model of apoptosis: what studies of retinal ganglion cell death tell us about the functions of the Bcl2 family proteins. Experimental eye research. 2010, 91: 2-8. 10.1016/j.exer.2010.03.004.
Novack DV, Korsmeyer SJ: Bcl-2 protein expression during murine development. Am J Pathol. 1994, 145: 61-73.
Cellerino A, Michaelidis T, Barski JJ, Bahr M, Thoenen H, Meyer M: Retinal ganglion cell loss after the period of naturally occurring cell death in bcl-2-/- mice. Neuroreport. 1999, 10: 1091-1095. 10.1097/00001756-199904060-00034.
Gonzalez-Garcia M, Garcia I, Ding L, O'Shea S, Boise LH, Thompson CB, Nunez G: bcl-x is expressed in embryonic and postnatal neural tissues and functions to prevent neuronal cell death. Proceedings of the National Academy of Sciences of the United States of America. 1995, 92: 4304-4308. 10.1073/pnas.92.10.4304.
Motoyama N, Wang F, Roth KA, Sawa H, Nakayama K, Negishi I, Senju S, Zhang Q, Fujii S, et al: Massive cell death of immature hematopoietic cells and neurons in Bcl-x-deficient mice. Science. 1995, 267: 1506-1510. 10.1126/science.7878471.
Yu J, Zhang L: PUMA, a potent killer with or without p53. Oncogene. 2008, 27 (Suppl 1): S71-83.
Marti A, Hafezi F, Lansel N, Hegi ME, Wenzel A, Grimm C, Niemeyer G, Reme CE: Light-induced cell death of retinal photoreceptors in the absence of p53. Invest Ophthalmol Vis Sci. 1998, 39: 846-849.
Jeyarasasingam G, Snider CJ, Ratto GM, Chalupa LM: Activity-regulated cell death contributes to the formation of ON and OFF alpha ganglion cell mosaics. J Comp Neurol. 1998, 394: 335-343. 10.1002/(SICI)1096-9861(19980511)394:3<335::AID-CNE5>3.0.CO;2-2.
Lipton SA: Blockade of electrical activity promotes the death of mammalian retinal ganglion cells in culture. Proc Natl Acad Sci USA. 1986, 83: 9774-9778. 10.1073/pnas.83.24.9774.
McKernan DP, Cotter TG: A Critical role for Bim in retinal ganglion cell death. J Neurochem. 2007, 102: 922-930. 10.1111/j.1471-4159.2007.04573.x.
Shacka JJ, Roth KA: Bcl-2 family and the central nervous system: from rheostat to real complex. Cell Death Differ. 2006, 13: 1299-1304. 10.1038/sj.cdd.4401974.
Jeffers JR, Parganas E, Lee Y, Yang C, Wang J, Brennan J, MacLean KH, Han J, Chittenden T, Ihle JN, et al: Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell. 2003, 4: 321-328. 10.1016/S1535-6108(03)00244-7.
Yin XM, Wang K, Gross A, Zhao Y, Zinkel S, Klocke B, Roth KA, Korsmeyer SJ: Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature. 1999, 400: 886-891. 10.1038/23730.
Libby RT, Anderson MG, Pang IH, Robinson ZH, Savinova OV, Cosma IM, Snow A, Wilson LA, Smith RS, Clark AF, John SW: Inherited glaucoma in DBA/2J mice: pertinent disease features for studying the neurodegeneration. Vis Neurosci. 2005, 22: 637-648.
Acknowledgements
The authors would like to thank Gerald Zambetti for generously providing Bbc3 null mice, Xiao-Ming Yin for generously providing the Bid null mice, and Thurma McDaniel, Siva Sugunan and Donna Shannon for technical help. This work was supported by EY018606 (RTL), T32 EY007125 (JMH), David Bryant Trust (RTL), Research to Prevent Blindness Career Development Award (RTL) and a Research to Prevent Blindness unrestricted grant to the Department of Ophthalmology.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
JH and RL designed the study and wrote the manuscript. JH carried out major parts of the experiments. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Harder, J.M., Libby, R.T. BBC3 (PUMA) regulates developmental apoptosis but not axonal injury induced death in the retina. Mol Neurodegeneration 6, 50 (2011). https://doi.org/10.1186/1750-1326-6-50
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1750-1326-6-50