Abstract
α-Synuclein is a small protein that has special relevance for understanding Parkinson disease and related disorders. Not only is α-synuclein found in Lewy bodies characteristic of Parkinson disease, but also mutations in the gene for α-synuclein can cause an inherited form of Parkinson disease and expression of normal α-synuclein can increase the risk of developing Parkinson disease in sporadic, or non-familial, cases. Both sporadic and familial Parkinson disease are characterized by substantial loss of several groups of neurons, including the dopaminergic cells of the substantia nigra that are the target of most current symptomatic therapies. Therefore, it is predicted that α-synuclein, especially in its mutant forms or under conditions where its expression levels are increased, is a toxic protein in the sense that it is associated with an increased rate of neuronal cell death. This review will discuss the experimental contexts in which α-synuclein has been demonstrated to be toxic. I will also outline what is known about the mechanisms by which α-synuclein triggers neuronal damage, and identify some of the current gaps in our knowledge about this subject. Finally, the therapeutic implications of toxicity of α-synuclein will be discussed.
Similar content being viewed by others
All neurodegenerative diseases share the common phenomenon that neurons, usually relatively specific groups, are lost progressively as the disease develops. In some cases, we can provide partial relief for patients by treating some of their symptoms. However, because we don't understand the underling mechanisms of why neurons die, degeneration continues inexorably and old symptoms often become unresponsive while new ones arrive. At the end of the disease process, we are left with only a few clues about what might have happened based on what we can glean from the pathology of the disease using post mortem samples. In general, the root cause of neurodegeneration remains obscure although rare genetic variants are helpful in that we can be certain that an inherited mutation acts as the trigger for disease in that specific family.
Here, I will discuss cell loss related to Parkinson disease (PD) in the context of one protein, α-synuclein, that has several links to the disorder. In doing so, I will outline what we know about the ways in which a protein can lead to cell death. Before doing so, it is worth discussing what PD is, and what it isn't.
Cell death in PD
It is very commonly said that PD is the second most common neurodegenerative disease and results from a loss of dopamine neurons. The first fact is dull and the second tells only part of the story. It is true that PD patients have a substantial loss of dopamine in the striatum resulting from a relatively selective loss of dopaminergic projection neurons in the substantia nigra pars compacta. Both biochemical measures and imaging modalities suggest that at least a 70% decrease in striatal dopamine occurs before the onset of clinical parkinsonism and progresses over time [1]. These estimates of the extent of striatal dopamine depletion, combined with the observation that the majority of dopaminergic neurons are lost by the end of the disease process, imply that there is substantial cell death throughout the PD disease process. It is not possible to show this directly, but measurements of nigral cell numbers in neurologically normal people and in non-human primates reveal a slow progressive loss of dopamine neurons with age [2]. In this view, parkinsonism is an accelerated, but still slow, cell death phenotype that would normally be seen with aging [3].
However, while there is relative vulnerability of dopaminergic neurons in the substantia nigra [4], not all dopamine cells are affected in PD. For example, although dopaminergic neurons in the ventral tegmental area that project to the nucleus accumbens do degenerate [5], compared to the dopaminergic neurons in the substantia nigra pars compacta these cells are relatively spared [6, 7].
Furthermore, not all affected neurons in PD are dopaminergic. Non-motor symptoms are a serious problem for many PD patients and are often untreated by replacement therapy with L-DOPA (3,4-dihydroxy-L-phenylalanine) [8]. A good example of non-dopaminergic cells that degenerate in PD is the cholinergic neurons in the dorsal vagal nucleus [9]. It has been suggested that involvement of non-nigral regions underlies the complex clinical picture in PD [10]. Therefore, although there is some specificity to cell death in PD, there is no absolute selectivity for any specific neurotransmitter group or anatomic region. It is also important to note that loss of nigral neurons occurs in diverse pathological situations [4] and that on its own, nigral cell loss defines the clinical term parkinsonism, not Parkinson disease.
This distinction is also important when discussing the other major pathological event in PD that appears alongside cell death, the formation of Lewy bodies and Lewy neurites. Lewy bodies are intracellular deposits of proteins and lipids [11] that were traditionally stained with eosin but now are more sensitively recognized by antibodies to specific marker proteins [12]. Using electron microscopy, Lewy bodies are fibrillar structures with a recognizable core and halo [13]. The range of Lewy pathology is now recognized as encompassing many regions of the diseased brain [14] including, for example, the olfactory bulb, raphe nucleus, locus coeruleus and the basal nucleus of Meynert. Additionally, some reports suggest that the nigra is not the first place where Lewy bodies form [15]. How this relates to the extent of cell loss in each region is not well defined. Lewy bodies are also seen in dementia with Lewy bodies (DLB, also known as Diffuse Lewy body Disease or DLBD), suggesting that PD and DLBD are related to one another by shared pathology and maybe by shared etiology.
Therefore, PD is a disease where substantial cell loss in the nigra occurs alongside the formation of Lewy bodies. Neither cell loss nor Lewy bodies is absolutely specific for PD but both are required for a diagnosis of PD under current definitions [16]. This review will focus on cell death, but it is important to understand a little more about the most commonly used marker for Lewy bodies; α-synuclein.
α-Synuclein is a marker of the PD process
The first member of the family of proteins for which α-synuclein is named was cloned from the neuromuscular junction of the electric eel [17]. Antibodies against that protein labeled both synapses and nuclei, leading to the naming of synuclein. A related protein was cloned from zebra finch as a protein upregulated during the process of song learning, a period of enormous synaptic plasticity [18]. In humans, there are three synuclein family members (α-,β-,γ-) and all synuclein genes are relatively well conserved both within and between species [19]. The synuclein genes are specific to the vertebrate lineage in that neither single cell organisms (including yeast) nor invertebrates (Drosophila melanogaster, Caenorhabditis elegans) have any apparent synuclein homologue. Additionally, primate α-synuclein sequences differ from other vertebrate synucleins by a substitution of Alanine for a Threonine at position 53 [20]. These two interesting facts about the evolutionary relationships in the synuclein family are important for understanding some of the experimental systems where synuclein has been explored.
The normal function of α-synuclein is poorly understood. Although it is expressed at high levels in the brain, relatively specifically within neurons, it is also found in other tissues, e.g., hematopoietic cells [21, 22]. α-Synuclein can bind to lipids [23] and, in neurons, is associated with presynaptic vesicles [24, 25] and the plasma membrane, possibly via lipid rafts [26]. The association of α-synuclein with vesicles is modulated by synaptic activity where the protein dissociates from vesicles after electrical stimulation of the neuron and only slowly re-associates [27]. However, α-synuclein knockout mice show only subtle abnormalities in neurotransmission [28–30], suggesting that α-synuclein plays a non-essential function at the synapse. There is some evidence that such a modulatory role may be more important under conditions where reactive oxygen species or nitric oxide are present [31, 32], although the mechanism(s) are not yet fully defined.
In the normal brain, α-synuclein immunostaining reveals a diffuse pattern of reactivity throughout the neuropil that would be consistent with a predominantly synaptic localization [25]. However, in PD brains, α-synuclein antibodies strongly stain Lewy bodies [33] and Lewy neurites [34]. Because of this sensitivity, α-synuclein staining is now more commonly used than eosin or ubiquitin staining for these structures. Biochemical analyses have shown that α-synuclein is a major protein component of Lewy bodies and may be part of the fibrillar structure of these structures [35]. The deposited, pathological forms of α-synuclein are aggregated and show lower solubility than the normal protein [36] and may be modified post-translationally by truncation, nitration, ubiquitylation and phosphorylation [37–40].
Therefore, α-synuclein protein deposition into Lewy bodies is a marker of the PD disease state. However, because we require Lewy bodies for a PD diagnosis this isn't an especially strong argument for involvement of α-synuclein in the disease process. It is also important to note that, although we cannot determine if Lewy bodies previously formed in the cells that eventually died, the individual neurons where Lewy bodies are found are the ones that have survived the disease process (though they still may be dysfunctional). Very recently, it has been shown that Lewy bodies form in functional dopaminergic neurons grafted in to brains of people with PD as a therapeutic intervention [41, 42], although this is not always seen [43]. These were embryonic cells that remained apparently healthy and were functional after grafting, which suggests that there is the environment of the PD brain predisposes even young cells to form Lewy bodies.
In summary, the available evidence identifies α-synuclein as a marker of the PD/DLB process but do not prove that it has a causal role. The evidence that it does comes from a variety of human genetic studies.
α-Synuclein can cause PD
A key discovery in understanding PD was the report that an A53T mutation in the α-synuclein gene was causal for dominantly inherited disease [44]. This was the first clear report that a Mendelian gene could be a cause of PD, which to that point had been thought of as a non-genetic disease. It is interesting that the first mutation found was A53T, i.e. a reversion of the human Alanine to the ancestral Threonine found in rodents and many other species. Since then, two other point mutations, A30P [45] and E46K [46], have been reported in different families. It is also important that while many cases are reported to have a phenotype of 'PD', in fact several patients in the A53T and E46K [46] families have a more diffuse involvement of synuclein deposition [47, 48] and clinical features that presumably result from this degree of involvement of non-dopaminergic systems [49].
A second group of important cases have multiplications of the normal wild type allele of SNCA, the gene that encodes for the α-synuclein protein. Cases with SNCA duplication have a brainstem-predominant PD phenotype [50], while cases with a triplication have a Lewy body disease that again involves several brain regions [51, 52]. Measurements of protein levels in triplication show the predicted doubling of α-synuclein in blood as well as increased levels and deposition of the protein in the cerebral cortex where Lewy bodies are found [21]. Therefore, even without sequence variants, α-synuclein dosage can be causal for Lewy body disease.
A third piece of genetic evidence comes from the reports common variants around the α-synuclein gene are associated with lifetime risk of sporadic PD. Both the promoter region, specifically the Rep1 polymorphic repeat [53], and polymorphisms towards the 3' end of the gene are associated with PD [54]. Although it is not known specifically how these risk variants influence lifetime incidence of PD, it seems likely that they increase α-synuclein protein levels in the brain.
Collectively, the human genetic data strongly support a causal role for α-synuclein in PD/DLBD. Whether Lewy bodies are causal or consequential is less clear, but they do support the idea that α-synuclein represents an important link between sporadic and inherited PD. The various lines of evidence identify α-synuclein as a potentially toxic protein, fulfilling the requirements of a causative agent in PD [55]. The question now is how, and in what context, is α-synuclein toxic, and can we do anything about it?
Where and when is α-synuclein toxic?
Given that cell loss is a major event in human PD, combined with the evidence that α-synuclein plays a causal role in disease, it is reasonable to infer that α-synuclein is toxic to human neurons. The time course is likely to be protracted, with the most likely explanation that there is asynchronous cell death that results in a slow depletion of the populations of relatively vulnerable neurons. However, it is not possible to watch cells die in the human brain and so we have to turn to experimental models to confirm or refute the idea that α-synuclein is toxic.
Yeast models are probably the simplest system used to show that expression of human α-synuclein evokes toxic events. In growing and stationary phase cultures, increased expression of α-synuclein limits cell growth [56–65]. These experiments are extraordinarily useful in defining pathways that underpin the toxic effects of the protein. α-Synuclein toxicity has also been demonstrated in Drosophila, where dopaminergic neuron cell loss has been reported [66–73], although this result is a little controversial [74] and the effects are modest. The worm C. elegans can also be used to show that α-synuclein can damage dopamine neurons in an intact, in vivo, setting [75–80]. What links these three model systems is that they all show a detrimental effect of expression of α-synuclein in organisms where the protein is not normally present. One reading of this data is that, at least in terms of toxicity occurring over days to weeks, the normal function of the protein is probably not relevant.
A situation where α-synuclein is normally present is in mammalian cell culture models. Two commonly used systems are primary neurons, including dopaminergic cultures of the ventral midbrain, or neuroblastoma derived cell lines. Experiments showing the most substantial effects of α-synuclein include those where the protein is transiently expressed, e.g. from viral vectors [81–86], or expression is controlled from an inducible promoter system [87–89], although some authors have reported a lack of toxicity in similar circumstances [90]. In midbrain cultures, toxicity is higher for dopamine neurons than other cells [81], which may be relevant to the relative vulnerability of nigral neurons in PD. Some experiments show nicely that the difference between wild type and mutant protein is really a matter of dose and that at increasing expression levels, the normal protein becomes as toxic as the dominant mutants [89].
Although potentially useful in for understanding mechanisms, these cell-based models are taken out of their in vivo context and tend to show cell loss over a few days, compared to the predicted years of progress in the disease. A more intact approach is to express α-synuclein using transgenic technology in various parts of the mouse CNS. Some of these models show toxicity, particularly in the spinal cord, but nigral cell loss is absent or modest [91–97]. Several models do show accumulation and insolubility of α-synuclein [e.g., [36, 91, 93, 98]], although whether true Lewy bodies are formed is uncertain. Therefore, most mouse models reported to date are better for understanding α-synuclein deposition than frank cellular toxicity. Why this is the case is unclear, but it is interesting that crossing transgenic models with mouse α-synuclein knockouts exacerbates phenotypes [99–101], suggesting that the presence of the murine protein limits damage in some undefined way. The lack of an ideal PD mouse model that more completely captures the human phenotype limits our current studies of α-synuclein toxicity. Though a goal worth pursuing, creation of such an ideal mouse model may be very challenging given the limitations of mouse lifespan and differences in physiology between mice and humans.
An alternate approach to traditional transgenics is to use viral vectors to deliver α-synuclein directly to the substantia nigra in mice [102], rats [103–106] or non-human primates [107–109]. In these approaches, a significant cell loss is noted along with deposition of α-synuclein protein. The extent of cell loss is less dramatic than in human PD and behavioral effects are similarly modest. However, the critical observation here is that α-synuclein can induce toxicity in vivo using vertebrate organisms, with a time course of several weeks, allowing for some dissection of mechanism.
Taken together, all of this evidence suggests that α-synuclein can induce toxicity in a variety of contexts, from simple organisms to dopamine neurons in the primate substantia nigra. It is less clear whether all of these situations are directly relevant to the human disease, where cell loss is probably more protracted, but as a practical matter such models at least afford an opportunity to examine mechanism(s) by which α-synuclein triggers neuronal death.
Why is α-synuclein toxic?
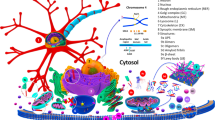
Some of the above model systems have been used to probe the mechanism(s) by which α-synuclein causes cell death. These can generally be sorted into aspects of the protein itself effects of the protein to the biological system (see figure 1). Appendix 1 highlights some of the key observations related to this critical question.
Events in α-synuclein toxicity. The central panel shows the major pathway for protein aggregation. Monomeric α-synuclein is natively unfolded in solution but can also bind to membranes in an α-helical form. It seems likely that these two species exist in equilibrium within the cell, although this is unproven. From in vitro work, it is clear that unfolded monomer can aggregate first into small oligomeric species that can be stabilized by β-sheet-like interactions and then into higher molecular weight insoluble fibrils. In a cellular context, there is some evidence that the presence of lipids can promote oligomer formation: α-synuclein can also form annular, pore-like structures that interact with membranes. The deposition of α-synuclein into pathological structures such as Lewy bodies is probably a late event that occurs in some neurons. On the left hand side are some of the known modifiers of this process. Electrical activity in neurons changes the association of α-synuclein with vesicles and may also stimulate polo-like kinase 2 (PLK2), which has been shown to phosphorylate α-synuclein at Ser129. Other kinases have also been proposed to be involved. As well as phosphorylation, truncation through proteases such as calpains, and nitration, probably through nitric oxide (NO) or other reactive nitrogen species that are present during inflammation, all modify synuclein such that it has a higher tendency to aggregate. The addition of ubiquitin (shown as a black spot) to Lewy bodies is probably a secondary process to deposition. On the right are some of the proposed cellular targets for α-synuclein mediated toxicity, which include (from top to bottom) ER-golgi transport, synaptic vesicles, mitochondria and lysosomes and other proteolytic machinery. In each of these cases, it is proposed that α-synuclein has detrimental effects, listed below each arrow, although at this time it is not clear if any of these are either necessary or sufficient for toxicity in neurons.
Aspects of protein chemistry of α-synuclein and toxicity
α-Synuclein has a strong tendency to self-associate in vitro [110, 111], and so a prime candidate for the underlying driving force for toxicity is the formation of aggregated species. One of the important questions about this idea is which species are present in cells/tissues. Oligomeric species can be isolated from cells [112–114] and from human [21] and mouse (both wild type and α-synuclein transgenic) brain [115]. In both cells and brain, oligomers are particularly found in membrane-enriched fractions [112, 115], suggesting a possible influence of the lipid environment on oligomer formation. Higher molecular weight forms have also been found in some models [116], especially after oxidative stress [117] or exposure to inflammatory triggers in mice [100]. Deposited α-synuclein immunoreactivity has been seen in transgenic [91–97] or viral models [102–109]. However, the observation of aggregated α-synuclein by and of itself does not prove that aggregation is important; as discussed for Lewy bodies, all this proves is that deposition occurs, not that it is causal.
Some recent studies have attempted to answer this question, mainly using cell-based approaches. For example, some oligomeric forms of α-synuclein trigger calcium entry and toxicity in SY5Y cells [118]. Interestingly, different species show differential toxicity, suggesting that not all oligomers are created equal. However, the nature of this experiment is to add α-synuclein to the outside of the cell, which may or may not be relevant to the pathophysiological situation. As α-synuclein is intracellular, it seems more likely that the protein would form aggregate inside cells. The presence of fibrils in Lewy bodies would support this contention. However, α-synuclein can end up in the extracellular media [119] and it is possible that the conditions for aggregation might be more suitable in a milieu free of cells. The relevance of extracellular α-synuclein is an important question, raised also by the observation of Lewy bodies in grafted neurons [41, 42] and the attendant hypothesis of 'host to graft transmission'.
Some studies have attempted to address whether intracellular aggregates of α-synuclein contribute to toxicity. For example, several imaging techniques shown that, in the context of a living cell, α-synuclein can form small oligomers, likely in an antiparallel configuration [114, 120] and such oligomers can be associated with cell toxicity.
These approaches have been used to show that overexpression of heat shock proteins (Hsps) can mitigate both oligomer formation and toxicity [114, 120, 121]. In vivo, Hsps can prevent toxic effects of α-synuclein in yeast [59] and in flies [67]. Whether these studies constitute formal proof that aggregation is required for toxicity is unclear as there are other theoretical interpretations of the data. For example, a formal possibility is that monomeric α-synuclein is toxic and, thus, any protein binding the protein directly could limit toxicity. It should be stated that the mechanism(s) by which monomers of α-synuclein could be toxic are relatively unexplored but, equally, there is an absence of proof that aggregation is absolutely required for toxicity. Alternatively, Hsps could be limiting a detrimental event downstream of the initial aggregation and thus may neither represent evidence for or against the role of aggregation in α-synuclein toxicity. Interestingly, Hsp expression in the fly model decreases neuronal toxicity without any change in the number of α-synuclein positive inclusions [67].
Overall, these considerations show that α-synuclein is capable of protein aggregation and can be deposited into inclusion bodies of various forms in vivo, but that there is insufficient evidence that aggregation or deposition is either necessary or sufficient for toxicity. In fact, several lines of evidence show that toxicity can be dissociated from deposition, including; the observation in cells of toxicity without deposition in some models [81]; differential effects on toxicity and inclusions of various manipulations of α-synuclein in fly models [66, 67]; and deposition of α-synuclein without clear toxic effects in some mouse models [e.g., [36]]. A key challenge for the field, therefore, is to understand whether protein aggregation is at all relevant for the toxic effects of α-synuclein. One way to potentially address this is to isolate various aggregated species of the protein and to express them within a neuron. This might be extraordinarily difficult from a technical standpoint and there is always possibility that the small aggregates would seed larger ones may confound interpretation. Another potential approach would be to develop reagents that limit the biological availability of specific aggregated species and use these to probe which agents are toxic in intact cells. As an example, recombinant single chain Fv antibody fragments against aggregated α-synuclein have been described [122, 123] that might be helpful.
α-Synuclein has many additional properties as well as the tendency to aggregate. Some of the post-translational modifications that have been reported have also been explored as possible mediators of toxicity. For example, antibodies against α-synuclein phosphorylated at Ser129 are very good at identifying Lewy pathology in the human brain [38], suggesting either that Ser129 phosphorylation is a causal event for deposition or represents a common modification of the protein after it is deposited. Several groups have therefore made versions of α-synuclein that cannot be modified at this residue (S129A) or pseudo-phosphorylation mimics (S129D, S129E) and determined the toxic effects of expression. In Drosophila models, S129A is less toxic but has an increased tendency to form inclusion bodies compared to wild type protein [66]. The S129D phosphomimic has the opposite effect, i.e. increased toxicity but fewer inclusions. In contrast, similar experiments using viral overexpression in rats show the opposite result, namely that S129A greatly increases the toxic effects of expression [124]. In mammalian cell culture, S129A has a diminished tendency to form inclusion bodies [125].
At first glance, these results seem to suggest that the behavior of α-synuclein as it relates to toxicity is opposite in mammals compared to invertebrates where, it is important to note, the protein is not normally present. However, interpretation is complicated by several considerations. First, the expression levels of α-synuclein are critical for toxicity, which is shown by the human case where a difference in protein levels is 2-fold in the triplication cases and 1.5-fold in the duplication cases. Second, recent data suggests that the phosphomimic S129D/E α-synuclein variants have different biophysical properties compared to authentically phosphorylated wild type protein [126]. Overall, these considerations raise some important caveats about comparison of properties of α-synuclein in terms of concentration-dependent behaviors of the protein such as aggregation and toxicity.
One alternate approach to understand α-synuclein phosphorylation is to identify the kinase that mediates the phosphotransfer event. Casein kinase II and GRK2/5 have been shown to phosphorylate α-synuclein in vitro or in cells and work in yeast [64] and flies [66] respectively shows that they are at least active in vivo. More recently, the polo-like kinase family, specifically PLK2, have been shown to be active both in vitro and in vivo in generating pS129 α-synuclein [127]. What is interesting about PLK2 is that it is known to respond to neuronal activity [128], suggesting a possible link between neuronal phenotype and α-synuclein toxicity. However, it is not yet known in PLK2 inhibitors or gene knockout will limit the toxic effects of α-synuclein in vivo. Such experiments are feasible in several species as PLK2 homologues are present in mice and flies, and there is at least one polo kinase in yeast.
There are a number of other modifications of α-synuclein that have been reported and some of these are found more often in pathological circumstances than under normal conditions, such as nitration or truncation. Truncation of α-synuclein is associated with a higher tendency for aggregation [129–131]. Transgenic mice expressing truncated α-synuclein have substantial cell loss [101] although in at least one line, this is a developmental and not degenerative phenotype [132]. Again, because the window for toxicity is quite narrow, comparison between different lines is difficult. One question that arises for truncation is where such species are generated. α-Synuclein is predominantly degraded by lysosomal pathways [133, 134], including chaperone-mediated autophagy [135], and the lysosomal cathepsins are important in proteolysis. Therefore, some truncated species are found in the lysosomes and it seems unlikely that they would cause damage to the cell. However, α-synuclein is also a substrate for cytoplasmic calpains [136–139], which are therefore more likely to generate cytoplasmic toxic truncated species. Some detail is therefore needed to prove which truncated species mediate toxicity, if any of them in fact do.
Oxidative stress, including the neurotransmitter dopamine, has been linked to increased α-synuclein aggregation [89, 140]. Dopamine itself may contribute to the toxic effects of α-synuclein in vitro [89], although such a mechanism cannot explain why non-dopaminergic neurons die early in the disease process. α-Synuclein expression can enhance sensitivity to oxidative and nitrative stressors [141, 142], although it can also be protective in some situations [143]. In most of these situations, the role of aggregation is unclear.
In summary, α-synuclein has properties, including the potential for aggregation and post-translational modifications, which may influence its toxic effects. Whether these are required for toxicity is unclear, and some results still need to be resolved, for example for the work on S129 phosphorylation. However, there is a larger question, which is: what effects synuclein has on neurons that are responsible for its toxic effects?
Mediators of α-synuclein toxicity in biological systems
Some of the relevant data from cellular systems has been reviewed previously [144] and will be discussed here in the context of examples across multiple models.
Presumably, α-synuclein might interact with other biomolecules to mediate toxicity. Because α-synuclein can associate with lipids, membranes are one possible target. In vitro, α-synuclein can form pore-like structures [145, 146], and annular rings of synuclein have been isolated from the brains of patients with multiple system atrophy, a synucleinopathy [147]. Cells expressing α-synuclein have increased cation permeability [148] and vesicles prepared from cultured cells or isolated from the adrenal medulla show leakage of catecholamines [149]. These events may be consistent with the formation of non-specific pores or similar structures at the plasma membrane or at a vesicle surface.
Because α-synuclein binds synaptic vesicles, it is possible that synaptic transmission would be directly or indirectly a target of synuclein toxicity. One example of this comes from work showing that A30P α-synuclein alters exocytosis of catecholamine containing vesicles in primary cells and in chromaffin cells [150]. The effect here is probably at a late stage of the exocytosis, before vesicle membrane fusion [150].
Further evidence for an effect of α-synuclein on vesicle function that may mediate toxicity comes from suppressor screens in yeast [63]. In the same organism, such defects can be localized to a block in endoplasmic reticulum (ER)-golgi vesicular trafficking [151]. Supporting this idea, there is evidence of ER stress [87] and golgi fragmentation [152] in mammalian cell systems.
Overexpression of Rab1, a GTPase that influences vesicle dynamics, was able to at least partially rescue the toxic effects of α-synuclein in yeast, worms and in mammalian cells [151]. Therefore, some of the toxic effects of α-synuclein that are conserved across species involve damage to vesicular transport, which might express itself as damage to presynaptic vesicle release in a neuron.
There are also suggestions that other membranous organelles are affected by α-synuclein, including mitochondria [87, 88, 153]. Recent data suggests that a portion of α-synuclein can localize to mitochondria, at least under some conditions [154–157]. Supporting this are observations that α-synuclein expression increases cellular organismal sensitivity to rotenone, a mitochondrial complex I inhibitor [78, 158]. Furthermore, intact mitochondrial function is required for a-synuclein toxicity in a yeast model, although it should also be noted that removal of mitochondria is also quite damaging in the same context [57]. The mechanism by which α-synuclein interacts with and causes damage to mitochondria is not fully resolved and, given the central role of mitochondria in apoptotic pathways, perhaps such effects are secondary to the induction of apoptosis. Increased levels of α-synuclein are reported to trigger apoptosis in various cell types [159–161]. Several apoptotic markers are also seen in yeast models of synuclein toxicity [59]. α-Synuclein toxicity can be rescued by caspase inhibitors or knock down of caspase-12 [87]. Activation of caspase-3 has been reported in transgenic mice [162] caspase-9 has been reported in viral models in mice [102] and rats [106]. However, these studies show only a few caspase positive cells, and so whether apoptosis is the only way in which cells expressing α-synuclein die remains unclear.
α-Synuclein can bind to the membranes of lysosomes [135] and inhibit lysosomal function [163] and chaperone-mediated autophagy [135]. Recent results suggest that CMA is implicated in the regulation of the transcription factor MEF2D and that this can be disrupted by expression of α-synuclein, leading to neuronal death [164]. As another example of misregulated protein turnover, α-synuclein (and specifically α-synuclein oligomers) can also inhibit the proteasome [81, 88, 163, 165–167], although it is not clear if the predicted altered turnover of proteasome substrates occurs in vivo [168].
The general principle is that multiple systems can be affected by α-synuclein expression and that if there is a common theme between them, it is likely to be that α-synuclein can binds lipids. Several lines of evidence suggest that lipid binding can promote the formation of oligomers [115, 145, 169]. Therefore, this interpretation links a primary protein abnormality to cellular targets of the protein. As discussed elsewhere [144], determining which events are truly primary and which are secondary remains a challenge. Although this distinction is an intellectual problem, it may also be relevant for deciding which aspects of cell death to target if we want to limit the disease process in PD.
Potential therapeutic approaches related to α-synuclein toxicity
One of the key questions here is to decide whether to try and target the protein or the process that mediates cellular damage. Both are attractive for different reasons, although both are also difficult (see figure 1 for where these might be utilized and Appendix 2 for the critical next steps).
If there were a pathogenic aggregated form of α-synuclein, then one tactic would be to target that species. If we propose that insoluble fibrils are toxic, then a 'fibril-buster' would be the way forward [reviewed in [111]], but if soluble oligomers damage cells then we would want to prevent their formation or encourage their turnover. As discussed above, both fibrils and oligomers can be found in different models and either alone, or both, could be toxic. For oligomers, the situation is more complicated if different oligomeric forms have different toxic properties [118], suggesting that we may need to be careful about which oligomers we target.
Alternatively, we could be agnostic about which species are important and try and decrease all α-synuclein expression. There are reports that increasing autophagy can help clear aggregation-prone proteins, including α-synuclein [170]. Antisense approaches might also be helpful, and have been reported to work in the rat [171] and mouse [172] brain. This approach is predicated on the idea that α-synuclein really is dispensable for CNS function in humans, as it appears to be in the mouse [28, 30], but perhaps even a modest decrease in protein levels would be enough to decrease PD progression.
We might also try to change the modifications of α-synuclein, especially if these are specific for pathogenic forms. For example, example of PLK2 as a kinase for Ser129 [127] may provide a way to test the idea that phosphorylation at this residue is key for pathogenesis, if sufficiently specific kinase inhibitors can be developed. Again, assuming specificity can be achieved, it might be interesting to block other modifications such as truncation or nitrosylation – the latter might be part of the general rubric of anti-inflammatory approaches. However, such approaches would only be helpful if the modification is truly specific for the pathogenic form and makes an active contribution to cellular toxicity, ie is not a bystander in the process.
Finally, we may target one or more of the cellular effects of α-synuclein that are associated with toxicity. This might have the advantage of leaving the protein alone, which may be useful if it turns out that α-synuclein has a specific function in the human brain. The difficulty, of course, is in understanding why the protein is toxic, although the work with Rab1 [151, 173] suggests that this is a tractable problem, at least in principle.
Conclusion
Cell death is a significant part of the pathology of PD. Although the process is a mysterious, the prime suspect for a toxic protein is α-synuclein. Assuming toxicity does indeed result from aberrant forms of the protein, including increased expression of the normal gene, there are two major aspects that might be targeted therapeutically. First, the protein is prone to aggregate and anti-aggregative compounds, or approaches to simply limit net expression levels, may be helpful. Second, there are a number of molecular events that largely revolve around membrane or organelle interactions that may contribute to toxicity, and these too may be targeted therapeutically. Future work should be directed at exploring these possibilities as well as at developing models that have a stronger cell death signal, to more accurately represent the substantive loss of neurons seen in PD.
Appendix 1: key observations
The role of α-synuclein in PD and related disease is highlighted by the convergence of pathological and genetic data. Because part of the pathological phenotype of PD involves cell death of neurons, particularly but not exclusively dopamine neurons in the substantia nigra pars compacta, this suggests that α-synuclein may be a toxic protein. The following key observations have been made in various experimental systems to support this contention:
- In pure in vitro assays, α-synuclein shows a lack of conformational restraint that tends to promote inappropriate aggregation. This can be enhanced by mutation, increasing concentration or any of several protein modifications associated with pathological deposition of the protein in vivo. α-Synuclein can also bind lipids and membranes in vitro
- In a variety of species, expression of α-synuclein can promote toxic events. These include organisms such as yeast, worms and flies, where no α-synuclein homologue is present, suggesting that irrespective of its normal function, the protein can be toxic.
- Data in mammalian cell culture also supports a toxic effect of α-synuclein, particularly to dopaminergic cells. Results in intact in vivo systems are mixed, with toxicity limited to the spinal cord in some transgenic mouse models and modest toxic effects to dopaminergic neurons using viral mediated overexpression in rodents and non-human primates.
- The mechanism(s) involved are currently unclear, but binding to several cellular membranes may contribute to toxic events.
Appendix 2: critical next steps
The following critical issues need to be addressed before our understanding of α-synuclein pathobiology can be applied to therapeutic development:
- We need to better understand normal function of α-synuclein, such that we can assess both what role it might play in toxicity in the mammalian CNS and so we can highlight potential detrimental effects of limiting expression or function of the protein.
- We need to clearly identify which cellular pathways contribute to the pathological effects of the protein. Some great work has been performed in yeast models that highlight interruption of vesicle transport, but it is important now to establish what the analogous process is in neurons and whether this is sufficient to explain α-synuclein toxicity in this system.
- We need to develop models where there is a lesion that better approximates the severity of cell loss seen in human PD. This will allow for a more rigorous test of pathways involved in toxicity as the disease progresses. An accelerated time course would be helpful, and may be necessary, but the pathology should be similar to human PD in that nigral neurons should be affected at some point in the model but not necessarily first or exclusively.
Abbreviations
- DLB/DLBD:
-
Dementia with Lewy bodies/Diffuse Lewy Body Disease
- ER:
-
endoplasmic reticulum
- L-DOPA:
-
3,4-dihydroxy-L-phenylalanine
- PD:
-
Parkinson disease.
References
Brooks DJ: The early diagnosis of Parkinson's disease. Ann Neurol. 1998, 44: S10-18. 10.1002/ana.410440107.
Chu Y, Kordower JH: Age-associated increases of alpha-synuclein in monkeys and humans are associated with nigrostriatal dopamine depletion: Is this the target for Parkinson's disease?. Neurobiol Dis. 2007, 25: 134-149. 10.1016/j.nbd.2006.08.021.
Thal DR, Del Tredici K, Braak H: Neurodegeneration in normal brain aging and disease. Sci Aging Knowledge Environ. 2004, 2004: pe26-10.1126/sageke.2004.23.pe26.
Dickson DW: Linking selective vulnerability to cell death mechanisms in Parkinson's disease. Am J Pathol. 2007, 170: 16-19. 10.2353/ajpath.2007.061011.
Hirsch EC: Biochemistry of Parkinson's disease with special reference to the dopaminergic systems. Mol Neurobiol. 1994, 9: 135-142. 10.1007/BF02816113.
Chung CY, Seo H, Sonntag KC, Brooks A, Lin L, Isacson O: Cell type-specific gene expression of midbrain dopaminergic neurons reveals molecules involved in their vulnerability and protection. Hum Mol Genet. 2005, 14: 1709-1725. 10.1093/hmg/ddi178.
Surmeier DJ: Calcium, ageing, and neuronal vulnerability in Parkinson's disease. Lancet Neurol. 2007, 6: 933-938. 10.1016/S1474-4422(07)70246-6.
Langston JW: The Parkinson's complex: parkinsonism is just the tip of the iceberg. Ann Neurol. 2006, 59: 591-596. 10.1002/ana.20834.
Gai WP, Blumbergs PC, Geffen LB, Blessing WW: Age-related loss of dorsal vagal neurons in Parkinson's disease. Neurology. 1992, 42: 2106-2111.
Jellinger KA: Post mortem studies in Parkinson's disease–is it possible to detect brain areas for specific symptoms?. J Neural Transm Suppl. 1999, 56: 1-29.
Gai WP, Yuan HX, Li XQ, Power JT, Blumbergs PC, Jensen PH: In situ and in vitro study of colocalization and segregation of alpha-synuclein, ubiquitin, and lipids in Lewy bodies. Exp Neurol. 2000, 166: 324-333. 10.1006/exnr.2000.7527.
Lennox G, Lowe J, Morrell K, Landon M, Mayer RJ: Anti-ubiquitin immunocytochemistry is more sensitive than conventional techniques in the detection of diffuse Lewy body disease. J Neurol Neurosurg Psychiatry. 1989, 52: 67-71. 10.1136/jnnp.52.1.67.
Gibb WR, Scott T, Lees AJ: Neuronal inclusions of Parkinson's disease. Mov Disord. 1991, 6: 2-11. 10.1002/mds.870060103.
Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E: Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003, 24: 197-211. 10.1016/S0197-4580(02)00065-9.
Del Tredici K, Rub U, De Vos RA, Bohl JR, Braak H: Where does parkinson disease pathology begin in the brain?. J Neuropathol Exp Neurol. 2002, 61: 413-426.
Hughes AJ, Daniel SE, Kilford L, Lees AJ: Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992, 55: 181-184. 10.1136/jnnp.55.3.181.
Maroteaux L, Campanelli JT, Scheller RH: Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J Neurosci. 1988, 8: 2804-2815.
George JM, Jin H, Woods WS, Clayton DF: Characterization of a novel protein regulated during the critical period for song learning in the zebra finch. Neuron. 1995, 15: 361-372. 10.1016/0896-6273(95)90040-3.
George JM: The synucleins. Genome Biol. 2002, 3: REVIEWS3002-
Hamilton BA: alpha-Synuclein A53T substitution associated with Parkinson disease also marks the divergence of Old World and New World primates. Genomics. 2004, 83: 739-742. 10.1016/j.ygeno.2003.09.016.
Miller DW, Hague SM, Clarimon J, Baptista M, Gwinn-Hardy K, Cookson MR, Singleton AB: Alpha-synuclein in blood and brain from familial Parkinson disease with SNCA locus triplication. Neurology. 2004, 62: 1835-1838.
Scherzer CR, Grass JA, Liao Z, Pepivani I, Zheng B, Eklund AC, Ney PA, Ng J, McGoldrick M, Mollenhauer B, Bresnick EH, Schlossmacher MG: GATA transcription factors directly regulate the Parkinson's disease-linked gene alpha-synuclein. Proc Natl Acad Sci USA. 2008, 105: 10907-10912. 10.1073/pnas.0802437105.
Jo E, McLaurin J, Yip CM, St George-Hyslop P, Fraser PE: alpha-Synuclein membrane interactions and lipid specificity. J Biol Chem. 2000, 275: 34328-34334. 10.1074/jbc.M004345200.
Withers GS, George JM, Banker GA, Clayton DF: Delayed localization of synelfin (synuclein, NACP) to presynaptic terminals in cultured rat hippocampal neurons. Brain Res Dev Brain Res. 1997, 99: 87-94. 10.1016/S0165-3806(96)00210-6.
Irizarry MC, Kim TW, McNamara M, Tanzi RE, George JM, Clayton DF, Hyman BT: Characterization of the precursor protein of the non-A beta component of senile plaques (NACP) in the human central nervous system. J Neuropathol Exp Neurol. 1996, 55: 889-895.
Fortin DL, Troyer MD, Nakamura K, Kubo S, Anthony MD, Edwards RH: Lipid rafts mediate the synaptic localization of alpha-synuclein. J Neurosci. 2004, 24: 6715-6723. 10.1523/JNEUROSCI.1594-04.2004.
Fortin DL, Nemani VM, Voglmaier SM, Anthony MD, Ryan TA, Edwards RH: Neural activity controls the synaptic accumulation of alpha-synuclein. J Neurosci. 2005, 25: 10913-10921. 10.1523/JNEUROSCI.2922-05.2005.
Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A: Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000, 25: 239-252. 10.1016/S0896-6273(00)80886-7.
Steidl JV, Gomez-Isla T, Mariash A, Ashe KH, Boland LM: Altered short-term hippocampal synaptic plasticity in mutant alpha-synuclein transgenic mice. Neuroreport. 2003, 14: 219-223. 10.1097/00001756-200302100-00012.
Cabin DE, Shimazu K, Murphy D, Cole NB, Gottschalk W, McIlwain KL, Orrison B, Chen A, Ellis CE, Paylor R, Lu B, Nussbaum RL: Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking alpha-synuclein. J Neurosci. 2002, 22: 8797-8807.
Martin ED, Gonzalez-Garcia C, Milan M, Farinas I, Cena V: Stressor-related impairment of synaptic transmission in hippocampal slices from alpha-synuclein knockout mice. Eur J Neurosci. 2004, 20: 3085-3091. 10.1111/j.1460-9568.2004.03801.x.
Liu S, Ninan I, Antonova I, Battaglia F, Trinchese F, Narasanna A, Kolodilov N, Dauer W, Hawkins RD, Arancio O: alpha-Synuclein produces a long-lasting increase in neurotransmitter release. Embo J. 2004, 23: 4506-4516. 10.1038/sj.emboj.7600451.
Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M: Alpha-synuclein in Lewy bodies. Nature. 1997, 388: 839-840. 10.1038/42166.
Takeda A, Mallory M, Sundsmo M, Honer W, Hansen L, Masliah E: Abnormal accumulation of NACP/alpha-synuclein in neurodegenerative disorders. Am J Pathol. 1998, 152: 367-372.
Crowther RA, Daniel SE, Goedert M: Characterisation of isolated alpha-synuclein filaments from substantia nigra of Parkinson's disease brain. Neurosci Lett. 2000, 292: 128-130. 10.1016/S0304-3940(00)01440-3.
Kahle PJ, Neumann M, Ozmen L, Muller V, Odoy S, Okamoto N, Jacobsen H, Iwatsubo T, Trojanowski JQ, Takahashi H, Wakabayashi K, Bogdanovic N, Riederer P, Kretzschmar HA, Haass C: Selective insolubility of alpha-synuclein in human Lewy body diseases is recapitulated in a transgenic mouse model. Am J Pathol. 2001, 159: 2215-2225.
Anderson JP, Walker DE, Goldstein JM, de Laat R, Banducci K, Caccavello RJ, Barbour R, Huang J, Kling K, Lee M, Diep L, Keim PS, Shen X, Chataway T, Schlossmacher MG, Seubert P, Schenk D, Sinha S, Gai WP, Chilcote TJ: Phosphorylation of Ser-129 is the dominant pathological modification of alpha-synuclein in familial and sporadic Lewy body disease. J Biol Chem. 2006, 281: 29739-29752. 10.1074/jbc.M600933200.
Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, Shen J, Takio K, Iwatsubo T: alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol. 2002, 4: 160-164. 10.1038/ncb841.
Giasson BI, Duda JE, Murray IV, Chen Q, Souza JM, Hurtig HI, Ischiropoulos H, Trojanowski JQ, Lee VM: Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science. 2000, 290: 985-989. 10.1126/science.290.5493.985.
Tofaris GK, Razzaq A, Ghetti B, Lilley KS, Spillantini MG: Ubiquitination of alpha-synuclein in Lewy bodies is a pathological event not associated with impairment of proteasome function. J Biol Chem. 2003, 278: 44405-44411. 10.1074/jbc.M308041200.
Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW: Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson's disease. Nat Med. 2008, 14: 504-506. 10.1038/nm1747.
Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, Lashley T, Quinn NP, Rehncrona S, Bjorklund A, Widner H, Revesz T, Lindvall O, Brundin P: Lewy bodies in grafted neurons in subjects with Parkinson's disease suggest host-to-graft disease propagation. Nat Med. 2008, 14: 501-503. 10.1038/nm1746.
Mendez I, Vinuela A, Astradsson A, Mukhida K, Hallett P, Robertson H, Tierney T, Holness R, Dagher A, Trojanowski JQ, Isacson O: Dopamine neurons implanted into people with Parkinson's disease survive without pathology for 14 years. Nat Med. 2008, 14: 507-509. 10.1038/nm1752.
Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL: Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997, 276: 2045-2047. 10.1126/science.276.5321.2045.
Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O: Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nat Genet. 1998, 18: 106-108. 10.1038/ng0298-106.
Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atares B, Llorens V, Gomez Tortosa E, del Ser T, Munoz DG, de Yebenes JG: The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004, 55: 164-173. 10.1002/ana.10795.
Duda JE, Giasson BI, Mabon ME, Miller DC, Golbe LI, Lee VM, Trojanowski JQ: Concurrence of alpha-synuclein and tau brain pathology in the Contursi kindred. Acta Neuropathol. 2002, 104: 7-11. 10.1007/s00401-002-0563-3.
Yamaguchi K, Cochran EJ, Murrell JR, Polymeropoulos MH, Shannon KM, Crowther RA, Goedert M, Ghetti B: Abundant neuritic inclusions and microvacuolar changes in a case of diffuse Lewy body disease with the A53T mutation in the alpha-synuclein gene. Acta Neuropathol. 2005, 110: 298-305. 10.1007/s00401-005-1042-4.
Markopoulou K, Dickson DW, McComb RD, Wszolek ZK, Katechalidou L, Avery L, Stansbury MS, Chase BA: Clinical, neuropathological and genotypic variability in SNCA A53T familial Parkinson's disease. Variability in familial Parkinson's disease. Acta Neuropathol. 2008, 116: 25-35. 10.1007/s00401-008-0372-4.
Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, Levecque C, Larvor L, Andrieux J, Hulihan M, Waucquier N, Defebvre L, Amouyel P, Farrer M, Destee A: Alpha-synuclein locus duplication as a cause of familial Parkinson's disease. Lancet. 2004, 364: 1167-1169. 10.1016/S0140-6736(04)17103-1.
Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K: alpha-Synuclein locus triplication causes Parkinson's disease. Science. 2003, 302: 841-10.1126/science.1090278.
Fuchs J, Nilsson C, Kachergus J, Munz M, Larsson EM, Schule B, Langston JW, Middleton FA, Ross OA, Hulihan M, Gasser T, Farrer MJ: Phenotypic variation in a large Swedish pedigree due to SNCA duplication and triplication. Neurology. 2007, 68: 916-922. 10.1212/01.wnl.0000254458.17630.c5.
Maraganore DM, de Andrade M, Elbaz A, Farrer MJ, Ioannidis JP, Kruger R, Rocca WA, Schneider NK, Lesnick TG, Lincoln SJ, Hulihan MM, Aasly JO, Ashizawa T, Chartier-Harlin MC, Checkoway H, Ferrarese C, Hadjigeorgiou G, Hattori N, Kawakami H, Lambert JC, Lynch T, Mellick GD, Papapetropoulos S, Parsian A, Quattrone A, Riess O, Tan EK, Van Broeckhoven C: Collaborative analysis of alpha-synuclein gene promoter variability and Parkinson disease. JAMA. 2006, 296: 661-670. 10.1001/jama.296.6.661.
Mueller JC, Fuchs J, Hofer A, Zimprich A, Lichtner P, Illig T, Berg D, Wullner U, Meitinger T, Gasser T: Multiple regions of alpha-synuclein are associated with Parkinson's disease. Ann Neurol. 2005, 57: 535-541. 10.1002/ana.20438.
Cookson MR: The biochemistry of Parkinson's disease. Annu Rev Biochem. 2005, 74: 29-52. 10.1146/annurev.biochem.74.082803.133400.
Brandis KA, Holmes IF, England SJ, Sharma N, Kukreja L, DebBurman SK: alpha-Synuclein fission yeast model: concentration-dependent aggregation without plasma membrane localization or toxicity. J Mol Neurosci. 2006, 28: 179-191. 10.1385/JMN:28:2:179.
Buttner S, Bitto A, Ring J, Augsten M, Zabrocki P, Eisenberg T, Jungwirth H, Hutter S, Carmona-Gutierrez D, Kroemer G, Winderickx J, Madeo F: Functional mitochondria are required for alpha-synuclein toxicity in aging yeast. J Biol Chem. 2008, 283: 7554-7560. 10.1074/jbc.M708477200.
Dixon C, Mathias N, Zweig RM, Davis DA, Gross DS: Alpha-synuclein targets the plasma membrane via the secretory pathway and induces toxicity in yeast. Genetics. 2005, 170: 47-59. 10.1534/genetics.104.035493.
Flower TR, Chesnokova LS, Froelich CA, Dixon C, Witt SN: Heat shock prevents alpha-synuclein-induced apoptosis in a yeast model of Parkinson's disease. J Mol Biol. 2005, 351: 1081-1100. 10.1016/j.jmb.2005.06.060.
Liang J, Clark-Dixon C, Wang S, Flower TR, Williams-Hart T, Zweig R, Robinson LC, Tatchell K, Witt SN: Novel suppressors of alpha-synuclein toxicity identified using yeast. Hum Mol Genet. 2008, 17: 3784-3795. 10.1093/hmg/ddn276.
Outeiro TF, Lindquist S: Yeast cells provide insight into alpha-synuclein biology and pathobiology. Science. 2003, 302: 1772-1775. 10.1126/science.1090439.
Sharma N, Brandis KA, Herrera SK, Johnson BE, Vaidya T, Shrestha R, Debburman SK: alpha-Synuclein budding yeast model: toxicity enhanced by impaired proteasome and oxidative stress. J Mol Neurosci. 2006, 28: 161-178. 10.1385/JMN:28:2:161.
Willingham S, Outeiro TF, DeVit MJ, Lindquist SL, Muchowski PJ: Yeast genes that enhance the toxicity of a mutant huntingtin fragment or alpha-synuclein. Science. 2003, 302: 1769-1772. 10.1126/science.1090389.
Zabrocki P, Bastiaens I, Delay C, Bammens T, Ghillebert R, Pellens K, De Virgilio C, Van Leuven F, Winderickx J: Phosphorylation, lipid raft interaction and traffic of alpha-synuclein in a yeast model for Parkinson. Biochim Biophys Acta. 2008, 1783: 1767-1780. 10.1016/j.bbamcr.2008.06.010.
Zabrocki P, Pellens K, Vanhelmont T, Vandebroek T, Griffioen G, Wera S, Van Leuven F, Winderickx J: Characterization of alpha-synuclein aggregation and synergistic toxicity with protein tau in yeast. FEBS J. 2005, 272: 1386-1400. 10.1111/j.1742-4658.2005.04571.x.
Chen L, Feany MB: Alpha-synuclein phosphorylation controls neurotoxicity and inclusion formation in a Drosophila model of Parkinson disease. Nat Neurosci. 2005, 8: 657-663. 10.1038/nn1443.
Auluck PK, Chan HY, Trojanowski JQ, Lee VM, Bonini NM: Chaperone suppression of alpha-synuclein toxicity in a Drosophila model for Parkinson's disease. Science. 2002, 295: 865-868. 10.1126/science.1067389.
Park SS, Lee D: Selective loss of dopaminergic neurons and formation of Lewy body-like aggregations in alpha-synuclein transgenic fly neuronal cultures. Eur J Neurosci. 2006, 23: 2908-2914. 10.1111/j.1460-9568.2006.04844.x.
Haywood AF, Staveley BE: Mutant alpha-synuclein-induced degeneration is reduced by parkin in a fly model of Parkinson's disease. Genome. 2006, 49: 505-510. 10.1139/G06-011.
Periquet M, Fulga T, Myllykangas L, Schlossmacher MG, Feany MB: Aggregated alpha-synuclein mediates dopaminergic neurotoxicity in vivo. J Neurosci. 2007, 27: 3338-3346. 10.1523/JNEUROSCI.0285-07.2007.
Kontopoulos E, Parvin JD, Feany MB: Alpha-synuclein acts in the nucleus to inhibit histone acetylation and promote neurotoxicity. Hum Mol Genet. 2006, 15: 3012-3023. 10.1093/hmg/ddl243.
Auluck PK, Bonini NM: Pharmacological prevention of Parkinson disease in Drosophila. Nat Med. 2002, 8: 1185-1186. 10.1038/nm1102-1185.
Feany MB, Bender WW: A Drosophila model of Parkinson's disease. Nature. 2000, 404: 394-398. 10.1038/35006074.
Pesah Y, Burgess H, Middlebrooks B, Ronningen K, Prosser J, Tirunagaru V, Zysk J, Mardon G: Whole-mount analysis reveals normal numbers of dopaminergic neurons following misexpression of alpha-Synuclein in Drosophila. Genesis. 2005, 41: 154-159. 10.1002/gene.20106.
Kuwahara T, Koyama A, Koyama S, Yoshina S, Ren CH, Kato T, Mitani S, Iwatsubo T: A systematic RNAi screen reveals involvement of endocytic pathway in neuronal dysfunction in alpha-synuclein transgenic C. elegans. Hum Mol Genet. 2008, 17: 2997-3009. 10.1093/hmg/ddn198.
van Ham TJ, Thijssen KL, Breitling R, Hofstra RM, Plasterk RH, Nollen EA: C. elegans model identifies genetic modifiers of alpha-synuclein inclusion formation during aging. PLoS Genet. 2008, 4: e1000027-10.1371/journal.pgen.1000027.
Kuwahara T, Koyama A, Gengyo-Ando K, Masuda M, Kowa H, Tsunoda M, Mitani S, Iwatsubo T: Familial Parkinson mutant alpha-synuclein causes dopamine neuron dysfunction in transgenic Caenorhabditis elegans. J Biol Chem. 2006, 281: 334-340. 10.1074/jbc.M504860200.
Ved R, Saha S, Westlund B, Perier C, Burnam L, Sluder A, Hoener M, Rodrigues CM, Alfonso A, Steer C, Liu L, Przedborski S, Wolozin B: Similar patterns of mitochondrial vulnerability and rescue induced by genetic modification of alpha-synuclein, parkin, and DJ-1 in Caenorhabditis elegans. J Biol Chem. 2005, 280: 42655-42668. 10.1074/jbc.M505910200.
Springer W, Hoppe T, Schmidt E, Baumeister R: A Caenorhabditis elegans Parkin mutant with altered solubility couples alpha-synuclein aggregation to proteotoxic stress. Hum Mol Genet. 2005, 14: 3407-3423. 10.1093/hmg/ddi371.
Lakso M, Vartiainen S, Moilanen AM, Sirvio J, Thomas JH, Nass R, Blakely RD, Wong G: Dopaminergic neuronal loss and motor deficits in Caenorhabditis elegans overexpressing human alpha-synuclein. J Neurochem. 2003, 86: 165-172. 10.1046/j.1471-4159.2003.01809.x.
Petrucelli L, O'Farrell C, Lockhart PJ, Baptista M, Kehoe K, Vink L, Choi P, Wolozin B, Farrer M, Hardy J, Cookson MR: Parkin protects against the toxicity associated with mutant alpha-synuclein: proteasome dysfunction selectively affects catecholaminergic neurons. Neuron. 2002, 36: 1007-1019. 10.1016/S0896-6273(02)01125-X.
Zach S, Bueler H, Hengerer B, Gillardon F: Predominant neuritic pathology induced by viral overexpression of alpha-synuclein in cell culture. Cell Mol Neurobiol. 2007, 27: 505-515. 10.1007/s10571-007-9141-5.
Zhou W, Hurlbert MS, Schaack J, Prasad KN, Freed CR: Overexpression of human alpha-synuclein causes dopamine neuron death in rat primary culture and immortalized mesencephalon-derived cells. Brain Res. 2000, 866: 33-43. 10.1016/S0006-8993(00)02215-0.
Zhou W, Schaack J, Zawada WM, Freed CR: Overexpression of human alpha-synuclein causes dopamine neuron death in primary human mesencephalic culture. Brain Res. 2002, 926: 42-50. 10.1016/S0006-8993(01)03292-9.
Zhou W, Freed CR: DJ-1 up-regulates glutathione synthesis during oxidative stress and inhibits A53T alpha-synuclein toxicity. J Biol Chem. 2005, 280: 43150-43158. 10.1074/jbc.M507124200.
Liu F, Hindupur J, Nguyen JL, Ruf KJ, Zhu J, Schieler JL, Bonham CC, Wood KV, Davisson VJ, Rochet JC: Methionine sulfoxide reductase A protects dopaminergic cells from Parkinson's disease-related insults. Free Radic Biol Med. 2008, 45: 242-255. 10.1016/j.freeradbiomed.2008.03.022.
Smith WW, Jiang H, Pei Z, Tanaka Y, Morita H, Sawa A, Dawson VL, Dawson TM, Ross CA: Endoplasmic reticulum stress and mitochondrial cell death pathways mediate A53T mutant alpha-synuclein-induced toxicity. Hum Mol Genet. 2005, 14: 3801-3811. 10.1093/hmg/ddi396.
Tanaka Y, Engelender S, Igarashi S, Rao RK, Wanner T, Tanzi RE, Sawa A, V LD, Dawson TM, Ross CA: Inducible expression of mutant alpha-synuclein decreases proteasome activity and increases sensitivity to mitochondria-dependent apoptosis. Hum Mol Genet. 2001, 10: 919-926. 10.1093/hmg/10.9.919.
Xu J, Kao SY, Lee FJ, Song W, Jin LW, Yankner BA: Dopamine-dependent neurotoxicity of alpha-synuclein: a mechanism for selective neurodegeneration in Parkinson disease. Nat Med. 2002, 8: 600-606. 10.1038/nm0602-600.
Ko LW, Ko HH, Lin WL, Kulathingal JG, Yen SH: Aggregates assembled from overexpression of wild-type alpha-synuclein are not toxic to human neuronal cells. J Neuropathol Exp Neurol. 2008, 67: 1084-1096. 10.1097/NEN.0b013e31818c3618.
Giasson BI, Duda JE, Quinn SM, Zhang B, Trojanowski JQ, Lee VM: Neuronal alpha-synucleinopathy with severe movement disorder in mice expressing A53T human alpha-synuclein. Neuron. 2002, 34: 521-533. 10.1016/S0896-6273(02)00682-7.
Kahle PJ, Neumann M, Ozmen L, Muller V, Jacobsen H, Schindzielorz A, Okochi M, Leimer U, Putten van Der H, Probst A, Kremmer E, Kretzschmar HA, Haass C: Subcellular localization of wild-type and Parkinson's disease-associated mutant alpha -synuclein in human and transgenic mouse brain. J Neurosci. 2000, 20: 6365-6373.
Lee MK, Stirling W, Xu Y, Xu X, Qui D, Mandir AS, Dawson TM, Copeland NG, Jenkins NA, Price DL: Human alpha-synuclein-harboring familial Parkinson's disease-linked Ala-53 --> Thr mutation causes neurodegenerative disease with alpha-synuclein aggregation in transgenic mice. Proc Natl Acad Sci USA. 2002, 99: 8968-8973. 10.1073/pnas.132197599.
Masliah E, Rockenstein E, Veinbergs I, Mallory M, Hashimoto M, Takeda A, Sagara Y, Sisk A, Mucke L: Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science. 2000, 287: 1265-1269. 10.1126/science.287.5456.1265.
Matsuoka Y, Vila M, Lincoln S, McCormack A, Picciano M, LaFrancois J, Yu X, Dickson D, Langston WJ, McGowan E, Farrer M, Hardy J, Duff K, Przedborski S, Di Monte DA: Lack of nigral pathology in transgenic mice expressing human alpha-synuclein driven by the tyrosine hydroxylase promoter. Neurobiol Dis. 2001, 8: 535-539. 10.1006/nbdi.2001.0392.
Richfield EK, Thiruchelvam MJ, Cory-Slechta DA, Wuertzer C, Gainetdinov RR, Caron MG, Di Monte DA, Federoff HJ: Behavioral and neurochemical effects of wild-type and mutated human alpha-synuclein in transgenic mice. Exp Neurol. 2002, 175: 35-48. 10.1006/exnr.2002.7882.
Putten van der H, Wiederhold KH, Probst A, Barbieri S, Mistl C, Danner S, Kauffmann S, Hofele K, Spooren WP, Ruegg MA, Lin S, Caroni P, Sommer B, Tolnay M, Bilbe G: Neuropathology in mice expressing human alpha-synuclein. J Neurosci. 2000, 20: 6021-6029.
Kahle PJ, Neumann M, Ozmen L, Muller V, Jacobsen H, Spooren W, Fuss B, Mallon B, Macklin WB, Fujiwara H, Hasegawa M, Iwatsubo T, Kretzschmar HA, Haass C: Hyperphosphorylation and insolubility of alpha-synuclein in transgenic mouse oligodendrocytes. EMBO Rep. 2002, 3: 583-588. 10.1093/embo-reports/kvf109.
Cabin DE, Gispert-Sanchez S, Murphy D, Auburger G, Myers RR, Nussbaum RL: Exacerbated synucleinopathy in mice expressing A53T SNCA on a Snca null background. Neurobiol Aging. 2005, 26: 25-35. 10.1016/j.neurobiolaging.2004.02.026.
Gao HM, Kotzbauer PT, Uryu K, Leight S, Trojanowski JQ, Lee VM: Neuroinflammation and oxidation/nitration of alpha-synuclein linked to dopaminergic neurodegeneration. J Neurosci. 2008, 28: 7687-7698. 10.1523/JNEUROSCI.0143-07.2008.
Tofaris GK, Garcia Reitbock P, Humby T, Lambourne SL, O'Connell M, Ghetti B, Gossage H, Emson PC, Wilkinson LS, Goedert M, Spillantini MG: Pathological changes in dopaminergic nerve cells of the substantia nigra and olfactory bulb in mice transgenic for truncated human alpha-synuclein(1–120): implications for Lewy body disorders. J Neurosci. 2006, 26: 3942-3950. 10.1523/JNEUROSCI.4965-05.2006.
St Martin JL, Klucken J, Outeiro TF, Nguyen P, Keller-McGandy C, Cantuti-Castelvetri I, Grammatopoulos TN, Standaert DG, Hyman BT, McLean PJ: Dopaminergic neuron loss and up-regulation of chaperone protein mRNA induced by targeted over-expression of alpha-synuclein in mouse substantia nigra. J Neurochem. 2007, 100: 1449-1457.
Kirik D, Rosenblad C, Burger C, Lundberg C, Johansen TE, Muzyczka N, Mandel RJ, Bjorklund A: Parkinson-like neurodegeneration induced by targeted overexpression of alpha-synuclein in the nigrostriatal system. J Neurosci. 2002, 22: 2780-2791.
Lauwers E, Debyser Z, Van Dorpe J, De Strooper B, Nuttin B, Baekelandt V: Neuropathology and neurodegeneration in rodent brain induced by lentiviral vector-mediated overexpression of alpha-synuclein. Brain Pathol. 2003, 13: 364-372.
Lo Bianco C, Ridet JL, Schneider BL, Deglon N, Aebischer P: alpha -Synucleinopathy and selective dopaminergic neuron loss in a rat lentiviral-based model of Parkinson's disease. Proc Natl Acad Sci USA. 2002, 99: 10813-10818. 10.1073/pnas.152339799.
Yamada M, Iwatsubo T, Mizuno Y, Mochizuki H: Overexpression of alpha-synuclein in rat substantia nigra results in loss of dopaminergic neurons, phosphorylation of alpha-synuclein and activation of caspase-9: resemblance to pathogenetic changes in Parkinson's disease. J Neurochem. 2004, 91: 451-461. 10.1111/j.1471-4159.2004.02728.x.
Eslamboli A, Romero-Ramos M, Burger C, Bjorklund T, Muzyczka N, Mandel RJ, Baker H, Ridley RM, Kirik D: Long-term consequences of human alpha-synuclein overexpression in the primate ventral midbrain. Brain. 2007, 130: 799-815. 10.1093/brain/awl382.
Kirik D, Annett LE, Burger C, Muzyczka N, Mandel RJ, Bjorklund A: Nigrostriatal alpha-synucleinopathy induced by viral vector-mediated overexpression of human alpha-synuclein: a new primate model of Parkinson's disease. Proc Natl Acad Sci USA. 2003, 100: 2884-2889. 10.1073/pnas.0536383100.
Yasuda T, Miyachi S, Kitagawa R, Wada K, Nihira T, Ren YR, Hirai Y, Ageyama N, Terao K, Shimada T, Takada M, Mizuno Y, Mochizuki H: Neuronal specificity of alpha-synuclein toxicity and effect of Parkin co-expression in primates. Neuroscience. 2007, 144: 743-753. 10.1016/j.neuroscience.2006.09.052.
Uversky VN: A protein-chameleon: conformational plasticity of alpha-synuclein, a disordered protein involved in neurodegenerative disorders. J Biomol Struct Dyn. 2003, 21: 211-234.
Uversky VN: Neuropathology, biochemistry, and biophysics of alpha-synuclein aggregation. J Neurochem. 2007, 103: 17-37.
Lee HJ, Choi C, Lee SJ: Membrane-bound alpha-synuclein has a high aggregation propensity and the ability to seed the aggregation of the cytosolic form. J Biol Chem. 2002, 277: 671-678. 10.1074/jbc.M107045200.
Esteves AR, Arduino DM, Swerdlow RH, Oliveira CR, Cardoso SM: Oxidative Stress involvement in alpha-synuclein oligomerization in Parkinsons disease cybrids. Antioxid Redox Signal. 2008
Outeiro TF, Putcha P, Tetzlaff JE, Spoelgen R, Koker M, Carvalho F, Hyman BT, McLean PJ: Formation of toxic oligomeric alpha-synuclein species in living cells. PLoS ONE. 2008, 3: e1867-10.1371/journal.pone.0001867.
Sharon R, Bar-Joseph I, Frosch MP, Walsh DM, Hamilton JA, Selkoe DJ: The formation of highly soluble oligomers of alpha-synuclein is regulated by fatty acids and enhanced in Parkinson's disease. Neuron. 2003, 37: 583-595. 10.1016/S0896-6273(03)00024-2.
Lee HJ, Lee SJ: Characterization of cytoplasmic alpha-synuclein aggregates. Fibril formation is tightly linked to the inclusion-forming process in cells. J Biol Chem. 2002, 277: 48976-48983. 10.1074/jbc.M208192200.
Ostrerova-Golts N, Petrucelli L, Hardy J, Lee JM, Farer M, Wolozin B: The A53T alpha-synuclein mutation increases iron-dependent aggregation and toxicity. J Neurosci. 2000, 20: 6048-6054.
Danzer KM, Haasen D, Karow AR, Moussaud S, Habeck M, Giese A, Kretzschmar H, Hengerer B, Kostka M: Different species of alpha-synuclein oligomers induce calcium influx and seeding. J Neurosci. 2007, 27: 9220-9232. 10.1523/JNEUROSCI.2617-07.2007.
Lee HJ, Patel S, Lee SJ: Intravesicular localization and exocytosis of alpha-synuclein and its aggregates. J Neurosci. 2005, 25: 6016-6024. 10.1523/JNEUROSCI.0692-05.2005.
Klucken J, Outeiro TF, Nguyen P, McLean PJ, Hyman BT: Detection of novel intracellular alpha-synuclein oligomeric species by fluorescence lifetime imaging. Faseb J. 2006, 20: 2050-2057. 10.1096/fj.05-5422com.
Yu F, Xu H, Zhuo M, Sun L, Dong A, Liu X: Impairment of redox state and dopamine level induced by alpha-synuclein aggregation and the prevention effect of hsp70. Biochem Biophys Res Commun. 2005, 331: 278-284. 10.1016/j.bbrc.2005.03.148.
Emadi S, Barkhordarian H, Wang MS, Schulz P, Sierks MR: Isolation of a human single chain antibody fragment against oligomeric alpha-synuclein that inhibits aggregation and prevents alpha-synuclein-induced toxicity. J Mol Biol. 2007, 368: 1132-1144. 10.1016/j.jmb.2007.02.089.
Emadi S, Liu R, Yuan B, Schulz P, McAllister C, Lyubchenko Y, Messer A, Sierks MR: Inhibiting aggregation of alpha-synuclein with human single chain antibody fragments. Biochemistry. 2004, 43: 2871-2878. 10.1021/bi036281f.
Gorbatyuk OS, Li S, Sullivan LF, Chen W, Kondrikova G, Manfredsson FP, Mandel RJ, Muzyczka N: The phosphorylation state of Ser-129 in human alpha-synuclein determines neurodegeneration in a rat model of Parkinson disease. Proc Natl Acad Sci USA. 2008, 105: 763-768. 10.1073/pnas.0711053105.
Smith WW, Margolis RL, Li X, Troncoso JC, Lee MK, Dawson VL, Dawson TM, Iwatsubo T, Ross CA: Alpha-synuclein phosphorylation enhances eosinophilic cytoplasmic inclusion formation in SH-SY5Y cells. J Neurosci. 2005, 25: 5544-5552. 10.1523/JNEUROSCI.0482-05.2005.
Paleologou KE, Schmid AW, Rospigliosi CC, Kim HY, Lamberto GR, Fredenburg RA, Lansbury PT, Fernandez CO, Eliezer D, Zweckstetter M, Lashuel HA: Phosphorylation at Ser-129 but not the phosphomimics S129E/D inhibits the fibrillation of alpha-synuclein. J Biol Chem. 2008, 283: 16895-16905. 10.1074/jbc.M800747200.
Inglis KJ, Chereau D, Brigham EF, Chiou SS, Schobel S, Frigon NL, Yu M, Caccavello RJ, Nelson S, Motter R, Wright S, Chian D, Santiago P, Soriano F, Ramos C, Powell K, Goldstein JM, Babcock M, Yednock T, Bard F, Basi GS, Sham H, Chilcote TJ, McConlogue L, Griswold-Prenner I, Anderson JP: Polo-like kinase 2 (PLK2) phosphorylates alpha-synuclein at serine 129 in central nervous system. J Biol Chem. 2008
Seeburg DP, Feliu-Mojer M, Gaiottino J, Pak DT, Sheng M: Critical role of CDK5 and Polo-like kinase 2 in homeostatic synaptic plasticity during elevated activity. Neuron. 2008, 58: 571-583. 10.1016/j.neuron.2008.03.021.
Hoyer W, Cherny D, Subramaniam V, Jovin TM: Impact of the acidic C-terminal region comprising amino acids 109–140 on alpha-synuclein aggregation in vitro. Biochemistry. 2004, 43: 16233-16242. 10.1021/bi048453u.
Li W, West N, Colla E, Pletnikova O, Troncoso JC, Marsh L, Dawson TM, Jakala P, Hartmann T, Price DL, Lee MK: Aggregation promoting C-terminal truncation of alpha-synuclein is a normal cellular process and is enhanced by the familial Parkinson's disease-linked mutations. Proc Natl Acad Sci USA. 2005, 102: 2162-2167. 10.1073/pnas.0406976102.
Liu CW, Giasson BI, Lewis KA, Lee VM, Demartino GN, Thomas PJ: A precipitating role for truncated alpha-synuclein and the proteasome in alpha-synuclein aggregation: implications for pathogenesis of Parkinson disease. J Biol Chem. 2005, 280: 22670-22678. 10.1074/jbc.M501508200.
Wakamatsu M, Ishii A, Iwata S, Sakagami J, Ukai Y, Ono M, Kanbe D, Muramatsu S, Kobayashi K, Iwatsubo T, Yoshimoto M: Selective loss of nigral dopamine neurons induced by overexpression of truncated human alpha-synuclein in mice. Neurobiol Aging. 2008, 29: 574-585. 10.1016/j.neurobiolaging.2006.11.017.
Paxinou E, Chen Q, Weisse M, Giasson BI, Norris EH, Rueter SM, Trojanowski JQ, Lee VM, Ischiropoulos H: Induction of alpha-synuclein aggregation by intracellular nitrative insult. J Neurosci. 2001, 21: 8053-8061.
Webb JL, Ravikumar B, Atkins J, Skepper JN, Rubinsztein DC: Alpha-Synuclein is degraded by both autophagy and the proteasome. J Biol Chem. 2003, 278: 25009-25013. 10.1074/jbc.M300227200.
Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D: Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science. 2004, 305: 1292-1295. 10.1126/science.1101738.
Dufty BM, Warner LR, Hou ST, Jiang SX, Gomez-Isla T, Leenhouts KM, Oxford JT, Feany MB, Masliah E, Rohn TT: Calpain-cleavage of alpha-synuclein: connecting proteolytic processing to disease-linked aggregation. Am J Pathol. 2007, 170: 1725-1738. 10.2353/ajpath.2007.061232.
Kim HJ, Lee D, Lee CH, Chung KC, Kim J, Paik SR: Calpain-resistant fragment(s) of alpha-synuclein regulates the synuclein-cleaving activity of 20S proteasome. Arch Biochem Biophys. 2006, 455: 40-47. 10.1016/j.abb.2006.08.019.
Mishizen-Eberz AJ, Guttmann RP, Giasson BI, Day GA, Hodara R, Ischiropoulos H, Lee VM, Trojanowski JQ, Lynch DR: Distinct cleavage patterns of normal and pathologic forms of alpha-synuclein by calpain I in vitro. J Neurochem. 2003, 86: 836-847. 10.1046/j.1471-4159.2003.01878.x.
Mishizen-Eberz AJ, Norris EH, Giasson BI, Hodara R, Ischiropoulos H, Lee VM, Trojanowski JQ, Lynch DR: Cleavage of alpha-synuclein by calpain: potential role in degradation of fibrillized and nitrated species of alpha-synuclein. Biochemistry. 2005, 44: 7818-7829. 10.1021/bi047846q.
Cappai R, Leck SL, Tew DJ, Williamson NA, Smith DP, Galatis D, Sharples RA, Curtain CC, Ali FE, Cherny RA, Culvenor JG, Bottomley SP, Masters CL, Barnham KJ, Hill AF: Dopamine promotes alpha-synuclein aggregation into SDS-resistant soluble oligomers via a distinct folding pathway. Faseb J. 2005, 19: 1377-1379.
Jiang H, Wu YC, Nakamura M, Liang Y, Tanaka Y, Holmes S, Dawson VL, Dawson TM, Ross CA, Smith WW: Parkinson's disease genetic mutations increase cell susceptibility to stress: Mutant alpha-synuclein enhances H(2)O(2)- and Sin-1-induced cell death. Neurobiol Aging. 2006
Prasad JE, Kumar B, Andreatta C, Nahreini P, Hanson AJ, Yan XD, Prasad KN: Overexpression of alpha-synuclein decreased viability and enhanced sensitivity to prostaglandin E(2), hydrogen peroxide, and a nitric oxide donor in differentiated neuroblastoma cells. J Neurosci Res. 2004, 76: 415-422. 10.1002/jnr.20058.
Quilty MC, King AE, Gai WP, Pountney DL, West AK, Vickers JC, Dickson TC: Alpha-synuclein is upregulated in neurones in response to chronic oxidative stress and is associated with neuroprotection. Exp Neurol. 2006, 199: 249-256. 10.1016/j.expneurol.2005.10.018.
Cookson MR, Brug van der M: Cell systems and the toxic mechanism(s) of alpha-synuclein. Exp Neurol. 2008, 209: 5-11. 10.1016/j.expneurol.2007.05.022.
Zhu M, Li J, Fink AL: The association of alpha-synuclein with membranes affects bilayer structure, stability, and fibril formation. J Biol Chem. 2003, 278: 40186-40197. 10.1074/jbc.M305326200.
Volles MJ, Lansbury PT: Vesicle permeabilization by protofibrillar alpha-synuclein is sensitive to Parkinson's disease-linked mutations and occurs by a pore-like mechanism. Biochemistry. 2002, 41: 4595-4602. 10.1021/bi0121353.
Pountney DL, Lowe R, Quilty M, Vickers JC, Voelcker NH, Gai WP: Annular alpha-synuclein species from purified multiple system atrophy inclusions. J Neurochem. 2004, 90: 502-512. 10.1111/j.1471-4159.2004.02533.x.
Furukawa K, Matsuzaki-Kobayashi M, Hasegawa T, Kikuchi A, Sugeno N, Itoyama Y, Wang Y, Yao PJ, Bushlin I, Takeda A: Plasma membrane ion permeability induced by mutant alpha-synuclein contributes to the degeneration of neural cells. J Neurochem. 2006, 97: 1071-1077. 10.1111/j.1471-4159.2006.03803.x.
Mosharov EV, Staal RG, Bove J, Prou D, Hananiya A, Markov D, Poulsen N, Larsen KE, Moore CM, Troyer MD, Edwards RH, Przedborski S, Sulzer D: Alpha-synuclein overexpression increases cytosolic catecholamine concentration. J Neurosci. 2006, 26: 9304-9311. 10.1523/JNEUROSCI.0519-06.2006.
Larsen KE, Schmitz Y, Troyer MD, Mosharov E, Dietrich P, Quazi AZ, Savalle M, Nemani V, Chaudhry FA, Edwards RH, Stefanis L, Sulzer D: Alpha-synuclein overexpression in PC12 and chromaffin cells impairs catecholamine release by interfering with a late step in exocytosis. J Neurosci. 2006, 26: 11915-11922. 10.1523/JNEUROSCI.3821-06.2006.
Cooper AA, Gitler AD, Cashikar A, Haynes CM, Hill KJ, Bhullar B, Liu K, Xu K, Strathearn KE, Liu F, Cao S, Caldwell KA, Caldwell GA, Marsischky G, Kolodner RD, Labaer J, Rochet JC, Bonini NM, Lindquist S: Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson's models. Science. 2006, 313: 324-328. 10.1126/science.1129462.
Gosavi N, Lee HJ, Lee JS, Patel S, Lee SJ: Golgi fragmentation occurs in the cells with prefibrillar alpha-synuclein aggregates and precedes the formation of fibrillar inclusion. J Biol Chem. 2002, 277: 48984-48992. 10.1074/jbc.M208194200.
Hsu LJ, Sagara Y, Arroyo A, Rockenstein E, Sisk A, Mallory M, Wong J, Takenouchi T, Hashimoto M, Masliah E: alpha-synuclein promotes mitochondrial deficit and oxidative stress. Am J Pathol. 2000, 157: 401-410.
Li WW, Yang R, Guo JC, Ren HM, Zha XL, Cheng JS, Cai DF: Localization of alpha-synuclein to mitochondria within midbrain of mice. Neuroreport. 2007, 18: 1543-1546.
Cole NB, Dieuliis D, Leo P, Mitchell DC, Nussbaum RL: Mitochondrial translocation of alpha-synuclein is promoted by intracellular acidification. Exp Cell Res. 2008, 314: 2076-2089. 10.1016/j.yexcr.2008.03.012.
Nakamura K, Nemani VM, Wallender EK, Kaehlcke K, Ott M, Edwards RH: Optical reporters for the conformation of alpha-synuclein reveal a specific interaction with mitochondria. J Neurosci. 2008, 28: 12305-12317. 10.1523/JNEUROSCI.3088-08.2008.
Devi L, Raghavendran V, Prabhu BM, Avadhani NG, Anandatheerthavarada HK: Mitochondrial import and accumulation of alpha-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J Biol Chem. 2008, 283: 9089-9100. 10.1074/jbc.M710012200.
Orth M, Tabrizi SJ, Schapira AH, Cooper JM: Alpha-synuclein expression in HEK293 cells enhances the mitochondrial sensitivity to rotenone. Neurosci Lett. 2003, 351: 29-32. 10.1016/S0304-3940(03)00941-8.
Saha AR, Ninkina NN, Hanger DP, Anderton BH, Davies AM, Buchman VL: Induction of neuronal death by alpha-synuclein. Eur J Neurosci. 2000, 12: 3073-3077. 10.1046/j.1460-9568.2000.00210.x.
Stefanova N, Klimaschewski L, Poewe W, Wenning GK, Reindl M: Glial cell death induced by overexpression of alpha-synuclein. J Neurosci Res. 2001, 65: 432-438. 10.1002/jnr.1171.
Kim S, Jeon BS, Heo C, Im PS, Ahn TB, Seo JH, Kim HS, Park CH, Choi SH, Cho SH, Lee WJ, Suh YH: Alpha-synuclein induces apoptosis by altered expression in human peripheral lymphocyte in Parkinson's disease. Faseb J. 2004, 18: 1615-1617. 10.1096/fj.03-1391com.
Martin LJ, Pan Y, Price AC, Sterling W, Copeland NG, Jenkins NA, Price DL, Lee MK: Parkinson's disease alpha-synuclein transgenic mice develop neuronal mitochondrial degeneration and cell death. J Neurosci. 2006, 26: 41-50. 10.1523/JNEUROSCI.4308-05.2006.
Stefanis L, Larsen KE, Rideout HJ, Sulzer D, Greene LA: Expression of A53T mutant but not wild-type alpha-synuclein in PC12 cells induces alterations of the ubiquitin-dependent degradation system, loss of dopamine release, and autophagic cell death. J Neurosci. 2001, 21: 9549-9560.
Yang Q, She H, Gearing M, Colla E, Lee M, Shacka JJ, Mao Z: Regulation of neuronal survival factor MEF2D by chaperone-mediated autophagy. Science. 2009, 323: 124-127. 10.1126/science.1166088.
Snyder H, Mensah K, Theisler C, Lee J, Matouschek A, Wolozin B: Aggregated and monomeric alpha-synuclein bind to the S6' proteasomal protein and inhibit proteasomal function. J Biol Chem. 2003, 278: 11753-11759. 10.1074/jbc.M208641200.
Lindersson E, Beedholm R, Hojrup P, Moos T, Gai W, Hendil KB, Jensen PH: Proteasomal inhibition by alpha-synuclein filaments and oligomers. J Biol Chem. 2004, 279: 12924-12934. 10.1074/jbc.M306390200.
Emmanouilidou E, Stefanis L, Vekrellis K: Cell-produced alpha-synuclein oligomers are targeted to, and impair, the 26S proteasome. Neurobiol Aging. 2008
Chen L, Thiruchelvam MJ, Madura K, Richfield EK: Proteasome dysfunction in aged human alpha-synuclein transgenic mice. Neurobiol Dis. 2006, 23: 120-126. 10.1016/j.nbd.2006.02.004.
Cole NB, Murphy DD, Grider T, Rueter S, Brasaemle D, Nussbaum RL: Lipid droplet binding and oligomerization properties of the Parkinson's disease protein alpha-synuclein. J Biol Chem. 2002, 277: 6344-6352. 10.1074/jbc.M108414200.
Sarkar S, Davies JE, Huang Z, Tunnacliffe A, Rubinsztein DC: Trehalose, a Novel mTOR-independent Autophagy Enhancer, Accelerates the Clearance of Mutant Huntingtin and {alpha}-Synuclein. J Biol Chem. 2007, 282: 5641-5652. 10.1074/jbc.M609532200.
Sapru MK, Yates JW, Hogan S, Jiang L, Halter J, Bohn MC: Silencing of human alpha-synuclein in vitro and in rat brain using lentiviral-mediated RNAi. Exp Neurol. 2006, 198: 382-390. 10.1016/j.expneurol.2005.12.024.
Lewis J, Melrose H, Bumcrot D, Hope A, Zehr C, Lincoln S, Braithwaite A, He Z, Ogholikhan S, Hinkle K, Kent C, Toudjarska I, Charisse K, Braich R, Pandey RK, Heckman M, Maraganore DM, Crook J, Farrer MJ: In vivo silencing of alpha-synuclein using naked siRNA. Mol Neurodegener. 2008, 3: 19-10.1186/1750-1326-3-19.
Gitler AD, Bevis BJ, Shorter J, Strathearn KE, Hamamichi S, Su LJ, Caldwell KA, Caldwell GA, Rochet JC, McCaffery JM, Barlowe C, Lindquist S: The Parkinson's disease protein alpha-synuclein disrupts cellular Rab homeostasis. Proc Natl Acad Sci USA. 2008, 105: 145-150. 10.1073/pnas.0710685105.
Acknowledgements
This research was supported by the Intramural Research Program of the NIH, National Institute on Aging.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The author declares that they have no competing interests.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Cookson, M.R. α-Synuclein and neuronal cell death. Mol Neurodegeneration 4, 9 (2009). https://doi.org/10.1186/1750-1326-4-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1750-1326-4-9