Abstract
More than a decade has passed since apolipoprotein E4 (APOE-ε4) was identified as a primary risk factor for Alzheimer 's disease (AD), yet researchers are even now struggling to understand how the apolipoprotein system integrates into the puzzle of AD etiology. The specific pathological actions of apoE4, methods of modulating apolipoprotein E4-associated risk, and possible roles of apoE in normal synaptic function are still being debated. These critical questions will never be fully answered without a complete understanding of the life cycle of the apolipoprotein receptors that mediate the uptake, signaling, and degradation of apoE. The present review will focus on apoE receptors as modulators of apoE actions and, in particular, explore the functions of soluble apoE receptors, a field almost entirely overlooked until now.
Similar content being viewed by others
Background
ApoE and apoE receptors
Apolipoprotein E (apoE) is a small (34-kDa) secreted glycoprotein that associates with lipoproteins and mediates uptake of these particles into target cells via receptor-mediated endocytosis by the low density lipoprotein (LDL) receptor family. Three commonly occurring isoforms have been identified in the human population due to single nucleotide polymorphisms on the APOE gene on chromosome 19. The apoE3 isoform (Cys112, Arg158) occurs at the highest frequency, followed by apoE4 (Arg112, Arg158) and apoE2 (Cys112, Cys158). ApoE is a ligand for the seven identified mammalian members of the evolutionarily conserved low density lipoprotein (LDL) receptor family: the low density lipoprotein receptor (LDLR), apoE receptor 2 (ApoER2), the very low density lipoprotein receptor (VLDLR), multiple epidermal growth factor (EGF) repeat-containing protein (MEGF7), megalin, LDL-related protein-1 (LRP1) and LDL-related protein-1b (LRP1b). APOE was initially recognized for its importance in lipoprotein metabolism and cardiovascular disease, however, more recently it has been studied for its role in several biological processes not directly related to lipoprotein transport. The following review will focus on the role of apoE and apoE receptors in the CNS with a focus on the processing and production of soluble apoE receptors.
Genetics of apoE and Alzheimer's disease
Alzheimer's disease (AD) is a complex neurodegenerative condition characterized neuropathologically by the presence of extracellular amyloid plaques, intraneuronal neurofibrillary tangles, and neuronal loss. While most AD is sporadic in nature, two classes of genetic risk factors for AD have been identified. The first class consists of mutations responsible for the rare, familial AD (FAD). Genes implicated in FAD include the β-amyloid protein precursor (APP), presenilin-1, and presenilin-2; mutations in these genes produce AD risk marked by autosomal dominance, early disease onset, high penetrance, and relative rarity, on the order of hundreds of families worldwide (reviewed in [1]). Each of the mutated forms of these genes enhances the production of amyloid-β (Aβ) peptide [2], particularly the 42 amino acid form (Aβ42). Because Aβ42 aggregates to form amyloid, the discovery of these genes strongly supports the "amyloid hypothesis" [2].
A second class of AD risk factor is genetic variation that modulates the sporadic late onset AD; at present, the sole member of this class is the APOE gene. APOE encodes a secreted protein of 299 amino acids important for the transport of cholesterol. Single nucleotide polymorphisms (SNP) define three common alleles (ε2, ε3, and ε4), which encode proteins that differ at two amino acids. APOE-ε2 is associated with reduced odds and delayed onset of AD while APOE-ε4 is associated with increased odds and earlier onset of AD [3]. APOE-ε4 appears to account for up to 40–50% of the genetic risk of AD [4, 5]. Furthermore, APOE-ε4 is associated with increased brain Aβ in affected individuals [6]. The effects of APOE on Aβ burden are seen in mice as well, with studies in mice deficient for apoE or transgenic for human apoE supporting a role for apoE in Aβ fibrillogenesis and neuritic plaque formation [7, 8]. Importantly, the risk associated with APOE-ε4 is modulated by other unknown genetic and environmental factors.
AD has a complex etiology that encompasses environmental factors as well as the genetic risk factors. The existence of environmental risk factors is demonstrated by multiple lines of evidence, including studies of twins. Interestingly, it was found that only about half of identical twins have concordance for AD [9, 10]. Among the environmental factors identified, cholesterol lowering treatments (reviewed in [11, 12]) and anti-inflammatory drugs (reviewed in [13]) may decrease risk of AD. Alternatively, analysis of post-mortem AD brains showed an increased level of traumatic brain injury compared to normal non-AD brains, suggesting that brain trauma may significantly increase the risk of AD [14]. ApoE is potentially involved in each of these environmental factors.
ApoE and apoE receptors in the CNS
ApoE and cholesterol transport in the CNS
In the periphery, there are numerous lipoprotein classes and apolipoproteins. However, in the CNS, lipoproteins are predominantly high density and do not include the large classes of lower density lipoproteins found in the plasma [15]. The two major apolipoproteins present in the cerebral spinal fluid (CSF) are apoE and apoAI; classes of high density lipoproteins in the CSF contain either or both of these apolipoproteins [16]. These lipoproteins can act either to deliver cholesterol to cells, or to remove excess cholesterol from cells [17]. The additional role of these proteins as signaling molecules will be discussed later in this review.
ApoE and Aβ
The strongest associations of APOE genotype with disease are with conditions containing amyloid deposition including AD, Down's syndrome, cerebral amyloid angiopathy and head trauma [6, 18–23]. In vivo, early evidence for an involvement of apoE in AD came from immunohistochemical localization of apoE to senile plaques [24]. ApoE4 increases levels of amyloid deposition in humans [6, 18] and accelerates amyloid deposition in transgenic mice [8, 25, 26]. Recent research has focused on soluble oligomeric assemblies of Aβ as the proximate cause of neuronal injury, synaptic loss and the eventual dementia associated with AD [27–31]. ApoE binds soluble Aβ oligomers found in the brain, plasma and CSF [32, 33]. In vitro, apoE forms stable complexes with Aβ [34–37], alters the aggregation of various Aβ peptides [38–40], modulates Aβ-induced neuroinflammation [41–44], and promotes Aβ clearance [45]. In addition, Aβ42 and apoE4 act synergistically to reduce neuronal viability in vitro and ex vivo as measured by neurotoxicity in primary cultures and impaired long term potentiation (LTP) in hippocampal slice cultures [46–51]. Each of these important functions is partially mediated by apoE receptors.
ApoE receptors
ApoE interacts with members of the LDL receptor family on the surface of cells. The LDLR family consists of over ten receptors that function in receptor-mediated endocytosis and cellular signaling (Figure 1) [52, 53]. In addition to the LDLR itself, the family includes LRP/LRP1 [54], megalin/LRP2 [55], VLDLR [56], ApoER2/LRP8 [57–59], SORLA-1/LR11 [60, 61], LRP4 [62], LRP5 [63, 64], LRP6 [65], and LRP1B [66]. The most characteristic structural component of the LDLR family is the cysteine-rich ligand-binding repeats forming ligand-binding domains [52, 67]. Additionally, most members of the LDLR family contain epidermal growth factor (EGF)-like repeats and YWTD motifs, which form β-propeller-like structures [68]. A common feature that is shared by most members of the LDLR family is their ability to bind the receptor-associated protein (RAP) [69]. RAP is an endoplasmic reticulum (ER)-resident protein that functions in receptor folding and trafficking along the early secretory pathway and universally antagonizes ligand-binding to all members of the family [69].
Many of the apoE receptors have been found in the CNS. Neurons express LDLR, LRP, ApoER2, and the VLDLR; astrocytes express LDLR and LRP; microglia express VLDLR and LRP [6, 57, 70–73]. It is unclear which receptors are expressed on oligodendrocytes. Soluble forms of each of these receptors have been detected (see below).
ApoE receptors and endocytosis
A major function of at least some of the apoE receptors is clathrin-mediated endocytosis. The rapid endocytosis rate of LRP is unique among LDLR family members. The dominant endocytosis signal for LRP is the Y XX L motif [74]. The two copies of the NPXY motif, which mediates LDLR endocytosis [75], do not play significant roles in LRP endocytosis but likely bridge interactions with adaptor proteins for signaling and intracellular trafficking. The initial endocytosis rates of individual LDLR family members are significantly different with half time for internalization ranging from <0.5 min (LRP) to > 8 min (ApoER2/VLDLR) ([76], see Table 1). These results suggest endocytic functions among LDLR family members are distinct. In addition to endocytosis, LDLR family members also exhibit efficient recycling. In particular, sorting nexin 17, a member of the PX-domain containing, sorting nexin family, interacts with the proximal NPXY motif of the LRP tail and promotes its recycling in the early endosome [77]. Other adaptor proteins, specifically Dab-1 and FE65, affect levels of surface apoE receptors [78]. Although significant cholesterol from the periphery does not get transported into the CNS and sufficient cholesterol is synthesized in the CNS [79], cholesterol redistribution is important for transport of cholesterol from glia to neurons [80] and for clearance of membrane debris after CNS damages [81].
ApoE receptors and intracellular signaling
Recently, several studies have demonstrated a role for some apoE receptors (specifically LRP, ApoER2, and VLDLR) as signaling molecules (see [53, 82] for review). VLDLR and ApoER2 transduce signals from the extracellular matrix molecule Reelin, affecting neuronal cell migration during development [83]. LRP activation by ligand binding affects NMDA receptor function [84–89]. These effects are transduced through the cytoplasmic domains of receptors binding various cytoplasmic adaptor and scaffold proteins containing PID or PDZ domains, including mammalian Disabled-1 (mDab1), mDab2, FE65, JNK-interacting protein JIP-1 and JIP-2, and PSD-95 [90–95]. ApoE receptor ligands also promote other intraneuronal signals via apoE receptors, including PI3 activation, ERK activation, and JNK inhibition [86, 96, 97] but exactly which receptors promote which signals is unknown. ApoE receptors on glia also affect signaling pathways, affecting the state of glial activation [41, 42, 98].
ApoE receptors and synaptic plasticity
These receptor-mediated processes defined in vitro are important for brain physiological functions. The apoE receptor antagonist RAP prevents induction of long-term potentiation (LTP) in hippocampal slices [69]. ApoER2 and VLDLR knock-out (KO) mice have normal baseline synaptic transmission, as measured in acute hippocampal slices, but have subtle impairment of hippocampal LTP [83, 99]. Moreover, Reelin application enhanced LTP induction, which was dependent on the presence of both ApoER2 and VLDLR [97]. A potential molecular mechanism for this function of ApoER2 is a 59 amino acid cytoplasmic domain that is alternatively spliced. This ApoER2 splice variant interacts with PSD-95, which is itself associated with NMDA receptor conductance [87, 95]. Knock-in mice exclusively expressing ApoER2 receptors that lack the 59 amino acid insert exhibit decreased LTP induction, and no enhancement of LTP in the presence of exogenous Reelin [87]. Thus, the role of ApoER2 in LTP appears to be in the capacity of NMDA receptor modulation by increasing NMDAR conductance and thus indirectly altering intracellular calcium levels.
ApoE isoforms and synaptic plasticity
Increasing evidence indicates that apoE4 itself impairs neuronal viability. Not all cell types are susceptible to apoE4-induced toxicity; glia are relatively resistant [100] and only cells with a neuronal phenotype appear vulnerable [101]. ApoE4 actually inhibits neurite outgrowth, overrides the neurite-stimulatory effect of apoE3 and is neurotoxic in vitro (reviewed by Teter [102]). Transgenic expression of human apoE4 has dominant negative behavioral effects [103–106], including deficits in memory tasks [103, 105]. In addition, apoE4 mice a exhibit greater memory impairment than apoE-knockout (apoE-KO) mice, suggesting that apoE4 confers a gain of negative function [106, 107].
Several studies utilizing genetically altered mice have begun to shed light on the roles of apoE in synaptic plasticity and memory formation. ApoE targeted replacement mice (apoE-TR mice) express one of the three human isoforms under the control of endogenous murine APOE promoter sequences in a conformation and at physiological levels in a temporal and spatial pattern comparable to endogenous mouse apoE [108]. ApoE-TR mice expressing the apoE3 isoform are identical to wild type mice in both LTP induction and spatial learning. In contrast, mice expressing the apoE4 isoform demonstrate compromised LTP induction and spatial learning. Importantly, the impaired spatial learning exhibited by apoE-deficient mice can be rescued by infusion of human apoE3 or apoE4 [109, 110]. Thus, apoE and its receptors influence NMDA receptor activity, LTP, and spatial memory.
ApoE, Aβ, and synaptic plasticity
In addressing the effect of apoE on Aβ-induced changes in neuronal viability, it is unclear precisely what form of the Aβ peptide was used in early studies because it has been difficult to isolate and determine the conformational species of Aβ responsible for its neural activity [50, 51]. In vitro, several recent studies have demonstrated that apoE2 and E3, but not E4, protect neurons against cell death induced by non-fibrillar Aβ, but have no effect on fibrillar-induced toxicity [111, 112]. In addition, oligomeric Aβ42-induced neurotoxicity is significantly greater in both Neuro-2A cells treated with apoE4 [112–114] and primary co-cultures of wild-type (WT) neurons and glia from apoE-TR mice expressing apoE4 [47, 112]. Using apoE-TR mice, oligomeric Aβ42-induced inhibition of LTP was greatest in hippocampal slice cultures from apoE4-TR mice, while apoE2 actually protected against LTP impairment [46]. In vivo, crossing apoE2 transgenic mice with APP transgenic mice prevented soluble Aβ-induced dendritic spine loss [115].
Summary
Of the two major apolipoproteins found in the CSF, apoE can associate to a number of extracellular molecules and bind to four major CNS apoE receptors; VLDLR, ApoER2, LDLR and LRP. The apoE4 isoform has garnered attention due to the genetic association of apoE4 inheritance and AD risk. ApoE receptors undergo rapid clathrin-mediated endocytosis following ligand binding and have the ability to link ligand binding to several signal transduction pathways. ApoE isoforms exhibit a differential affect on synaptic function and VLDLR and ApoER2 are shown to play a role in synaptic plasticity and memory formation.
ApoE receptors and AD
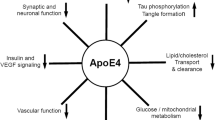
ApoE receptors are an integral part of normal apoE metabolism, potentially mediating and/or modulating the effects of apoE isoforms on AD pathological processes. They are also important for the cellular homeostasis of cholesterol, which may also affect Aβ production from APP [12]. Several lines of research have implicated apoE receptors directly in AD pathophysiology through several mechanisms (Figure 2).
ApoE receptors and AD pathophysiology. ApoER2 and LRP receptors can mediate several intracellular signaling kinases including PI3K, CDK-5, JnK and MAPK. The splice variant of ApoER2 expressing exon 19 can influence calcium influx in response to ligand binding by modulating NMDA receptor-mediated currents via a Src-dependent mechanism. Signaling can also be influenced by apoE receptor – APP interaction and specific apoE isoform or Reelin binding.
ApoE receptors endocytose Aβ
Almost from the initial observations that apoE bound Aβ [24, 34], apoE receptors have been suggested to act as clearance mechanisms for Aβ. Since then, apoE receptors have been found to help transport Aβ across the endothelial cells forming the blood brain barrier [116] or clear Aβ into astrocytes as a degradative process [45]. ApoE is found on most, but not all Aβ deposits in the AD brain [117, 118]. LRP is expressed on activated astrocytes [6] and closely associated with Aβ deposits [119]. The importance of Aβ clearance via apoE receptors is also supported by the significant increase in amyloid deposition observed in transgenic APP mice deficient in the RAP gene [120], which has increased levels of several LDLR family members [121, 122]. Thus, the interactions of apoE complexes with the apoE receptors in the CNS vitally affect not only the metabolism of apoE, but of Aβ as well.
LRP alters APP trafficking and processing
ApoE receptors also have been implicated in the production of Aβ. LRP interacts with APP through the intracellular adaptor protein FE65 or via direct binding to the KPI domain [90, 123–126]. Functionally, LRP's rapid endocytosis facilitates APP endocytic trafficking and Aβ production [127–129]. Overexpression of a functional LRP minireceptor in vivo resulted in an increase of soluble Aβ in the brain [130].
Other members of the LDLR family alter APP trafficking and processing
The apoE receptor LRP1B, which undergoes a slow endocytosis, interacts with APP. However, unlike LRP, expression of LRP1B decreases APP endocytic trafficking and processing to Aβ [131]. ApoER2 also interacts with APP, through an extracellular matrix molecule F-spondin [132] and the intracellular adaptor protein FE65 [78]. These studies suggest that conditions that stabilize APP on the cell surface can increase α-cleavage of APP and decrease Aβ production. Finally, several recent studies have shown that SorLA/LR11 alters APP trafficking to discrete compartments such that APP processing by β/γ-secretases is decreased [133–136]. Together these studies indicate that binding to APP is a common event for the LDLR family members and expression and proteolytic processing of these receptors can impact APP trafficking and processing.
Summary
ApoE receptors are believed to act as a clearance mechanisms for extracellular Aβ and apoE is often associated with Aβ deposits in post mortem AD brains. The apoE receptors ApoER2, LRP and LRP1B can directly interact with and stabilize amyloid precursor causing increased α-cleavage and reduced Aβ producing cleavage. Thus, apoE and apoE receptors can influence both levels and production of Aβ.
Soluble apoE receptors
In addition to the transmembrane forms of apoE receptors, soluble forms of these receptors have been observed in vitro and in vivo (Figure 3). Soluble receptors can be generated by cleavage of transmembrane forms of the receptors (also called "ectodomain shedding"). Extracellular proteinases responsible for the release of soluble receptors are commonly metalloproteinases, either membrane bound (A Disintegrin and Metalloproteinase, ADAMs) or secreted (Matrix Metalloproteinase, MMPs) [137]. Alternately, soluble receptors can be expressed from alternately spliced mRNAs that lack a transmembrane domain [138]. Both of these processes are important for regulation of soluble apoE receptors and functions of membrane bound apoE receptors.
Soluble apoE receptors. Processing by γ-secretase cleaves ApoER2, VLDLR, and LRP1 at the membrane. Cleavage by furin can release soluble forms of ApoER2 and LRP. In addition, sheddase cleavage can result in soluble forms of all four receptors. Soluble ApoER2 fragments from furin or sheddase cleavage contain the ligand-binding regions necessary for interaction with Reelin and apoE. These soluble fragments can block endogenous ligand binding to full length receptor acting in a dominant negative fashion.
Soluble LRP
LRP is synthesized as a single polypeptide chain of ~600 kDa and then cleaved in the trans-Golgi network by furin into a 515-kDa ligand-binding subunit and an 85-kDa transmembrane subunit that remain non-covalently associated with one another as they traffic to the cell surface [139]. The LRP extracellular region undergoes shedding from a region close to the membrane by metalloproteinases, releasing a soluble LRP (sLRP) capable of binding ligands [140, 141]. sLRP is detected in human plasma at nanomolar concentrations [140, 141] and in human CSF [142]. Recent studies have also shown that the cell associated fragment of LRP can be cleaved at a third, intramembranous site by the γ-secretase, releasing its intracellular domain (ICD) [143, 144]. These sequential cleavage events by furin, metalloproteinases, and γ-secretase closely resemble those of Notch family proteins [145–147].
Soluble ApoER2 and VLDLR
Like LRP, ApoER2 and VLDLR undergo extracellular cleavages by metalloproteinases to release soluble receptors as well as C-terminal, cell-associated fragments, and these events are induced by Phorbol esters [148]. Furthermore, the C-terminal fragments are cleaved by γ-secretase [149]. ApoER2 and VLDLR proteolytic events are also increased by extracellular ligand binding [148]. Interestingly, the different apoE alleles induced different degrees of release; both ApoER2 and VLDLR show greatest cleavage following apoE2 activation, less with apoE3 and relatively little with apoE4 [148]. The release of soluble forms of ApoER2 and VLDLR is affected by the presence of splice variants. ApoER2 and VLDLR both have prominent splice variants that lack the exon encoding an O-linked glycosylation site. This region is important in the regulated cleavage of transmembrane proteins [150]. In addition, some ApoER2 splice variants contain an exon that encodes a furin cleavage site in the extracellular domain [59, 151]. Furin-dependent cleavage results in extrusion of a soluble fragment of the receptor [152]. Thus, cleavages of ApoER2 and VLDLR are regulated in part by alternate splicing events.
Soluble LDLR
Like the other family members, LDLR exists as a soluble form [153, 154]. LDLR shedding from the cell surface is enhanced by several stimuli, including interferon and phorbol ester. As for the other apoE receptors, this effect is dependent upon a cell surface metalloproteinase [154]. Inefficient LDLR exon splicing may also contribute to soluble LDLR isoforms because LDLR ESTs have been reported which lack (i) exon 12, which causes a frameshift in the extracellular domain, resulting in a premature termination codon in exon 13, or (ii) exon 15, which encodes the LDLR O-linked glycosylation domain (BG945931 and BQ685399, respectively).
Release of soluble receptors
Numerous transmembrane proteins in addition to the apoE receptors have soluble forms, including growth factors and their receptors, cytokine precursors and receptors, cell adhesion molecules, enzymes, and differentiation factors [155]. To gain insight into the possible mechanisms of soluble apoE receptors, we will briefly consider the functions of released extracellular domains of various other transmembrane proteins.
1. Protein function at a distance from the cell
Certain transmembrane proteins have functions that are inactive as long as the protein is tethered to the cell surface. Upon cleavage, these proteins become active and their activities can be carried out at a distance from the cell. For example, TNF-α is synthesized as a membrane-bound, inactive protein but is activated by cleavage by the metalloproteinase TNF-α converting enzyme (TACE) [156]. Soluble TNF-α can then act as a cytokine in the maintenance of inflammation. Similarly, transforming growth factor-α (TGF-α) has a biologically active form on the cell surface, but its activity is limited to the cell surface. However, upon surface cleavage, an active TGF-α is secreted and acts at a distance [157]. Another example of membrane bound protein cleavage is APP. Isoforms of APP containing the Kunitz proteinase inhibitor (KPI) domain can act as serine proteinase inhibitors at the cell surface but, once released from the cell surface, act as soluble proteinase inhibitors [158].
2. An initial step in cell signaling
Ligand binding to a cell surface receptor can transduce a signal inside the cell through several general mechanisms. One of these involves sequential cleavage of the surface receptor after ligand binding, releasing extracellular (soluble) and intracellular (membrane-bound) domains. A subsequent intramembranous (i.e. γ-secretase) cleavage of the membrane-associated protein then releases the cytoplasmic domain for intracellular effects [159]. Thus, release of the soluble receptor is a required step in the signal transduction pathway. The large number of proteins identified as γ-secretase substrates undergo this series of proteolytic events [160]. One well-studied example of this mechanism is the Notch receptor [161]. Notch is a receptor for cell surface proteins on adjacent cells (Delta, Jagged, etc.). After ligand binding, Notch undergoes sequential cleavages to release of its intracellular domain (NICD), which acts as a transcription factor of genes important to development. No function has been assigned the soluble fragment of Notch generated by the initial cleavage. Another example is the proteolysis of SREBP (sterol responsive element binding protein) at luminal and intramembranous sites [162]. These cleavages result in the release of the cytoplasmic fragments of SREBPs that act as transcriptional activators of specific genes important in cholesterol homeostasis.
3. Inhibition of cell signaling
The soluble form of receptors can bind to soluble ligands and prevent them from interacting with membrane-bound receptors, thus preventing their effects on cells. High affinity receptors for several interleukins (IL-1, IL-4, IL-15) have shed forms that bind the specific interleukins and block their actions on cells [163–165]. A soluble version of the receptor for advanced glycation end products (RAGE) can block the interactions of ligands with the membrane bound forms of RAGE, preventing their endocytosis [166], and a soluble version of the leptin receptor blocks leptin signaling [167]. Receptor cleavage can also inhibit signaling that is already occurring on the cell surface. For example, cell surface ephrins interact with Eph receptors on adjacent cells, promoting both forward and reverse cell signaling cascades important in development [168]. The formation of the ligand receptor complex promotes cleavage of the ephrin from the cell surface by ADAMs, thus ceasing both forward and reverse signaling events [169].
4. Release of cell adhesion
Transmembrane proteins are vital for establishing stable connections of a cell with adjacent cells or with the extracellular matrix. When proteolysis of these membrane proteins occurs, the cellular binding to the extracellular matrix is broken, allowing a cell to migrate, or allowing portions of a cell to form new interactions. A component of the cleavage of the ephrin-Eph receptor complex is that this cleavage allows for the induced axonal repulsion [170]. Other examples include the cleavage of cell adhesion molecules L1 that disrupts cell-cell adhesion [171], and cleavage of the discoidan domain receptor 1, that disrupts cell-collagen adhesion [172].
5. Protein turnover
Secreted proteins are often degraded by soluble proteinases, or internalized by cells and degraded in endosomes and lysosomes. Cytoplasmic proteins can be ubiquitinated and degraded by the proteosome [173]. However, transmembrane proteins cannot be sufficiently degraded by either of these mechanisms as long as they are membrane bound. Therefore, turnover of transmembrane proteins requires a combination of proteolytic events. Cleavage at the cell surface would release soluble proteins for extracellular degradation or clearance. Subsequent cleavages within the membrane would generate small protein fragments that could be removed from the membrane, as well as cytoplasmic fragments that could be degraded by intracellular pathways. The urokinase receptor uPAR undergoes a series of cleavages that may be responsible for this type of protein turnover[174]. CD44 is another example of a protein that undergoes these sequential cleavages for degradation [175].
Functions of soluble apoE receptors
These potential functions of soluble receptors each apply to apoE receptors. One of the most profound implications of the production of soluble apoE receptors is the possible dominant negative effect on apoE receptor function. This action is observed with the production of soluble ApoER2. The expression of the ApoER2 variant containing the furin consensus site results in the production of soluble receptor consisting of the ligand binding domain [152]. Soluble ApoER2 can effectively block Reelin binding to both ApoER2 and VLDLR and subsequent Reelin-dependent signaling in primary neuronal cells. Thus, inhibition of normal Reelin signaling through ApoER2 and VLDLR can acutely modulate other signaling mechanisms through changes in NMDA receptor activity and intracellular signaling pathways [84–89]. The effect on apoE-dependent signaling has yet to be determined, but these studies suggest that selected apoE receptor shedding would affect both specific ligand binding, as in the case of soluble ApoER2 and Reelin signaling, as well as overall apoE binding and signaling.
Other soluble apoE receptors are shown to act in the same capacity as soluble ApoER2. Soluble LRP can bind RAP in ligand blots [140] and soluble derivatives of LRP, LDLR, and VLDLR have each been shown to mediate receptor-ligand interactions [140, 152, 176, 177]. A physiologic function has yet to be ascribed to the production of soluble apoE receptors. However, in light of the essential roles these receptors play in synaptic function, integration into numerous signal transduction pathways and their wide-range of their ligands, it is likely that this type of negative feedback would be necessary in modulating the activity of specific or multiple apoE receptor subtypes. In addition, cleavage of apoE receptors could be a necessary step in receptor turnover, affecting receptor half-lives, and in preventing uptake of apoE-containing lipoproteins. In summary, release of soluble apoE receptors from the cell surface may modulate the cell surface apoE receptor pathway through multiple mechanisms, including ligand binding away from the cell, altered cell signaling, and differences in receptor degradation.
Summary
Numerous transmembrane receptors undergo proteolytic processing. Soluble apoE receptors have also been identified for VLDLR, ApoER2, LDLR and LRP. The specific physiologic function for apoE receptor processing has yet to be elucidated. However, a dominant negative effect has been attributed to the processing of ApoER2 and subsequent production of soluble receptor. Thus, specific apoE receptor shedding may represent a novel mechanism for modulating individual apoE receptor-ligand interactions and overall apoE receptor function.
Conclusion
Soluble apoE receptors are generated by two mechanisms, i.e., proteolysis of transmembrane receptors and by expression of alternately spliced isoforms of the proteins. Moreover, splicing also modulates cell surface proteolysis because exons encoding the O-linked glycosylation domains of VLDLR, ApoER2 and LDLR are alternatively spliced, and this glycosylation domain modulates susceptibility to cell surface proteolysis. These soluble receptors bind their ligands, including apoE, and affect their function and metabolism. The mechanisms and regulation of the processes generating soluble apoE receptors, mediating the actions of these receptors, and controlling the eventual clearance of the soluble receptors are just now being examined. Thus, soluble apoE receptors overall may represent an area of rapid growth in our understanding of AD-related processes. The importance of receptor shedding as a general regulatory mechanism is being recognized in many fields, with shedding of molecules important, for example, in development (Notch, ephrins), immunology (TNF-α, IL-1 receptor, CD44), cell signaling (SREBP, leptin receptors), and cell adhesion (L1, discoidan domain receptor 1). Soluble apoE receptors could play roles as dominant negative regulators of apoE, and thus understanding their generation and actions are important for understanding normal apoE functions in the CNS.
References
Tanzi RE, Bertram L: Twenty years of the Alzheimer's disease amyloid hypothesis: a genetic perspective. Cell. 2005, 120 (4): 545-555. 10.1016/j.cell.2005.02.008.
Selkoe DJ: Alzheimer's disease: genotypes, phenotypes, and treatments. Science. 1997, 275 (5300): 630-631. 10.1126/science.275.5300.630.
Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA: Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993, 261 (5123): 921-923. 10.1126/science.8346443.
Lahiri DK, Sambamurti K, Bennett DA: Apolipoprotein gene and its interaction with the environmentally driven risk factors: molecular, genetic and epidemiological studies of Alzheimer's disease. Neurobiol Aging. 2004, 25 (5): 651-660. 10.1016/j.neurobiolaging.2003.12.024.
Rocchi A, Pellegrini S, Siciliano G, Murri L: Causative and susceptibility genes for Alzheimer's disease: a review. Brain Res Bull. 2003, 61 (1): 1-24. 10.1016/S0361-9230(03)00067-4.
Rebeck GW, Reiter JS, Strickland DK, Hyman BT: Apolipoprotein E in sporadic Alzheimer's disease: allelic variation and receptor interactions. Neuron. 1993, 11 (4): 575-580. 10.1016/0896-6273(93)90070-8.
Holtzman DM, Bales KR, Tenkova T, Fagan AM, Parsadanian M, Sartorius LJ, Mackey B, Olney J, McKeel D, Wozniak D, Paul SM: Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2000, 97 (6): 2892-2897. 10.1073/pnas.050004797.
Holtzman DM, Bales KR, Wu S, Bhat P, Parsadanian M, Fagan AM, Chang LK, Sun Y, Paul SM: Expression of human apolipoprotein E reduces amyloid-beta deposition in a mouse model of Alzheimer's disease. J Clin Invest. 1999, 103 (6): R15-R21.
Breitner JC, Murphy EA: Twin studies of Alzheimer disease: II. Some predictions under a genetic model. Am J Med Genet. 1992, 44 (5): 628-634. 10.1002/ajmg.1320440520.
Bergem AL, Engedal K, Kringlen E: The role of heredity in late-onset Alzheimer disease and vascular dementia. A twin study. Arch Gen Psychiatry. 1997, 54 (3): 264-270.
Miller LJ, Chacko R: The role of cholesterol and statins in Alzheimer's disease. Ann Pharmacother. 2004, 38 (1): 91-98. 10.1345/aph.1D104.
Wolozin B: Cholesterol, statins and dementia. Curr Opin Lipidol. 2004, 15 (6): 667-672. 10.1097/00041433-200412000-00007.
McGeer PL, Schulzer M, McGeer EG: Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer's disease: a review of 17 epidemiologic studies. Neurology. 1996, 47 (2): 425-432.
Guo Z, Cupples LA, Kurz A, Auerbach SH, Volicer L, Chui H, Green RC, Sadovnick AD, Duara R, DeCarli C, Johnson K, Go RC, Growdon JH, Haines JL, Kukull WA, Farrer LA: Head injury and the risk of AD in the MIRAGE study. Neurology. 2000, 54 (6): 1316-1323.
Pitas RE, Boyles JK, Lee SH, Hui D, Weisgraber KH: Lipoproteins and their receptors in the central nervous system. Characterization of the lipoproteins in cerebrospinal fluid and identification of apolipoprotein B,E(LDL) receptors in the brain. J Biol Chem. 1987, 262 (29): 14352-14360.
Koch S, Donarski N, Goetze K, Kreckel M, Stuerenburg HJ, Buhmann C, Beisiegel U: Characterization of four lipoprotein classes in human cerebrospinal fluid. J Lipid Res. 2001, 42 (7): 1143-1151.
Rebeck GW, Alonzo NC, Berezovska O, Harr SD, Knowles RB, Growdon JH, Hyman BT, Mendez AJ: Structure and functions of human cerebrospinal fluid lipoproteins from individuals of different APOE genotypes. Exp Neurol. 1998, 149 (1): 175-182. 10.1006/exnr.1997.6710.
Schmechel DE, Saunders AM, Strittmatter WJ, Crain BJ, Hulette CM, Joo SH, Pericak-Vance MA, Goldgaber D, Roses AD: Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc Natl Acad Sci U S A. 1993, 90 (20): 9649-9653. 10.1073/pnas.90.20.9649.
Greenberg SM, Briggs ME, Hyman BT, Kokoris GJ, Takis C, Kanter DS, Kase CS, Pessin MS: Apolipoprotein E epsilon 4 is associated with the presence and earlier onset of hemorrhage in cerebral amyloid angiopathy. Stroke. 1996, 27 (8): 1333-1337.
Hyman BT, West HL, Rebeck GW, Buldyrev SV, Mantegna RN, Ukleja M, Havlin S, Stanley HE: Quantitative analysis of senile plaques in Alzheimer disease: observation of log-normal size distribution and molecular epidemiology of differences associated with apolipoprotein E genotype and trisomy 21 (Down syndrome). Proc Natl Acad Sci U S A. 1995, 92 (8): 3586-3590. 10.1073/pnas.92.8.3586.
Nicoll JA, Roberts GW, Graham DI: Apolipoprotein E epsilon 4 allele is associated with deposition of amyloid beta-protein following head injury. Nat Med. 1995, 1 (2): 135-137. 10.1038/nm0295-135.
Nicoll JA, Burnett C, Love S, Graham DI, Ironside JW, Vinters HV: High frequency of apolipoprotein E epsilon 2 in patients with cerebral hemorrhage due to cerebral amyloid angiopathy. Ann Neurol. 1996, 39 (5): 682-683. 10.1002/ana.410390521.
Lai F, Kammann E, Rebeck GW, Anderson A, Chen Y, Nixon RA: APOE genotype and gender effects on Alzheimer disease in 100 adults with Down syndrome. Neurology. 1999, 53 (2): 331-336.
Namba Y, Tomonaga M, Kawasaki H, Otomo E, Ikeda K: Apolipoprotein E immunoreactivity in cerebral amyloid deposits and neurofibrillary tangles in Alzheimer's disease and kuru plaque amyloid in Creutzfeldt-Jakob disease. Brain Res. 1991, 541 (1): 163-166. 10.1016/0006-8993(91)91092-F.
Bales KR, Verina T, Dodel RC, Du Y, Altstiel L, Bender M, Hyslop P, Johnstone EM, Little SP, Cummins DJ, Piccardo P, Ghetti B, Paul SM: Lack of apolipoprotein E dramatically reduces amyloid beta-peptide deposition. Nat Genet. 1997, 17 (3): 263-264. 10.1038/ng1197-263.
Fryer JD, Taylor JW, DeMattos RB, Bales KR, Paul SM, Parsadanian M, Holtzman DM: Apolipoprotein E markedly facilitates age-dependent cerebral amyloid angiopathy and spontaneous hemorrhage in amyloid precursor protein transgenic mice. J Neurosci. 2003, 23 (21): 7889-7896.
Klein WL, Krafft GA, Finch CE: Targeting small Abeta oligomers: the solution to an Alzheimer's disease conundrum?. Trends Neurosci. 2001, 24 (4): 219-224. 10.1016/S0166-2236(00)01749-5.
Lansbury PT: Evolution of amyloid: what normal protein folding may tell us about fibrillogenesis and disease. Proc Natl Acad Sci U S A. 1999, 96 (7): 3342-3344. 10.1073/pnas.96.7.3342.
Small DH: The amyloid cascade hypothesis debate: emerging consensus on the role of A beta and amyloid in Alzheimer's disease. Amyloid. 1998, 5 (4): 301-304.
Terry RD: An honorable compromise regarding amyloid in Alzheimer disease. Ann Neurol. 2001, 49 (5): 684-10.1002/ana.1042.
Haass C, Steiner H: Protofibrils, the unifying toxic molecule of neurodegenerative disorders?. Nat Neurosci. 2001, 4 (9): 859-860. 10.1038/nn0901-859.
Wisniewski T, Golabek A, Matsubara E, Ghiso J, Frangione B: Apolipoprotein E: binding to soluble Alzheimer's beta-amyloid. Biochem Biophys Res Commun. 1993, 192 (2): 359-365. 10.1006/bbrc.1993.1423.
Kuo YM, Emmerling MR, Vigo-Pelfrey C, Kasunic TC, Kirkpatrick JB, Murdoch GH, Ball MJ, Roher AE: Water-soluble Abeta (N-40, N-42) oligomers in normal and Alzheimer disease brains. J Biol Chem. 1996, 271 (8): 4077-4081. 10.1074/jbc.271.8.4077.
Strittmatter WJ, Weisgraber KH, Huang DY, Dong LM, Salvesen GS, Pericak-Vance M, Schmechel D, Saunders AM, Goldgaber D, Roses AD: Binding of human apolipoprotein E to synthetic amyloid beta peptide: isoform-specific effects and implications for late-onset Alzheimer disease. Proc Natl Acad Sci U S A. 1993, 90 (17): 8098-8102. 10.1073/pnas.90.17.8098.
LaDu MJ, Falduto MT, Manelli AM, Reardon CA, Getz GS, Frail DE: Isoform-specific binding of apolipoprotein E to beta-amyloid. J Biol Chem. 1994, 269 (38): 23403-23406.
LaDu MJ, Pederson TM, Frail DE, Reardon CA, Getz GS, Falduto MT: Purification of apolipoprotein E attenuates isoform-specific binding to beta-amyloid. J Biol Chem. 1995, 270 (16): 9039-9042. 10.1074/jbc.270.16.9039.
Tokuda T, Calero M, Matsubara E, Vidal R, Kumar A, Permanne B, Zlokovic B, Smith JD, Ladu MJ, Rostagno A, Frangione B, Ghiso J: Lipidation of apolipoprotein E influences its isoform-specific interaction with Alzheimer's amyloid beta peptides. Biochem J. 2000, 348 Pt 2: 359-365. 10.1042/0264-6021:3480359.
Ma J, Yee A, Brewer HB, Das S, Potter H: Amyloid-associated proteins alpha 1-antichymotrypsin and apolipoprotein E promote assembly of Alzheimer beta-protein into filaments. Nature. 1994, 372 (6501): 92-94. 10.1038/372092a0.
Sanan DA, Weisgraber KH, Russell SJ, Mahley RW, Huang D, Saunders A, Schmechel D, Wisniewski T, Frangione B, Roses AD, et al: Apolipoprotein E associates with beta amyloid peptide of Alzheimer's disease to form novel monofibrils. Isoform apoE4 associates more efficiently than apoE3. J Clin Invest. 1994, 94 (2): 860-869.
Wisniewski T, Castano EM, Golabek A, Vogel T, Frangione B: Acceleration of Alzheimer's fibril formation by apolipoprotein E in vitro. Am J Pathol. 1994, 145 (5): 1030-1035.
LaDu MJ, Shah JA, Reardon CA, Getz GS, Bu G, Hu J, Guo L, van Eldik LJ: Apolipoprotein E receptors mediate the effects of beta-amyloid on astrocyte cultures. J Biol Chem. 2000, 275 (43): 33974-33980. 10.1074/jbc.M000602200.
Laskowitz DT, Thekdi AD, Thekdi SD, Han SK, Myers JK, Pizzo SV, Bennett ER: Downregulation of microglial activation by apolipoprotein E and apoE-mimetic peptides. Exp Neurol. 2001, 167 (1): 74-85. 10.1006/exnr.2001.7541.
Lynch JR, Tang W, Wang H, Vitek MP, Bennett ER, Sullivan PM, Warner DS, Laskowitz DT: APOE genotype and an ApoE-mimetic peptide modify the systemic and central nervous system inflammatory response. J Biol Chem. 2003, 278 (49): 48529-48533. 10.1074/jbc.M306923200.
Guo L, LaDu MJ, Van Eldik LJ: A dual role for apolipoprotein e in neuroinflammation: anti- and pro-inflammatory activity. J Mol Neurosci. 2004, 23 (3): 205-212. 10.1385/JMN:23:3:205.
Koistinaho M, Lin S, Wu X, Esterman M, Koger D, Hanson J, Higgs R, Liu F, Malkani S, Bales KR, Paul SM: Apolipoprotein E promotes astrocyte colocalization and degradation of deposited amyloid-beta peptides. Nat Med. 2004, 10 (7): 719-726. 10.1038/nm1058.
Trommer BL, Shah C, Yun SH, Gamkrelidze G, Pasternak ES, Stine WB, Manelli A, Sullivan P, Pasternak JF, LaDu MJ: ApoE isoform-specific effects on LTP: blockade by oligomeric amyloid-beta1-42. Neurobiol Dis. 2005, 18 (1): 75-82. 10.1016/j.nbd.2004.08.011.
Manelli AM, Bulfinch LC, Sullivan PM, Ladu MJ: Abeta42 neurotoxicity in primary co-cultures: Effect of apoE isoform and Abeta conformation. Neurobiol Aging. 2006
Whitson JS, Mims MP, Strittmatter WJ, Yamaki T, Morrisett JD, Appel SH: Attenuation of the neurotoxic effect of A beta amyloid peptide by apolipoprotein E. Biochem Biophys Res Commun. 1994, 199 (1): 163-170. 10.1006/bbrc.1994.1209.
Ma J, Brewer HB, Potter H: Alzheimer A beta neurotoxicity: promotion by antichymotrypsin, ApoE4; inhibition by A beta-related peptides. Neurobiol Aging. 1996, 17 (5): 773-780. 10.1016/0197-4580(96)00112-1.
Miyata M, Smith JD: Apolipoprotein E allele-specific antioxidant activity and effects on cytotoxicity by oxidative insults and beta-amyloid peptides. Nat Genet. 1996, 14 (1): 55-61. 10.1038/ng0996-55.
Jordan J, Galindo MF, Miller RJ, Reardon CA, Getz GS, LaDu MJ: Isoform-specific effect of apolipoprotein E on cell survival and beta-amyloid-induced toxicity in rat hippocampal pyramidal neuronal cultures. J Neurosci. 1998, 18 (1): 195-204.
Herz J, Bock HH: Lipoprotein receptors in the nervous system. Annu Rev Biochem. 2002, 71: 405-434. 10.1146/annurev.biochem.71.110601.135342.
May P, Herz J, Bock HH: Molecular mechanisms of lipoprotein receptor signalling. Cell Mol Life Sci. 2005, 62 (19-20): 2325-2338. 10.1007/s00018-005-5231-z.
Herz J, Hamann U, Rogne S, Myklebost O, Gausepohl H, Stanley KK: Surface location and high affinity for calcium of a 500-kd liver membrane protein closely related to the LDL-receptor suggest a physiological role as lipoprotein receptor. Embo J. 1988, 7 (13): 4119-4127.
Raychowdhury R, Niles JL, McCluskey RT, Smith JA: Autoimmune target in Heymann nephritis is a glycoprotein with homology to the LDL receptor. Science. 1989, 244 (4909): 1163-1165. 10.1126/science.2786251.
Takahashi S, Kawarabayasi Y, Nakai T, Sakai J, Yamamoto T: Rabbit very low density lipoprotein receptor: a low density lipoprotein receptor-like protein with distinct ligand specificity. Proc Natl Acad Sci U S A. 1992, 89 (19): 9252-9256. 10.1073/pnas.89.19.9252.
Kim DH, Iijima H, Goto K, Sakai J, Ishii H, Kim HJ, Suzuki H, Kondo H, Saeki S, Yamamoto T: Human apolipoprotein E receptor 2. A novel lipoprotein receptor of the low density lipoprotein receptor family predominantly expressed in brain. J Biol Chem. 1996, 271 (14): 8373-8380. 10.1074/jbc.271.14.8373.
Novak S, Hiesberger T, Schneider WJ, Nimpf J: A new low density lipoprotein receptor homologue with 8 ligand binding repeats in brain of chicken and mouse. J Biol Chem. 1996, 271 (20): 11732-11736. 10.1074/jbc.271.20.11732.
Brandes C, Novak S, Stockinger W, Herz J, Schneider WJ, Nimpf J: Avian and murine LR8B and human apolipoprotein E receptor 2: differentially spliced products from corresponding genes. Genomics. 1997, 42 (2): 185-191. 10.1006/geno.1997.4702.
Jacobsen L, Madsen P, Moestrup SK, Lund AH, Tommerup N, Nykjaer A, Sottrup-Jensen L, Gliemann J, Petersen CM: Molecular characterization of a novel human hybrid-type receptor that binds the alpha2-macroglobulin receptor-associated protein. J Biol Chem. 1996, 271 (49): 31379-31383. 10.1074/jbc.271.49.31379.
Yamazaki H, Bujo H, Kusunoki J, Seimiya K, Kanaki T, Morisaki N, Schneider WJ, Saito Y: Elements of neural adhesion molecules and a yeast vacuolar protein sorting receptor are present in a novel mammalian low density lipoprotein receptor family member. J Biol Chem. 1996, 271 (40): 24761-24768. 10.1074/jbc.271.40.24761.
Johnson EB, Hammer RE, Herz J: Abnormal development of the apical ectodermal ridge and polysyndactyly in Megf7-deficient mice. Hum Mol Genet. 2005, 14 (22): 3523-3538. 10.1093/hmg/ddi381.
Kim DH, Inagaki Y, Suzuki T, Ioka RX, Yoshioka SZ, Magoori K, Kang MJ, Cho Y, Nakano AZ, Liu Q, Fujino T, Suzuki H, Sasano H, Yamamoto TT: A new low density lipoprotein receptor related protein, LRP5, is expressed in hepatocytes and adrenal cortex, and recognizes apolipoprotein E. J Biochem (Tokyo). 1998, 124 (6): 1072-1076.
Hey PJ, Twells RC, Phillips MS, Yusuke N, Brown SD, Kawaguchi Y, Cox R, Guochun X, Dugan V, Hammond H, Metzker ML, Todd JA, Hess JF: Cloning of a novel member of the low-density lipoprotein receptor family. Gene. 1998, 216 (1): 103-111. 10.1016/S0378-1119(98)00311-4.
Brown SD, Twells RC, Hey PJ, Cox RD, Levy ER, Soderman AR, Metzker ML, Caskey CT, Todd JA, Hess JF: Isolation and characterization of LRP6, a novel member of the low density lipoprotein receptor gene family. Biochem Biophys Res Commun. 1998, 248 (3): 879-888. 10.1006/bbrc.1998.9061.
Liu CX, Musco S, Lisitsina NM, Forgacs E, Minna JD, Lisitsyn NA: LRP-DIT, a putative endocytic receptor gene, is frequently inactivated in non-small cell lung cancer cell lines. Cancer Res. 2000, 60 (7): 1961-1967.
Li Y, Cam J, Bu G: Low-density lipoprotein receptor family: endocytosis and signal transduction. Mol Neurobiol. 2001, 23 (1): 53-67. 10.1385/MN:23:1:53.
Jeon H, Meng W, Takagi J, Eck MJ, Springer TA, Blacklow SC: Implications for familial hypercholesterolemia from the structure of the LDL receptor YWTD-EGF domain pair. Nat Struct Biol. 2001, 8 (6): 499-504. 10.1038/88556.
Bu G: Receptor-associated protein: a specialized chaperone and antagonist for members of the LDL receptor gene family. Curr Opin Lipidol. 1998, 9 (2): 149-155. 10.1097/00041433-199804000-00012.
Christie RH, Chung H, Rebeck GW, Strickland D, Hyman BT: Expression of the very low-density lipoprotein receptor (VLDL-r), an apolipoprotein-E receptor, in the central nervous system and in Alzheimer's disease. J Neuropathol Exp Neurol. 1996, 55 (4): 491-498.
Clatworthy AE, Stockinger W, Christie RH, Schneider WJ, Nimpf J, Hyman BT, Rebeck GW: Expression and alternate splicing of apolipoprotein E receptor 2 in brain. Neuroscience. 1999, 90 (3): 903-911. 10.1016/S0306-4522(98)00489-8.
Moestrup SK, Gliemann J, Pallesen G: Distribution of the alpha 2-macroglobulin receptor/low density lipoprotein receptor-related protein in human tissues. Cell Tissue Res. 1992, 269 (3): 375-382. 10.1007/BF00353892.
Swanson LW, Simmons DM, Hofmann SL, Goldstein JL, Brown MS: Localization of mRNA for low density lipoprotein receptor and a cholesterol synthetic enzyme in rabbit nervous system by in situ hybridization. Proc Natl Acad Sci U S A. 1988, 85 (24): 9821-9825. 10.1073/pnas.85.24.9821.
Li Y, Marzolo MP, van Kerkhof P, Strous GJ, Bu G: The YXXL motif, but not the two NPXY motifs, serves as the dominant endocytosis signal for low density lipoprotein receptor-related protein. J Biol Chem. 2000, 275 (22): 17187-17194. 10.1074/jbc.M000490200.
Chen WJ, Goldstein JL, Brown MS: NPXY, a sequence often found in cytoplasmic tails, is required for coated pit-mediated internalization of the low density lipoprotein receptor. J Biol Chem. 1990, 265 (6): 3116-3123.
Li Y, Lu W, Marzolo MP, Bu G: Differential functions of members of the low density lipoprotein receptor family suggested by their distinct endocytosis rates. J Biol Chem. 2001, 276 (21): 18000-18006. 10.1074/jbc.M101589200.
van Kerkhof P, Lee J, McCormick L, Tetrault E, Lu W, Schoenfish M, Oorschot V, Strous GJ, Klumperman J, Bu G: Sorting nexin 17 facilitates LRP recycling in the early endosome. Embo J. 2005, 24 (16): 2851-2861. 10.1038/sj.emboj.7600756.
Hoe HS, Magill LA, Guenette S, Fu Z, Vicini S, Rebeck GW: FE65 Interaction with the apoE receptor ApoEr2. J Biol Chem. 2006
Dietschy JM, Turley SD: Thematic review series: brain Lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J Lipid Res. 2004, 45 (8): 1375-1397. 10.1194/jlr.R400004-JLR200.
Bjorkhem I, Meaney S: Brain cholesterol: long secret life behind a barrier. Arterioscler Thromb Vasc Biol. 2004, 24 (5): 806-815. 10.1161/01.ATV.0000120374.59826.1b.
Poirier J: Apolipoprotein E in animal models of CNS injury and in Alzheimer's disease. Trends Neurosci. 1994, 17 (12): 525-530. 10.1016/0166-2236(94)90156-2.
Herz J: The LDL receptor gene family: (un)expected signal transducers in the brain. Neuron. 2001, 29 (3): 571-581. 10.1016/S0896-6273(01)00234-3.
Trommsdorff M, Gotthardt M, Hiesberger T, Shelton J, Stockinger W, Nimpf J, Hammer RE, Richardson JA, Herz J: Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell. 1999, 97 (6): 689-701. 10.1016/S0092-8674(00)80782-5.
Bacskai BJ, Xia MQ, Strickland DK, Rebeck GW, Hyman BT: The endocytic receptor protein LRP also mediates neuronal calcium signaling via N-methyl-D-aspartate receptors. Proc Natl Acad Sci U S A. 2000, 97 (21): 11551-11556. 10.1073/pnas.200238297.
Qiu Z, Strickland DK, Hyman BT, Rebeck GW: alpha 2-Macroglobulin exposure reduces calcium responses to N-methyl-D-aspartate via low density lipoprotein receptor-related protein in cultured hippocampal neurons. J Biol Chem. 2002, 277 (17): 14458-14466. 10.1074/jbc.M112066200.
Hoe HS, Harris DC, Rebeck GW: Multiple pathways of apolipoprotein E signaling in primary neurons. J Neurochem. 2005, 93 (1): 145-155. 10.1111/j.1471-4159.2004.03007.x.
Beffert U, Weeber EJ, Durudas A, Qiu S, Masiulis I, Sweatt JD, Li WP, Adelmann G, Frotscher M, Hammer RE, Herz J: Modulation of synaptic plasticity and memory by Reelin involves differential splicing of the lipoprotein receptor Apoer2. Neuron. 2005, 47 (4): 567-579. 10.1016/j.neuron.2005.07.007.
Ohkubo N, Mitsuda N, Tamatani M, Yamaguchi A, Lee YD, Ogihara T, Vitek MP, Tohyama M: Apolipoprotein E4 stimulates cAMP response element-binding protein transcriptional activity through the extracellular signal-regulated kinase pathway. J Biol Chem. 2001, 276 (5): 3046-3053. 10.1074/jbc.M005070200.
Aono M, Bennett ER, Kim KS, Lynch JR, Myers J, Pearlstein RD, Warner DS, Laskowitz DT: Protective effect of apolipoprotein E-mimetic peptides on N-methyl-D-aspartate excitotoxicity in primary rat neuronal-glial cell cultures. Neuroscience. 2003, 116 (2): 437-445. 10.1016/S0306-4522(02)00709-1.
Trommsdorff M, Borg JP, Margolis B, Herz J: Interaction of cytosolic adaptor proteins with neuronal apolipoprotein E receptors and the amyloid precursor protein. J Biol Chem. 1998, 273 (50): 33556-33560. 10.1074/jbc.273.50.33556.
Howell BW, Lanier LM, Frank R, Gertler FB, Cooper JA: The disabled 1 phosphotyrosine-binding domain binds to the internalization signals of transmembrane glycoproteins and to phospholipids. Mol Cell Biol. 1999, 19 (7): 5179-5188.
Gotthardt M, Trommsdorff M, Nevitt MF, Shelton J, Richardson JA, Stockinger W, Nimpf J, Herz J: Interactions of the low density lipoprotein receptor gene family with cytosolic adaptor and scaffold proteins suggest diverse biological functions in cellular communication and signal transduction. J Biol Chem. 2000, 275 (33): 25616-25624. 10.1074/jbc.M000955200.
Stockinger W, Brandes C, Fasching D, Hermann M, Gotthardt M, Herz J, Schneider WJ, Nimpf J: The reelin receptor ApoER2 recruits JNK-interacting proteins-1 and -2. J Biol Chem. 2000, 275 (33): 25625-25632. 10.1074/jbc.M004119200.
Oleinikov AV, Zhao J, Makker SP: Cytosolic adaptor protein Dab2 is an intracellular ligand of endocytic receptor gp600/megalin. Biochem J. 2000, 347 Pt 3: 613-621. 10.1042/0264-6021:3470613.
Hoe HS, Pocivavsek A, Chakraborty G, Fu Z, Vicini S, Ehlers MD, Rebeck GW: Apolipoprotein E receptor 2 interactions with the N-methyl-D-aspartate receptor. J Biol Chem. 2006, 281 (6): 3425-3431. 10.1074/jbc.M509380200.
Beffert U, Morfini G, Bock HH, Reyna H, Brady ST, Herz J: Reelin-mediated signaling locally regulates protein kinase B/Akt and glycogen synthase kinase 3beta. J Biol Chem. 2002, 277 (51): 49958-49964. 10.1074/jbc.M209205200.
Weeber EJ, Beffert U, Jones C, Christian JM, Forster E, Sweatt JD, Herz J: Reelin and ApoE receptors cooperate to enhance hippocampal synaptic plasticity and learning. J Biol Chem. 2002, 277 (42): 39944-39952. 10.1074/jbc.M205147200.
LaDu MJ, Shah JA, Reardon CA, Getz GS, Bu G, Hu J, Guo L, Van Eldik LJ: Apolipoprotein E and apolipoprotein E receptors modulate A beta-induced glial neuroinflammatory responses. Neurochem Int. 2001, 39 (5-6): 427-434. 10.1016/S0197-0186(01)00050-X.
Frykman PK, Brown MS, Yamamoto T, Goldstein JL, Herz J: Normal plasma lipoproteins and fertility in gene-targeted mice homozygous for a disruption in the gene encoding very low density lipoprotein receptor. Proc Natl Acad Sci U S A. 1995, 92 (18): 8453-8457. 10.1073/pnas.92.18.8453.
Crutcher KA, Clay MA, Scott SA, Tian X, Tolar M, Harmony JA: Neurite degeneration elicited by apolipoprotein E peptides. Exp Neurol. 1994, 130 (1): 120-126. 10.1006/exnr.1994.1191.
Hashimoto Y, Jiang H, Niikura T, Ito Y, Hagiwara A, Umezawa K, Abe Y, Murayama Y, Nishimoto I: Neuronal apoptosis by apolipoprotein E4 through low-density lipoprotein receptor-related protein and heterotrimeric GTPases. J Neurosci. 2000, 20 (22): 8401-8409.
Teter B: Apolipoprotein E isotype-specific effects in neurodegeneration. Alzheimer's Reports. 2000, 3 (4): 199-212.
Raber J, Wong D, Buttini M, Orth M, Bellosta S, Pitas RE, Mahley RW, Mucke L: Isoform-specific effects of human apolipoprotein E on brain function revealed in ApoE knockout mice: increased susceptibility of females. Proc Natl Acad Sci U S A. 1998, 95 (18): 10914-10919. 10.1073/pnas.95.18.10914.
Raber J, Wong D, Yu GQ, Buttini M, Mahley RW, Pitas RE, Mucke L: Apolipoprotein E and cognitive performance. Nature. 2000, 404 (6776): 352-354. 10.1038/35006165.
Hartman RE, Wozniak DF, Nardi A, Olney JW, Sartorius L, Holtzman DM: Behavioral phenotyping of GFAP-apoE3 and -apoE4 transgenic mice: apoE4 mice show profound working memory impairments in the absence of Alzheimer's-like neuropathology. Exp Neurol. 2001, 170 (2): 326-344. 10.1006/exnr.2001.7715.
Buttini M, Akeefe H, Lin C, Mahley RW, Pitas RE, Wyss-Coray T, Mucke L: Dominant negative effects of apolipoprotein E4 revealed in transgenic models of neurodegenerative disease. Neuroscience. 2000, 97 (2): 207-210. 10.1016/S0306-4522(00)00069-5.
Buttini M, Orth M, Bellosta S, Akeefe H, Pitas RE, Wyss-Coray T, Mucke L, Mahley RW: Expression of human apolipoprotein E3 or E4 in the brains of Apoe-/- mice: isoform-specific effects on neurodegeneration. J Neurosci. 1999, 19 (12): 4867-4880.
Sullivan PM, Mezdour H, Aratani Y, Knouff C, Najib J, Reddick RL, Quarfordt SH, Maeda N: Targeted replacement of the mouse apolipoprotein E gene with the common human APOE3 allele enhances diet-induced hypercholesterolemia and atherosclerosis. J Biol Chem. 1997, 272 (29): 17972-17980. 10.1074/jbc.272.29.17972.
Grootendorst J, Bour A, Vogel E, Kelche C, Sullivan PM, Dodart JC, Bales K, Mathis C: Human apoE targeted replacement mouse lines: h-apoE4 and h-apoE3 mice differ on spatial memory performance and avoidance behavior. Behav Brain Res. 2005, 159 (1): 1-14. 10.1016/j.bbr.2004.09.019.
Trommer BL, Shah C, Yun SH, Gamkrelidze G, Pasternak ES, Ye GL, Sotak M, Sullivan PM, Pasternak JF, LaDu MJ: ApoE isoform affects LTP in human targeted replacement mice. Neuroreport. 2004, 15 (17): 2655-2658. 10.1097/00001756-200412030-00020.
Drouet B, Fifre A, Pincon-Raymond M, Vandekerckhove J, Rosseneu M, Gueant JL, Chambaz J, Pillot T: ApoE protects cortical neurones against neurotoxicity induced by the non-fibrillar C-terminal domain of the amyloid-beta peptide. J Neurochem. 2001, 76 (1): 117-127. 10.1046/j.1471-4159.2001.00047.x.
Manelli AM, Stine WB, Van Eldik LJ, LaDu MJ: ApoE and Abeta1-42 interactions: effects of isoform and conformation on structure and function. J Mol Neurosci. 2004, 23 (3): 235-246. 10.1385/JMN:23:3:235.
Ji ZS, Miranda RD, Newhouse YM, Weisgraber KH, Huang Y, Mahley RW: Apolipoprotein E4 potentiates amyloid beta peptide-induced lysosomal leakage and apoptosis in neuronal cells. J Biol Chem. 2002, 277 (24): 21821-21828. 10.1074/jbc.M112109200.
Ji ZS, Mullendorff K, Cheng IH, Miranda RD, Huang Y, Mahley RW: Reactivity of apolipoprotein E4 and amyloid beta peptide: lysosomal stability and neurodegeneration. J Biol Chem. 2006, 281 (5): 2683-2692. 10.1074/jbc.M506646200.
Lanz TA, Carter DB, Merchant KM: Dendritic spine loss in the hippocampus of young PDAPP and Tg2576 mice and its prevention by the ApoE2 genotype. Neurobiol Dis. 2003, 13 (3): 246-253. 10.1016/S0969-9961(03)00079-2.
Zlokovic BV: Clearing amyloid through the blood-brain barrier. J Neurochem. 2004, 89 (4): 807-811. 10.1111/j.1471-4159.2004.02385.x.
Gearing M, Schneider JA, Robbins RS, Hollister RD, Mori H, Games D, Hyman BT, Mirra SS: Regional variation in the distribution of apolipoprotein E and A beta in Alzheimer's disease. J Neuropathol Exp Neurol. 1995, 54 (6): 833-841.
Cho HS, Hyman BT, Greenberg SM, Rebeck GW: Quantitation of apoE domains in Alzheimer disease brain suggests a role for apoE in Abeta aggregation. J Neuropathol Exp Neurol. 2001, 60 (4): 342-349.
Arelin K, Kinoshita A, Whelan CM, Irizarry MC, Rebeck GW, Strickland DK, Hyman BT: LRP and senile plaques in Alzheimer's disease: colocalization with apolipoprotein E and with activated astrocytes. Brain Res Mol Brain Res. 2002, 104 (1): 38-46. 10.1016/S0169-328X(02)00203-6.
Van Uden E, Mallory M, Veinbergs I, Alford M, Rockenstein E, Masliah E: Increased extracellular amyloid deposition and neurodegeneration in human amyloid precursor protein transgenic mice deficient in receptor-associated protein. J Neurosci. 2002, 22 (21): 9298-9304.
Willnow TE, Rohlmann A, Horton J, Otani H, Braun JR, Hammer RE, Herz J: RAP, a specialized chaperone, prevents ligand-induced ER retention and degradation of LDL receptor-related endocytic receptors. Embo J. 1996, 15 (11): 2632-2639.
Willnow TE, Armstrong SA, Hammer RE, Herz J: Functional expression of low density lipoprotein receptor-related protein is controlled by receptor-associated protein in vivo. Proc Natl Acad Sci U S A. 1995, 92 (10): 4537-4541. 10.1073/pnas.92.10.4537.
Kounnas MZ, Moir RD, Rebeck GW, Bush AI, Argraves WS, Tanzi RE, Hyman BT, Strickland DK: LDL receptor-related protein, a multifunctional ApoE receptor, binds secreted beta-amyloid precursor protein and mediates its degradation. Cell. 1995, 82 (2): 331-340. 10.1016/0092-8674(95)90320-8.
Knauer MF, Orlando RA, Glabe CG: Cell surface APP751 forms complexes with protease nexin 2 ligands and is internalized via the low density lipoprotein receptor-related protein (LRP). Brain Res. 1996, 740 (1-2): 6-14. 10.1016/S0006-8993(96)00711-1.
Kinoshita A, Whelan CM, Smith CJ, Mikhailenko I, Rebeck GW, Strickland DK, Hyman BT: Demonstration by fluorescence resonance energy transfer of two sites of interaction between the low-density lipoprotein receptor-related protein and the amyloid precursor protein: role of the intracellular adapter protein Fe65. J Neurosci. 2001, 21 (21): 8354-8361.
Pietrzik CU, Yoon IS, Jaeger S, Busse T, Weggen S, Koo EH: FE65 constitutes the functional link between the low-density lipoprotein receptor-related protein and the amyloid precursor protein. J Neurosci. 2004, 24 (17): 4259-4265. 10.1523/JNEUROSCI.5451-03.2004.
Ulery PG, Beers J, Mikhailenko I, Tanzi RE, Rebeck GW, Hyman BT, Strickland DK: Modulation of beta-amyloid precursor protein processing by the low density lipoprotein receptor-related protein (LRP). Evidence that LRP contributes to the pathogenesis of Alzheimer's disease. J Biol Chem. 2000, 275 (10): 7410-7415. 10.1074/jbc.275.10.7410.
Pietrzik CU, Busse T, Merriam DE, Weggen S, Koo EH: The cytoplasmic domain of the LDL receptor-related protein regulates multiple steps in APP processing. Embo J. 2002, 21 (21): 5691-5700. 10.1093/emboj/cdf568.
Cam JA, Zerbinatti CV, Li Y, Bu G: Rapid endocytosis of the low density lipoprotein receptor-related protein modulates cell surface distribution and processing of the beta-amyloid precursor protein. J Biol Chem. 2005, 280 (15): 15464-15470. 10.1074/jbc.M500613200.
Zerbinatti CV, Wozniak DF, Cirrito J, Cam JA, Osaka H, Bales KR, Zhuo M, Paul SM, Holtzman DM, Bu G: Increased soluble amyloid-beta peptide and memory deficits in amyloid model mice overexpressing the low-density lipoprotein receptor-related protein. Proc Natl Acad Sci U S A. 2004, 101 (4): 1075-1080. 10.1073/pnas.0305803101.
Cam JA, Zerbinatti CV, Knisely JM, Hecimovic S, Li Y, Bu G: The low density lipoprotein receptor-related protein 1B retains beta-amyloid precursor protein at the cell surface and reduces amyloid-beta peptide production. J Biol Chem. 2004, 279 (28): 29639-29646. 10.1074/jbc.M313893200.
Hoe HS, Wessner D, Beffert U, Becker AG, Matsuoka Y, Rebeck GW: F-spondin interaction with the apolipoprotein E receptor ApoEr2 affects processing of amyloid precursor protein. Mol Cell Biol. 2005, 25 (21): 9259-9268. 10.1128/MCB.25.21.9259-9268.2005.
Andersen OM, Schmidt V, Spoelgen R, Gliemann J, Behlke J, Galatis D, McKinstry WJ, Parker MW, Masters CL, Hyman BT, Cappai R, Willnow TE: Molecular dissection of the interaction between amyloid precursor protein and its neuronal trafficking receptor SorLA/LR11. Biochemistry. 2006, 45 (8): 2618-2628. 10.1021/bi052120v.
Spoelgen R, von Arnim CA, Thomas AV, Peltan ID, Koker M, Deng A, Irizarry MC, Andersen OM, Willnow TE, Hyman BT: Interaction of the cytosolic domains of sorLA/LR11 with the amyloid precursor protein (APP) and beta-secretase beta-site APP-cleaving enzyme. J Neurosci. 2006, 26 (2): 418-428. 10.1523/JNEUROSCI.3882-05.2006.
Andersen OM, Reiche J, Schmidt V, Gotthardt M, Spoelgen R, Behlke J, von Arnim CA, Breiderhoff T, Jansen P, Wu X, Bales KR, Cappai R, Masters CL, Gliemann J, Mufson EJ, Hyman BT, Paul SM, Nykjaer A, Willnow TE: Neuronal sorting protein-related receptor sorLA/LR11 regulates processing of the amyloid precursor protein. Proc Natl Acad Sci U S A. 2005, 102 (38): 13461-13466. 10.1073/pnas.0503689102.
Offe K, Dodson SE, Shoemaker JT, Fritz JJ, Gearing M, Levey AI, Lah JJ: The lipoprotein receptor LR11 regulates amyloid beta production and amyloid precursor protein traffic in endosomal compartments. J Neurosci. 2006, 26 (5): 1596-1603. 10.1523/JNEUROSCI.4946-05.2006.
Garcia-Touchard A, Henry TD, Sangiorgi G, Spagnoli LG, Mauriello A, Conover C, Schwartz RS: Extracellular proteases in atherosclerosis and restenosis. Arterioscler Thromb Vasc Biol. 2005, 25 (6): 1119-1127. 10.1161/01.ATV.0000164311.48592.da.
Xing Y, Xu Q, Lee C: Widespread production of novel soluble protein isoforms by alternative splicing removal of transmembrane anchoring domains. FEBS Lett. 2003, 555 (3): 572-578. 10.1016/S0014-5793(03)01354-1.
Herz J, Kowal RC, Goldstein JL, Brown MS: Proteolytic processing of the 600 kd low density lipoprotein receptor-related protein (LRP) occurs in a trans-Golgi compartment. Embo J. 1990, 9 (6): 1769-1776.
Quinn KA, Grimsley PG, Dai YP, Tapner M, Chesterman CN, Owensby DA: Soluble low density lipoprotein receptor-related protein (LRP) circulates in human plasma. J Biol Chem. 1997, 272 (38): 23946-23951. 10.1074/jbc.272.38.23946.
Grimsley PG, Quinn KA, Owensby DA: Soluble low-density lipoprotein receptor-related protein. Trends Cardiovasc Med. 1998, 8 (8): 363-368. 10.1016/S1050-1738(98)00029-2.
Qiu Z, Strickland DK, Hyman BT, Rebeck GW: Elevation of LDL receptor-related protein levels via ligand interactions in Alzheimer disease and in vitro. J Neuropathol Exp Neurol. 2001, 60 (5): 430-440.
May P, Reddy YK, Herz J: Proteolytic processing of low density lipoprotein receptor-related protein mediates regulated release of its intracellular domain. J Biol Chem. 2002, 277 (21): 18736-18743. 10.1074/jbc.M201979200.
Kinoshita A, Shah T, Tangredi MM, Strickland DK, Hyman BT: The intracellular domain of the low density lipoprotein receptor-related protein modulates transactivation mediated by amyloid precursor protein and Fe65. J Biol Chem. 2003, 278 (42): 41182-41188. 10.1074/jbc.M306403200.
Blaumueller CM, Qi H, Zagouras P, Artavanis-Tsakonas S: Intracellular cleavage of Notch leads to a heterodimeric receptor on the plasma membrane. Cell. 1997, 90 (2): 281-291. 10.1016/S0092-8674(00)80336-0.
Logeat F, Bessia C, Brou C, LeBail O, Jarriault S, Seidah NG, Israel A: The Notch1 receptor is cleaved constitutively by a furin-like convertase. Proc Natl Acad Sci U S A. 1998, 95 (14): 8108-8112. 10.1073/pnas.95.14.8108.
Mumm JS, Schroeter EH, Saxena MT, Griesemer A, Tian X, Pan DJ, Ray WJ, Kopan R: A ligand-induced extracellular cleavage regulates gamma-secretase-like proteolytic activation of Notch1. Mol Cell. 2000, 5 (2): 197-206. 10.1016/S1097-2765(00)80416-5.
Hoe HS, Rebeck GW: Regulation of ApoE receptor proteolysis by ligand binding. Brain Res Mol Brain Res. 2005, 137 (1-2): 31-39. 10.1016/j.molbrainres.2005.02.013.
May P, Bock HH, Nimpf J, Herz J: Differential glycosylation regulates processing of lipoprotein receptors by gamma-secretase. J Biol Chem. 2003, 278 (39): 37386-37392. 10.1074/jbc.M305858200.
Iijima H, Miyazawa M, Sakai J, Magoori K, Ito MR, Suzuki H, Nose M, Kawarabayasi Y, Yamamoto TT: Expression and characterization of a very low density lipoprotein receptor variant lacking the O-linked sugar region generated by alternative splicing. J Biochem (Tokyo). 1998, 124 (4): 747-755.
Brandes C, Kahr L, Stockinger W, Hiesberger T, Schneider WJ, Nimpf J: Alternative splicing in the ligand binding domain of mouse ApoE receptor-2 produces receptor variants binding reelin but not alpha 2-macroglobulin. J Biol Chem. 2001, 276 (25): 22160-22169. 10.1074/jbc.M102662200.
Koch S, Strasser V, Hauser C, Fasching D, Brandes C, Bajari TM, Schneider WJ, Nimpf J: A secreted soluble form of ApoE receptor 2 acts as a dominant-negative receptor and inhibits Reelin signaling. Embo J. 2002, 21 (22): 5996-6004. 10.1093/emboj/cdf599.
Fischer DG, Tal N, Novick D, Barak S, Rubinstein M: An antiviral soluble form of the LDL receptor induced by interferon. Science. 1993, 262 (5131): 250-253. 10.1126/science.8211145.
Begg MJ, Sturrock ED, van der Westhuyzen DR: Soluble LDL-R are formed by cell surface cleavage in response to phorbol esters. Eur J Biochem. 2004, 271 (3): 524-533. 10.1046/j.1432-1033.2003.03953.x.
Ehlers MR, Riordan JF: Membrane proteins with soluble counterparts: role of proteolysis in the release of transmembrane proteins. Biochemistry. 1991, 30 (42): 10065-10074. 10.1021/bi00106a001.
Wong ST, Winchell LF, McCune BK, Earp HS, Teixido J, Massague J, Herman B, Lee DC: The TGF-alpha precursor expressed on the cell surface binds to the EGF receptor on adjacent cells, leading to signal transduction. Cell. 1989, 56 (3): 495-506. 10.1016/0092-8674(89)90252-3.
Arribas J, Massague J: Transforming growth factor-alpha and beta-amyloid precursor protein share a secretory mechanism. J Cell Biol. 1995, 128 (3): 433-441. 10.1083/jcb.128.3.433.
Van Nostrand WE, Wagner SL, Suzuki M, Choi BH, Farrow JS, Geddes JW, Cotman CW, Cunningham DD: Protease nexin-II, a potent antichymotrypsin, shows identity to amyloid beta-protein precursor. Nature. 1989, 341 (6242): 546-549. 10.1038/341546a0.
Wolfe MS, Kopan R: Intramembrane proteolysis: theme and variations. Science. 2004, 305 (5687): 1119-1123. 10.1126/science.1096187.
Steiner H, Haass C: Intramembrane proteolysis by presenilins. Nat Rev Mol Cell Biol. 2000, 1 (3): 217-224. 10.1038/35043065.
Mumm JS, Kopan R: Notch signaling: from the outside in. Dev Biol. 2000, 228 (2): 151-165. 10.1006/dbio.2000.9960.
Brown MS, Goldstein JL: A proteolytic pathway that controls the cholesterol content of membranes, cells, and blood. Proc Natl Acad Sci U S A. 1999, 96 (20): 11041-11048. 10.1073/pnas.96.20.11041.
Arend WP, Malyak M, Smith MF, Whisenand TD, Slack JL, Sims JE, Giri JG, Dower SK: Binding of IL-1 alpha, IL-1 beta, and IL-1 receptor antagonist by soluble IL-1 receptors and levels of soluble IL-1 receptors in synovial fluids. J Immunol. 1994, 153 (10): 4766-4774.
Fernandez-Botran R, Chilton PM, Ma Y: Soluble cytokine receptors: their roles in immunoregulation, disease, and therapy. Adv Immunol. 1996, 63: 269-336.
Mortier E, Bernard J, Plet A, Jacques Y: Natural, proteolytic release of a soluble form of human IL-15 receptor alpha-chain that behaves as a specific, high affinity IL-15 antagonist. J Immunol. 2004, 173 (3): 1681-1688.
Bierhaus A, Humpert PM, Morcos M, Wendt T, Chavakis T, Arnold B, Stern DM, Nawroth PP: Understanding RAGE, the receptor for advanced glycation end products. J Mol Med. 2005, 83 (11): 876-886. 10.1007/s00109-005-0688-7.
Yang G, Ge H, Boucher A, Yu X, Li C: Modulation of direct leptin signaling by soluble leptin receptor. Mol Endocrinol. 2004, 18 (6): 1354-1362. 10.1210/me.2004-0027.
Pasquale EB: Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol. 2005, 6 (6): 462-475. 10.1038/nrm1662.
Janes PW, Saha N, Barton WA, Kolev MV, Wimmer-Kleikamp SH, Nievergall E, Blobel CP, Himanen JP, Lackmann M, Nikolov DB: Adam meets Eph: an ADAM substrate recognition module acts as a molecular switch for ephrin cleavage in trans. Cell. 2005, 123 (2): 291-304. 10.1016/j.cell.2005.08.014.
Hattori M, Osterfield M, Flanagan JG: Regulated cleavage of a contact-mediated axon repellent. Science. 2000, 289 (5483): 1360-1365. 10.1126/science.289.5483.1360.
Maretzky T, Schulte M, Ludwig A, Rose-John S, Blobel C, Hartmann D, Altevogt P, Saftig P, Reiss K: L1 is sequentially processed by two differently activated metalloproteases and presenilin/gamma-secretase and regulates neural cell adhesion, cell migration, and neurite outgrowth. Mol Cell Biol. 2005, 25 (20): 9040-9053. 10.1128/MCB.25.20.9040-9053.2005.
Slack BE, Siniaia MS, Blusztajn JK: Collagen type I selectively activates ectodomain shedding of the discoidin domain receptor 1: Involvement of Src tyrosine kinase. J Cell Biochem. 2006, 98 (3): 672-684. 10.1002/jcb.20812.
Nandi D, Tahiliani P, Kumar A, Chandu D: The ubiquitin-proteasome system. J Biosci. 2006, 31 (1): 137-155.
Montuori N, Visconte V, Rossi G, Ragno P: Soluble and cleaved forms of the urokinase-receptor: degradation products or active molecules?. Thromb Haemost. 2005, 93 (2): 192-198.
Lammich S, Okochi M, Takeda M, Kaether C, Capell A, Zimmer AK, Edbauer D, Walter J, Steiner H, Haass C: Presenilin-dependent intramembrane proteolysis of CD44 leads to the liberation of its intracellular domain and the secretion of an Abeta-like peptide. J Biol Chem. 2002, 277 (47): 44754-44759. 10.1074/jbc.M206872200.
Ronacher B, Marlovits TC, Moser R, Blaas D: Expression and folding of human very-low-density lipoprotein receptor fragments: neutralization capacity toward human rhinovirus HRV2. Virology. 2000, 278 (2): 541-550. 10.1006/viro.2000.0636.
Bajari TM, Strasser V, Nimpf J, Schneider WJ: LDL receptor family: isolation, production, and ligand binding analysis. Methods. 2005, 36 (2): 109-116. 10.1016/j.ymeth.2004.11.007.
Author information
Authors and Affiliations
Corresponding author
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Rebeck, G.W., LaDu, M.J., Estus, S. et al. The generation and function of soluble apoE receptors in the CNS. Mol Neurodegeneration 1, 15 (2006). https://doi.org/10.1186/1750-1326-1-15
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1750-1326-1-15