Abstract
Mature natural killer (NK) cell neoplasms are classified by the World Health Organization into NK/T cell lymphoma, nasal type (NKTCL), aggressive NK-cellleukemia (ANKCL) and chronic lymphoproliferative disorders of NK-cells, thelatter being considered provisionally. NKTCL and ANKCL are rare diseases, withhigher prevalence in Asia, Central and South America. Most NKTCL presentextranodal, as a destructive tumor affecting the nose and upper aerodigestivetract (nasal NKTCL) or any organ or tissue (extranasal NKTCL) whereas ANKCLmanifests as a systemic disease with multiorgan involvement and naturallyevolutes to death in a few weeks. The histopathological hallmark of theseaggressive NK-cell tumors is a polymorphic neoplastic infiltrate withangiocentricity, angiodestruction and tissue necrosis. The tumor cells havecytoplasmatic azurophilic granules and usually show a CD45+bright,CD2+, sCD3-, cytCD3epsilon+,CD56+bright, CD16−/+, cytotoxic granulesmolecules+ phenotype. T-cell receptor genes are in germ-lineconfiguration. Epstein-Barr virus (EBV) -encoded membrane proteins and earlyregion EBV RNA are usually detected on lymphoma cells, with a pattern suggestiveof a latent viral infection type II. Complex chromosomal abnormalities arefrequent and loss of chromosomes 6q, 11q, 13q, and 17p are recurrentaberrations. The rarity of the NK-cell tumors limits our ability to standardizethe procedures for the diagnosis and clinical management and efforts should bemade to encourage multi-institutional registries.
Resumo
As neoplasias de células natural killer (NK) maduras foramclassificadas pela Organização Mundial de Saúde em trêsentidades: o linfoma de células NK/T tipo nasal (NKTCL), a leucemiaagressiva de células NK (ANKCL) e as doenças linfoproliferativascrónicas de células NK, estas últimas consideradas umaentidade provisória. Os NKTCL e a ANKCL são doenças raras,mais prevalentes na Ásia, na América Central e na América doSul. A maioria dos NKTCL tem uma apresentação extra-ganglionar, naforma de tumor destrutivo que atinge o nariz e o trato aerodigestivo alto(forma nasal) ou qualquer órgão ou tecido (forma extranasal). AANKCL manifesta-se como uma doença sistémica que evolui para amorte em poucas semanas. Do ponto de vista histopatológico, estasneoplasias caraterizam-se por um infiltrado polimórfico, comangiocentricidade, destruição vascular e necrose tecidular. Ascélulas tumorais têm grânulos azurófilos no citoplasma eo seu imunofenótipo (CD45+forte, CD2+,sCD3-, cytCD3epsilon+, CD56+forte,CD16−/+, proteínas dos grânuloscitotóxicos+) é caraterístico. Os genes quecodificam para o recetor das células T estão emconfiguração nativa. As células tumorais expressam geralmenteproteínas da membrana e ARN do vírus Epstein Barr, com umpadrão sugestivo de uma infecção vírica latente tipo II.As alterações cromossómicas são complexas, e algumas,como deleções nos braços longos dos cromossomas 6, 11 e 13 edo braço curto do cromossoma 17, ocorrem de forma recorrente. Araridade dos tumores de células NK limita a nossa capacidade parauniformizar os procedimentos de diagnóstico e a abordagem clínica,sendo necessário desenvolver esforços para promover os registosmulticêntricos.
Similar content being viewed by others
Introduction
Lymphoproliferative disorders of natural killer (NK) cells are rare diseases whichaccount for less than 5% of all lymphoid neoplasms and comprise differentclinical entities [1–17].
The World Health Organization (WHO) classification of tumors of hematopoietic andlymphoid tissues, updated in 2008, has made advances in their classification.Accordingly, three disease conditions originating from mature NK-cells were proposedbased on their distinct clinical and pathological features [18]. These include two aggressive mature NK-cell neoplasms – extranodalNK/T cell lymphoma, nasal type (NKTCL) [19], and aggressive NK-cell leukemia (ANKCL) [20] – and one provisional entity – chronic lymphoproliferativedisorders of NK-cells (CLPD-NK) [21] (Table 1). The first two entities are indexedindividually in the 10th revision of the International Classification of Diseases(ICD-10) [22] and in the 3rd edition of the ICD for Oncology (ICD-O-3) [23], as well as in Orphanet databases [24]. In addition, two diseases were proposed in the past as originating fromNK-cell precursors, based mainly on the blastic appearance and the CD56+immature immunophenotype of the neoplastic cells. The first, NK-cell lymphoblasticleukemia / lymphoma [25], in fact comprise an heterogeneous group of immature disordersoriginating from NK-, T- and/or myeloid cell precursors, and is now being consideredin the group of the acute leukemia of ambiguous lineage; the other, blasticplasmacytoid dendritic cell neoplasm, previously designed blastic NK-cell lymphoma,arises from plasmacytoid dendritic cells and should no longer be considered aNK-cell malignancy [26].
Nasal type NKTCL, originates in nasal and extranasal organs and tissues and accountfor the majority of cases, with only exceptional cases presenting primarily in thelymph nodes. ANKCL manifests as a systemic disease with multiorgan failure andrapidly evolutes to death. The diagnosis of these aggressive NK-cell neoplasms isoften difficult and requires both clinical suspicion and a differentiatedlaboratorial approach based in morphological, immunophenotypic and molecularstudies.
We review the epidemiology and the clinical and laboratorial criteria for thediagnosis of NKTCL and ANKCL, with emphasis on tissue histology and on themorphological, immunophenotypic and genetic features of the neoplastic cells.
Review
Epidemiology
Both NKTCL and ANKCL are relatively frequent in Asia, Central and South America,but extremely rare in Europe and North America [1–17].
Extranodal NK/T-cell lymphoma, nasal type, and ANKCL are relatively frequent inCentral American (e.g. Mexico, Guatemala), South American (e.g. Argentina,Brazil, Peru, Chile) and Eastern (e.g. Hong Kong, Japan, Korea) countries, wherethey may account for up to 10% of the non Hodgkin’s lymphoma (NHL),whereas very uncommon in North America and Europe, where they represent lessthan 1% of the NHLa[1, 2, 27–29]. Moreover, in series from the United States in which the ethnicbackground was recorded, most patients with NKTCL were of Asian or Hispanicdescent [30]. Few epidemiological data is available in Europe, where itsprevalence has been estimated to be lower than 1–9 cases / 1.000.000inhabitants.
Aggressive NK-cell neoplasms are almost always associated to Epstein Barr Virus(EBV) and similarly to that occurring in Hodgkin’s lymphoma andnasopharyngeal carcinoma, the neoplastic NK-cells usually have a type II latencypattern, expression of EBV nuclear antigens (EBNA) and latent membrane proteins(LMP) being limited to EBNA-1, LMP-1, and LMP-2 [31]. In Asia, increase in the risk of developing nasal NKTCL have beendescribed among crop producers and individuals exposed to pesticides [32], also having an increased risk to develop NHL in general [33, 34].
The International Peripheral T-cell Lymphoma Project (IPTCLP) group reported afour-fold higher relative frequency of NKTCL among lymphomas in Asian countriescompared to Western countries, ANKCL being rarer than NKTCL (Table 2). From the 136 cases of NK-cell neoplasms analyzed by thisgroup, collected in different centers from various countries in North America,Europe, and Asia, only 2 (1.5%) corresponded to ANKCL, as compared to 127cases of NKTCL, the remaining 7 cases being unclassifiable according to the WHOschema [35]. Comparatively, based on the Japanese survey of NK-cell neoplasmsdiagnosed from 1994 to 1998 [36], the NK-cell Tumor Study Group reported on a Japanese series of 172NK-cell tumors, which included 22 ANKCL (12.8%) [37]. Few European series of NKTCL were published to date [38, 39] and, in Europe, reports on ANKCL are limited to sporadic cases [40–43].
Two variants of extranodal NKTCL, have been described, the nasal and theextranasal forms, the first being more frequent in nearly all reported series.In the register from the Japanese survey, only 18% of the NKTCL wereextranasal [37], a higher percentage of extranasal cases (28%) being found amongthe NKTCL reported by the IPTCLP group [35]; in addition, a Brazilian and an Italian series of NKTCL included19% and 12% of extranasal lymphomas, respectively [39, 44] (Table 2).
Clinical features
Extranodal NK/T cell lymphomas, nasal type
The nasal and extranasal forms of NKTCL differ from each other from theclinical point of view (Table 3) [1, 10, 14, 45].
Nasal NK/T cell lymphomas
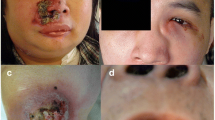
In contrast to that observed in Occidental countries, where the majority ofthe sinonasal lymphomas are B-cell lymphomas, in Asia more than 40% ofthese lymphomas originate from NK-cells. This neoplasm (also known as“lethal midline granuloma” or “midline malignantreticulosis”) commonly affects males and generally manifests as alocalized disease, with mid-facial and/or upper airway destructive lesions [1, 10, 14, 45]. Patients with nasal NKTCL present with nasal signals andsymptoms, including mass, obstruction swelling, or bleeding. The tumor islocally invasive and often infiltrates the surrounding tissues, such as thenasopharynx, the oropharynx, the palate and the orbits; dissemination toother organs may occur in advanced disease stages.
Extranasal NK/T cell lymphomas
The extranasal form is frequently disseminated at the time of the diagnosis,most patients having multiple organs and tissues involved, usually withoutadenopathies [1, 10, 14, 45]. Patients with extranasal NKTCL often have more adverse clinicalfeatures such as an advanced stage and poor performance status, and are morelikely to have cytopenias, when compared to patients with nasal lymphoma [1, 10, 14]. The tumor may involve any anatomic site at the diseasepresentation or during disease progression, including the skin, thegastrointestinal tract, the testis, the lungs, the eyes, the soft tissues,the adrenal glands, the brain, the breast and the tongue [1, 10, 14]. The diagnosis of an extranasal NKTCL requires the exclusion ofoccult nasal disease, which may require nasal endoscopy with randombiopsies.
Bone marrow involvement at the diagnosis is uncommon in NKTCL, in both nasal(<3.5%) and extranasal (<7%) cases [4, 46]. In contrast, the hemophagocytic syndrome is relatively frequent,and often occurs in advanced disease [47].
Nodal NK-cell lymphomas, nasal type
Although nodal NKTCL are not being considered separately in the WHOclassification, a few cases of NKTCL presenting primarily in the lymph nodeshave been described [44, 48–52]. Some of these cases were included in extranodal NKTCL series [44, 50] and in series of patients with cytotoxic lymphomas [51]. For instance, in a review of 49 Asian cases of CD56+ neoplasmsfrom which 34 were NKTCL, one had a primary nodal presentation [50] and a Brazilian series of 122 cases NKTCL, from which 23 caseswere extranasal, included 6 nodal cases [44]. In another series of 66 patients with nodal cytotoxic celllymphomas, one had the classic NKTCL phenotype [51]. In addition, cases of nodal lymphomas with a typical NKTCLphenotype and T-cell receptor (TCR) gamma (TCRG) generearrangements in germ-line configuration were described as case reports [48, 49]. However, in other cases, the tumor probably originates fromcytotoxic T-lymphocytes, as in the series of nodal lymphoma with a typicalNKTCL phenotype reported by Takashi et al., from which 4 cases had clonalTCRG gene rearrangements [52]. Nodal NKTCL have a poor prognosis, most patients surviving forless than one year; they usually affect the cervical lymph nodes and thehistology and phenotype are similar to those of extranodal NKTCL.
Aggressive NK-cell leukemia
Aggressive NK-cell leukemia is a very rare and extremely aggressive neoplasm,also with a higher prevalence among Asians [2, 35, 36, 53–55]. Men and women are equally affected and the disease usuallymanifest in the third or four decades. Patients usually present extremelyill, with fever and other systemic symptoms, hepatosplenomegaly,pancytopenia and abnormal liver function. Serum levels of lacticdehydrogenase (LDH) and Fas Ligand (FasL) are often markedly increased. Thehemophagocytic syndrome is frequent at diagnosis or during the diseasecourse, resulting from uncontrolled monocyte/macrophage activation inresponse to cytokines produced by the neoplastic NK-cells [56–61]. The natural disease course is fulminant, with multiorgan failureand disseminated intravascular coagulation, death occurring usually within afew weeks [62].
Clinical staging
The Ann-Arbor staging system, originally designed for Hodgkin’s lymphoma,is used for clinical staging of the NHL in general (Table 4) [63, 64]. However, this system is not completely satisfactory for NKTCL, as itdoes not take into account the tumor size and the invasion to contiguousstructures, which may be important prognostic features. Consequently, a modifiedtumor-staging system originally proposed for sinonasal B-cell lymphoma wasadopted, which takes into account the local involvement [65] (Table 4).
In order to perform disease staging, patients should be evaluated with routinehematological and biochemical analysis, bilateral bone marrow trephine biopsy,chest radiography, computerized tomography, and digestive endoscopy. Inaddition, magnetic resonance imaging helps to define the local involvement innasal lymphoma, being superior to computerized tomography in determining theextent of soft-tissue infiltration, in differentiating inflamed from neoplastictissue, and in clarifying bone lesions [66]. Positron emission tomography using fluorine-18-fluoro-deoxy-glucoseis useful to investigate systemic spread and to distinguishing lymphoma frominflammatory masses [67].
The ratio of patients presenting limited extranodal disease stages (IEor IIE) versus those with presenting with advanced disease stages(III or IV) is 7:3 for nasal NKTCL and 4:6 for extranasal NKTCL [36].
Laboratorial diagnosis
Histology and cytology
Natural killer/T cell lymphoma, nasal type, are histologically characterizedby angiocentricity and invasion of the blood vessels by lymphoma cells,resulting in ischemic necrosis; the neoplastic cells have a variable sizeand usually appear morphologically immature and the cytological features areheterogeneous, with a variable mixture of inflammatory cells. Azurophilicgranules are usually observed in the cytoplasm of the lymphoma cells usingimprint smears [6]. Aggressive NK-cell leukemia cells are larger than normal largegranular lymphocytes and often have a pale or slightly basophilic cytoplasmwith azurophilic granules and a nucleus with slightly immature chromatin andinconspicuous or distinct nucleoli. As in NKTCL, necrosis, apoptosis,angioinvasion, and angiodestruction are common findings in tissue biopsies [6]. Also frequent is hemophagocytosis, which results fromuncontrolled macrophage stimulation by inflammatory cytokines produced bythe neoplastic cells and may result in the development of a hemophagocyticsyndrome.
Immunophenotype
Most of our knowledge on the immunophenotype of the neoplastic NK-cells inNKTCL [30, 35, 37, 50, 68–70] as in ANKCL [2, 50, 53–55, 71–75], is based on immunohistochemistry studies.
Like normal NK-cells, NKTCL and ANKCL tumor cells do not express CD3 and theTCR on their surface [1]. Nevertheless, they often have the epsilon chain of CD3 in thecytoplasm and, therefore, they may stain positively for CD3 inimmunohistochemistry of paraffin sections, imposing the differentialdiagnosis with T-cell lymphoma [30]. Also as normal NK-cells, tumor NK-cells do not rearrange TCRgenes, which can be shown to be in germ-line configuration by polymerasechain reaction (PCR) analysis. In addition, NKTCL and ANKCL tumor cellsnearly always express CD2 and less often CD7 and CD8, but not CD4 and CD5.From the markers usually used to identify NK-cells, CD56 is the mostfrequently positive, whereas CD16 expression is variable, and CD57 is almostnever found. In addition, cytotoxic granule–associated proteins suchas T-cell–restricted intracellular antigen (TIA-1), granzyme B, andperforin are frequently expressed. The immunophenotypic characteristics ofNKTCL and ANKCL cells seems to be similar, except for a higher frequency ofcyCD3epsilon+ and a lower frequency of CD16+ casesin NKTCL [37].
Only a limited number of studies have evaluated the expression of killerreceptors on the neoplastic NK-cells from patients with NKTCL and ANKCL [76–80]. Reverse transcriptase PCR techniques, revealed that most NKTCLtumor cells do express killer lectin type receptors (KLR) transcripts,including those for the CD94 and the NKG2 (most frequently NKG2A/B andNKG2D) receptors [80], a result that was confirmed by immunohistochemical stainings ontissue biopsies [76, 77] and flow cytometry immunophenotyping of NKTCL and ANKCL cells [78]. The expression of killer immunoglobulin like receptors (KIR)seems to be more variable [76–78].
Linker for activation of T cells (LAT), a membrane protein that plays animportant role in T-cell activation, which appears early during T-celldevelopment and is present on T- and NK-cells, among others, is expressed inthe great majority of T- and NK-cell neoplasms [81]. CD70, the receptor for CD27, was also found to be expressed bothon NK-cell lines and on NKTCL lymphoma cells [82].
Ki67 is also frequently positive and the percentage of Ki67+ cellshas proven to be a prognostic factor in extranodal NKTCL [83]. The Ki-67 protein (also known as MKI67) is a nuclear markerstrictly associated with cell proliferation, which is present during allactive phases of the cell cycle (G1, S, G2, and mitosis), but is absent fromresting cells (G0) [84].
The fact that interactions between chemokines and chemokine receptors areinvolved in migration of lymphoma cells and tissue invasion have lead to theinvestigation of chemokine receptor expression on NKTCL cells [69, 70, 74, 75]. These studies revealed that NKTCL cells usually express CXCR3,whose main ligand is CXCL11 (IP9, IFN-gamma inducible protein type 9) [68–70]. In addition, ANKL cells are simultaneously positive for CXCR1and CCR5, whose major ligands are CXCL1 (interleukin-8, IL-18) and the CCL3(MIP-1alpha, macrophage inflammatory protein type 1 alpha), CCL4 (MIP-1beta)and CCL5 (RANTES, regulated on activation, normal T cell expressed andsecreted) chemokines, respectively [74, 75].
Primary nodal NKTCL have the same phenotypic and genotypic characteristics asextranodal NKTCL, at least for the markers that are frequently tested, mostof them being described as being CD2+, sCD3-,cytCD3epsilon+, CD56+, EBV+ and ashaving the TCR genes in germ-line configuration; moreover, they also usuallyhave a CD4-, CD5-, CD7-, cytotoxicmolecules+ phenotype [48, 49, 52].
In overall, the immunophenotypic features of the neoplastic NK-cells frompatients with NKTCL and ANKCL are different from those of normal peripheralblood NK-cells [85], reactive NK-cells from patients with acute and chronic NK-celllymphocytosis associated with viral infections and tumors [86, 87], and monoclonal CLPD-NK [88].
Chromosomal and genomic abnormalities
Cytogenetic analyses of the NK-cell neoplasms are difficult because of thescarcity of specimens, small-size samples, tissue necrosis and the presenceof inflammatory cells. Despite these difficulties, several studies wereperformed to date [89–96], some of them using comparative genomic hybridization and loss ofheterozygosity techniques. Currently, genetic abnormalities specific forNKTCL and ANKL have not yet been identified, although complex chromosomalaberrancies occur in a large fraction of cases, abnormalities of thechromosome 6 being the most frequent finding [89]. In overall, cytogenetic aberrancies are seen in up to 77% ofcases and karyotypic abnormalities observed include pseudodiploidy(57%), hyperdiploidy (30%), and hypodiploidy (13%) [89]. Recurrent abnormal chromosomal losses are 6q16–q25,11q23.1, 11q24-q25, 13q14.11 and 17p13.3, among others; chromosomal gainsinclude 1q21–q44, 2q13-q14, 2q31.1–q32.2, 6p25–p11.1,7q11.2–q34, 7q35-q36 and 17q21.1 [89–96]. A common deletion on 6q in the target area 6q21-25 wasidentified, affecting multiple genes that are probably involved inoncogenesis and disease progression [90–96].
Concluding remarks
Mature NK cell neoplasms comprise a wide spectrum of entities, from cases with anindolent disease course (chronic NK-cell lymphocytosis) to cases with aggressiveclinical behavior (NKTCL and ANKCL). Aggressive NK-cell neoplasms areEBV-related diseases with a particular geographic distribution, have a typicalimmunophenotype and complex karyotypic abnormalities, often affecting extranodalorgans and tissues, invading and destroying the adjacent structures, causinghemophagocytosis and disseminating thought the body. Due to their rarity, theyare difficult to diagnose and to manage, and except for nasal NKTCL in earlystages, they are refractory to the available therapies and have a very poorprognosis. Thus, multicentric registering studies and clinical trials are neededin order to better understand the disease biology and to develop new therapeuticagents.
Endnotes
a The estimated incidence of NHL varies worldwide, with the highest ratesbeing reported in the most economically developed regions of the world (e.g.Northern America, Australia/New Zealand, and Northern Europe) and the lowest ratesin the least developed regions (e.g. South-Central and Eastern Asia, and theCaribbean). According to data provided by the Cancer Research, UK(http://www.cancerresearchuk.org/, accessed in 10 September 2011),the crude incidence rate in the UK in 2009 was 22 new NHL cases for every 100,000males and 18 for every 100,000 females and within the countries of the EuropeanUnion, the highest age standardized incidence rates for 2008 were estimated to be inLuxembourg for men (around 19 cases per 100,000) and Ireland for women (more than 13cases per 100,000), while the lowest rates were found in Greece for both sexes (eacharound 3 cases per 100,000).
Author’s information
ML is a senior medical doctor, responsible for the Laboratory of Cytometry of theDepartment of Hematology, Hospital de Santo António, Centro Hospitalar doPorto, Porto, Portugal. The Laboratory of Cytometry is a reference laboratory forthe diagnosis of T- and NK-cell disorders. The author has been dedicated to thediagnosis and treatment of T- and NK-cell lymphoproliferative disorders and hasalready published a large number of papers in this field.
Abbreviations
- ANKCL:
-
Aggressive NK-cell leukemia
- CCL3:
-
C-C motif chemokine ligand type 3, alsoknown as MIP-1alpha
- CCL4:
-
C-C motif chemokine ligand type 4, also known asMIP-1beta
- CCL5:
-
C-C motif chemokine ligand type 5, also known as RANTES
- CCR5:
-
C-Cmotif chemokine ligand type 5 (CD195)
- CLPD-NK:
-
Chronic lymphoproliferativedisorders of NK-cells
- CXCL1:
-
C-X-C motif chemokine ligand type 1, also known asIL-18
- CXCL11:
-
C-X-C motif chemokine ligand type 3, also known as IP9
- CXCR1:
-
C-X-Cmotif chemokine ligand receptor type 1 (CD181)
- CXCR3:
-
C-X-C motif chemokine ligandreceptor type 3 (CD183)
- EBNA:
-
Epstein Barr virus nuclear antigens
- EBV:
-
Epstein-Barr virus
- FasL:
-
Fas ligand
- ICD:
-
International Classification of Diseases(now International Statistical Classification of Diseases and Related HealthProblems)
- ICD-O:
-
International Classification of Diseases for Oncology
- IL-8:
-
Interleukin-8 (CXCL1)
- IP9:
-
IFN-gamma inducible protein type 9 (CXCL11)
- IPTCLP:
-
International Peripheral T-cell Lymphoma Project
- KIR:
-
Killer immunoglobulin-likereceptors
- KLR:
-
C-type lectin-like receptors
- LAT:
-
Linker for activation of T cells
- LDH:
-
Lactate dehydrogenase
- LGL:
-
Large Granular Lymphocytes
- LMP:
-
EBV-encoded latentmembrane protein
- MIP-1alpha:
-
Macrophage inflammatory protein type 1 alpha (CCL3)
- MIP-1beta:
-
Macrophage inflammatory protein type 1 beta (CCL4)
- NHL:
-
Non-Hodgkin’s lymphoma
- NK:
-
Natural killer
- NKTCL:
-
NK/T cell lymphoma
- PCR:
-
Polymerase chain reaction
- RANTES:
-
Regulated on activation, normal T cell expressedand secreted (CCL5)
- TCR:
-
T cell receptor
- TIA-1:
-
T-cell–restrictedintracellular antigen
- WHO:
-
World Health Organization.
References
Cheung MMC, Chan JKC, Wong K-F: Natural killer cell neoplasms: a distinctive group of highly aggressivelymphomas/leukemias. Semin Hematol. 2003, 40: 221-232. 10.1016/S0037-1963(03)00136-7.
Suzuki R, Suzumiya J, Nakamura S, Aoki S, Notoya A, Ozaki S, Gondo H, Hino N, Mori H, Sugimori H, Kawa K, Oshimi K: Aggressive natural killer-cell leukemia revisited: large granular lymphocyteleukemia of cytotoxic NK cells. Leukemia. 2004, 18: 763-770. 10.1038/sj.leu.2403262.
Hasserjian RP, Harris NL: NK-cell lymphomas and leukemias: a spectrum of tumors with variablemanifestations and immunophenotype. Am J Clin Pathol. 2007, 127: 860-868. 10.1309/2F39NX1AL3L54WU8.
Oshimi K: Progress in understanding and managing natural killer-cell malignancies. Br J Haematol. 2007, 139: 532-544. 10.1111/j.1365-2141.2007.06835.x.
Aozasa K, Takakuwa T, Hongyo T, Yang W-I: Nasal NK/T-cell lymphoma: epidemiology and pathogenesis. Int J Hematol. 2008, 87: 110-117. 10.1007/s12185-008-0021-7.
Liang X, Graham DK: Natural killer cell neoplasms. Cancer. 2008, 112: 1425-1436. 10.1002/cncr.23316.
Greer JP, Mosse CA: Natural killer-cell neoplasms. Curr Hematol Malig Rep. 2009, 4: 245-252. 10.1007/s11899-009-0032-3.
Harabuchi Y, Takahara M, Kishibe K, Moriai S, Nagato T, Ishii H: Nasal natural killer (NK)/T-cell lymphoma: clinical, histological,virological, and genetic features. Int J Clin Oncol. 2009, 14: 181-190. 10.1007/s10147-009-0882-7.
Kohrt H, Advani R: Extranodal natural killer/T-cell lymphoma: current concepts in biology andtreatment. Leuk Lymphoma. 2009, 50: 1773-1784. 10.3109/10428190903186502.
Gill H, Liang RHS, Tse E: Extranodal natural-killer/t-cell lymphoma, nasal type. Adv Hematol. 2010, 2010: 627401.
Suzuki R: Treatment of advanced extranodal NK/T cell lymphoma, nasal-type andaggressive NK-cell leukemia. Int J Hematol. 2010, 92: 697-701. 10.1007/s12185-010-0726-2.
Aozasa K, Zaki MAA: Epidemiology and pathogenesis of nasal NK/T-cell lymphoma: a mini-review. ScientificWorldJournal. 2011, 11: 422-428.
Kobayashi S: Natural killer cell leukemia: diagnosis. InTech: Pathogenesis and Treatment. In Novel Aspects in AcuteLymphoblastic Leukemia. Edited by Faderl S; 2011.
Yook-Lam K: The diagnosis and management of extranodal NK/T-cell lymphoma, nasal-type andaggressive NK-cell leukemia. J Clin Exp Hematop. 2011, 51: 21-28. 10.3960/jslrt.51.21.
Tse E, Kwong Y-L: Treatment algorithms for mature T-cell and natural killer-cell neoplasms. Future Oncol. 2011, 7: 1101-1112. 10.2217/fon.11.84.
Jaccard A, Hermine O: Extranodal natural killer/T-cell lymphoma: advances in the management. Curr Opin Oncol. 2011, 23: 429-435. 10.1097/CCO.0b013e328349aba6.
Semenzato G, Marino F, Zambello R: State of the art in natural killer cell malignancies. Int J Lab Hematol. 2012, 34: 117-128. 10.1111/j.1751-553X.2011.01374.x.
Swerdlow SH, International Agency for Research on Cancer: WHO classification of Tumours of Haematopoietic and lymphoidtissues. Lyon: International Agency for Research on Cancer; 2008.
Chan JKC, Quintanilla-Martinez L, Ferry JA, Peh S-C: Extranodal NK/T-cell lymphoma, nasal type. World Health Organization classification of Tumours of Haematopoietic andlymphoid tissues. 4th edition. Edited by: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J,Vardiman JW. Lyon, France: International Agency for Research on Cancer (IARC)Press; 2008:285-288.
Chan JKC, Jane ES, Ralfkiaer E, Ko Y-H: Aggressive NK-cell leukaemia. World Health Organization classification of Tumours of Haematopoietic andlymphoid tissues. 4th edition. Edited by: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J,Vardiman JW. Lyon, France: International Agency for Research on Cancer (IARC)Press; 2008:276-277.
Villamor N, Morice WG, Chan WC, Foucar KK: Chronic Iymphoproliferative disorders of NK cells. World Health Organization Classification of tumours of haematopoietic andlymphoid tissues. Edited by: Swerdlow SH SH, Campo EE, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J,Vardiman JW. Lyon: International Agency for Research on Cancer (IARC)Press; 2008, 274-275. 4,
World Health Organization: The ICD-10 Classification of Mental and Behavioural Disorders: ClinicalDescriptions and Diagnostic Guidelines. 1992, Geneva: World Health Organization, Available at:http://apps.who.int/classifications/icd10/browse/2010/en AccessedFebruary 2, 2013,
World Health Organization: International classification of diseases for oncology, 3rd edition((ICD-O-3). Edited by: Fritz A, Jack A, Parkin DM, Percy C, Shanmugarathan S, Sobin L, WhelanL. 2000, Geneva: World Health Organisation, Available at:http://www.who.int/classifications/icd/adaptations/oncology/en/.Accessed February 2, 2013, 3,
Orphanet: an online database of rare diseases and orphan drugs. Copyright, INSERM 1997. Available at:http://www.orpha.net/consor/cgi-bin/Disease_Classif.php. AccessedFebruary 2, 2013,
Borowitz MJ, Bene ME, Harris NL, Porwit A, Matures E: Acute leukaemias of ambiguous lineage. World Health Organization Classification of tumours of haematopoietic andlymphoid tissues. 4th edition. Edited by: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J,Vardiman J. Lyon, France: International Agency for Research on Cancer (IARC)Press; 2008:150-155.
Pacchem F, Jones DM, Petrella T: Blastic plasmacytoid dendritic cell neoplasms. World Health Organization Classification of Tumours of Haematopoietic andlymphoid tissues. 4th edition. Edited by: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J,Vardiman JW. Lyon, France: International Agency for Research on Cancer (IARC)Press; 2008:145-147.
Kwong Y-L, Chan AC, Liang R, Chiang AK, Chim CS, Chan TK, Todd D, Ho FC: CD56+ NK lymphomas: clinicopathological features and prognosis. Br J Haematol. 1997, 97: 821-829. 10.1046/j.1365-2141.1997.1462962.x.
Au W-Y, Ma S-Y, Chim C-S, Choy C, Loong F, Lie AKW, Lam CCK, Leung AYH, Tse E, Yau C-C, Liang R, Kwong Y-L: Clinicopathologic features and treatment outcome of mature T-cell and naturalkiller-cell lymphomas diagnosed according to the World Health Organizationclassification scheme: a single center experience of 10 years. Ann Oncol. 2005, 16: 206-214. 10.1093/annonc/mdi037.
Liu J, Song B, Fan T, Huang C, Xie C, Li J, Zhong W, Li S, Yu J: Pathological and clinical characteristics of 1,248 non-Hodgkin’slymphomas from a regional cancer hospital in Shandong, China. Asian Pac J Cancer Prev. 2011, 12: 3055-3061.
Gaal K, Sun NC, Hernandez AM, Arber DA: Sinonasal NK/T-cell lymphomas in the United States. Am J Surg Pathol. 2000, 24: 1511-1517. 10.1097/00000478-200011000-00006.
Chiang AK, Tao Q, Srivastava G, Ho FC: Nasal NK- and T-cell lymphomas share the same type of Epstein-Barr viruslatency as nasopharyngeal carcinoma and Hodgkin’s disease. Int J Cancer. 1996, 68: 285-290. 10.1002/(SICI)1097-0215(19961104)68:3<285::AID-IJC3>3.0.CO;2-Y.
Xu J-X, Hoshida Y, Yang W-I, Inohara H, Kubo T, Kim G-E, Yoon J-H, Kojya S, Bandoh N, Harabuchi Y, Tsutsumi K, Koizuka I, Jia X-S, Kirihata M, Tsukuma H, Aozasa K: Life-style and environmental factors in the development of nasal NK/T-celllymphoma: a case–control study in East Asia. Int J Cancer. 2007, 120: 406-410. 10.1002/ijc.22313.
Miligi L, Costantini AS, Bolejack V, Veraldi A, Benvenuti A, Nanni O, Ramazzotti V, Tumino R, Stagnaro E, Rodella S, Fontana A, Vindigni C, Vineis P: Non-Hodgkin’s lymphoma, leukemia, and exposures in agriculture: resultsfrom the Italian multicenter case–control study. Am J Ind Med. 2003, 44: 627-636. 10.1002/ajim.10289.
Chiu BC-H, Weisenburger DD, Zahm SH, Cantor KP, Gapstur SM, Holmes F, Burmeister LF, Blair A: Agricultural pesticide use, familial cancer, and risk of non-Hodgkinlymphoma. Cancer Epidemiol Biomarkers Prev. 2004, 13: 525-531.
Au W-Y, Weisenburger D, Intragumtornchai T, Nakamura S, Kim W-S, Sng I, Vose J, Armitage J, Liang R: Clinical differences between nasal and extranasal natural killer/T-celllymphoma: a study of 136 cases from the International Peripheral T-CellLymphoma Project. Blood. 2009, 113: 3931-3937. 10.1182/blood-2008-10-185256.
Oshimi K, Kawa K, Nakamura S, Suzuki R, Suzumiya J, Yamaguchi M, Kameoka J, Tagawa S, Imamura N, Ohshima K, Kojya S, Iwatsuki K, Tokura Y, Sato E, Sugimori H: NK-cell neoplasms in Japan. Hematology. 2005, 10: 237-245. 10.1080/10245330400026162.
Suzuki R, Suzumiya J, Yamaguchi M, Nakamura S, Kameoka J, Kojima H, Abe M, Kinoshita T, Yoshino T, Iwatsuki K, Kagami Y, Tsuzuki T, Kurokawa M, Ito K, Kawa K, Oshimi K: Prognostic factors for mature natural killer (NK) cell neoplasms: aggressiveNK cell leukemia and extranodal NK cell lymphoma, nasal type. Ann Oncol. 2010, 21: 1032-1040. 10.1093/annonc/mdp418.
Kanavaros P, Lescs MC, Brière J, Divine M, Galateau F, Joab I, Bosq J, Farcet JP, Reyes F, Gaulard P: Nasal T-cell lymphoma: a clinicopathologic entity associated with peculiarphenotype and with Epstein-Barr virus. Blood. 1993, 81: 2688-2695.
Pagano L, Gallamini A, Trapè G, Fianchi L, Mattei D, Todeschini G, Spadea A, Cinieri S, Iannitto E, Martelli M, Nosari A, Bona ED, Tosti ME, Petti MC, Falcucci P, Montanaro M, Pulsoni A, Larocca LM, Leone G: NK/T-cell lymphomas “nasal type”: an Italian multicentricretrospective survey. Ann Oncol. 2006, 17: 794-800. 10.1093/annonc/mdl015.
Murdock J, Jaffe ES, Wilson WH, McManus DT, Alexander HD, Morris TCMC: Aggressive natural killer cell leukemia/lymphoma: case report, use oftelesynergy and review of the literature. Leuk Lymphoma. 2004, 45: 1269-1273. 10.1080/10428190310001646879.
Guerrero AAM, Lira VVP, Bertin CP, Galleguillos VM, Ocqueteau TM: Natural killer cell leukemia. Case report. Rev Med Chil. 2005, 133: 457-460.
Castelli R, Molteni M, Gianelli U, Cro L, Grimoldi MG, Cortelezzi A: Aggressive natural killer cell leukaemia with a complex karyotype: a casereport. Ann Hematol. 2006, 85: 66-68. 10.1007/s00277-005-0001-4.
Sousa J, Cabezuelo L, Almeida S, Filipe C, Simão A, Carvalho A, Nascimento Costa J: Aggressive NK/T cell leukemia/lymphoma associated with EBV. Acta Med Port. 2011, 24 (Suppl 3): 649-652.
Gualco G, Domeny-Duarte P, Chioato L, Barber G, Natkunam Y, Bacchi CE: Clinicopathologic and molecular features of 122 Brazilian cases of nodal andextranodal NK/T-cell lymphoma, nasal type, with EBV subtyping analysis. Am J Surg Pathol. 2011, 35: 1195-1203. 10.1097/PAS.0b013e31821ec4b5.
Suzuki R, Suzumiya J, Oshimi K: Differences between nasal and extranasal NK/T-cell lymphoma. Blood. 2009, 113: 6260-6261. author reply 6261–6262
Wong KF, Chan JK, Cheung MM, So JC: Bone marrow involvement by nasal NK cell lymphoma at diagnosis isuncommon. Am J Clin Pathol. 2001, 115: 266-270. 10.1309/E5PR-6A9R-Q02N-8QVW.
Takahashi N, Miura I, Chubachi A, Miura AB, Nakamura S: A clinicopathological study of 20 patients with T/natural killer (NK)-celllymphoma-associated hemophagocytic syndrome with special reference to nasaland nasal-type NK/T-cell lymphoma. Int J Hematol. 2001, 74: 303-308. 10.1007/BF02982065.
Chim C-S, Ma ESK, Loong F, Kwong Y-L: Diagnostic cues for natural killer cell lymphoma: primary nodal presentationand the role of in situ hybridisation for Epstein-Barr virus encoded earlysmall RNA in detecting occult bone marrow involvement. J Clin Pathol. 2005, 58: 443-445. 10.1136/jcp.2004.022608.
Chang S-T, Liao Y-L, Lin S-H, Chuang S-S: NK-cell lymphoma with nodal presentation and expression of cutaneouslymphocyte-associated antigen. Pathol Res Pract. 2010, 206: 463-466. 10.1016/j.prp.2009.07.001.
Chan JK, Sin VC, Wong KF, Ng CS, Tsang WY, Chan CH, Cheung MM, Lau WH: Nonnasal lymphoma expressing the natural killer cell marker CD56: aclinicopathologic study of 49 cases of an uncommon aggressive neoplasm. Blood. 1997, 89: 4501-4513.
Kagami Y, Suzuki R, Taji H, Yatabe Y, Takeuchi T, Maeda S, Kondo E, Kojima M, Motoori T, Mizoguchi Y, Okamoto M, Ohnishi K, Yamabe H, Seto M, Ogura M, Koshikawa T, Takahashi T, Kurita S, Morishima Y, Suchi T, Nakamura S: Nodal cytotoxic lymphoma spectrum: a clinicopathologic study of 66patients. Am J Surg Pathol. 1999, 23: 1184-1200. 10.1097/00000478-199910000-00003.
Takahashi E, Asano N, Li C, Tanaka T, Shimada K, Shimada S, Yoshino T, Kojima M, Hara K, Eimoto T, Nakamura S: Nodal T/NK-cell lymphoma of nasal type: a clinicopathological study of sixcases. Histopathology. 2008, 52: 585-596. 10.1111/j.1365-2559.2008.02997.x.
Ruskova A, Abizanda R, Chan G: Aggressive Natural Killer-Cell Leukemia: report of five cases and review ofthe literature. Leuk Lymphoma. 2004, 45: 2427-2438. 10.1080/10428190400004513.
Liu EB, Chen HS, Zhang PH, Li ZQ, Sun Q, Yang QY, Fang LH, Sun FJ: Aggressive NK-cell leukemia: report of nine cases and review ofliterature. Zhonghua Xue Ye Xue Za Zhi. 2006, 27: 116-119.
Ryder J, Wang X, Bao L, Gross SA, Hua F, Irons RD: Aggressive natural killer cell leukemia: report of a Chinese series andreview of the literature. Int J Hematol. 2007, 85: 18-25. 10.1532/IJH97.A10612.
Okuda T, Sakamoto S, Deguchi T, Misawa S, Kashima K, Yoshihara T, Ikushima S, Hibi S, Imashuku S: Hemophagocytic syndrome associated with aggressive natural killer cellleukemia. Am J Hematol. 1991, 38: 321-323. 10.1002/ajh.2830380412.
Akashi K, Mizuno S: Epstein-Barr virus-infected natural killer cell leukemia. Leuk Lymphoma. 2000, 40: 57-66. 10.3109/10428190009054881.
Kaizu K, Maeda M, Ohkawa T, Hayashida M, Nakajima S, Sugisaki Y, Fukunaga Y: Marked elevation of soluble fas ligand and cytokine secretion aftersplenectomy in aggressive natural killer cell leukemia/lymphoma. Leuk Lymphoma. 2004, 45: 2291-2294. 10.1080/10428190412331283233.
Choi Y-L, Park J-H, Kim W-S, Lee D-Y, Lee J-H, Yang J-M, Lee E-S: Aggressive NK-cell leukaemia associated with reactive haemophagocyticsyndrome. Clin Exp Dermatol. 2006, 31: 83-85. 10.1111/j.1365-2230.2005.01986.x.
Petterson TE, Bosco AA, Cohn RJ: Aggressive natural killer cell leukemia presenting with hemophagocyticlymphohistiocytosis. Pediatr Blood Cancer. 2008, 50: 654-657. 10.1002/pbc.21358.
Suzuki S, Uozumi K, Utsunomiya A, Ishitsuka K, Masamoto I, Owatari S, Makino T, White Y, Arima N: Aggressive NK cell leukaemia after splenectomy: association withCD95-resistant memory T-cell proliferation and recalcitrant clinical courseof haemophagocytic syndrome. Eur J Haematol. 2008, 81: 236-241. 10.1111/j.1600-0609.2008.01097.x.
Han A-R, Lee HR, Park B-B, Hwang IG, Park S, Lee SC, Kim K, Lim HY, Ko YH, Kim SH, Kim WS: Lymphoma-associated hemophagocytic syndrome: clinical features and treatmentoutcome. Ann Hematol. 2007, 86: 493-498. 10.1007/s00277-007-0278-6.
Carbone PP, Kaplan HS, Musshoff K, Smithers DW, Tubiana M: Report of the committee on Hodgkin’s disease staging classification. Cancer Res. 1971, 31: 1860-1861.
Lister TA, Crowther D, Sutcliffe SB, Glatstein E, Canellos GP, Young RC, Rosenberg SA, Coltman CA, Tubiana M: Report of a committee convened to discuss the evaluation and staging ofpatients with Hodgkin’s disease: Cotswolds meeting. J Clin Oncol. 1989, 7: 1630-1636.
Robbins KT, Fuller LM, Vlasak M, Osborne B, Jing BS, Velasquez WS, Sullivan JA: Primary lymphomas of the nasal cavity and paranasal sinuses. Cancer. 1985, 56: 814-819. 10.1002/1097-0142(19850815)56:4<814::AID-CNCR2820560419>3.0.CO;2-P.
Ooi GC, Chim CS, Liang R, Tsang KW, Kwong YL: Nasal T-cell/natural killer cell lymphoma: CT and MR imaging features of anew clinicopathologic entity. AJR Am J Roentgenol. 2000, 174: 1141-1145. 10.2214/ajr.174.4.1741141.
Khong P-L, Pang CBY, Liang R, Kwong Y-L, Au W-Y: Fluorine-18 fluorodeoxyglucose positron emission tomography in mature T-celland natural killer cell malignancies. Ann Hematol. 2008, 87: 613-621. 10.1007/s00277-008-0494-8.
Schwartz EJ, Molina-Kirsch H, Zhao S, Marinelli RJ, Warnke RA, Natkunam Y: Immunohistochemical characterization of nasal-type extranodal NK/T-celllymphoma using a tissue microarray: an analysis of 84 cases. Am J Clin Pathol. 2008, 130: 343-351. 10.1309/V561QTM6854W4WAV.
Ishida T, Inagaki H, Utsunomiya A, Takatsuka Y, Komatsu H, Iida S, Takeuchi G, Eimoto T, Nakamura S, Ueda R: CXC chemokine receptor 3 and CC chemokine receptor 4 expression in T-cell andNK-cell lymphomas with special reference to clinicopathological significancefor peripheral T-cell lymphoma, unspecified. Clin Cancer Res. 2004, 10: 5494-5500. 10.1158/1078-0432.CCR-04-0371.
Yagi H, Seo N, Ohshima A, Itoh T, Itoh N, Horibe T, Yoshinari Y, Takigawa M, Hashizume H: Chemokine receptor expression in cutaneous T cell and NK/T-cell lymphomas:immunohistochemical staining and in vitro chemotactic assay. Am J Surg Pathol. 2006, 30: 1111-1119.
Falcão RP, Rizzatti EG, Saggioro FP, Garcia AB, Marinato AF, Rego EM: Flow cytometry characterization of leukemic phase of nasal NK/T-cell lymphomain tumor biopsies and peripheral blood. Haematologica. 2007, 92: e24-e25. 10.3324/haematol.10654.
Yoo E-H, Kim H-J, Lee S-T, Kim W-S, Kim S-H: Frequent CD7 antigen loss in aggressive natural killer-cell leukemia: auseful diagnostic marker. Korean J Lab Med. 2009, 29: 491-496. 10.3343/kjlm.2009.29.6.491.
Liu E-B, Chen H-S, Zhang P-H, Li Z-G, Sun Q, Yang Q-Y, Fang L-H, Sun F-J: Clinicopathologic features of aggressive natural killer cell leukemia. Zhonghua Bing Li Xue Za Zhi. 2011, 40: 810-814.
Makishima H, Ito T, Asano N, Nakazawa H, Shimodaira S, Kamijo Y, Nakazawa Y, Suzuki T, Kobayashi H, Kiyosawa K, Ishida F: Significance of chemokine receptor expression in aggressive NK cellleukemia. Leukemia. 2005, 19: 1169-1174. 10.1038/sj.leu.2403732.
Makishima H, Ito T, Momose K, Nakazawa H, Shimodaira S, Kamijo Y, Nakazawa Y, Ichikawa N, Ueno M, Kobayashi H, Kitano K, Saito H, Kiyosawa K, Ishida F: Chemokine system and tissue infiltration in aggressive NK-cell leukemia. Leuk Res. 2007, 31: 1237-1245. 10.1016/j.leukres.2006.10.020.
Haedicke W, Ho FC, Chott A, Moretta L, Rüdiger T, Ott G, Müller-Hermelink HK: Expression of CD94/NKG2A and killer immunoglobulin-like receptors in NK cellsand a subset of extranodal cytotoxic T-cell lymphomas. Blood. 2000, 95: 3628-3630.
Dukers DF, Vermeer MH, Jaspars LH, Sander CA, Flaig MJ, Vos W, Willemze R, Meijer CJ: Expression of killer cell inhibitory receptors is restricted to true NK celllymphomas and a subset of intestinal enteropathy-type T cell lymphomas witha cytotoxic phenotype. J Clin Pathol. 2001, 54: 224-228. 10.1136/jcp.54.3.224.
Mori KL, Egashira M, Oshimi K: Differentiation stage of natural killer cell-lineage lymphoproliferativedisorders based on phenotypic analysis. Br J Haematol. 2001, 115: 225-228. 10.1046/j.1365-2141.2001.03038.x.
Sawada A, Sato E, Koyama M, Higuchi B, Kusuki S, Kim JY, Takeshita Y, Sakata A, Sakata N, Okamura T, Yasui M, Inoue M, Kawa K: NK-cell repertoire is feasible for diagnosing Epstein-Barr virus-infectedNK-cell lymphoproliferative disease and evaluating the treatment effect. Am J Hematol. 2006, 81: 576-581. 10.1002/ajh.20659.
Nong L, Zhang S, Li Y, Zhang Y, Wang Y, Li T: Study on expression of natural killer (NK) cell C-type lectin-like receptorsin nasal NK/T-cell lymphomas. Zhonghua Bing Li Xue Za Zhi. 2010, 39: 319-324.
Facchetti F, Chan JK, Zhang W, Tironi A, Chilosi M, Parolini S, Notarangelo LD, Samelson LE: Linker for activation of T cells (LAT), a novel immunohistochemical markerfor T cells, NK cells, mast cells, and megakaryocytes: evaluation in normaland pathological conditions. Am J Pathol. 1999, 154: 1037-1046. 10.1016/S0002-9440(10)65356-4.
Yoshino K, Kishibe K, Nagato T, Ueda S, Komabayashi Y, Takahara M, Harabuchi Y: Expression of CD70 in nasal natural killer/T cell lymphoma cell lines andpatients; its role for cell proliferation through binding to solubleCD27. Br J Haematol. 2013, 160: 331-342. 10.1111/bjh.12136.
Kim SJ, Kim BS, Choi CW, Choi J, Kim I, Lee Y-H, Kim JS: Ki-67 expression is predictive of prognosis in patients with stage I/IIextranodal NK/T-cell lymphoma, nasal type. Ann Oncol. 2007, 18: 1382-1387. 10.1093/annonc/mdm183.
Scholzen T, Gerdes J: The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000, 182: 311-322. 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9.
Lima M, Teixeira MA, Queirós ML, Leite M, Santos AH, Justiça B, Orfão A: Immunophenotypic characterization of normal blood CD56 + loversus CD56 + hi NK-cell subsets and its impact on theunderstanding of their tissue distribution and functional properties. Blood Cells Mol Dis. 2001, 27: 731-743. 10.1006/bcmd.2001.0443.
Lima M, Almeida J, dos Anjos Teixeira M, Queirós ML, Justiça B, Orfão A: The “ex vivo” patterns of CD2/CD7, CD57/CD11c, CD38/CD11b,CD45RA/CD45RO, and CD11a/HLA-DR expression identify acute/early andchronic/late NK-cell activation states. Blood Cells Mol Dis. 2002, 28: 181-190. 10.1006/bcmd.2002.0506.
Lima M, Almeida J, Teixeira MA, Santos AH, Queirós ML, Fonseca S, Moura J, Gonçalves M, Orfão A, Pinto Ribeiro AC: Reactive phenotypes after acute and chronic NK-cell activation. J Biol Regul Homeost Agents. 2004, 18: 331-334.
Lima M, Almeida J, Montero AG, Teixeira M d A, Queirós ML, Santos AH, Balanzategui A, Estevinho A, Algueró M d C, Barcena P, Fonseca S, Amorim ML, Cabeda JM, Pinho L, Gonzalez M, San Miguel J, Justiça B, Orfão A: Clinicobiological, immunophenotypic, and molecular characteristics ofmonoclonal CD56−/+dim chronic natural killer cell large granularlymphocytosis. Am J Pathol. 2004, 165: 1117-1127. 10.1016/S0002-9440(10)63373-1.
Wong KF, Zhang YM, Chan JK: Cytogenetic abnormalities in natural killer cell lymphoma/leukaemia–isthere a consistent pattern?. Leuk Lymphoma. 1999, 34: 241-250.
Siu LL, Wong KF, Chan JK, Kwong YL: Comparative genomic hybridization analysis of natural killer celllymphoma/leukemia. Recognition of consistent patterns of geneticalterations. Am J Pathol. 1999, 155: 1419-1425. 10.1016/S0002-9440(10)65454-5.
Ko YH, Ree HJ, Kim WS, Choi WH, Moon WS, Kim SW: Clinicopathologic and genotypic study of extranodal nasal-type naturalkiller/T-cell lymphoma and natural killer precursor lymphoma amongKoreans. Cancer. 2000, 89: 2106-2116. 10.1002/1097-0142(20001115)89:10<2106::AID-CNCR11>3.0.CO;2-G.
Siu LL, Chan V, Chan JK, Wong KF, Liang R, Kwong YL: Consistent patterns of allelic loss in natural killer cell lymphoma. Am J Pathol. 2000, 157: 1803-1809. 10.1016/S0002-9440(10)64818-3.
Zhang Y, Matthiesen P, Harder S, Siebert R, Castoldi G, Calasanz MJ, Wong KF, Rosenwald A, Ott G, Atkin NB, Schlegelberger B: A 3-cM commonly deleted region in 6q21 in leukemias and lymphomas delineatedby fluorescence in situ hybridization. Genes Chromosomes Cancer. 2000, 27: 52-58. 10.1002/(SICI)1098-2264(200001)27:1<52::AID-GCC7>3.0.CO;2-X.
Ko YH, Choi KE, Han JH, Kim JM, Ree HJ: Comparative genomic hybridization study of nasal-type NK/T-cell lymphoma. Cytometry. 2001, 46: 85-91. 10.1002/cyto.1069.
Sun HS, Su I-J, Lin Y-C, Chen J-S, Fang S-Y: A 2.6 Mb interval on chromosome 6q25.2-q25.3 is commonly deleted in humannasal natural killer/T-cell lymphoma. Br J Haematol. 2003, 122: 590-599. 10.1046/j.1365-2141.2003.04419.x.
Nakashima Y, Tagawa H, Suzuki R, Karnan S, Karube K, Ohshima K, Muta K, Nawata H, Morishima Y, Nakamura S, Seto M: Genome-wide array-based comparative genomic hybridization of natural killercell lymphoma/leukemia: different genomic alteration patterns of aggressiveNK-cell leukemia and extranodal Nk/T-cell lymphoma, nasal type. Genes Chromosomes Cancer. 2005, 44: 247-255. 10.1002/gcc.20245.
Acknowledgements
The author thanks to the medical doctors (Catarina Lau, Maria dos Anjos Teixeira)and other professionals (Ana Helena Santos, João Rodrigues, LurdesOliveira, Maria Luís Queirós, Marlene Santos, Marta Gonçalves andSónia Fonseca) and collaborators (Magdalena Leander) of the CytometryLaboratory, for the support and collaboration concerning NK-cellimmunophenotyping and diagnosis of NK-cells lymphoproliferative disorders. Shealso thanks to the medical doctors who have referred patients for study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The author discloses any financial and non-financial competing interests that mayinfluence the interpretation of data or the presentation of information in themanuscript.
Author’s contributions
ML reviewed the literature on the subject, had write and approved the finalmanuscript.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative CommonsAttribution License (http://creativecommons.org/licenses/by/2.0), whichpermits unrestricted use, distribution, and reproduction in any medium, provided theoriginal work is properly cited.
About this article
Cite this article
Lima, M. Aggressive mature natural killer cell neoplasms: from epidemiology todiagnosis. Orphanet J Rare Dis 8, 95 (2013). https://doi.org/10.1186/1750-1172-8-95
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1750-1172-8-95