Abstract
Background
Focal adhesion kinase (FAK) functions in cell migration and signaling through activation of the mitogen-activated protein kinase (MAPK) signaling cascade. Neuronal function of FAK has been suggested to control axonal branching; however, the underlying mechanism in this process is not clear.
Results
We have generated mutants for the Drosophila FAK gene, Fak56. Null Fak56 mutants display overgrowth of larval neuromuscular junctions (NMJs). Localization of phospho-FAK and rescue experiments suggest that Fak56 is required in presynapses to restrict NMJ growth. Genetic analyses imply that FAK mediates the signaling pathway of the integrin αPS3βν heterodimer and functions redundantly with Src. At NMJs, Fak56 downregulates ERK activity, as shown by diphospho-ERK accumulation in Fak56 mutants, and suppression of Fak56 mutant NMJ phenotypes by reducing ERK activity.
Conclusion
We conclude that Fak56 is required to restrict NMJ growth during NMJ development. Fak56 mediates an extracellular signal through the integrin receptor. Unlike its conventional role in activating MAPK/ERK, Fak56 suppresses ERK activation in this process. These results suggest that Fak56 mediates a specific neuronal signaling pathway distinct from that in other cellular processes.
Similar content being viewed by others
Background
Formation and stabilization of neuronal synapses demands communication between pre- and post-synaptic partners, as well as signals from the extracellular matrix (ECM). These signals can reorganize local cytoskeletal structures or be transduced into the nucleus to regulate transcription, thereby modulating neuronal plasticity [1–3]. One major receptor family for ECM signals comprises the transmembrane protein integrins, which have been shown to play critical roles in sequential steps of neuronal wiring, such as in neurite outgrowth, axon guidance, and synaptic formation and maturation [4–7]. In Drosophila, various integrin subunits have been shown to function in motor axon pathfinding and target recognition, and synaptic morphogenesis at neuromuscular junctions (NMJs) [8–10]. Mutant analyses for the integrin subunits αPS3 and βPS indicate that integrin signaling is involved in synaptic growth and arborization of larval NMJs [8–10]. Although specific ECM signals for these integrin receptors are not clear, dynamic NMJ growth is regulated by heparan sulfate proteoglycans [11]. Also, the N-glycosaminoglycan-binding protein MTG (encoded by mind the gap), a pre-synaptic secreted ECM molecule, has been shown to shape the synaptic cleft and modulate post-synaptic differentiation [12].
Integrin signaling activities in cell adhesion, spreading and migration can be mediated by the non-receptor tyrosine kinase focal adhesion kinase (FAK) [13–15]. In these processes, FAK becomes activated when phosphorylated at tyrosine 397 (Y397) and associates with Src to form a dual kinase complex [14, 16]. Activated Src phosphorylates FAK thereby creating a signaling cascade through Ras and mitogen-activated protein kinase (MAPK)/ERK [17–19]. Activated ERK can modulate focal contact dynamics during cell migration, as well as promote cell proliferation and survival. In Drosophila larval NMJ growth regulation, ERK is specifically activated by Ras and its activation downregulates the protein levels of the cell adhesion molecule Fasciclin II (FasII) at NMJs [20].
The significance of FAK in development has been revealed by fak knockout mice that are embryonic lethal at embryonic day 8.5 during gastrulation, consistent with its role in cell adhesion and migration [21]. FAK proteins are highly enriched in developing nervous systems, in particular in axonal tracks and growth cones [22–25]. Neuronal-specific depletion of fak leads to cortical abnormalities, revealing the requirement of FAK in neural development [26]. At the cellular level, ablation of fak in Purkinje cells induces axonal branching and synapse formation, and this FAK activity is suggested to be partially mediated through p190RhoGEF, which modulates cytoskeletal structure [27]. Inactivation of the only Drosophila FAK gene, Fak56, however, permits normal development and transduction of integrin signaling pathways [28]. A requirement for Fak56 in glial cells of the optic stalk has recently been reported, suggesting for the first time a role for FAK family kinase activity in Drosophila [29].
We have generated Fak56 mutants and identified a role for Fak56 in restricting NMJ growth. Analyses of genetic interactions suggest that Fak56 plays a conventional role in cooperation with Src to transduce integrin signaling. Fak56 is activated at NMJs, as shown by immunostaining for its phosphorylated form and this activation depends on the presence of the integrin βν subunit. ERK activation and FasII protein downregulation were observed at Fak56 mutant NMJs. The NMJ overgrowth phenotype and FasII downregualtion in Fak56 mutants can be suppressed by reducing ERK activity. The physiological output of the enlarged NMJ in Fak56 mutant displays increased synaptic response by nerve stimulation. These results suggest that Fak56 negatively regulates ERK activity and modulates synaptic plasticity at NMJs.
Results
Larval NMJ overgrowth in Drosophila Fak56 null mutants
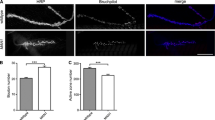
The Fak56 protein is highly expressed in the ventral nerve cord during embryonic stages [22, 25]. To examine whether Fak56 has a role in NMJ formation, we dissected late third instar larvae from a transheterozygous Fak56N 30/K 24mutant that deletes the Fak56 gene and lacks Fak56 mRNA expression (Additional file 1A, B, and Additional file 1 legend for the generation of Fak56 mutants). This Fak56 null mutant was immunostained with horseradish peroxidase (HRP) in order to label axonal processes [30], and phalloidin (Pha) to label muscle fibers. No abnormality of motor axonal tracts could be detected, and the pattern and size of muscles were normal, in agreement with earlier observations [28]. However, a more detailed examination revealed that Fak56 null mutant NMJs were overelaborated in comparison to wild-type ones (Figure 1A, B). NMJs innervating muscles 6 and 7 (NMJ 6/7s) of abdominal segment 3 (A3) were analyzed by immunostaining for HRP and synaptotagmin (Syt) to label presynaptic boutons [31]. Altered branching patterns and ectopic synaptic boutons were observed, with increases in both Ib and Is boutons (arrows and arrowheads, respectively, in Figure 1C, D). Quantitatively, the number of synaptic boutons was increased by 44% and the total branch length increased by 22% when normalized to the total area of muscles 6 and 7 (quantified in Figure 1E). The Fak56 activity was not limited to NMJ 6/7s since NMJ 4s also displayed overgrowth phenotypes in both total branch length (62% increase) and bouton number (101% increase) (Figure 1F–H). Furthermore, previous reported Fak56CG 1null mutants [28] also displayed a significant NMJ overgrowth phenotype when compared to wild-type control (Additional file 1C–E).
NMJ overelaboration in Fak56 mutants. (A, B) Muscular and axonal patterns in A3 segments shown by horseradish peroxidase (HRP)-labeled axonal branches of motor neurons (green), and Pha-labeled muscular pattern (magenta). NMJs 6/7, 12/13 and 4 are shown for wild-type (A) and Fak56N 30/K 24(B). In these and all other figures, scale bars represent 20 μm unless specifically indicated. (C, D) NMJ 6/7 phenotypes in A3 segments shown by HRP-labeled axons (green), Syt-labeled synaptic boutons (red) and Pha-labeled muscles (not shown). Arrows and arrowheads indicate type Ib and Is boutons, respectively. (E) Quantification of bouton numbers and total branch length that are normalized to total muscle 6/7 areas for wild-type, Fak56N 30/K 24, Fak56N 30/K 24;elav>Fak56, and Fak56N 30/K 24;MHC>Fak56. In this and all following quantifications, values are mean ± SEM, asterisks indicate p < 0.05 by Student's t test and sample numbers are within each bar. (F-H) NMJ 4 in A3 segments of wild-type (F) and Fak56N 30/K 24(G) are labeled as in (C, D), and quantifications of bouton number and total branch length are shown in (H). (I) Quantification for the number of branches at NMJ 6/7s of wild-type and Fak56N 30/K 24. Branches originating from the nerve entry point are primary (1°), and subsequent branches with at least three boutons are defined progressively with one higher order (2°, 3° or >3°). (J, K) Electrophysiological recording of postsynaptic currents in wild-type and Fak56N 30/K 24in 0.2 mM [Ca2+]. (J) Cumulative frequency plot to compare amplitudes of mEJPs in wild-type and Fak56N 30/K 24. (K) Representative traces (left panel) and mean peak amplitude (right panel) of EJPs in wild-type and Fak56N 30/K 24. Calibration: 30 ms, 5 mV for evoked release.
When scored for NMJ 6/7s, the altered branching pattern in Fak56N 30/K 24mutants showed secondary branch reduction by 21% but increases in higher-order branches (73% for tertiary branches and 424% for beyond tertiary; Figure 1I). The increase in higher-order branches was not caused by extension of multiple branches from single boutons, since a normal bifurcating pattern was observed.
To confirm that NMJ overgrowth phenotypes in the Fak56N 30/K 24mutant are due to the absence of Fak56 activity, a UAS-Fak56 transgene [25] was introduced. We found that neuronal expression of Fak56 with elav-GAL4 in the Fak56N 30/K 24mutant completely suppressed the NMJ phenotypes, as shown in assays for total branch length and bouton number of NMJ 6/7s. In contrast, Fak56 expression with the muscle-specific MHC-GAL4 failed to rescue Fak56 mutant phenotypes (Figure 1E). Taken together, these results suggest that Fak56 is specifically required in presynaptic neurons but not postsynaptic muscles to restrict NMJ growth. The exuberant NMJs in Fak56 null mutants were constructed normally, since molecular markers for various synaptic structures were expressed in a wild-type pattern (Additional file 2). Synaptic ultrastructure analyzed by transmission electron microscopy revealed no significant alternations in pre- and post-synaptic structures (Additional file 3).
Synaptic transmission is affected in the Fak56 null mutant
To examine whether the enlarged NMJ in Fak56 null mutants is associated with functional changes in transmitter release, postsynaptic currents were recorded. In the null Fak56N 30/K 24mutant, no alteration was observed in the amplitude of spontaneous release of neurotransmitter or miniature junctional potentials (mEJPs) at a low Ca2+ concentration (0.2 mM), as shown in the cumulative frequency plot (Figure 1J). Similar skews of distributions were measured for wild type and Fak56N 30/K 24(1.5 ± 0.1 in wild type and 1.7 ± 0.2 in Fak56N 30/K 24, 0.25 <p < 0.5 by Kruskal-Wallis h test). The variance/mean of mEJP amplitudes were also similar (0.28 ± 0.04 in wild type and 0.25 ± 0.04 in Fak56N 30/K 24, 0.25 <p < 0.5 by Kruskal-Wallis H test). The frequency of mEJP was not changed significantly (1.2 ± 0.2 Hz in wild type and 1.8 ± 0.3 Hz in Fak56N 30/K 24, p = 0.16, Student's t-test). Resting membrane potentials were similar in these measurements (-69.1 ± 1.9 mV in wild-type and -66.6 ± 1.5 mV in Fak56N 30/K 24, p = 0.32 by Student's t-test). However, the mean amplitude of nerve-evoked EJPs was significantly enhanced at Fak56 mutant NMJs compared to wild type (p = 0.026 by Student's t-test, Figure 1K; measurements were also performed at 1 mM [Ca2+]; Additional file 4). These data demonstrate a role of Fak56 in modulating the electrophysiological behavior of Drosophila NMJs.
Involvement of integrin subunits αPS3 and βν in Fak56-regulated NMJ growth
We then tested whether Fak56 mediates specific integrin activities at NMJs by genetic analysis. Integrin receptors are composed of heterodimeric α and β subunits [32]. In the Drosophila genome, there are five α subunits: αPS1 (encoded by multiple edematous wings, mew), αPS2 (inflated, if), αPS3 (Vol or scb), αPS4 and αPS5 (both αPS4 and αPS5 uncharacterized), and two β subunits (βPS (myospheroid, mys) and βν(βν)) [33–38]. We tested for possible genetic interactions between the available mutant alleles of integrin subunits and Fak56. In Fak56N 30/KGhypomorphic animals, expression of Fak56 mRNA was reduced, but the NMJ appeared phenotypically normal (Additional file 1A, B; Figure 2A). However, when single mutant alleles of scb2 and βν1 were introduced into the Fak56N 30/KGbackground, significant NMJ overgrowth was induced (Figure 2B, C). This overgrowth phenotype was not detected when mew1, ifk 27eand mys1 were introduced (quantified in Figure 2G). As controls, larvae that were heterozygous for the scb2 or βν1 mutant alleles displayed normal NMJ bouton number and length (quantified in Figure 2G). These results suggest that compromised αPS3 or βν integrin signaling demands the full-strength of Fak56 activity to constrain NMJ growth. Since NMJ overgrowth has been observed for αPS3 but not βν mutants [9], we examined NMJ phenotypes in the viable βν1/2 mutant. Strikingly, significant increases in both branch length and bouton number were detected, similar to those observed in Fak56 null mutant larvae (Figure 2D). In summary, these genetic analyses suggest that αPS3 and βν are the main integrin subunits in regulating Fak56 activity during NMJ growth.
Genetic interactions between Fak56 and integrin signaling pathway components during neuromuscular junction (NMJ) growth. (A-F) Images of NMJ 6/7 are shown as described for Figure 1C, D. Hypomorphic Fak56N 30/KGmutants showed a normal morphology (A), but one allele of scb2 (B), βν1 (C) or LanA9–32 (E) in Fak56N 30/KGinduced dramatic NMJ growth. Overelaborated NMJs in transheterozygotes βν1/2 (D) and βν1/+;LanA9–32/+ (F) mutants are shown. (G) Quantification of NMJ 6/7 phenotypes for Fak56N 30/KG, mew1/+;Fak56N 30/KG, ifk 27e/+;Fak56N 30/KG, scb2/+ Fak56N 30/KG, mys1/+;Fak56N 30/KG, βν1/+Fak56N 30/KG, Fak56N 30/KG;LanA9–32/+, wb4Y 18/+Fak56N 30/KG, scb2/+, βν1/+, βν1/2, LanA9–32/216 and βν1/+;LanA9–32/+. Asterisks indicate significant difference by Student's t test (p < 0.05) and error bars represent the standard error of the mean (SEM).
The laminins are ECM components composed of heterotrimers of α,β and γ subunits, and are major signals for integrin receptors [39]. In Drosophila, LanA and wing blister (wb) encode two different α chains. We performed genetic interaction for both α chain mutants to test their involvement in Fak56 activity. Introducing one mutant allele of LanA9–32 but not wb4Y 18into the Fak56N 30/KGhypomorphic background promoted a significant increase in the number of synaptic boutons (Figure 2E, G). The total NMJ length was also increased, although it was not significant (p = 0.37). While the hypomorphic LanA9–32/216 mutant displayed normal NMJ phenotypes, transheterozygous βν1/+;LanA9–32/+ displayed strong overgrowth phenotypes, with 61% increase in the bouton number and 32% increase in the total length compared to wild-type NMJs (Figure 2F, G). These results are consistent with a role for the α subunit LanA as a component of laminins to signal integrins during NMJ growth.
Participation of Src in Fak56-regulated NMJ growth
Activated FAK forms a complex with Src, and the dual FAK-Src kinase complex induces downstream signaling [40]. To test whether Src is involved in Fak56-regulated NMJ growth, we performed genetic interactions between Fak56 and the Drosophila Src genes Src42A and Src64B. Reducing one gene dosage of either Src42A (Src42AE 1) or Src64B (Src64BPI) in the Fak56KG/N 30background displayed significant NMJ overgrowth, as scored for total branch length and bouton number (Figure 3A, B, E). Controls of Src42AE 1/+;Src64B+/+ and Src42AE 1/+; Src64BPI/+in a wild-type background displayed no significant NMJ overgrowth (quantified in Figure 3E), suggesting that the efficiency of Src signaling at NMJs is dependant upon Fak56 activity in a dose-dependant manner. These results are consistent with a role for a FAK-Src complex in the restriction of NMJ growth.
Role of Src and its genetic interaction with Fak56 during neuromuscular junction (NMJ) growth. (A-D) Images of NMJ 6/7 are shown as in Figure 1. Fak56N 30/KGmutants carrying one allele of Src42AE 1(A) or Src64BPI(B) displayed NMJ overgrowth phenotype. (C) NMJ phenotype in the severe Src mutant Src42AE 1/+;Src64BPI/PI. (D) NMJ phenotype of the Fak56N 30/K 24null mutant was enhanced by removing both one Src42AE 1and one Src64BPIallele. (E) Quantification of NMJ 6/7 phenotypes for Fak56N 30/KG(the same set of data as in Figure 2G), Src42AE 1/+Fak56N 30/KG, Fak56N 30/KG;Src64BPI/+, Src42AE 1/+, Src42AE 1/+;Src64BPI/+and Src42AE 1/+;Src64BPI/PI. Note that Src42AE 1/+and Src42AE 1/+;Src64BPI/+show no significant alteration in NMJ phenotypes when compared to wild-type. Asterisks indicate significant difference by Student's t test (p < 0.05) and error bars represent the standard error of the mean (SEM).
We then tested whether severe Src mutants display NMJ growth defects. In the viable Src42AE 1/+; Src64BPI/PImutant that generates the least Src activity [41], the number of boutons was significantly increased and the total branch length was slightly enhanced (Figure 3C, E). To test whether Src has any contribution in the complete absence of Fak56 activity, we generated the combinatorial mutant Src42AE 1/+Fak56N 30/K 24;Src64BPI/+and found that reducing the gene dosage of Src further increased the number of boutons in the Fak56 null mutant by 21%. In comparison to wild-type animal controls, Src42AE 1/+Fak56N 30/K 24;Src64BPI/+mutants displayed an 80% increase in the bouton number and 25% increase in total branch length (Figure 3D). In summary, these genetic analyses suggest that Fak56 and Src have overlapping and distinct contributions in inhibiting NMJ growth.
Activation of Fak56 at NMJs
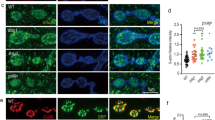
In mammals, activation of FAK and the FAK homolog Pyk2 proceeds with an auto-phosphorylation step at the conserved Y397 of FAK and Y402 of Pyk2 [16, 40, 42], which corresponds to Y430 in Fak56 [22, 25, 43]. To examine the activation of Fak56 at NMJs, we immunostained larval NMJs with the anti-FAK [pY397] antibody, which detects Fak56 activation at muscle attachment sites [28]. As shown for NMJ 12/13, phospho-FAK (pFAK) was expressed strongly in Ib boutons (white arrows in Figure 4A1) and weakly in Is boutons (white arrowheads). Expression at NMJ 4 was also prominent (Figure 4B1). In co-staining for HRP, the pFAK signals could be found within boutons and inter-bouton tracks (Figure 4A1, B1), suggesting a presynaptic activation of Fak56. Strong pFAK expression was also detected within the incoming axons that were co-labeled by HRP (yellow arrowhead and inset image in Figure 4B1). Cytosolic punctate staining was also present in muscles. In Fak56N 30/K 24null mutants, pFAK signals in axons, presynapses and muscles were completely absent (Figure 4C1, C2), confirming the specificity of the anti-pFAK antibody in detecting Fak56 activation signals.
Distribution and requirement of phospho-FAK (pFAK) at presynapses of neuromuscular junctions (NMJs). (A-D) Active Fak56 (pFAK in magenta) recognized by anti-FAK [pY397] antibodies localized at presynapses of NMJ 12/13 (A) and NMJ 4 (B) in wild-type larvae, and was absent in Fak56N 30/K 24(C), and reduced in βν1/1 (D), with co-stained horseradish peroxidase (HRP) in green. White arrows and arrowheads indicate Ib and Is boutons, respectively. Images in (A-D) come from a single section of the Z-stack confocal scanning. Note punctate distribution in muscles and strong expression in axonal trunks (yellow arrowhead in B1) surrounded by HRP membrane staining. The inset image in B1 is the inclusion of pFAK inside the incoming axon from a Z-stack section crossing the middle of the axon. (A2-D2) are diphospho-ERK (dpERK) images in white. (E-F) Images of NMJ 6/7 are shown as for Figure 1. Neuronal overexpression of LacZ control (E) or Fak56Y 430F(F) by elav-GAL4 displays a NMJ overgrowth phenotype. (G) Quantification of NMJ 6/7 phenotypes for elav>LacZ, elav>Fak56 and elav>Fak56Y430F. Note that elav>Fak56 shows slight reduction in bouton number and NMJ length when compared to elav>LacZ control (E) but these reductions were not statistically significant (p = 0.413 for bouton number and p = 0.125 for branch length in Student's t-test). Asterisks indicate significant difference by Student's t test (p < 0.05) and error bars represent the standard error of the mean (SEM).
In the βν1/1 integrin mutant, the pFAK staining in presynapses was dramatically reduced while the muscle punctate staining pattern was still retained (Figure 4D1, D2), indicating that integrin signaling mediated by the βν subunit is required for Fak56 activation in presynapses of NMJs. Taken together with the requirement of βν in restricting NMJ growth, these results suggest the presynaptic activation of Fak56 in restricting NMJ growth. To test this, the autoactivation site Y430 in Fak56 was mutated to phenylalanine to generate the UAS-Fak56Y 430Ftransgene. When ectopically expressed in neurons by elav-GAL4, Fak56Y 430Finduced significant NMJ overgrowth phenotypes (Figure 4F). As a control, the wild-type Fak56 transgene caused slight but no significant reduction in NMJ growth (quantified in Figure 4G). This dominant negative effect by the Fak56Y 430Fmutant suggests that phosphorylation at Y430 in the presynapse is critical for normal Fak56 function to constrain NMJ growth.
Fak56 suppresses MAPK/ERK activation at NMJs
To further investigate the role of Fak56 at the presynapse, we generated an RNAi transgene to deplete Fak56 expression (see Materials and methods and Additional file 1F). Expression of the Fak56RNAi transgene in presynapses (elav>Fak56RNAi) resulted in an increase in both total branch length and bouton number of NMJs compared to the elav>LacZ control (Figure 5E, G). In contrast, Fak56 depletion in muscles using MHC-GAL4 retained normal NMJ phenotypes (not shown).
Suppression of ERK activity by Fak56 during neuromuscular junction (NMJ) growth. (A, B) Immunostaining of elav>LacZ (A1) and elav>Fak56RNAi (B1) for diphospho-ERK (dpERK; green) and horseradish peroxidase (HRP; magenta). A single section of image is shown. Punctate expression of dpERK was observed in presynaptic boutons and enhanced in the enlarged NMJ 4 in elav>Fak56RNAi. (A2, B2) Only dpERK expression is shown. Scale bars are 10 μm. (C) Quantification of relative immunoreactivities of dpERK to HRP within the presynaptic zone. Note that elav>Fak56RNAi and elav>Fak56Y 430Fhad 3.3- and 3.1-fold increases compared to elav>LacZ. (D, E) Images of NMJ 6/7 are shown as described in Figure 1. (E) Depletion of Fak56 activity in elav>Fak56RNAi results in NMJ overgrowth, which can be suppressed by the rlEMS 698allele (F). (F) Quantification of NMJ 6/7 phenotypes for the control elav>LacZ, elav>Fak56RNAi, elav>Fak56RNAi;rlEMS 698/+and rlEMS 698/+. Note that the rlEMS 698allele suppressed NMJ phenotypes in elav>Fak56RNAi. Asterisks indicate significant difference by Student's t test (p < 0.05) and error bars represent the standard error of the mean (SEM).
It has been shown that presynaptic ERK activation promotes larval NMJ growth [20]. We then tested whether Fak56 had an effect on ERK activation at NMJs, which can be monitored by immunostaining for diphospho-ERK (dpERK) [44]. The expression of dpERK was detected in punctate patterns in some but not all boutons (Figure 5A1, A2) [20].
We then examined whether dpERK expression at NMJs was altered by presynaptic depletion of Fak56 using RNA interference (RNAi). In elav>Fak56RNAi, dpERK expression was highly enriched in almost all boutons at the enlarged NMJ (Figure 5B, B1). To quantify the difference among wild-type and Fak56 mutants, the level of dpERK immuno-reactivity within the presynaptic region was normalized to that of co-stained HRP. We found that in elav>Fak56RNAi the ratio was increased by 3.3-fold when compared to that in elav>lacZ. Consistently, neuronal expression of the dominant-negative Fak56Y430F also resulted in strongly enhanced dpERK expression to 3.1-fold (Figure 5C). The enhancement in dpERK expression levels in both approaches to block Fak56 function suggests that Fak56 activation suppresses ERK signaling in presynaptic boutons.
To test whether NMJ overgrowth phenotypes in Fak56 mutants were caused by the increased ERK activity, one wild-type allele of the ERK gene rolled (rl) [45] was replaced with the null allele rlEMS 698[46] in elav>Fak56RNAi larvae. The control heterozygous rlEMS 698/+larvae displayed normal NMJ phenotypes. However, reduction of ERK gene dosage by 50% completely suppressed the NMJ overgrowth phenotypes observed in elav>Fak56RNAi (Figure 5D–F). The BMP/Gbb signaling pathway also promotes NMJ growth [47]. We then tested whether the BMP/Gbb pathway would have a similar regulation in Fak56 mutant NMJs. Three mutants in the BMP/Gbb signaling pathway components were tested for potential genetic interactions with Fak56 but failed to significantly modify NMJ phenotypes in elav>Fak56RNAi larvae (Additional file 5). Taken together, these results suggest that Fak56 specifically downregulates the growth-promoting ERK signaling during NMJ growth.
Fak56 modulates IgCAM FasII levels at NMJs
It has been shown that ERK signaling regulates NMJ growth through the modulation of the protein levels of the cell-adhesion protein FasII [20]. At NMJs, FasII protein levels are inversely correlated with ERK activation. In elav>Fak56RNAi mutants, the NMJ FasII level was reduced (Figure 6B1). Using the elav>LacZ as the reference, a 33.5% reduction in the ratio of the FasII level to the HRP level was detected (Figure 6A1, D). Comparison of FasII expression between wild type and Fak56N 30/K 24also revealed a 26.6% reduction in the Fak56 mutant (images not shown). Analyses of these two mutants suggest that Fak56 activity in presynapses is required for the full expression of FasII at NMJs. To examine whether Fak56-regulated FasII expression is mediated through ERK, the FasII protein level was examined in elav>Fak56RNAi;rlEMS 698/+. We found that the FasII protein level at NMJs of elav>Fak56RNAi was significantly restored by introducing the rlEMS 698allele, with only 14.9% reduction compared to elav>LacZ (Figure 6C1, D). These results suggest that Fak56 regulation of FasII expression at NMJs is at least partially mediated by ERK.
Modulation of Fasciclin II (FasII) levels by Fak56. (A-C) Expression of FasII (green) at neuromuscular junction (NMJ) 6/7 in elav>LacZ (A1), elav>Fak56RNAi (B1), and elav>Fak56RNAi;rlEMS 698/+(C1). Co-stained horseradish peroxidase (HRP) is in magenta. (A2-C2) Only FasII staining is shown. Images in (A-C) come from a single section of the Z-stack confocal image. (D) Quantification of FasII levels relative to HRP immunoreactivity shown in (A1-C1). Note that elav>Fak56RNAi had a 33.5% reduction compared to elav>LacZ, which was restored significantly by removing one copy of rl in elav>Fak56RNAi;rlEMS 698/+. Asterisks indicate significant difference by Student's t test (p < 0.05) and error bars represent the standard error of the mean (SEM).
Discussion
Growth of the stereotypical NMJs during larval stages is tightly regulated by signaling pathways that either promote or inhibit terminal branching, bouton addition and active zone formation [20, 47–50]. In this study, we have identified an inhibitory role of the non-receptor tyrosine kinase FAK in the regulation of NMJ growth. The Drosophila FAK is required in presynaptic boutons for the growth process, where it functions in concert with the non-receptor tyrosine kinase Src. As evidenced by our genetic analysis, Fak56 plays a conventional role in mediating signal from the integrin receptors that mainly consist of αPS3 and βν subunits. Noncanonically, Fak56 suppresses MAPK/ERK activity in restricting synaptic elaboration. In support of this context-specificity of FAK activity, we have noted no gross changes in the dynamic patterns of ERK activation during Fak56 mutant embryogenesis (Additional file 6). Our data suggest that Fak56 activity inhibits ERK signaling in restricting synapse growth (Figure 7).
Model to depict Fak56 and ERK signaling and Fasciclin II (FasII) protein levels at neuromuscular junctions (NMJs). In restricting NMJ growth, the extracellular matrix signal laminin including the α subunit LanA is received by integrin receptors, including αPS3 and βν subunits. This signal is transduced through the association between Fak56 and Src, and in this process phosphorylation of Y430 Fak56 is essential. Activated Fak56 mediates signaling through suppressing ERK activation at NMJs and consequently upregulates the FasII protein level at NMJs, leading to the inhibition of NMJ growth. Those molecules (shown in grey) were not tested in this study.
The importance of FAK in regulating axonal branching of motor neurons in Drosophila is revealed in this study and has been shown in Purkinje cells [27]. FAK activity in Purkinje cells has been attributed partially to the recruitment of p190RhoGEF during axonal branching and growth. In integrin-mediated cell adhesion, Rho activity is initially downregulated and followed by sustained activation, leading to actin reorganization [51]. In response to integrin signaling, the initial downregulation of Rho activity requires the activation of p190RhoGAP by tyrosine phosphorylation and association with SH2 domain-containing p120RasGAP, thus providing an alternative link between FAK and the Ras-MAPK pathway. Future studies on the characterization of the p190RhoGAP-p120RasGAP complex in NMJ development should illuminate how FAK regulates synaptic growth and plasticity.
ERK signaling regulates the protein levels of the cell adhesion molecule FasII at NMJs [20]. Homophilic interaction of FasII-like IgCAMs regulates axon pathfinding, target recognition, and synapse formation and remodeling [52–57]. At Drosophila NMJs, FasII is involved in synaptic formation and maintenance [52, 53, 56, 57]. Different levels of FasII play different roles in NMJ formation. While the basal level is essential to form the synaptic structure, a higher-level of FasII protein restricts NMJ growth. We found that Fak56 regulates the high level of FasII at NMJs and this regulation could be accounted for by a suppression of ERK activity. Therefore, in NMJ growth regulation, the cell-matrix interaction mediated by integrin signaling cross-talks with FasII-dependent cell-cell adhesion between pre- and post-synaptic partners (Figure 7).
Previous analysis of the activity of the Drosophila integrin αPS3 in the viable Vol allele suggested that αPS3 regulates NMJ elaboration, synaptic transmission and plasticity [9]. Lack of αPS3 induces moderate NMJ overgrowth with increases in higher-order branches and boutons, similar to what were observed in Fak56 mutants. In our analysis, βν genetically interacts with the Fak56 mutant and the βν mutant NMJ displays an overgrowth phenotype as well, suggesting that βν may be the major β subunit forming integrin heterodimers with αPS3 to restrict NMJ growth. The integrin subunits αPS1, αPS2 and βPS are also expressed at NMJs, and alteration of βPS activity affects NMJ morphology [10]; it is thus foreseeable that multiple modes of integrin signaling pathways regulate NMJ growth.
Laminins are the major component of the ECM and are involved in NMJ synaptic formation and maintenance [58]. Functional laminins are heterotrimers composed of α, β and γ chains, and different chain combinations contribute to laminin diversity. Laminins 4, 9 and 11 are composed of the same β2 and γ1 chain but differ in the α chain (α2, α4 and α5, respectively) and have been shown to localize in synaptic clefts of the mammalian neuromuscular system [59]. In an in vitro culture system, laminin 11 with the α5 subunit serves as a stop signal in motor axon outgrowth [59]. In Drosophila, LanA is most homologous to mammalian α3 and α5 subunits. LanA genetically interacts with Fak56 and βν mutants and may serve as the conserved component of the stop signal to restrict NMJ elaboration.
Conclusion
FAK activation by integrins regulates various cellular processes, and in many cases can be accounted for by an activation of Ras through the recruitment of the GRB2-SOS complex [14]. In our study, Fak56 activity restricts NMJ synaptic elaboration by inhibiting the ERK signaling cascade. This noncanonical link between FAK activity and ERK signaling might be cell-context specific, such as in neurons, or even subcellular site-specific, such as at synapses. Vol (αPS3) functions in the process of learning and memory [35], and can act as the FAK upstream regulator with the same regulatory link proposed here (Figure 7). FAK has been suggested as a putative therapeutic target for its role in tumor cell invasion and metastasis [13, 15, 60–62]. The neuronal-specific nonconventional link between FAK and ERK proposed in this study may have implications in cancer biology and therapy.
Materials and methods
Fly stocks
Flies were reared at 25°C except where specifically indicated. Wild-type flies used in this study were the w1118 strain. Mutant alleles Fak56KG 00304, mew1, ifk 27e, scb2, mys1, Src42AE 1, Src64BPIand rlEMS 698were obtained from the Bloomington stock center. βν1, βν2 [34], LanA9–32, LanA216 [63] and wb4Y 18[64] have been previously described. The various Fak56 alleles used in this study are described in detail in Additional file 1. The transgenic lines elav-GAL4 (X) (used in neuronal Fak56 knockdown and overexpression), elav-GAL4 (III) (used in neuronal Fak56 rescue), and UAS-LacZ were obtained from the Bloomington stock center.UAS-Fak56 [28] and MHC-GAL4 [65] have been described previously. The pUAST-Fak56RNAi construct was generated by subcloning two inverted Fak56 cDNA fragments (base pairs 629–1177) into the pUAST vector and the knockdown effect was examined (Additional file 1F).pUAST-Fak56Y 430Fflies were generated from pUAST-Fak56 by PCR based site-directed mutagenesis. To enhance the Fak56RNAi transgene expression, embryos from the elav-GAL4 (X) and pUAST-Fak56RNAi cross were collected for 6 hours, kept at 25°C for 45 hours and shifted to 30°C until late third instar.
Immunostaining
In all experiments, wandering late third instar larvae were dissected for analysis of NMJ phenotypes. After dissection, tissues were incubated in fixative solution (4% formaldehyde in 1× phosphate-buffered saline) for 20 minutes. For immunostaining, primary antibodies used were against synaptotagmin (mouse, 1:25; DHSB, Iowa City, IA, USA), HRP conjugated with TRITC (rabbit, 1:100; Jackson ImmunoResearch, West Grove, PA, USA), FAK [pY397] (rabbit, 1:50; Biosource-Invitrogen, Carlsbad, CA, USA), FasII (1D4, 1:100; DHSB) and dp-ERK-1/2 (mouse, 1:20; Sigma-Aldrich, St. Louis, MO, USA). Alexa 488-, Cy3- and Cy5-conjugated secondary antibodies and TRITC-phalloidin were used (Jackson ImmunoResearch).
Image processing and presentation
Confocal images were acquired using a Zeiss LSM 510 Meta and processed using Adobe Photoshop CS. Images for quantification of NMJ branch length and bouton number were from a projection of 10 z-sections of 6.5–8 μm in total. To quantify the NMJ length and muscle area, the images were analyzed by a measurement tool in Zeiss LSM Image Examiner. For quantification of signal intensity at NMJs, images were acquired under the same scanning parameters. NMJs were outlined and the signal intensity was calculated by histogram analysis in Adobe Photoshop CS.
Electrophysiological recording
For sample preparation, dissected larval body walls (including the central nervous system and motor axons) were exposed in cold (4°C) HL3.1 Ca2+ free saline (70 mM NaCl, 5 mM KCl, 4 mM MgCl2, 10 mM NaHCO3, 5 mM trehalose, 115 mM sucrose, 5 mM HEPES pH 7.2) [66]. Experiments were performed on muscle 6 of segment A3 in late third instar larvae. The segmental nerve was cut near the ventral ganglion. Preparations were then incubated in HL3.1 saline containing 0.2 or 1 mM CaCl2 for electrophysiological experiments at room temperature (22°C). For stimulation and recording, a glass microelectrode (30–50 MO in resistance) filled with 3 M KCl was impaled in the sixth muscle of the third abdominal segment to record the EJPs. The mEJPs occurring in the background within 200 seconds were obtained without any stimulation on the segmental nerve. To evoke an EJP, the segmental nerve was stimulated every 30 seconds through the cut end with a suction electrode with 0.1 ms of pulse duration at 2 times the threshold voltage. Once the threshold voltage was reached, the size of EJPs remained unchanged despite the increase in stimulating voltage. Signals were digitized at 64 KHz by a PCI-6221 data-acquisition card (National Instrument, Austin, Texas, USA), and saved on an IBM compatible PC for analysis.
Abbreviations
- dpERK:
-
diphospho-ERK
- ECM:
-
extracellular matrix
- FAK:
-
focal adhesion kinase
- FasII:
-
Fasciclin II
- HRP:
-
horseradish peroxidase
- MAPK:
-
mitogen-activated protein kinase
- mEJP:
-
miniature junctional potential
- NMJ:
-
neuromuscular junction
- pFAK:
-
phospho-FAK
- RNAi:
-
RNA interference.
References
Nishimune H, Sanes JR, Carlson SS: A synaptic laminin-calcium channel interaction organizes active zones in motor nerve terminals. Nature. 2004, 432: 580-587. 10.1038/nature03112.
Dityatev A, Schachner M: The extracellular matrix and synapses. Cell Tissue Res. 2006, 326: 647-654. 10.1007/s00441-006-0217-1.
Dityatev A, Schachner M: Extracellular matrix molecules and synaptic plasticity. Nat Rev Neurosci. 2003, 4: 456-468. 10.1038/nrn1115.
Huber AB, Kolodkin AL, Ginty DD, Cloutier JF: Signaling at the growth cone: ligand-receptor complexes and the control of axon growth and guidance. Annu Rev Neurosci. 2003, 26: 509-563. 10.1146/annurev.neuro.26.010302.081139.
Clegg DO: Novel roles for integrins in the nervous system. Mol Cell Biol Res Commun. 2000, 3: 1-7. 10.1006/mcbr.1999.0175.
Nakamoto T, Kain KH, Ginsberg MH: Neurobiology: New connections between integrins and axon guidance. Curr Biol. 2004, 14: R121-123.
Milner R, Campbell IL: The integrin family of cell adhesion molecules has multiple functions within the CNS. J Neurosci Res. 2002, 69: 286-291. 10.1002/jnr.10321.
Hoang B, Chiba A: Genetic analysis on the role of integrin during axon guidance in Drosophila. J Neurosci. 1998, 18: 7847-7855.
Rohrbough J, Grotewiel MS, Davis RL, Broadie K: Integrin-mediated regulation of synaptic morphology, transmission, and plasticity. J Neurosci. 2000, 20: 6868-6878.
Beumer KJ, Rohrbough J, Prokop A, Broadie K: A role for PS integrins in morphological growth and synaptic function at the postembryonic neuromuscular junction of Drosophila. Development. 1999, 126: 5833-5846.
Johnson KG, Tenney AP, Ghose A, Duckworth AM, Higashi ME, Parfitt K, Marcu O, Heslip TR, Marsh JL, Schwarz TL: The HSPGs Syndecan and Dallylike bind the receptor phosphatase LAR and exert distinct effects on synaptic development. Neuron. 2006, 49: 517-531. 10.1016/j.neuron.2006.01.026.
Rohrbough J, Rushton E, Woodruff E, Fergestad T, Vigneswaran K, Broadie K: Presynaptic establishment of the synaptic cleft extracellular matrix is required for post-synaptic differentiation. Genes Dev. 2007, 21: 2607-2628. 10.1101/gad.1574107.
Cohen LA, Guan JL: Mechanisms of focal adhesion kinase regulation. Curr Cancer Drug Targets. 2005, 5: 629-643. 10.2174/156800905774932798.
Mitra SK, Hanson DA, Schlaepfer DD: Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005, 6: 56-68. 10.1038/nrm1549.
Schlaepfer DD, Mitra SK, Ilic D: Control of motile and invasive cell phenotypes by focal adhesion kinase. Biochim Biophys Acta. 2004, 1692: 77-102.
Avraham H, Park SY, Schinkmann K, Avraham S: RAFTK/Pyk2-mediated cellular signalling. Cell Signal. 2000, 12: 123-133. 10.1016/S0898-6568(99)00076-5.
Schlaepfer DD, Hanks SK, Hunter T, Geer van der P: Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature. 1994, 372: 786-791.
Schlaepfer DD, Hunter T: Focal adhesion kinase overexpression enhances ras-dependent integrin signaling to ERK2/mitogen-activated protein kinase through interactions with and activation of c-Src. J Biol Chem. 1997, 272: 13189-13195. 10.1074/jbc.272.20.13189.
Schlaepfer DD, Jones KC, Hunter T: Multiple Grb2-mediated integrin-stimulated signaling pathways to ERK2/mitogen-activated protein kinase: summation of both c-Src- and focal adhesion kinase-initiated tyrosine phosphorylation events. Mol Cell Biol. 1998, 18: 2571-2585.
Koh YH, Ruiz-Canada C, Gorczyca M, Budnik V: The Ras1-mitogen-activated protein kinase signal transduction pathway regulates synaptic plasticity through fasciclin II-mediated cell adhesion. J Neurosci. 2002, 22: 2496-2504.
Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T: Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995, 377: 539-544. 10.1038/377539a0.
Fox GL, Rebay I, Hynes RO: Expression of DFak56, a Drosophila homolog of vertebrate focal adhesion kinase, supports a role in cell migration in vivo. Proc Natl Acad Sci USA. 1999, 96: 14978-14983. 10.1073/pnas.96.26.14978.
Menegon A, Burgaya F, Baudot P, Dunlap DD, Girault JA, Valtorta F: FAK+ and PYK2/CAKbeta, two related tyrosine kinases highly expressed in the central nervous system: similarities and differences in the expression pattern. Eur J Neurosci. 1999, 11: 3777-3788. 10.1046/j.1460-9568.1999.00798.x.
Contestabile A, Bonanomi D, Burgaya F, Girault JA, Valtorta F: Localization of focal adhesion kinase isoforms in cells of the central nervous system. Int J Dev Neurosci. 2003, 21: 83-93. 10.1016/S0736-5748(02)00126-0.
Palmer RH, Fessler LI, Edeen PT, Madigan SJ, McKeown M, Hunter T: DFak56 is a novel Drosophila melanogaster focal adhesion kinase. J Biol Chem. 1999, 274: 35621-35629. 10.1074/jbc.274.50.35621.
Beggs HE, Schahin-Reed D, Zang K, Goebbels S, Nave KA, Gorski J, Jones KR, Sretavan D, Reichardt LF: FAK deficiency in cells contributing to the basal lamina results in cortical abnormalities resembling congenital muscular dystrophies. Neuron. 2003, 40: 501-514. 10.1016/S0896-6273(03)00666-4.
Rico B, Beggs HE, Schahin-Reed D, Kimes N, Schmidt A, Reichardt LF: Control of axonal branching and synapse formation by focal adhesion kinase. Nat Neurosci. 2004, 7: 1059-1069. 10.1038/nn1317.
Grabbe C, Zervas CG, Hunter T, Brown NH, Palmer RH: Focal adhesion kinase is not required for integrin function or viability in Drosophila. Development. 2004, 131: 5795-5805. 10.1242/dev.01462.
Murakami S, Umetsu D, Maeyama Y, Sato M, Yoshida S, Tabata T: Focal adhesion kinase controls morphogenesis of the Drosophila optic stalk. Development. 2007, 134: 1539-1548. 10.1242/dev.001529.
Jan LY, Jan YN: Antibodies to horseradish peroxidase as specific neuronal markers in Drosophila and in grasshopper embryos. Proc Natl Acad Sci USA. 1982, 79: 2700-2704. 10.1073/pnas.79.8.2700.
Littleton JT, Bellen HJ, Perin MS: Expression of synaptotagmin in Drosophila reveals transport and localization of synaptic vesicles to the synapse. Development. 1993, 118: 1077-1088.
Geiger B, Bershadsky A, Pankov R, Yamada KM: Transmembrane crosstalk between the extracellular matrix – cytoskeleton crosstalk. Nat Rev Mol Cell Biol. 2001, 2: 793-805. 10.1038/35099066.
Schock F, Perrimon N: Retraction of the Drosophila germ band requires cell-matrix interaction. Genes Dev. 2003, 17: 597-602. 10.1101/gad.1068403.
Devenport D, Brown NH: Morphogenesis in the absence of integrins: mutation of both Drosophila beta subunits prevents midgut migration. Development. 2004, 131: 5405-5415. 10.1242/dev.01427.
Grotewiel MS, Beck CD, Wu KH, Zhu XR, Davis RL: Integrin-mediated short-term memory in Drosophila. Nature. 1998, 391: 455-460. 10.1038/35079.
Brower DL, Jaffe SM: Requirement for integrins during Drosophila wing development. Nature. 1989, 342: 285-287. 10.1038/342285a0.
MacKrell AJ, Blumberg B, Haynes SR, Fessler JH: The lethal myospheroid gene of Drosophila encodes a membrane protein homologous to vertebrate integrin beta subunits. Proc Natl Acad Sci USA. 1988, 85: 2633-2637. 10.1073/pnas.85.8.2633.
Bogaert T, Brown N, Wilcox M: The Drosophila PS2 antigen is an invertebrate integrin that, like the fibronectin receptor, becomes localized to muscle attachments. Cell. 1987, 51: 929-940. 10.1016/0092-8674(87)90580-0.
Fessler JH, Fessler LI: Drosophila extracellular matrix. Annu Rev Cell Biol. 1989, 5: 309-339. 10.1146/annurev.cb.05.110189.001521.
Schaller MD, Hildebrand JD, Shannon JD, Fox JW, Vines RR, Parsons JT: Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol Cell Biol. 1994, 14: 1680-1688.
Takahashi M, Takahashi F, Ui-Tei K, Kojima T, Saigo K: Requirements of genetic interactions between Src42A, armadillo and shotgun, a gene encoding E-cadherin, for normal development in Drosophila. Development. 2005, 132: 2547-2559. 10.1242/dev.01850.
Dikic I, Tokiwa G, Lev S, Courtneidge SA, Schlessinger J: A role for Pyk2 and Src in linking G-protein-coupled receptors with MAP kinase activation. Nature. 1996, 383: 547-550. 10.1038/383547a0.
Fujimoto J, Sawamoto K, Okabe M, Takagi Y, Tezuka T, Yoshikawa S, Ryo H, Okano H, Yamamoto T: Cloning and characterization of Dfak56, a homolog of focal adhesion kinase, in Drosophila melanogaster. J Biol Chem. 1999, 274: 29196-29201. 10.1074/jbc.274.41.29196.
Gabay L, Seger R, Shilo BZ: In situ activation pattern of Drosophila EGF receptor pathway during development. Science. 1997, 277: 1103-1106. 10.1126/science.277.5329.1103.
Biggs WH, Zavitz KH, Dickson B, Straten van der A, Brunner D, Hafen E, Zipursky SL: The Drosophila rolled locus encodes a MAP kinase required in the sevenless signal transduction pathway. EMBO J. 1994, 13: 1628-1635.
Wilson DP, Wan ZK, Xu WX, Kirincich SJ, Follows BC, Joseph-McCarthy D, Foreman K, Moretto A, Wu J, Zhu M: Structure-based optimization of protein tyrosine phosphatase 1B inhibitors: from the active site to the second phosphotyrosine binding site. J Med Chem. 2007, 50: 4681-4698. 10.1021/jm0702478.
Keshishian H, Kim YS: Orchestrating development and function: retrograde BMP signaling in the Drosophila nervous system. Trends Neurosci. 2004, 27: 143-147. 10.1016/j.tins.2004.01.004.
Deschenes MR, Tenny KA, Wilson MH: Increased and decreased activity elicits specific morphological adaptations of the neuromuscular junction. Neuroscience. 2006, 137: 1277-1283. 10.1016/j.neuroscience.2005.10.042.
Budnik V, Ruiz C: The fly neuromuscular junction: structure and function second edition. Int Rev Neurobiol. 2006, 75: 1-406. 10.1016/S0074-7742(06)75015-2.
Packard M, Mathew D, Budnik V: Wnts and TGF beta in synaptogenesis: old friends signalling at new places. Nat Rev Neurosci. 2003, 4: 113-120. 10.1038/nrn1036.
Clark EA, King WG, Brugge JS, Symons M, Hynes RO: Integrin-mediated signals regulated by members of the rho family of GTPases. J Cell Biol. 1998, 142: 573-586. 10.1083/jcb.142.2.573.
Thomas U, Kim E, Kuhlendahl S, Koh YH, Gundelfinger ED, Sheng M, Garner CC, Budnik V: Synaptic clustering of the cell adhesion molecule fasciclin II by discs-large and its role in the regulation of presynaptic structure. Neuron. 1997, 19: 787-799. 10.1016/S0896-6273(00)80961-7.
Ashley J, Packard M, Ataman B, Budnik V: Fasciclin II signals new synapse formation through amyloid precursor protein and the scaffolding protein dX11/Mint. J Neurosci. 2005, 25: 5943-5955. 10.1523/JNEUROSCI.1144-05.2005.
Hebbar S, Fernandes JJ: A role for Fas II in the stabilization of motor neuron branches during pruning in Drosophila. Dev Biol. 2005, 285: 185-199. 10.1016/j.ydbio.2005.06.015.
Fushima K, Tsujimura H: Precise control of fasciclin II expression is required for adult mushroom body development in Drosophila. Dev Growth Differ. 2007, 49: 215-227.
Schuster CM, Davis GW, Fetter RD, Goodman CS: Genetic dissection of structural and functional components of synaptic plasticity. I. Fasciclin II controls synaptic stabilization and growth. Neuron. 1996, 17: 641-654. 10.1016/S0896-6273(00)80197-X.
Schuster CM, Davis GW, Fetter RD, Goodman CS: Genetic dissection of structural and functional components of synaptic plasticity. II. Fasciclin II controls presynaptic structural plasticity. Neuron. 1996, 17: 655-667. 10.1016/S0896-6273(00)80198-1.
Patton BL: Laminins of the neuromuscular system. Microsc Res Tech. 2000, 51: 247-261. 10.1002/1097-0029(20001101)51:3<247::AID-JEMT5>3.0.CO;2-Z.
Patton BL, Miner JH, Chiu AY, Sanes JR: Distribution and function of laminins in the neuromuscular system of developing, adult, and mutant mice. J Cell Biol. 1997, 139: 1507-1521. 10.1083/jcb.139.6.1507.
Zeng ZZ, Jia Y, Hahn NJ, Markwart SM, Rockwood KF, Livant DL: Role of focal adhesion kinase and phosphatidylinositol 3'-kinase in integrin fibronectin receptor-mediated, matrix metalloproteinase-1-dependent invasion by metastatic prostate cancer cells. Cancer Res. 2006, 66: 8091-8099. 10.1158/0008-5472.CAN-05-4400.
van Nimwegen MJ, Water van de B: Focal adhesion kinase: a potential target in cancer therapy. Biochem Pharmacol. 2007, 73: 597-609. 10.1016/j.bcp.2006.08.011.
Mitra SK, Lim ST, Chi A, Schlaepfer DD: Intrinsic focal adhesion kinase activity controls orthotopic breast carcinoma metastasis via the regulation of urokinase plasminogen activator expression in a syngeneic tumor model. Oncogene. 2006, 25: 4429-4440. 10.1038/sj.onc.1209482.
Henchcliffe C, Garcia-Alonso L, Tang J, Goodman CS: Genetic analysis of laminin A reveals diverse functions during morphogenesis in Drosophila. Development. 1993, 118: 325-337.
Martin D, Zusman S, Li X, Williams EL, Khare N, DaRocha S, Chiquet-Ehrismann R, Baumgartner S: wing blister, a new Drosophila laminin alpha chain required for cell adhesion and migration during embryonic and imaginal development. J Cell Biol. 1999, 145: 191-201. 10.1083/jcb.145.1.191.
DiAntonio A, Petersen SA, Heckmann M, Goodman CS: Glutamate receptor expression regulates quantal size and quantal content at the Drosophila neuromuscular junction. J Neurosci. 1999, 19: 3023-3032.
Feng Y, Ueda A, Wu CF: A modified minimal hemolymph-like solution, HL3.1, for physiological recordings at the neuromuscular junctions of normal and mutant Drosophila larvae. J Neurogenet. 2004, 18: 377-402. 10.1080/01677060490894522.
Acknowledgements
We thank S-P Lee of IMB TEM core facility for technical supports, members of the CT Chien and RH Chen labs for discussion and comments on the manuscript, and NH Brown and N Harden for providing reagents. CTC is supported by a National Science Council Frontier Research Grant and an Academia Sinica Sn-Gn Research Grant of Taiwan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
PIT designed the study, wrote the manuscript, and performed and participated in all experiments. HHK, YTL and SRY participated in the electrophysiological experiments and analysis. CG and RHP designed, manufactured, and supplied the Fak56 point mutation and Fak56RNAi constructs, performed dpERK embryonic staining, and contributed to manuscript revisions. AG and DVV helped to characterize the NMJ phenotype of Fak56CG 1. TTL and KPP helped to analyze the synaptic markers at NMJ of Fak56 mutants. RHC helped revise the manuscript and provided suggestions with regard to signal transduction and intellectual input for the study. CTC participated in the overall design and coordination of the study and helped to write the manuscript. All authors have read and approved the final manuscript.
Electronic supplementary material
13064_2008_58_MOESM1_ESM.pdf
Additional file 1: Fak56 mutant alleles and expression. This file describes (A) the Fak56 locus and the generation of N30 and K24 deletions from KG00304 P-element insertion, (B) the expression of Fak56 mRNA in different Fak56 allele combinations, (C, D) HRP staining for wild-type and Fak56CG 1NMJs, (E) quantifications for NMJ phenotypes, and (F) the knock-down effect of Fak56RNAi. (PDF 357 KB)
13064_2008_58_MOESM2_ESM.pdf
Additional file 2: Expressions of NMJ proteins in Fak56null. This file describes identical expressions of Dlg (A, B), Brp (C, D), dPak (E, F), GluIIA (G, H) and Fustch (I, J) at wild-type and Fak56N 30/K 24NMJs. (PDF 568 KB)
13064_2008_58_MOESM3_ESM.pdf
Additional file 3: Ultrastructures of Fak56N 30/K 24synapses. Electron micrographs of cross-sections through a type-I bouton of muscle 6/7 in wild-type (A) and Fak56N 30/K 24(B) larvae. Quantitative analyses reveal no difference for synaptic unltrastructures (C). (PDF 2 MB)
13064_2008_58_MOESM4_ESM.pdf
Additional file 4: Electrophysiological recording of postsynaptic currents from wild-type and Fak56N 30/K 24in 1 mM [Ca2+]. (A) Cumulative frequency plot reveals a significant shift in the distribution of mEJP amplitudes. (B) Representative traces and mean amplitudes of EJPs in wild-type and Fak56N 30/K 24. (PDF 91 KB)
13064_2008_58_MOESM5_ESM.pdf
Additional file 5: BMP/Gbb signaling-independent mechanism of Fak56 in NMJ growth. No alternations of NMJ phenotypes were detected by introducing mutant alleles (sax4, witA 12and med13) for BMP signaling components into elav>Fak56RNAi. (PDF 69 KB)
13064_2008_58_MOESM6_ESM.pdf
Additional file 6: ERK phosphorylation in Fak56CG 1mutant embryos. Expressions of phospho-ERK appear grossly normal during Drosophila embryogenesis. (PDF 838 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Tsai, PI., Kao, HH., Grabbe, C. et al. Fak56 functions downstream of integrin alphaPS3betanu and suppresses MAPK activation in neuromuscular junction growth. Neural Dev 3, 26 (2008). https://doi.org/10.1186/1749-8104-3-26
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1749-8104-3-26