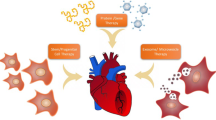
Abstract
Atherosclerotic disease of the arteries is a major cause of coronary artery disease, peripheral vascular disease and stroke. Some patients are however not candidate for the standard treatment of angioplasty or bypass surgery. Hence there is tremendous enthusiasm for the utilization of angiogenesis as a therapeutic modality for atherosclerotic arterial disease. This augmentation of physiological neo-vascularization in cardiovascular disease can be achieved through different pathways. In this article we are reviewing the Use of Gene therapy, Protein therapy and cellular therapy.
Similar content being viewed by others
Introduction
In the 20th century, the number of patients affected by atherosclerosis increased dramatically. Cardiovascular disease due to atherosclerosis had become the first cause of death worldwide as compared to infectious diseases at the beginning of the century. The natural history of atherosclerosis can be divided into three stages:
-
Stage I: Asymptomatic fatty streak and fibrous plaque formation.
-
Stage II: Symptomatic: Formation of fibrous plaque with medial calcification; enlargement of the fibrous plaque and thrombus formation; significant homodynamic occlusion of the vessel and then complete occlusion.
-
Stage III: Where three major complications are seen:
-
Myocardial infarction.
-
Brain infarction.
-
Gangrene.
-
Risk factors and disease management
The risk factors for having atherosclerotic disease are age, hyperlipidemia, smoking, hypertension, physical inactivity, obesity and hormone status. The conventional management of atherosclerotic arterial insufficiency can be divided into three major parts:
-
Risk factors modification and medical treatment in order to restore the balance between the oxygen demand and oxygen supply and prevention of acute thrombotic episodes, these can be achieved by: Lifestyle modification (exercise, smoking cessation, weight reduction), and Cholesterol lowering. Another risk factor modification is Blood pressure management through the following therapies like Aspirin therapy, Beta- blocker, ACE inhibitors. And the last is through interventional therapy which is divided into: Surgical: Includes aorto-coronary bypass, transmyocardial revascularization (TMR) and peripheral vascular bypass and catheter-based: which include percutanous trans-coronary angioplasty and stenting or coronary atherectomy.
Despite the improvement of these interventions and outcomes there are many patients that are not suitable candidates because of their diffuse and severe atherosclerotic CAD and/or because of the risky nature of the intervention. Hence the idea came of promoting and stimulating the process of angiogenesis with newly created vessels which could serve as a biological bypass of the atherosclerotic vessel.
Neovascularization
During the development of the embryo and the physio-logical repair of any tissue damage, the formation of new blood vessels plays a major role [1]. Some of this new blood vessel formation is normal and beneficial as can be seen in wound healing after trauma, ischemic tissue restoration and in the endometrium during the menstrual cycle. Abnormal formation of blood vessels however, also occur and contributes to a number of important disease processes such as diabetic retinopathy, malignancy, and diseases like rheumatoid arthritis, systemic lupus erythematosus and in atherosclerotic disease[2–5]. Stimulation of new blood vessel growth must therefore be site specific and organ specific.
There are three major processes that contribute to the growth of new blood vessels that are termed globally as neovascularization:
-
Vasculogenesis: At the beginning it was thought that this happens during embryogenesis in which there is a differentiation of embryonic mesenchymal cells (such as the endothelial precursor cells or angioblasts) into endothelial cells and the de novo development of blood vessels but now it is well known that it contribute to the adult neovasculirization as well [6]. This process is believed to be induced by FGF, VEGF and other angiogenic factors or/and tissue ischemia. This process leads to the development of new blood vessels that are formed are composed mainly of endothelial cells and are referred to as the capillary plexus. The smooth muscle layer that incorporates in the plexus results from migration and proliferation of different cell types like pericytes, smooth muscles cells and fibroblasts [7, 8].
-
Angiogenesis: It is the formation of new blood vessels by sprouting from pre existing small vessels in adult and embryonic tissue or by intravascular subdivision process called intussusception [9, 10]. These new blood vessels are usually lacking developed media. It occurs as the level of local angiogenic stimuli increases; this will lead to the activation of the endothelial cells found in pre-existing vessels, with subsequent vasodilatation, increase permeability and the disruption of the basement membrane encompassing endothelial cells of the pre-existing capillaries via proteolytic degradation. Disturbance of the basement membrane allow the cytoplasmic process to extend from the activated endothelial cells, directing their migration and sprouting to the extra vascular space towards the angiogenic stimuli [11]. The cells elongate and aligned to form capillary sprouts and then these sprouts will form a lumen and connect with the neighboring vessels, these capillaries are very small in size and their contribution to the actual blood flow with no or little significance to the ischemic tissue.
-
Arteriogenesis: It is defined as a rapid proliferation of pre-existing collateral vessels and these vessels have fully developed tunica media [9, 12]. It is a mature type of neovascularization which can restore perfusion to an ischemic area.
The occurrence of both angiogenesis and arteriogenesis has been shown in many animal models and in patients with coronary artery disease. Tissue ischemia is one of the key factors that stimulate neovascularization [9].
Folkman has described a series of morphological and biochemical events involved in angiogenesis as, Vasodilatation of parent vessel, degradation of basement membrane, endothelial migration, endothelial cell proliferation, lumen formation, loop formation, formation of new basement membrane, and incorporation of pericytes [13].
Angiogenesis triggering factors
It is a very complex process. Conceptually the major triggers of this process can be simplified into three broad categories: mechanical, chemical and molecular factors.
1. Mechanical influences
and this can by explained by two factors either through homodynamic or the shear stress:
-
Hemodynamic: Augmentation of blood flow during exercise, the hyperthyroid state (Thyrotoxicosis) and administration of certain drugs have all been shown to stimulate vascular sprouting[14–17]. It also has been shown that large vessels with low flow tend to reduce or obliterate their endovascular diameter and the lumens of the small vessels with chronically increased high blood flow tend to enlarge [18]. Augmentation of blood flow therefore may both stimulate vascular sprouting and maintain patency of the newly formed collateral vessels and so providing blood flow to the ischemic area.
-
Shear Stress: Shear stress has an important influence on the development of collaterals in the ischemic tissues. Shear stress and stretch of the myocardium (i.e. increase left ventricular end diastolic volume) can lead to up-regulation of adhesion molecules in the endothelium, attraction of the inflammatory cells and stimulation of the endothelial cells to produce angiogenic factors[19–22].
2. Chemical influence
this can happen either through hypoxia or through the production of nitric oxide:
-
Hypoxia and oxygen tension gradient: Hypoxia stimulates macrophages to release various factors including platelets derived growth factor and fibroblast growth factor 1 and 2 [23]. Hypoxia has been shown to upregulate vascular endothelial growth factor owing both by increasing transcription mediated by hypoxia inducible factor 1 and by increasing the stability of vascular endothelial growth factor mRNA [24–26]. Hypoxia also increase the expression of vascular endothelial growth factor receptors(FLK and FLT) this renders the endothelium susceptible to either the systematic or the paracrine effect of pre-existing vascular endothelial growth factor [27, 28].
-
Nitric oxide: VEGF is known to induce the release of Nitric oxide from endothelial cells, and vascular endothelium inducible NO synthase (iNOS) production is amplified during VEGF- induced angiogenesis [28]. The role of Nitric Oxide in VEGF induced angiogenesis have been shown in NOS knocked out mice as well as after NOS inhibition, both result in reduction of angiogenesis [29–32]. Nitric Oxide has also a regulatory effect on VEGF production.
3. Molecular influences
-
Effect of glucose on VEGF expression: Hypoglycemia increases VEGF expression, and after equilibration of the glucose concentration the level of VEGF return back to normal [33, 34]. This most likely happen when intracellular CA2+ levels increases in glucose deprived environment leads to activation of protein kinase C. this process induces the activation of Angiopoiten -1, leading to an increase of VEGF expression [35]. Not only hypoglycemia increases VEGF expression but also hyperglycemia do the same thing both of which can result an increase in the production of VEGF[36, 37].
-
Inflammation: Animal studies have shown that the presence of inflammatory cells, like macrophages and neutrophils is sufficient to produce angiogenesis. Following myocardial necrosis, the influx of inflammatory cells including macrophages, monocytes, and platelets results in the release of cytokines that are capable of stimulating fibroblast growth factor and vascular endothelial growth factor expression [38, 39]. Vascular endothelial growth factor by itself can stimulate and recruit other macrophages to stimulate inflammation response and further stimulate angiogenesis.
-
Angiogenic growth factors: The existence of angiogenic factors were first observed with the isolation of tumor factors that generate mitogenic activities in endothelial cells [40–42]. They are induced by a many varieties of cells and their functions are development as well as tumor angiogenesis. Angiogenic growth factors are called so because of their ability to induce the proliferation of various cells in vitro, which contribute to the process of angiogenesis in vivo as shown by studies of animal models. We will describe briefly couple of these growth factors:
-
a.
Fibroblast growth factor(FGF): The first angiogenic growth factor to be discovered as a member of a family currently comprises at least 20 molecules with extensive mitogenic potentials representing some of the most potent angiogenic peptide [41–43]. They are produced by vascular endothelial cell and smooth muscle cells. One characteristic of the FGF family is the ability to interact with heparin- like glycosaminoglycan of the extracellular matrix [44]. The two most widely researched isoforms are FGF-1 and FGF-2[45–48].
-
b.
Vascular endothelial growth factor (VEGF): Was initially purified as vascular permeability factor from tumor cell ascites [49]. It is now known as a multifunctional peptide capable of inducing receptor- mediated endothelial cell proliferation and angiogenesis both in vivo and in vitro. It has a crucial role in embryonic vascular development and in adult pathophysiology. VEGF is a family composed of five members and their effect mediated through three receptors (VEGFR)[50–53].
-
c.
Placenta growth factor: Got its name because of it predominance in placental tissue. It has little angiogenic effect in vitro but it seems that both PLGF and VEGF maybe co expressed in vivo [54–56].
-
d.
Angiopoietin: is a recently discovered family of growth factors and angiopoietin1 (Ang1) is expressed in tissue close to the blood vessels, suggesting a paracrine mode of action. Angiopoietin 2 (ANG2) is found at sites of tissue remodeling [57–60].
-
e.
VEGF receptors: In humans, the effect of VEGF on endothelial cells is mediated via two membrane spanning receptors, VEGFR1 and VEGFR2. Both receptors have high affinity for VEGF [61–63]. VEGFR1 Promotes cell migration, paly a role in organization of blood vessels and gene expression of monocytes and macrophages. While VEGFR2 play a major role in mitogenesis, differentiation of endothelail cells, promotion of cell migration and enhancement of vascular permeability.
Therapeutic angiogenesis
Therapeutic angiogenesis is the clinical use of methods to enhance or promote the development of collateral blood vessels in ischemic tissue. Angiogenic treatment is potentially important as we see more patients that are having ischmia or vascular insufficiency that are not amenable to revascularization. This new form of treatment is an alternative to high risk percutaneous coronary intervention or coronary artery bypass surgery. It can also be used in combination with surgery to provide more complete revascularization.
The ideal agent for therapeutic angiogenesis should have a potent effect, with sustained clinical benefit [64]. It should be specific for the targeted ischemic tissue, with no chronic side effects. It should maintain a high local concentration and adequate exposure time. It should be that the ability of readministration feasible and non invasive way of delivering the agent should be considered.
There are three major ways to promote angiogenesis: protein therapy, gene therapy and cellular therapy.
1. Protein therapy
angiogenesis can be achieved by growth factor proteins. These factors include VEGF, bFGF through different routes [65]. There are conflicting results reported in the literature as to the efficacy of these agents [66]. The argument in favor of protein therapy are Finite temporal exposure to angiogenic factor, titratable dosing of angiogenic factor exposure, no exposure to exogenous genetic material, no exposure to viral vector, readministration may be easier; decreased risks of inflammatory response or immune inactivation at the time of repeat dosing, modulation of protein structure and/or combination with slow- release delivery system may abrogate issue of protein stability.
The argument against Protein Therapy is that these proteins have short serum half life and so tissue half life and that would lead to the need of frequent administration and higher doses.
2. Gene therapy
Gene therapy is known as any manipulation of gene expression or function for the treatment of specific disease [66–68]. The advantages of gene therapy are, sustained production of angiogenic factor resulting in prolonged exposure to elevated level, the ability for local delivery and so less systemic exposure. This all can be done with one dose as well and the production of angiogenic factor can be directed to specific cell type and so less side effects.
The disadvantages for gene therapy include:
-
Introduction of foreign genetic material.
-
Exposure to viral vectors with concomitant risk of inflammatory response, viral persistence, and in vivo recombination.
-
Potential for non- targeted cell gene delivery.
-
Inability to precisely modulate gene expression and thus angiogenic factor levels with present vector system.
-
Potential for inactivation and/or inflammatory response at readministration.
-
Potential for long term, low level systemic exposure to secreted angiogenic factors.
-
Vascular malformation in the sense of hemangioma formation[71–73]
-
Neoplasm: there is a raise in concern regarding stimulating carcinogenesis[41].
-
Edema: It has been shown that the presence of high VEGF levels lead to augmentation of vascular permeability [76, 77].
3. Cellular therapy
this is a new approach in which cells that are known to produce angiogenic factors are used to promote angiogenesis in ischemic tissue [78]. Many cell types have been used for this purpose as monocytes and endothelial progenator cells and marrow stromal cells. These cells have been shown to stimulate angiogenesis through expression of growth factors stimulated by local paracrine stimuli from ischemia. These cells can also participate as cellular precursors for vasculogenesis and they can act as a vehicle for delivery of therapeutic genes coding for angiogenic factors.
Clinical trials in therapeutic angiogenesis
The results from the preclinical studies about the growing new blood vessels in the ischemic areas in the heart and the peripheral parts of the body had led to several clinical trials which are mainly phase 1 trials investigating the efficacy of different routs of delivery of protein, gene or cellular therapy.
Few studies were carried out by injecting VEGF protein through intracoronary and intravenous routs in different dose scales and showed improvement in symptoms and in perfusion [79, 80]. The problem came with it was the results were dose dependent and hypotension was quite a clear complication. The VIVA study was a randomized double blinded, placebo controlled study that showed no difference between the two groups in improvement of symptoms, quality of life And exercise tolerance [81]. The FGF-2 protein was also studied using intracoronary and intravenous route in no option patients. Laham et al had studied in fifty two patients and they received intracoronary protein and there was a significant improvement in quality of life and exercise time and the infracted defect size, but the control group was lacking in this study [82]. The FIRST trial was a multicentric randomized double blinded placebo controlled trial and was designed to examine the safety, pharmacokinetics and efficacy of FGF-2 protein but did not show any improvement in exercise tolerance or myocardial perfusion.
As for the gene therapy, few studies with small numbers of patients were published till the Angiogenic Gene Therapy trial (AGENT) with intracoronary infusion of adenovirus encoding FGF-2. This study stopped enrollment when the interim analysis showed no possibility of detected efficacy. even though the subgroup analysis with the exercise tolerance test of less then 10 minutes did show an improvement [83].
For the cellular therapy, a number of clinical trials for the endothelial progenitor cells therapy were published in the last few years. Most of those studies had similar results which showed a 7–9% improvement in global left ventricular function and remodeling of left ventricle [84]. Other studies for the intramyocardial percutanouse delivery of autologus bone marrow had shown that this procedure is safe and it improved Angina symptoms and improve myocardial perfusion and increase left ventricular function[85–87]. Long term studies are not available.
Conclusion
Therapeutic angiogenesis seems to be the promising hope for patients with advanced vascular diseases not responding to the conventional treatment. The different methods that have been approached in the last two decades keep us with hope and caution. Couscous measures have to be taken into consideration in applying any the treatment options as the unwanted side effects can be prominent. The present trials in the literature right now do not have the full answer and more trials will be needed in order to document these methods as routine alterative methods. Till then we should stick with our conventional methodology whenever it is feasible.
References
Walting J, Christ J: Emberyonic angiogenesis: a review. Naturwissenschaften. 1996, 83: 153-164. 10.1007/BF01143056.
Gibbons GH, Dzau VJ: The emerging concept of vascular remodeling. New Eng J Med. 1994, 330: 1431-1438. 10.1056/NEJM199405193302008.
Liotta LA, Steeg PS, Stetler-Stevenson WG: Cancer Metastesis Angiogenesis. An imbalance of positive and negative regulation. Cell. 1991, 64: 327-336. 10.1016/0092-8674(91)90642-C.
Adamis AP, Miller JW, Bernal MT: Increased vascular endothelial growth factor in the vitreous of eyes with proliferative diabetic retinopathy. Am J Oph. 1994, 118: 445-50.
Folkman J: Angiogenesis in cancer, vascular, rheumatoid and other diseases. Nat Med. 1995, 1: 27-31. 10.1038/nm0195-27.
Risau W, Flamme L: Vasculogenesis. Ann Rev Cell Div Biology. 1995, 11: 73-91. 10.1146/annurev.cb.11.110195.000445.
Murasawa S, Asahara T: Endothelial Progenitor Cells for Vasculogenesis. Physiology. 2005, 20: 36-42. 10.1152/physiol.00033.2004.
Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM: Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999, 85: 221-8.
Carmeliet P: Mechanisms of angiogenesis and arteriogenesis. Nature Med. 2000, 6: 389-395. 10.1038/74651.
Ausprunk DH, Folkman J: Migration and proliferation of endothelial cells in preformed and newly formed blood vessels during tumor angiogenesis. Microvasc Res. 1997, 14: 53-65. 10.1016/0026-2862(77)90141-8.
Gross JL, Moscatelli D, Rifkin DB: Increased capillary endothelial cell protease activity in response to angiogenic stimuli in vitro. Proc Nat Acad Sci USA. 1983, 80: 2623-2627. 10.1073/pnas.80.9.2623.
Buschmann I, Schaper W: Arteriogenesis versus angiogenesis: Two mechanisms of vessels growth. News Physiol Sci. 1999, 14: 121-125.
Moulton K, Folkman J: Molecular Basis of Cardiovascular Disease. Edited by: Chien K. 1998, Saunders WB, 393-409.
Tomanek RJ: Exercise-induced coronary angiogenesis: A review. Med Sci Sports Exer. 1994, 26: 1245-1251.
Hudlicka O, Brown M, Eggenton S: Angiogenesis in skeletal and cardiac muscles. Physiol Rev. 1992, 72: 369-417.
Hudlicka O, Brown MD, Walter H, Weiss JB, Bate A: Factors involved in capillary growth in the heart. Mol Cell Biochem. 1995, 147: 57-68. 10.1007/BF00944784.
Gibaldi M: Regulating angiogenesis: A new therapeutic strategy. J Clin Pharma. 1998, 38: 898-903.
Schiper W, Ito WD: Molecular mechanism of coronary collateral vessel growth. Circ Res. 1996, 79: 911-919.
Nagel T, Resnick N, Atkinson WJ, Dewey CF, Gimbrone MA: Shear stress selectively upregulate intracellular adhesion molecule-1 expression in cultured human vascular endotherlial cells. J Clin Invest. 1994, 94: 885-891.
Clauss M, Gerlach M, Gerlach H, Brett J, Wang F, Familletti PC, Pan YC, Olander JV, Connolly DT, Stern D: Vascular permeability factor: A tumor-derived polypeptide that induces endothelial cell and monocyte procoagulant activity and promotes monocyte migration. J Exp Med. 1990, 172: 1535-45. 10.1084/jem.172.6.1535.
Li J, Hampton T, Morgan JP, Simons M: Stretch induced VEGF expression in the heart. J Clin Invest. 1997, 100: 18-24.
Ando J, Kamiya A: Flow independent regulation of the gene expression in vascular endothelial cells. Jap Heart J. 1996, 37: 19-32.
Kuwabara K, Ogawa S, Matsumoto M: Hypoxia mediated induction of acidic basic fibroblast growth factor and platlet-derived growth factor in mono nuclear phagocytes stimulates growth of hypoxic endothelial cells. Proc Nat Acad Sci USA. 1995, 92: 4606-4610. 10.1073/pnas.92.10.4606.
Shweiki D, Itin A, Soffer D, Keshet E: Vascular endothelial growth factor may mediate hypoxic initiated angiogenesis. Nature. 1992, 359: 843-845. 10.1038/359843a0.
Namiki A, Brogi E, Kearney M: Hypoxia induces vascular endothelial growth factor in cultured human endothelial cells. J Biol Chem. 1995, 270: 31189-31195. 10.1074/jbc.270.52.31189.
Semenza GL, Agani F, Iyer N: Regulation of cardiovascular development and physiology by hypoxia-inducible factor 1. Ann NY Acad Sci. 1999, 874: 262-268. 10.1111/j.1749-6632.1999.tb09241.x.
Li J, Brown LF, Hibberd MG, Grossman JD, Morgan JP, Simons M: VEGFflk-1 and flt-1 expression in a rat myocardial infarction model of angiogenesis. Am J Physiol. 1996, 270: H1803-1811.
Tuder RM, Flook BE, Voelkle NF: Increased gene expression for VEGF and VEGF receptors KDR/Flk and flt in lungs exposed to acute or to chronic hypoxia. Modulation of gene expression by nitric oxide. J Clin Invest. 1995, 95: 1798-1807.
Murohara T, Horowitz JR, Silver M, Tsurumi Y, Chen D, Sullivan A, Isner JM: Vascular endothelial growth factor/vascular permeability factor. 1998, 97: 99-107.
Ziche M, Morbidelli L, Choudhuri R, Zhang HT, Donnini S, Granger HJ, Bicknell R: Nitric oxide synthase lies downstream from vascular endothelial growth factor-induced but not basic fibroblast growth factor-induced angiogenesis. J Clin Invest. 1997, 99: 2625-34.
Miyazaki H, Matsuoka H, Cooke JP, Usui M, Ueda S, Okuda S, Imaizumi T: Endogenous nitric oxide synthase inhibitor: A novel marker of atherosclerosis. Circulation. 1999, 99: 1141-6.
Tsurumi Y, Murohara T, Krasinski K, Chen D, Witzenbichler B, Kearney M, Couffinhal T, Isner JM: Reciprocal relation between VEGF and NO in the regulation of endothelial integrity. Nat Med. 1997, 3: 879-86. 10.1038/nm0897-879.
Sone H, Kawakami Y, Okuda Y, Kondo S, Hanatani M, Suzuki H, Yamashita K: Vascular endothelial growth factor is induced by long-term high glucose concentration and up-regulated by acute glucose deprivation in cultured bovine retinal pigmented epithelial cells. Biochem Biophys Res Com. 1996, 221: 193-8. 10.1006/bbrc.1996.0568.
Satake S, Kuzuya M, Miura H, Asai T, Ramos MA, Muraguchi M, Ohmoto Y, Iguchi X: Up-regulation of vascular endothelial growth factor in response to glucose deprivation. Biol Cell. 1998, 90: 161-8. 10.1016/S0248-4900(98)80337-7.
Park SH, Kim KW, Lee YS, Baek JH, Kim MS, Lee YM, Lee MS, Kim YJ: Hypoglycemia-induced VEGF expression is mediated by intracellular Ca2+ and protein kinase C signaling pathway in HepG2 human hepatoblastoma cells. Int J Mol Med. 2001, 7: 91-6.
Kim NH, Jung HH, Yoon JW: Expression of vascular endothelial growth factor in response to high glucose in rat mesangial cells. J Endo. 2000, 165: 617-624.
Hoshi S, Nomoto Ki, Kuromitsu J: High glucose induced VEGF expression via PKC and ERK in glomular podocytes. Biochem Biophys Res Com. 2002, 290: 177-184. 10.1006/bbrc.2001.6138.
Sunderkotter C, Beil W, Roth J, Sorg C: Cellular events associated with inflammatory angiogenesis in the mouse cornea. Am J Pathol. 1991, 138: 931-939.
Sunderkotter C, Goebeler M, Schulze-Othoff K, Bhardwaj R: Macrophage-derived angiogenesis factors. Pharmacol Ther. 1991, 51: 195-216. 10.1016/0163-7258(91)90077-Y.
Folkman J, Shing Y: Angiogenesis. J Biol Chem. 1992, 267: 10931-10934.
Folkman J: Tumor angiogenesis: Therapuitic implications. New Eng J Med. 1971, 285: 1182-1186.
Friesel RE, Maciag T: Molecular mechanism of angiogenesis: Fibroblast growth factor signal transducing. FASEB J. 1995, 9: 919-925.
Folkman J, Klagsbrun M: Angiogenic factors. Science. 1987, 235: 442-447. 10.1126/science.2432664.
Wadzinski MG, Folkman J, Sasse J, Devey K, Ingber D, Klagsbrun M: Heparin-binding angiogenesis factors detection by immunological methods. Clin Phys Biochem. 1987, 5: 200-9.
Jaye M, Howk R, Burgess W, Ricca GA, Chiu IM, Ravera MW, O'Brien SJ, Modi WS, Maciag T, Drohan WN: Human endothelial cell growth factor: Cloning, nucleotide sequence, and chromosome localization. Science. 1986, 233: 541-5. 10.1126/science.3523756.
Imamura T, Engleka K, Zhan X: Recovery of mitogenic activity of a growth factor mutant with a nuclear translocation sequence. Science. 1990, 49: 1567-1570. 10.1126/science.1699274.
Banai S, Jaklitsch MT, Casscells W, Shou M, Shrivastav S, Correa R, Epstein SE, Unger EF: Effects of acidic fibroblast growth factor on normal and ischemic myocardium. Circ Res. 1991, 69: 76-85.
Engelman GL, Dionne CA, Jaye MC: Acidic fibroblast growth factor and heart development. Role in myocytes proliferation and capillary angiogenesis. Cir Res. 1993, 72: 7-19.
Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF: Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983, 219: 983-5. 10.1126/science.6823562.
Ferrara N, Houk K, Jackman L: Molecular and biological properties of vascular endothelial growth factor family of proteins. Endocr Rev. 1992, 13: 18-32. 10.1210/er.13.1.18.
Tischer E, Gospodarowicz D, Mitchell R, Silva M, Schilling J, Lau K, Crisp T, Fiddes JC, Abraham JA: Vascular endothelial growth factor: A new member of the platelet-derived growth factor gene family. Biochem Biophys Res Com. 1989, 165: 1198-206. 10.1016/0006-291X(89)92729-0.
Carmeliet P, Collen D: Molecular analysis of blood vessel formation and disease. Am J Physiol. 1997, 273: H2091-104.
Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z: Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999, 13: 9-22.
Cao Y, Ji WR, Qi P, Rosin A, Cao Y: Placenta growth factor: Identification and characterization of a novel isoform generated by RNA alternative splicing. Biochem Biophys Res Com. 1997, 235: 493-8. 10.1006/bbrc.1997.6813.
Takahashi A, Sasaki H, Kim SJ, Tobisu K, Kakizoe T, Tsukamoto T, Kumamoto Y, Sugimura T, Terada M: Markedly increased amounts of messenger RNAs for vascular endothelial growth factor and placenta growth factor in renal cell carcinoma associated with angiogenesis. Cancer Res. 1994, 54: 4233-7.
Cao Y, Linden P, Shima D, Browne F, Folkman J: In vivo angiogenic activity and hypoxia induction of heterodimers of placenta growth factor/vascular endothelial growth factor. J Clin Invest. 1996, 98: 2507-11.
Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, Ryan TE, Bruno J, Radziejewski C, Maisonpierre PC, Yancopoulos GD: Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996, 87: 1161-9. 10.1016/S0092-8674(00)81812-7.
Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD: Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997, 277: 55-60. 10.1126/science.277.5322.55.
Koblizek TI, Weiss C, Yancopoulos GD, Deutsch U, Risau W: Angiopoietin-1 induces sprouting angiogenesis in vitro. Curr Biol. 1998, 8: 529-32. 10.1016/S0960-9822(98)70205-2.
Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD: Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996, 87: 1171-80. 10.1016/S0092-8674(00)81813-9.
Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M: Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998, 92: 735-45. 10.1016/S0092-8674(00)81402-6.
Li J, Perrella MA, Tsai JC, Yet SF, Hsieh CM, Yoshizumi M, Patterson C, Endege WO, Zhou F, Lee ME: Induction of vascular endothelial growth factor gene expression by interleukin-1 beta in rat aortic smooth muscle cells. J Biol Chem. 1995, 270: 308-12. 10.1074/jbc.270.1.308.
Warren RS, Yuan H, Matli MR, Ferrara N, Donner DB: Induction of vascular endothelial growth factor by insulin-like growth factor 1 in colorectal carcinoma. J Biol Chem. 1996, 271: 29483-8. 10.1074/jbc.271.46.29483.
Brindle NP, McCarthy MJ, Bell PR: Angiogenic revascularisation in ischaemic disease. Molecular techniques hold promise, though they are still some way off. BMJ. 1999, 318: 1500-1.
Post MJ, Laham R, Sellke FW, Simons M: Therapeutic angiogenesis in cardiology using protein formulations. Cardiovasc Res. 2001, 49: 522-31. 10.1016/S0008-6363(00)00216-9.
Simons M, Bonow RO, Chronos NA, Cohen DJ, Giordano FJ, Hammond HK, Laham RJ, Li W, Pike M, Sellke FW, Stegmann TJ, Udelson JE, Rosengart TK: Clinical trials in coronary angiogenesis: issues, problems, consensus: An expert panel summary. Circulation. 2000, 102: E73-86.
Isner JM, Pieczek A, Schainfeld R, Blair R, Haley L, Asahara T, Rosenfield K, Razvi S, Walsh K, Symes JF: Clinical evidence of angiogenesis after arterial gene transfer of phVEGF165 in patient with ischaemic limb. Lancet. 1996, 348: 370-4. 10.1016/S0140-6736(96)03361-2.
Mühlhauser J, Jones M, Yamada I, Cirielli C, Lemarchand P, Gloe TR, Bewig B, Signoretti S, Crystal RG, Capogrossi MC: Safety and efficacy of in vivo gene transfer into the porcine heart with replication-deficient, recombinant adenovirus vectors. Gene Ther. 1996, 3: 145-53.
Inoue M, Itoh H, Ueda M, Naruko T, Kojima A, Komatsu R, Doi K, Ogawa Y, Tamura N, Takaya K, Igaki T, Yamashita J, Chun TH, Masatsugu K, Becker AE, Nakao K: Vascular endothelial growth factor (VEGF) expression in human coronary atherosclerotic lesions: Possible pathophysiological significance of VEGF in progression of atherosclerosis. Circulation. 1998, 98: 2108-16.
Celletti FL, Waugh JM, Amabile PG, Brendolan A, Hilfiker PR, Dake MD: Vascular endothelial growth factor enhances atherosclerotic plaque progression. Nat Med. 2001, 7: 425-9. 10.1038/86490.
Springer ML, Chen AS, Kraft PE, Bednarski M, Blau HM: VEGF gene delivery to muscle: potential role for vasculogenesis in adults. Mol Cell. 1998, 2: 549-58. 10.1016/S1097-2765(00)80154-9.
Lee RJ, Springer ML, Blanco-Bose WE, Shaw R, Ursell PC, Blau HM: VEGF gene delivery to myocardium: deleterious effects of unregulated expression. Circulation. 2000, 102: 898-901.
Schwarz ER, Speakman MT, Patterson M, Hale SS, Isner JM, Kedes LH, Kloner RA: Evaluation of the effects of intramyocardial injection of DNA expressing vascular endothelial growth factor (VEGF) in a myocardial infarction model in the rat: Angiogenesis and angioma formation. J Am Coll Cardiol. 2000, 35: 1323-30. 10.1016/S0735-1097(00)00522-2.
Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA, Park JE: Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994, 331: 1480-7. 10.1056/NEJM199412013312203.
Miller JW, Adamis AP, Shima DT, D'Amore PA, Moulton RS, O'Reilly MS, Folkman J, Dvorak HF, Brown LF, Berse B: Vascular endothelial growth factor/vascular permeability factor is temporally and spatially correlated with ocular angiogenesis in a primate model. Am J Pathol. 1994, 145: 574-84.
Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF: Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983, 219: 983-5. 10.1126/science.6823562.
Dvorak HF, Nagy JA, Feng D, Brown LF, Dvorak AM: Vascular permeability factor/vascular endothelial growth factor and the significance of microvascular hyperpermeability in angiogenesis. Curr Top Micro Imm. 1999, 237: 97-132.
Suzuki K, Murtuza B, Smolenski RT, Sammut IA, Suzuki N, Kaneda Y, Yacoub MH: Cell transplantation for the treatment of acute myocardial infarction using vascular endothelial growth factor-expressing skeletal myoblasts. Circulation. 2001, 104: I207-12. 10.1161/hc3601.093987.
Hendel RC, Henry TD, Rocha-Singh K, Isner JM, Kereiakes DJ, Giordano FJ, Simons M, Bonow RO: Effect of intracoronary recombinant human vascular endothelial growth factor on myocardial perfusion: evidence for a dose-dependent effect. Circulation. 2000, 101: 118-121.
Henry TD, Rocha-Singh K, Isner JM, Kereiakes DJ, Giordano FJ, Simons M, Losordo DW, Hendel RC, Bonow RO, Eppler SM, Zioncheck TF, Holmgren EB, McCluskey ER: Intracoronary administration of recombinant human vascular endothelial growth factor to patients with coronary artery disease. Am Heart J. 2001, 142: 872-880. 10.1067/mhj.2001.118471.
Henry TD, Annex BH, McKendall GR, Azrin MA, Lopez JJ, Giordano FJ, Shah PK, Willerson JT, Benza RL, Berman DS, Gibson CM, Bajamonde A, Rundle AC, Fine J, McCluskey ER: The VIVA trial: Vascular endothelial growth factor in Ischemia for Vascular Angiogenesis. Circulation. 2003, 107: 1359-1365. 10.1161/01.CIR.0000061911.47710.8A.
Laham RJ, Chronos NA, Pike M, Leimbach ME, Udelson JE, Pearlman JD, Pettigrew RI, Whitehouse MJ, Yoshizawa C, Simons M: Intracoronary basic fibroblast growth factor (FGF-2) in patients with severe ischemic heart disease: results of a phase I open-label dose escalation study. J Am Coll Cardiol. 2000, 36: 2132-2139. 10.1016/S0735-1097(00)00988-8.
Grines C, Watkins M, Helmer G: Angiogenic Gene Therapy Trial (Agent) inpatients with stable angina pectoris. Circulation. 2002, 105: 1291-1297. 10.1161/hc1102.105595.
Caplice NM: The future of cell therapy for acute myocardial infarction. Nature Clinical Practice and Cardiovascular Medicine. 2006, 3 (Suppl 1): S129-S132. 10.1038/ncpcardio0432.
Kajiguchi M, Kondo T, Izawa H, Kobayashi M, Yamamoto K, Shintani S, Numaguchi Y, Naoe T, Takamatsu J, Komori K, Murohara T: Safety and efficacy of autologous progenitor cell transplantation for therapeutic angiogenesis in patients with critical limb ischemia. Circ J. 2007, 71 (2): 196-201. 10.1253/circj.71.196.
Beeres SL, Bax JJ, Kaandorp TA, Zeppenfeld K, Lamb HJ, Dibbets-Schneider P, Stokkel MP, Fibbe WE, de Roos A, van der Wall EE, Schalij MJ, Atsma DE: Usefulness of intramyocardial injection of autologous bone marrow-derived mononuclear cells in patients with severe angina pectoris and stress-induced myocardial ischemia. Am J Cardiol. 2006, 97 (9): 1326-31. 10.1016/j.amjcard.2005.11.068.
Briguori C, Reimers B, Sarais C, Napodano M, Pascotto P, Azzarello G, Bregni M, Porcellini A, Vinante O, Zanco P, Peschle C, Condorelli G, Colombo A: Direct intramyocardial percutaneous delivery of autologous bone marrow in patients with refractory myocardial angina. Am Heart J. 2006, 151 (3): 674-80. 10.1016/j.ahj.2005.04.033.
Acknowledgements
the authors report no financial interest related to this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors' contributions
HA: Acquisition of data, design of audit, Literature search and preparation of draft of manuscript
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Al Sabti, H. Therapeutic angiogenesis in cardiovascular disease. J Cardiothorac Surg 2, 49 (2007). https://doi.org/10.1186/1749-8090-2-49
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1749-8090-2-49