Abstract
Background
Ischemic mitral regurgitation often complicates severe ischemic heart disease and adversely affects the prognosis in these patients. There is wide variation in the clinical spectrum of ischemic mitral regurgitation due to varying location and chronicity of ischemia and anomalies in annular and ventricular remodeling. As a result, there is lack of consensus in treating these patients. Treatment has to be individualized for each patient. Most of the available surgical options do not consistently correct this condition in all the patients. Chordal cutting is one of the newer surgical approaches in which cutting a limited number of critically positioned basal chordae have found success by relieving the leaflet tethering and thereby improving the coaptation of leaflets. Three-dimensional echocardiography is a potentially valuable tool in identifying the specific pattern of tethering and thus the suitability of this procedure in a given clinical scenario.
Case Presentation
A 66-year-old man with cardiomyopathy and ischemic mitral regurgitation presented to us with the features of congestive heart failure. The three-dimensional echocardiography revealed severe mitral regurgitation associated with the tethering of the lateral (P1) and medial (P3) scallops of the posterior leaflet of the mitral valve due to secondary chordal attachments. The ejection fraction was only 15% with severe global systolic and diastolic dysfunction. Mitral regurgitation was successfully corrected with mitral annuloplasty and resection of the secondary chordae tethering the medial and lateral scallops of the posterior leaflet of the mitral valve.
Conclusion
Cutting the second order chordae along with mitral annuloplasty could be a novel method to remedy Ischemic mitral regurgitation by relieving the tethering of the valve leaflets. The preoperative three-dimensional echocardiography should be considered in all patients with Ischemic mitral regurgitation to assess the complex three-dimensional interactions between the mitral valve apparatus and the left ventricle. This aids in timely surgical planning.
Similar content being viewed by others
Background
Ischemic Mitral regurgitation (IMR) continues to be a complex surgical problem. Choosing the most optimal therapy remains a challenging issue in cardiac surgery. There is wide variation in the clinical spectrum of IMR due to varying location and chronicity of ischemia and anomalies in annular and ventricular remodeling. As a result, there is lack of consensus in treating these patients. Treatment has to be individualized for each patient. Three-dimensional (3-D) echocardiography is a potentially valuable tool in gaining the mechanistic insights into the pathogenesis of IMR by precise evaluation of the complex three-dimensional interactions between the mitral valve apparatus and the left ventricle. Noting the exact geometric perturbations in each individual clinical setting will enable us in the rational design of the specific repair methods and in making the choice amongst the different techniques and devices that are currently available. We present a case of severe IMR in which ring annuloplasty combined with chordal cutting was planned based on the findings on 3-D echocardiography with a good result.
Case Presentation
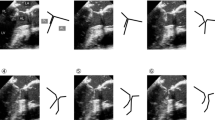
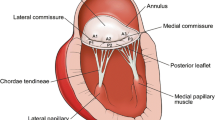
A 66-year-old African-American male with cardiomyopathy and IMR, presented to the emergency room with a 3 day history of breathlessness and lower extremity edema. Chest radiography displayed marked cardiomegaly. A 2-dimensional echocardiography demonstrated severe (3–4+) mitral regurgitation, moderately severe aortic valve insufficiency, tricuspid regurgitation (3+) with moderate pulmonary hypertension (right ventricular systolic pressure was 58 mm of Hg). The LV was markedly dilated with the end diastolic diameter of 6.7 cm and end systolic diameter of 6.0 cm. The ejection fraction was only 15% with severe global systolic and diastolic dysfunction of the LV. Cardiac catheterization showed triple vessel disease with severe diffuse stenosis of the right coronary artery, left coronary artery and first diagonal artery. Intra-operatively, transesophageal 3-D echocardiography (TEE) was performed by multiplane TEE probe using Sonos® 7500 (Philips Medical Systems, N.A, Bothel, WA, USA). The 3-D processing was performed with 4-D Cardio-view®1.3 (TomTec Imaging Systems, Munich, Germany). The three-dimensional left ventricular model was generated. It produced a shell reconstruction of the LV allowing to calculate total and segmental left ventricular volumes during the cardiac cycle. The end systole frame was determined as frame with a closed aortic valve just prior to the opening of the mitral valve. This time point was used to make all valve measurements. Frame by frame analysis yielded the valvular area, circumference, commissure-to-commissure (c-c) diameter, antero-posterior (a-p) diameter (Figure 1) and mitral annular volume (measured from annulus to cusp) (Figure 2). The 3-D reconstruction of the mitral annular volume showed tethering of the lateral scallop (P1) and medial scallop (P3) of the posterior leaflet of the mitral valve due to the secondary chordal attachments. There was an eccentric jet due to inadequate apposition of the mitral leaflets. (Figure 3).
The surgical plan was to increase the zone of coaptation by chordal cutting to relieve the P1 and P3 tethering by the secondary chordae and performing a mitral annuloplasty. Coronary revascularization was performed along with the aortic valve replacement with a bioprosthetic valve, resection of the secondary chordae tethering the P1 and P3 scallops, mitral annuloplasty with a saddle ring and closure of a patent foramen ovale. In addition to intra-operative visualization and testing, the site of chordal resection was determined by the 3-D echocardiographic analysis of the tethered portions of the posterior leaflet of the mitral valve. At the conclusion of the operation and in the follow up period echocardiography demonstrated a competent mitral valve. There was a significant decrease in the mitral valvular area, circumference, commissure-to-commissure (c-c) diameter, antero-posterior (a-p) diameter (Figure 4) and mitral annular volume (Figure 5) post operatively.
The numerical data comparing the pre- and post-operative measurements of mitral valve is presented in Table 1.
Discussion
Ischemic mitral regurgitation (IMR) often complicates severe coronary artery disease and has a negative impact on the long term survival of these patients. Several anatomical and pathophysiological changes play a role in the genesis of IMR. The initiating event is an ischemic insult to the three dimensional interaction between the leaflets, annulus, subvalvular apparatus and left ventricular wall. The remodeling of the ischemic left ventricle results in increase in the sphericity index [1, 2], mitral annular dilatation [3, 4], and outward displacement of the papillary muscles with the subsequent tethering and tenting of the mitral leaflets into the left ventricle [5–8]. All these have been implicated in the pathogenesis of IMR. In the setting of left ventricular systolic dysfunction, there is also reduction in the force available to close the leaflets in opposition to the increased tethering which further complicates the IMR [9–11]. Mitral regurgitation in turn leads to a vicious cycle of volume overload, with further progression of annular dilation, LV remodeling and increased severity of symptoms of congestive heart failure. Mitral regurgitation is an independent predictor of mortality and adversely affects the survival and functional status [12–15].
Normal mitral valve has a unique morphology with a non-planner saddle shape and curved mitral leaflets. [16–18]. The 3-dimensional configuration of the curved mitral annulus is difficult to investigate using conventional 2-D echocardiography as the images represent a single plane which requires mental reconstruction of the 2-dimensional views [16, 17, 19]. Characterization of the mitral valve apparatus using 3-D echocardiography has shed a new light on the pathophysiology of mitral regurgitation [5–9], [20–23]. One of the important predictors of IMR is tenting of the mitral valve [5–9, 24]. Tenting results from tethering of the leaflets, which is an end result of the perturbation in the complex three-dimensional interactions between the mitral valve apparatus and the left ventricle. The accuracy and variability of conventional 2-D echocardiography in these patients is often limited as it requires geometrical assumptions of 2-dimensional views. As a result some spatial relations and different structural features can be perceived erroneously even by the experienced echocardiographers. This would call for an unexpected modification in the technique of repair in the operating room, where the surgeon is challenged by limited time, operating field and non-physiological condition of the heart being devoid of blood which does not mimic the normal hemodynamic situation. In this clinical setting, the 3-D echocardiography is a potentially valuable tool as it creates images, resembling the true anatomy of the heart. By choosing a cutting plane and reconstructing the image beyond this plane the heart can be opened as if by a surgeon. This enhances the ability to assess the spatial relations between cardiac structures and also the specific tethering patterns.
Remodeling anunuloplasty using an undersized ring is the standard therapeutic approach for MR. It reduces antero-posterior valve dimensions by bringing the posterior annulus forward thus increases coaptating surface of the leaflets [1, 25–27]. However, several studies have demonstrated that MR can persist or recur even after ring annuloplasty. This could be due to the fact that annuloplasty doesn't sufficiently address the tethering of the leaflets by the remodeled ventricle [28–31]. Chordal cutting, papillary muscle imbrication in combination with LV volume reduction, Septal anterior ventricular exclusion (SAVE), papillary muscle sling, ventricular reconstruction, pericardial patch enlargement of the restricted PML are some of the strategies that have shown success in limited number of patients [32, 33]. Chordal cutting approaches championed by Messas et al [34] have shown promising results in several experimental and clinical studies [32, 34–36]. In these studies, cutting a limited number of critically positioned basal chordae has shown to improve the coaptation by relieving the leaflet tethering and thereby reduce chronic persistent IMR even in the remodeled ventricle. Subsequently, this therapeutic option was challenged by another experimental study by Rodriguez et al [37] in which the chordal cutting did not relieve IMR. This controversy could be better understood by examining the specific valvular geometry through 3-D echocardiography.
Technical limitations of 3-D echocardiography
The 3-D images were actually reconstructed from the multiplanar 2-D images taken over several cardiac cycles. Therefore, 3-D TEE reconstructions depend critically on the quality of the original 2D sectional images. There is a risk of introducing artifacts from patient respiration, motion and irregular movements of the heart during arrhythmias. Analysis was performed off-line and required tedious manual tracing of endocardial borders. The time required for reconstruction is an important factor limiting its applicability in the operating room [38]. However, the past years have seen significant development in transducer technology, allowing pyramidal shaped blocks to be acquired in real time. It is now possible to image an entire ventricle or valve and analyze it online, thus obviating the need for complicated and time-consuming reconstructions. However, Images obtained by Real-time 3-D echocardiography are of poorer quality compared to conventional 2-D echocardiography [21–23, 39].
Conclusion
All patients with IMR should be considered for pre-operative 3D TEE evaluation for timely surgical planning and also for intra-operative 3D TEE imaging and reconstruction to assess the dynamic changes that occur intra-operatively.
References
Filsoufi F, Salzberg SP, Adams DH: Current management of ischemic mitral regurgitation. Mt Sinai J Med. 2005, 72: 105-115.
Sabbah HN, Kono T, Rosman H, Jafri S, Stein PD, Goldstein S: Left ventricular shape: a factor in the etiology of functional mitral regurgitation in heart failure. Am Heart J. 1992, 123: 961-966. 10.1016/0002-8703(92)90703-X.
Boltwood CM, Tei C, Wong M, Shah PM: Quantitative echocardiography of the mitral complex in dilated cardiomyopathy: The mechanism of functional mitral regurgitation. Circulation. 1983, 68: 498-508.
Izumi S, Miyatake K, Beppu S, Park Y, Nagata S, KinoshJta N, Sakakibara H, Nimura Y: Mechanism of mitral regurgitation in patients with myocardial infarction: a study using real-time two-dimensional Doppler flow imaging and echocardiography. Circulation. 1987, 76: 777-785.
Otsuji Y, Handschumacher MD, Schwammenthal E, Jiang L, Song JK, Guerrero JL, Vlahakes GJ, Levine RA: Insights from three-dimensional echocardiography into the mechanism of functional mitral regurgitation: direct in vivo demonstration of altered leaflet tethering geometry. Circulation. 1997, 96: 1999-2008.
Liel-Cohen N, Guerrero JL, Otsuji Y, Handschumacher MD, Rudski LG, Hunziker PR, Tanabe H, Scherrer-Crosbie M, Sulivan S, Levine RA: Design of a new surgical approach for ventricular remodeling to relieve ischemic mitral regurgitation: insights from three-dimensional echocardiography. Circulation. 2000, 101: 2756-2763.
Agricola E, Oppizzi M, Maisano F, De Bonis M, Schinkel AF, Torracca L, Margonato A, Melisurgo G, Alfieri O: Echocardiographic classification of chronic ischemic mitral regurgitation caused by restricted motion according to tethering pattern. Eur J Echocardiogr. 2004, 5: 326-334. 10.1016/j.euje.2004.03.001.
Otsuji Y, Handschumacher MD, Liel-Cohen N, Tanabe H, Jiang L, Schwammenthal E, Guerrero JL, Nicholls LA, Vlahakes GJ, Levine RA: Mechanism of ischemic mitral regurgitation with segmental left ventricular dysfunction: three-dimensional echocardiographic studies in models of acute and chronic progressive regurgitation. J Am Coll Cardiol. 2001, 37: 641-648. 10.1016/S0735-1097(00)01134-7.
He S, Fontaine AA, Schwammenthal E, Yoganathan AP, Levine RA: An integrated mechanism for functional mitral regurgitation: leaflet restriction vs. coapting force. In vitro studies. Circulation. 1997, 96: 1826-1834.
Kaul S, Spotnitz WD, Glasheen WP, Touchstone DA: Mechanism of ischemic mitral regurgitation: An experimental evaluation. Circulation. 1991, 84: 2167-2180.
Dent JM, Spotniz WD, Nolan SP, Jayaweera AR, Glasheen WP, Kaul S: Mechanism of mitral leaflet excursion. Am J Physiol. 1995, 269: H2100-2108.
Trichon BH, Felker GM, Shaw LK, Cabell CH, O'Connor CM: Relation of frequency and severity of mitral regurgitation to survival among patients with left ventricular systolic dysfunction and heart failure. Am J Card. 2003, 91: 538-543. 10.1016/S0002-9149(02)03301-5.
Koelling TM, Aaronson KD, Cody RJ, Bach DS, Armstrong WF: Prognostic significance of mitral regurgitation and tricuspid regurgitation in patients with left ventricular systolic dysfunction. Am Heart J. 2002, 144: 524-529. 10.1067/mhj.2002.123575.
Lamas GA, Mitchell GF, Flaker GC, Smith SC, Gersh BJ, Basta L, Moye L, Braunwald E, Pfeffer MA: Clinical significance of mitral regurgitation after acute myocardial infarction. Survival and Ventricular Enlargement Investigators. Circulation. 1997, 96: 827-833.
Grigioni F, Enriquez-Sarano M, Zehr KJ, Bailey KR, Tajik AJ: Ischemic mitral regurgitation: long-term outcome and prognostic implications with quantitative Doppler assessment. Circulation. 2001, 103: 1759-1764.
Levine RA, Handschumacher MD, Sanfilippo AJ, Hagege AA, Harrigan P, Marshall JE, Weyman AE: Three dimensional echocardiographic reconstruction of the mitral valve, with implications for the diagnosis of mitral valve prolapse. Circulation. 1989, 80: 589-598.
Kaplan SR, Bashein G, Sheehan FH, Legget ME, Munt B, Li XN, Sivarajan M, Bolson EL, Zeppa M, Arch MZ, Martin RW: Three-dimensional echocardiographic assessment of annular shape changes in the normal and regurgitant mitral valve. Am Heart J. 2000, 139: 378-387. 10.1016/S0002-8703(00)90077-2.
Flachskampf FA, Chandra S, Gaddipatti A, Levine RA, Weyman AE, Ameling W, Hanrath P, Thomas JD: Analysis of shape and motion of the mitral annulus in subjects with and without cardiomyopathy by echocardiographic 3-dimensional reconstruction. J Am Soc Echocardiogr. 2000, 13: 277-287. 10.1067/mje.2000.103878.
Macnab A, Jenkins NP, Bridgewater BJM, Hooper TL, Greenhaigh DL, Patrick MR, Ray SG: Three-dimensional echocardiography is superior to multiplane transesophageal echo in the assessment of regurgitant mitral valve morphology. Eur J Echocardiogr. 2004, 5: 212-222. 10.1016/j.euje.2004.01.005.
Kaji S, Nasu M, Yamamuro A, Tanabe K, Nagai K, Tani T, Tamita K, Shiratori K, Kinoshita M, Senda M, Okada Y, Morioka S: Annular geometry in patients with chronic ischemic mitral regurgitation: three-dimensional magnetic resonance imaging study. Circulation. 2005, 112 (9 Suppl): I409-I414.
Watanabe N, Ogasawara Y, Yamaura Y, Kawamoto T, Toyota E, Akasaka T, Yoshida K: Quantitation of mitral valve tenting in ischemic mitral regurgitation by transthoracic real-time three-dimensional echocardiography. J Am Coll Cardiol. 2005, 45: 763-776. 10.1016/j.jacc.2004.11.048.
Watanabe N, Ogasawara Y, Yamaura Y, Kawamoto T, Akasaka T, Yoshida K: Geometric deformity of the mitral annulus in patients with ischemic mitral regurgitation: a real-time three-dimensional echocardiographic study. J Heart Valve Dis. 2005, 14: 447-452.
Watanabe N, Ogasawara Y, Yamaura Y, Wada N, Kawamoto T, Toyota E, Akasaka T, Yoshida K: Mitral annulus flattens in ischemic mitral regurgitation: geometric differences between inferior and anterior myocardial infarction: a real-time 3-dimensional echocardiographic study. Circulation. 2005, 112 (9 Suppl): I458-I462.
Yiu SF, Enriquez-Sarano M, Tribouilloy C, Seward JB, Tajik AJ: Determinants of the degree of functional mitral regurgitation in patients with systolic left ventricular dysfunction: A quantitative clinical study. Circulation. 2000, 102: 1400-1406.
Badhwar V, Bolling SF: Mitral valve surgery in the patient with the left ventricular dysfunction. Semin Thoracic Cardiovasc Surg. 2002, 14: 133-136. 10.1053/stcs.2002.32314.
Szalay ZA, Civelek A, Hohe S, Brunner-LaRocca HP, Klovekorn WP, Knez I, Vogt PR, Bauer EP: Mitral annuloplasty in patients with ischemic versus dilated cardiomyopathy. Eur J Cardiothorac Surg. 2003, 23: 567-572. 10.1016/S1010-7940(02)00864-3.
Gillinov AM, Cosgrove DM, Shiota T, Qin J, Tsujino H, Stewart WJ, Thomas JD, Porqueddu M, White JA, Blackstone EH: Cosgrove-Edwards Annuloplasty System midterm results. Ann Thorac Surg. 2000, 69: 717-721. 10.1016/S0003-4975(99)01543-X.
Hung J, Handschumacher MD, Rudski L, Chow CM, Guerrero JL, Levine RA: Persistence of ischemic mitral regurgitation despite annular ring reduction: mechanistic insights from 3D echocardiography. Circulation. 1999, 100: I-73-
Calafiore AM, Gallina S, DiMauro M, Gaeta F, Iaco AL, D'Allesandro S, Mazzei V, Di Giammarco G: Mitral valve procedure in dilated cardiomyopathy: repair or replacement. Ann Thorac Surg. 2001, 71: 1146-1152. 10.1016/S0003-4975(00)02650-3.
Tahta SA, Oury JH, Maxwell JM, Hiro SP, Duran CM: Outcome after mitral valve repair for functional ischemic mitral regurgitation. J Heart Valve Dis. 2002, 11: 11-18.
Hung J, Papakostas L, Tahta SA, Hardy BG, Bollen BA, Duran CM, Levine RA: Mechanism of Recurrent Ischemic Mitral Regurgitation After Annuloplasty: Continued LV Remodeling as a Moving Target. Circulation. 2004, 85-11. Suppl 1
Terai H, Tao K, Sakata R: Surgical treatment for ischemic mitral regurgitation: strategy for a tethered valve. Ann Thorac Cardiovasc Surg. 2005, 11: 288-292.
Dobre M, Koul B, Roger A: Anatomic and physiologic correction of the restricted posterior mitral leaflet motion in chronic ischemic mitral regurgitation. J Thorac Cardiovasc Surg. 2000, 120: 409-411. 10.1067/mtc.2000.106521.
Messas E, Pouzet B, Touchot B, Guerrero JL, Vlahakes GJ, Desnos M, Menasche P, Hagege A, Levine RA: Efficacy of chordal cutting to relieve chronic persistent ischemic mitral regurgitation. Circulation. 2003, 108 (Suppl 1): II111-II115.
Wakiyama H, Okada Y, Kitamura A, Tsuda S, Shomura Y, Shinkai M, Fujiwara H, Handa N, Nasu M, Tanabe K, Tani T, Morioka S: Chordal cutting for the treatment of ischemic mitral regurgitation: two case reports. J Cardiol. 2004, 44: 113-117. 10.1159/000076864.
Yamamoto H, Iguro Y, Sakata R, Arata K, Yotsumoto G: Effectively treating ischemic mitral regurgitation with chordal cutting in combination with ring annuloplasty and left ventricular reshaping approach. J Thorac Cardiovasc Surg. 2005, 130: 589-590. 10.1016/j.jtcvs.2005.04.006.
Rodriguez F, Langer F, Harrington KB, Tibayan FA, Zasio MK, Liang D, Daughters GT, Ingels NB, Miller DC: Cutting second-order chords does not prevent acute ischemic mitral regurgitation. Circulation. 2004, 91-97. Suppl 1
Fabricius Alexander, Walther Thomas, Falk Volkmar, Friedrich W: Three-Dimensional Echocardiography for Planning of Mitral Valve Surgery: Current Applicability. Ann Thorac Surg. 2004, 78: 575-578. 10.1016/j.athoracsur.2003.10.031.
Shiota T, McCarthy PM, White RD, Qin JX, Greenberg NL, Flamm SD, Wong J, Thomas JD: Initial clinical experience of real-time three-dimensional echocardiography in patients with ischemic and idiopathic dilated cardiomyopathy. Am J Cardiol. 1999, 84: 1068-1073. 10.1016/S0002-9149(99)00500-7.
Acknowledgements
Written consent was obtained from the patient for publication of the study. We have no funding for this study as it is a case report.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
RV contributed to the conception and design of the manuscript, review of literature and drafted the manuscript, TA, NN gave the anesthesia for the case, TA, NN and SS did the echocardiography of the case and its analysis and obtained the 3-D images and revised the manuscript for its important intellectual content, NN gave the final approval of the version to be published, KB participated in the data acquisition and writing the paper along with the first author. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Sai-Sudhakar, C.B., Vandse, R., Armen, T.A. et al. Efficacy of chordal cutting in alleviating ischemic mitral regurgitation: insights from 3-dimensional echocardiography. J Cardiothorac Surg 2, 39 (2007). https://doi.org/10.1186/1749-8090-2-39
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1749-8090-2-39