Abstract
Background and purpose
The aim of the present analysis was to assess the feasibility, toxicity, and the tumor control of reirradiation as a salvage treatment for progressive pediatric non-pontine high-grade gliomas (HGG).
Patients and methods
The database of the Reference Center for Radiation Oncology of the German HIT (HIT = German acronym for brain tumor) treatment network for childhood brain tumors was screened for children who were reirradiated for progressive non-pontine HGG.
Results
We identified eight patients (WHO grade III: n = 5; WHO grade IV: n = 3) who underwent reirradiation between April 2006 and July 2012. Median age was 13.5 years at primary diagnosis and 14.8 years at first progression. All patients initially underwent surgery (incomplete resection, n = 7; biopsy, n = 1) followed by radiochemotherapy. Relapses occurred inside (n = 2), at the margin (n = 4), and outside of the preirradiated area (n = 2). In all patients, reirradiation was tolerated well without significant acute toxicity. Temporary clinical improvement and tumor regression on magnetic resonance imaging (MRI) following reirradiation was reported (n = 3). However, all patients finally died by disease progression. Median survival time was 26.2 months from initial diagnosis and 11.4 months after first progression. Median time interval between initial radiotherapy and first reirradiation was 9.0 months. In six patients, all macroscopic tumor deposits were reirradiated. In these patients, median progression-free (overall) survival from the start of reirradiation was 2.4 (4.6) months.
Conclusion
Our analysis, although based on a limited patient number, suggests that reirradiation of progressive non-pontine HGG is feasible in children. Benefit in terms of quality of life and/or survival needs to be assessed in a prospective and ideally in a randomized manner.
Similar content being viewed by others
Background
Until present, prognosis of progressive high-grade gliomas (HGG) has remained dismal. Based on numerous retrospective studies reporting on encouraging survival rates and favorable toxicity profile, reirradiation has been widely accepted as an useful therapeutic option for adult patients with relapsed HGG [1–3]. However, as tumor and molecular biology differs between adult and pediatric HGG and as the juvenile brain is particularly vulnerable to radiation-induced injury, results from the above mentioned studies cannot be extrapolated to children [4]. In this analysis we specifically address the feasibility, outcome, and radiation-induced toxicity in a small cohort of pediatric patients with progressive non-pontine HGG who underwent a salvage treatment containing a second course of radiation therapy.
Patients & methods
The database of the Reference Center for Radiation Oncology of the German HIT (HIT = German acronym for brain tumor) treatment network for childhood brain tumors was screened, between 2006–2012, for children who underwent a second course of radiation therapy for progressive non-pontine HGG. Clinical information was extracted from patient medical charts. The date of initial diagnosis was defined as the date of the first tumor resection or biopsy. The date of progression was determined by neuroradiological imaging. Progression-free (PFS) and overall survival (OS) were assessed using the Kaplan–Meier method. All statistical analyses were performed with SPSS, version 20 (SPSS Inc., Chicago, IL, USA). General informed consent had been given for data acquisition and analyses in the context of the corresponding clinical trials of the first-line treatment.
Results
We identified eight pediatric HGG patients (male, n = 5) who underwent a second course of radiation therapy between April 2006 and July 2012. Median age was 13.5 years (range, 10.3 – 16.7 years) at initial diagnosis and 14.8 years (range, 11.0 - 17.5 years) at first progression after initial treatment.
First-line treatment
Surgery included incomplete resection (n = 7), or biopsy (#6). All patients were treated by adjuvant radiochemotherapy (median total dose 59.4 Gy, range, 54.0 Gy – 60.0 Gy) according to the German HIT-GBM D (EU-205100, NCT00278278) (n = 4) or HIT-HGG 2007 (EudraCT 2007-000128-42; ISRCTN19852453) (n = 4) protocols. One patient (#2) received methotrexate (MTX) prior to radiochemotherapy (according to the HIT-GBM D protocol, arm M), and second look surgery of the irradiated tumor was performed. Another patient (#6) received chemotherapy consisting of carboplatin, etoposide and vincristine (VCR) prior to radiochemotherapy as a supratentorial primitive neuroectodermal tumor (CNS-PNET) had been initially suspected before central review confirmed a HGG. All patients received maintenance chemotherapy consisting of temozolomide (TMZ) (n = 5) or lomustine (CCNU), VCR and steroids (n = 3) (Table 1).
Histology
Histological assessment initially revealed five WHO grade III (anaplastic oligodendroglioma (AOD) n = 1, anaplastic astrocytoma (AA) n = 4) and three WHO grade IV tumors (glioblastoma multiforme (GBM)). In addition, the tumor tissue from one patient with an AA (#2) showed at the time of the first relapse histopathological features fulfilling the diagnostic criteria of a GBM (AA → GBM). All cases were centrally reviewed (German Brain Tumor Reference Center, Bonn, Germany).
Second-line treatment
Salvage treatment after first relapse differed considerably between the eight patients.
Patient #1 (AA) relapsed at the margin of the preirradiated area 9.5 months after surgery. Subsequently, he received stereotactic hypofractionated reirradiation (single doses of 4 × 5 Gy and 1 × 4.2 Gy) to a total dose of 24.2 Gy and concurrent TMZ. Prior exposition to radiotherapy in the reirradiated area was approximately 24 Gy. Salvage treatment was tolerated well without major complications. After reirradiation, two additional cycles of PCV chemotherapy consisting of procarbazine, CCNU and VCR were administered. Three months after the start of reirradiation, a deterioration of the patient’s general condition was observed. A cranial MRI showed clear evidence for a progressive disease. The patient died 4.9 months after the relapse.
Patient #2 (AA) relapsed at the margin of the preirradiated area 17.4 months after surgery. Prior radiation exposure was approximately 30 Gy. An incomplete tumor resection was performed. Interestingly, whereas the histopathological assessment of the initial tumor revealed an AA, the tissue of the relapsed tumor showed clear evidence for a GBM. Subsequently, a treatment with TMZ (orally) and intraventricular MTX was administrated. Approximately 13 months after the relapse tumor resection cranial MRI demonstrated again a progressive disease. A reirradiation (single dose 1.8 Gy, total dose 55.8 Gy) with concurrent TMZ was performed. Apart from a focal alopecia and moderate erythema no acute radiation-induced adverse reactions or toxicities were reported. Hematotoxicity was mild. Of note, the boy was able to attend the school during the treatment. Following re-radiochemotherapy high-dose TMZ was administered. Regrettably, the child did experience disease progression only 2.4 months after the start of reirradiation. Despite a further salvage treatment attempt with nimotuzumab, the patient died of disease progression 20.5 months after the first recurrence.
Patient #3 (AOD) relapsed at the margin of the preirradiated area 9.4 months after the surgery. Following partial resection, the girl received reirradiation with 6 × 5 Gy without any complications. Then, the girl received dendritic cell vaccination, 1.6 months after start of the reirradiation, the cranial MRI again revealed disease progression. The child finally died due to tumor progression 5.3 months after the first relapse.
Patient #4 (GBM) recurred at the margin of the radiation field 5.0 months after the initial surgery. Salvage treatment consisted of reirradiation (17 × 1.8 Gy) with concurrent TMZ, and this treatment was tolerated well. Subsequently, the girl received metronomic chemotherapy with the COMBAT regimen [5]. The tumor responded and the neurological status of the patient significantly improved. Unfortunately, 35.9 months after the start of reirradiation the patient experienced a multilocular second relapse. The girl died 39.5 months after the first relapse despite the girl was treated with a salvage chemotherapy consisting of CCNU and trofosfamide.
Patient #5 (GBM) experienced a relapse within the preirradiated area 49.9 months after primary surgery. The salvage chemotherapy with TMZ was initiated and this resulted initially in a tumor regression. Due to a second tumor progression the patient finally received reirradiation with 17 × 1.8 Gy. This treatment was tolerated remarkably well, despite a relatively large radiation field due to the large tumor volume of 125 cm3. Moreover, the patient received bevacizumab every second weeks. Four weeks after the end of the reirradiation, a cranial MRI showed a very good partial response of the relapsed tumor (Figure 1). At the same time, the neurological status of the boy improved. Unfortunately, 2.9 months after the start of reirradiation, the patient experienced a multilocular tumor progression within and outside of the reirradiated area. Despite an intensification of the ongoing bevacizumab treatment by addition of irinotecan, the patient died 11.5 months after the first relapse.
Patient #6 (AA) had a multilocular relapse outside of the preirradiated volume 12.2 months after initial surgery. With a palliative objective, he received reirradiation focused on a symptomatic tumor deposit in the left cerebellar peduncle (25 × 1.8 Gy). Metronomic TMZ was administered simultaneously. Treatment was effective. Neurological status, especially the gait pattern, significantly improved. Six weeks after the end of irradiation, cranial MRI demonstrated a tumor regression within and progressive disease outside of the reirradiated area. There were no radiation-induced side effects reported requiring any supportive treatment. Approximately two months later, the boy received radiation therapy for the third time. The radiation therapy was directed at a tumor deposit in the area of the right lateral ventricle (17 × 1.8 Gy). The treatment was well tolerated. Two months after the end of the third irradiation, the MRI again showed disseminated tumor progression, however not in the preirradiated areas. Subsequently, high-dose TMZ (5 days on/5 days off) was started. Finally, a fourth course of stereotactic radiation therapy to a tumor deposit in the right cerebellopontine angle (3 × 5 Gy) was given without any acute side effects. The patient finally died 11.3 months after the first relapse.
Patient #7 (GBM) relapsed locally 2.5 months after the initial surgery. The relapsed tumor was incompletely resected. The boy received dendritic cell vaccination [6]. The patient finally experienced a multilocular relapse with leptomeningeal spread along the entire craniospinal axis. He subsequently underwent palliative craniospinal irradiation with 10 × 3 Gy with concurrent TMZ. Nevertheless, the disease progressed and the boy finally died 10.6 months after the first relapse.
Patient #8 (AA) experienced a local relapse, 9.0 months after the initial surgery. The relapsed tumor progressed slowly. In the further course of the disease, a cerebellar metastasis with brain stem infiltration occurred. This tumor manifestation was subsequently irradiated with conventionally fractionated reirradiation to a total dose of 54 Gy. There were no supportive treatments requiring radiation-induced side effects. Shortly after, the patient suffered from a multilocular progression and refused further treatment. She finally died 9.2 months after the beginning of reirradiation and 20 months after first relapse (Table 2).
Survival
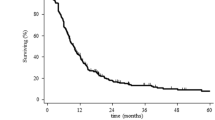
The median survival time from initial diagnosis was 26.2 months (range, 13.1 – 61.4 months) (Figure 2) and 11.4 months (range, 4.9 – 39.5 months) from the time of the first recurrence. Median time interval between the end of the initial radiotherapy and the beginning of the reirradiation was 9.0 months (range, 3.8 – 52.1 months). The tumor control of the six patients, who received reirradiation of all visible tumor lesions was moderate with a median progression-free (overall) survival after the start of reirradiation of 2.4 ± 0.8 months (4.6 ± 1.4 months) (Figure 3).
Discussion
General aspects
In the present analysis, we retrospectively assessed eight pediatric patients with non-pontine HGG who underwent reirradiation as part of their salvage treatment after disease progression. Our series is small, however, to the best of our knowledge, this is the first report on reirradiated pediatric non-pontine HGG.
Surprisingly, the idea of reirradiating progressive childhood HGG dates back to the 1980ies. In the Children’s Cancer Group (CCG) study 943, patients who showed tumor recurrences 12 months after the diagnosis could optionally receive irradiation to the recurrent tumor sites up to a dose of 3000 rad in 15–18 fractions. However, in none of the 43 relapsed patients the possibility of reirradiation was exhausted [7]. Apparently, pediatric radiation oncologists have been reluctant to reirradiate relapsed or progressive HGG due to their increased vulnerability of the CNS to radiation therapy. Hence, the fear of an unacceptably high incidence of neurological and other radiotherapy-induced toxicity and sequelae may have limited the use of a second course of radiation therapy. Moreover, even in adult patients, the benefit of reirradiation in terms of survival is still an issue of debate. To assess the impact of reirradiation in adult recurrent GBM patients, the Radiation Therapy Oncology Group (RTOG) 1205 randomized phase II trial is currently validating the survival benefit of reirradiation in a prospective randomized setting.
Feasibility and safety of reirradiation in children
In our small series, we report on children receiving a second course of radiation therapy. In four cases (patients #1 – #4) the target volumes for reirradiation were located at the margins of and in two cases (patients #5 and #7) even within the former radiotherapy fields. Hence, significant ionizing preloads to the surrounding normal brain tissue ranging between 25 Gy (see patients #1 and #2) and almost 60 Gy had to be expected. Moreover, at least in patient #4, the time interval between the end of initial and the beginning of the reirradiation was rather short (3.8 months). Most experts in the field suggest a radiation-free interval of at least six months between the first and second irradiation [8–10]. However, our very limited experience might provide evidence that some patients may tolerate reirradiation within a shorter (<6 months) radiation-free interval.
The reirradiation was tolerated remarkably well in our small cohort. Alopecia and reddening of the skin were reported. No major unexpected toxicities, and no treatment-related deaths were reported. Moreover, no reirradiation-induced necrosis which would have required treatment did occur in any patient during the further course of the disease. This is in accordance with the conclusions of two important reviews on normal brain tissue tolerance which stated that radiation-induced necrosis has to be expected only at cumulative normalized total doses (single dose, 2 Gy; α/β, 3 Gy) > 102 Gy and just 1–2 years after irradiation, i.e. after the expected survival time in progressive HGG patients [11, 12].
Tumor response following reirradiation
In three of our patients, temporary but significant improvement of the neurological status and regression of the (re-) irradiated tumor deposits were reported (patient #4, #5, and #6). These findings are in agreement with the experiences reported by Fontanilla and colleagues. They published a series of five children with diffuse intrinsic pontine gliomas whose disease progressed after initial radiochemotherapy and subsequent salvage chemotherapy. A second course of radiotherapy was given with concurrent chemotherapy up to a dose of 20 Gy or 18 Gy. Four patients had substantial clinical improvement of their symptoms (improvement in speech, ataxia, and swallowing). Three patients showed an improved mobility after reirradiation. Four patients had decreased tumor size on posttreatment MRI. Acute radiation-related toxicities were fatigue (n = 2), alopecia (n = 2), and reduced appetite (n = 1). No grade 3 or 4 toxicities were reported [13].
Impact of reirradiation on survial
Regrettably, in our small series, reirradiation did not demonstrate convincing efficacy in terms of long-term tumor control and survival. In six patients the target volume of reirradiation contained all macroscopic tumor deposits. Only one of these patients (patient #4) showed sustained stable disease for 35.9 months after reirradiation. Surprisingly, the histology of the tumor was a GBM, and the child had progressed only five months after initial diagnosis despite intense first-line treatment consisting of surgery, followed by adjuvant radiochemotherapy (TMZ) and TMZ maintenance chemotherapy. The other five patients did progress less than 3.5 months after the start of reirradiation. Median progression-free (overall) survival from the start of reirradiation was 2.4 ± 0.8 months (4.6 ± 1.4 months) and hence by far less than hoped on the basis of prior experiences gained in adults who had undergone a second course of radiation therapy for refractory HGG. Scholtyssek et al. recently reported a median progression-free (overall) survival of 4.3 (7.7) months in a cohort of 64 adult HGG patients (53 × WHO grade IV, 11 × WHO grade III), who had been treated at progression using a second series of radiotherapy with or without concurrent/subsequent chemotherapy [14]. Other authors even reported much more favorable outcomes with median overall survivals ranging between 9.5 and 11 months [15–17]. Of note, the impact of reirradiation on survival appears to be difficult to interpret in our series as we report on a limited number of patients with differing primary treatment and heterogeneous salvage therapy approaches. In addition, three of our patients (patients #2, #5 and #7) received a second course of radiation therapy at the time of the second relapse thereby influencing the survival data of the present study (Table 2).
Conclusions
In summary, our findings, although based on a limited patient number, suggest that reirradiation might be feasible in progressive pediatric non-pontine HGG. The recommended radiation dose depends on the location of the relapse, the age of the patients, and the ionizing preloads of the relapse area. Further evaluation of such cases is necessary in order to better delineate feasibility, toxicity, and impact on outcome of this new treatment approach in pediatric non-pontine HGG patients with relapsed tumors.
Ethical standards
This manuscript is in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its subsequent amendments.
References
Combs SE, Edler L, Rausch R, Welzel T, Wick W, Debus J: Generation and validation of a prognostic score to predict outcome after re-irradiation of recurrent glioma. Acta Oncol 2012. doi:10.3109/0284186X.2012.692882
Weller M, Cloughesy T, Perry JR, Wick W: Standards of care for treatment of recurrent glioblastoma–are we there yet? Neuro Oncol 2013,15(1):4-27. doi:10.1093/neuonc/nos273 10.1093/neuonc/nos273
Niyazi M, Siefert A, Schwarz SB, Ganswindt U, Kreth FW, Tonn JC, Belka C: Therapeutic options for recurrent malignant glioma. Radiother Oncol 2011,98(1):1-14. doi:10.1016/j.radonc.2010.11.006 10.1016/j.radonc.2010.11.006
MacDonald TJ, Aguilera D, Kramm CM: Treatment of high-grade glioma in children and adolescents. Neuro Oncol 2011,13(10):1049-1058. doi:10.1093/neuonc/nor092 10.1093/neuonc/nor092
Zapletalova D, Andre N, Deak L, Kyr M, Bajciova V, Mudry P, Dubska L, Demlova R, Pavelka Z, Zitterbart K, Skotakova J, Husek K, Martincekova A, Mazanek P, Kepak T, Doubek M, Kutnikova L, Valik D, Sterba J: Metronomic chemotherapy with the COMBAT regimen in advanced pediatric malignancies: a multicenter experience. Oncology 2012,82(5):249-260. doi:10.1159/000336483 10.1159/000336483
De Vleeschouwer S, Fieuws S, Rutkowski S, Van Calenbergh F, Van Loon J, Goffin J, Sciot R, Wilms G, Demaerel P, Warmuth-Metz M, Soerensen N, Wolff JE, Wagner S, Kaempgen E, Van Gool SW: Postoperative adjuvant dendritic cell-based immunotherapy in patients with relapsed glioblastoma multiforme. Clin Cancer Res 2008,14(10):3098-3104. doi:10.1158/1078-0432.CCR-07-4875 10.1158/1078-0432.CCR-07-4875
Sposto R, Ertel IJ, Jenkin RD, Boesel CP, Venes JL, Ortega JA, Evans AE, Wara W, Hammond D: The effectiveness of chemotherapy for treatment of high grade astrocytoma in children: results of a randomized trial. A report from the childrens cancer study group. J Neurooncol 1989,7(2):165-177. 10.1007/BF00165101
Combs SE, Debus J, Schulz-Ertner D: Radiotherapeutic alternatives for previously irradiated recurrent gliomas. BMC Cancer 2007, 7: 167. doi:10.1186/1471-2407-7-167 10.1186/1471-2407-7-167
Ernst-Stecken A, Ganslandt O, Lambrecht U, Sauer R, Grabenbauer G: Survival and quality of life after hypofractionated stereotactic radiotherapy for recurrent malignant glioma. J Neurooncol 2007,81(3):287-294. doi:10.1007/s11060-006-9231-0 10.1007/s11060-006-9231-0
Fokas E, Wacker U, Gross MW, Henzel M, Encheva E, Engenhart-Cabillic R: Hypofractionated stereotactic reirradiation of recurrent glioblastomas: a beneficial treatment option after high-dose radiotherapy? Strahlentherapie und Onkologie: Organ der Deutschen Rontgengesellschaft [et al]. 2009,185(4):235-240.
Mayer R, Sminia P: Reirradiation tolerance of the human brain. Int J Radiat Oncol Biol Phys 2008,70(5):1350-1360. doi:10.1016/j.ijrobp.2007.08.015 10.1016/j.ijrobp.2007.08.015
Lawrence YR, Li XA, el Naqa I, Hahn CA, Marks LB, Merchant TE, Dicker AP: Radiation dose-volume effects in the brain. Int J Radiat Oncol Biol Phys 2010,76(3 Suppl):S20-S27. doi:10.1016/j.ijrobp.2009.02.091
Fontanilla HP, Pinnix CC, Ketonen LM, Woo SY, Vats TS, Rytting ME, Wolff JE, Mahajan A: Palliative reirradiation for progressive diffuse intrinsic pontine glioma. Am J Clin Oncol 2012,35(1):51-57. doi:10.1097/COC.0b013e318201a2b7 10.1097/COC.0b013e318201a2b7
Scholtyssek F, Zwiener I, Schlamann A, Seidel C, Meixensberger J, Bauer M, Hoffmann KT, Combs SE, von Bueren AO, Kortmann RD, Muller K: Reirradiation in progressive high-grade gliomas: outcome, role of concurrent chemotherapy, prognostic factors and validation of a new prognostic score with an independent patient cohort. Radiat Oncol 2013,8(1):161. doi:10.1186/1748-717X-8-161 10.1186/1748-717X-8-161
Ciammella P, Podgornii A, Galeandro M, D’Abbiero N, Pisanello A, Botti A, Cagni E, Iori M, Iotti C: Hypofractionated stereotactic radiation therapy for recurrent glioblastoma: single institutional experience. Radiat Oncol 2013,8(1):222. doi:10.1186/1748-717X-8-222 10.1186/1748-717X-8-222
Fogh SE, Andrews DW, Glass J, Curran W, Glass C, Champ C, Evans JJ, Hyslop T, Pequignot E, Downes B, Comber E, Maltenfort M, Dicker AP, Werner-Wasik M: Hypofractionated stereotactic radiation therapy: an effective therapy for recurrent high-grade gliomas. J Clin Oncol 2010,28(18):3048-3053. doi:10.1200/JCO.2009.25.6941 10.1200/JCO.2009.25.6941
Henke G, Paulsen F, Steinbach JP, Ganswindt U, Isijanov H, Kortmann RD, Bamberg M, Belka C: Hypofractionated reirradiation for recurrent malignant glioma. Strahlentherapie und Onkologie: Organ der Deutschen Rontgengesellschaft [et al]. 2009,185(2):113-119.
Acknowledgments
We thank all the participating centers for their valuable cooperation. The HIT-HGG trial office and the reference institutions (Neuropathology, Prof. Dr. Torsten Pietsch et al. / Neuro-Radiology: Prof. Dr. Monika Warmuth-Metz et al./Radio-Oncology: Prof. Dr. Rolf-Dieter Kortmann et al.) are supported by the German Children’s Cancer Foundation (Deutsche Kinderkrebsstiftung). We thank Michael Josef Kerber for proofreading and linguistic revision of the manuscript. We would like to thank Gudrun Leyerer for administrative support. We acknowledge the contributions by Drs. Torsten Pietsch (central pathologic review), Monika Warmuth-Metz (central review of imaging), Uwe Kordes and Annette Sander (provision of study patients). We also acknowledge support by the Open Access Publication Funds of the Goettingen University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors indicate no potential conflicts of interest.
Authors’ contributions
Conception and design: KM, AOvB. Collection and assembly of data: KM, HS, AOvB. Data analysis and interpretation: KM. Manuscript writing: KM. Manuscript editing: HS, AOvB, SP, CMK, RDK. Final approval of manuscript: All authors.
Klaus Müller, Heike Scheithauer contributed equally to this work.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Müller, K., Scheithauer, H., Pietschmann, S. et al. Reirradiation as part of a salvage treatment approach for progressive non-pontine pediatric high-grade gliomas: preliminary experiences from the German HIT-HGG study group. Radiat Oncol 9, 177 (2014). https://doi.org/10.1186/1748-717X-9-177
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1748-717X-9-177