Abstract
Background
Computerized clinical decision support systems (CCDSSs) for drug therapy management are designed to promote safe and effective medication use. Evidence documenting the effectiveness of CCDSSs for improving drug therapy is necessary for informed adoption decisions. The objective of this review was to systematically review randomized controlled trials assessing the effects of CCDSSs for drug therapy management on process of care and patient outcomes. We also sought to identify system and study characteristics that predicted benefit.
Methods
We conducted a decision-maker-researcher partnership systematic review. We updated our earlier reviews (1998, 2005) by searching MEDLINE, EMBASE, EBM Reviews, Inspec, and other databases, and consulting reference lists through January 2010. Authors of 82% of included studies confirmed or supplemented extracted data. We included only randomized controlled trials that evaluated the effect on process of care or patient outcomes of a CCDSS for drug therapy management compared to care provided without a CCDSS. A study was considered to have a positive effect (i.e., CCDSS showed improvement) if at least 50% of the relevant study outcomes were statistically significantly positive.
Results
Sixty-five studies met our inclusion criteria, including 41 new studies since our previous review. Methodological quality was generally high and unchanged with time. CCDSSs improved process of care performance in 37 of the 59 studies assessing this type of outcome (64%, 57% of all studies). Twenty-nine trials assessed patient outcomes, of which six trials (21%, 9% of all trials) reported improvements.
Conclusions
CCDSSs inconsistently improved process of care measures and seldomly improved patient outcomes. Lack of clear patient benefit and lack of data on harms and costs preclude a recommendation to adopt CCDSSs for drug therapy management.
Similar content being viewed by others
Background
Computerized clinical decision support systems (CCDSSs) algorithmically apply an electronic knowledge base to individual patient data to generate and present suggested actions intended to enhance health and healthcare [1–3]. CCDSSs for drug therapy management are used to facilitate evidence-informed medication use [4], reduce the incidence of harmful medication errors [5], and improve healthcare system efficiency [2, 4, 6]. In this review, we considered any CCDSS that provides recommendations to healthcare providers regarding the initiation, modification, monitoring, or discontinuation of drug therapy, based on the patient's characteristics. Systems designed solely to provide advice on the management of narrow therapeutic index drugs based on in vivo monitoring and pharmacokinetic principles (therapeutic drug monitoring [7]) were excluded because they are in a complementary in-depth review on therapeutic drug monitoring and dosing (submitted to Implementation Science). Variety in the structure and function of CCDSSs complicates methodologically sound investigations and comparisons of these interventions. A CCDSS may be integrated with one or more of electronic medical records (EMR), computerized provider order entry systems (CPOE) or electronic transmission of prescriptions to the point of dispensing. CCDSSs require input of patient data to deliver advice, and this may be accomplished via integration with patient information repositories or by manual entry. Optimally, the knowledge base of a CCDSS used to generate recommendations is evidence-informed, though this may not always be the case. Advice may be delivered to many kinds of providers through a variety of media across diverse settings of care. Systems may be developed 'in house' to meet the requirements of a specific organization or acquired from a commercial vendor.
Decision makers, clinicians, and patients should require sound evidence of CCDSS benefits, risks and costs prior to general adoption, as for any health intervention. Randomized controlled trials (RCTs) represent the gold standard for unbiased comparisons of alternative interventions [8].
Our previous review [2] included 24 RCTs of a CCDSS for drug therapy. Of the 13 trials measuring patient-important outcomes, only one detected benefit with a CCDSS. Small study size limited detection of change in patient-important clinical endpoints.
Lack of data on which to base overall conclusions on the effects of CCDSSs together with the increased pace of research in the field prompted this update of our previous review. To aid decision makers and providers, we evaluated the effects of CCDSSs on process of care and patient outcomes via cumulative synthesis of relevant RCTs. This review is one of a series of reviews considering the effects of CCDSSs across multiple application areas (therapeutic drug monitoring and dosing, primary preventive care, diagnostic test ordering, acute care management, and chronic disease management).
Methods
This review was conducted in accord with a published protocol http://www.implementationscience.com/content/5/1/12[9]. Some trials have been included in more than one review because they were relevant to more than one CCDSS intervention area. Specific details for the drug therapy review follow.
Research questions
For this review, we were primarily interested in determining: 1) Do CCDSSs improve performance on drug-related process of care measures or patient outcomes compared to usual care? 2) What features or characteristics of studies or systems are associated with improved process or patient measures? Based partly on our previous review [2], we expected studies demonstrating benefit from CCDSSs would: a) be integrated with an existing EMR or CPOE system (versus a standalone system); b) deliver decision support before or during a patient care encounter where the decision that is being supported was taken (versus supply of decision support at any other time); c) actively suggest treatments or other actions (versus supply general information or access to general information); d) be used in a patient care setting affiliated with an academic institution (versus any other setting); e) have developers of the CCDSS who were also the study investigators (versus study investigators not associated with developers); f) measure intermediate/surrogate patient outcomes (versus patient-important outcomes); g) describe higher rates of user satisfaction (versus no or low rates of user satisfaction).
Partnering with decision makers
This review was conducted in partnership with senior hospital managers and clinical leaders with an academic research team in the field of knowledge translation, from healthcare research to clinical practice. Decision makers provided key input as to the kind of data needed about CCDSS to drive effective choices and these needs were incorporated into the research plan where feasible.
Search strategy
The search methods employed have been described in detail elsewhere [9]. Briefly, a comprehensive search (2004 to 2010) of major biomedical databases (MEDLINE, EMBASE, Ovid's EBM Reviews, and Inspec) yielded citations for screening. Pairs of reviewers independently evaluated each citation and abstract. A third reader resolved disagreements where necessary. Inter-reviewer agreement on study eligibility was measured via unweighted Cohen's kappa (κ). Studies from our previous reviews were carried forward to this review if they met the inclusion criteria, effectively extending our search from database inception to 2010.
Study selection
Studies were included for review if they described an RCT comparing outcomes for a group of providers or patients using a CCDSS compared with care without the CCDSS. Non-experimental or quasi-experimental investigations were excluded. For inclusion, we required that independent providers or post-graduate trainee (e.g. medical residents) providers be identified as primary users of the CCDSS. The intervention CCDSS was required to provide patient-specific output in the form of assessments, management options, or recommendations to the clinical user. Studies were excluded if the system was used solely by students, only provided summaries of information for patients, only provided feedback on groups of patients without feedback about individual patients, only provided computer-aided instruction, or were used for image analysis. The six CCDSS intervention areas in this series of reviews used a common eligibility screening process [9] to identify reports of trials of CCDSSs for any purpose. Studies were then further screened to determine if the system provided advice regarding drug therapy.
Data extraction
Independent reviewers extracted key data concerning study methods, CCDSS and population characteristics, possible sources of bias, and outcomes in duplicate. Primary authors of each study were asked to review the extracted data for their study and offer comments on the extracted data.
Assessment of study quality
Included studies were evaluated on five dimensions of quality--including concealment of allocation, appropriate unit of allocation, appropriate adjustment for baseline differences, adequate follow-up, and appropriate outcome assessment--to yield a 10-point methods score [9].
Assessment of CCDSS intervention effects
Outcome selection and improvement determinations
Each included trial describing a CCDSS that provided advice exclusively or predominantly about drug therapy was classified as drug therapy management only (Rx-only). Systems that gave advice on drug therapy as part of a more complex intervention were categorised as 'multi-faceted' CCDSSs. Improvement was considered to have occurred where 50% or more of the selected outcomes showed a benefit with a CCDSS compared to control. To determine whether improvement occurred, all outcomes were selected from the first of: primary, then pre-specified, then any outcome(s), as defined by study authors (i.e., if a primary outcome was reported for a trial this was used to determine improvement to the exclusion of any other reported outcomes). Where no outcomes were defined as primary, but the study reported a sample size calculation for an outcome, we defined that outcome as primary. These criteria are more specific than those used in our previous review [2]; therefore, the assignment of effect was adjusted for some studies included in the 2005 review. Process of care outcomes for multi-faceted CCDSS studies were selected only if they were clearly drug-related. Multi-faceted systems that reported a patient outcome but did not report a drug-related process of care outcome intermediary were excluded as non-responsive to our research questions. Where there were multiple intervention arms, the arm testing the most sophisticated CCDSS was used to determine improvement. Two reviewers, working independently and blinded to study results, classified trials as drug treatment-only or multi-faceted, and initially identified the outcomes used to determine improvement, with disagreements resolved by consensus.
Data synthesis and analysis
Data were summarized using descriptive summary measures, including proportions for categorical variables and means (± SD) for continuous variables. For interpretation, a 2-sided p < 0.05 indicated statistical significance. For individual studies we report the measures of association and p -values reported in the studies.
We did not attempt a meta-analysis because of differences across studies of participants, settings, disease conditions, interventions, and outcomes. Tests of association between study and CCDSS factors and improved outcomes were tested using the univariate Fisher's exact test. Multivariate analyses were conducted using multinomial logistic regression. All analyses were conducted using SPSS v. 17.
Sensitivity analyses were conducted to determine if the class of outcome selected to judge improvement affected our results. We also identified cluster randomized trials where units of allocation and units of analysis were appropriately matched or mismatched. The proportions of successful trials with matched versus mismatched units were compared.
Results
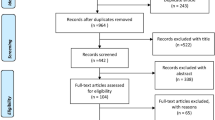
A total of 14,952 possibly relevant records were identified [9]. After excluding duplicate records, 14,188 records were screened to yield 329 articles eligible for full-text screening. Of those, 166 trials met our criteria for a CCDSS; Cohen's κ for reviewer agreement on trial eligibility was 0.93 (95% confidence interval [CI], 0.91 to 0.94). Initially, 71 trials were judged relevant to drug therapy management. Six of these trials [10–15] were excluded because they studied a multi-faceted CCDSS that included drug therapy, but did not report any drug-related process outcomes. A total of 65 trials reported in 74 papers were included [16–89] (see Figure 1). Twenty-four RCTs [60–62, 65–67, 69, 71–76, 78, 80–89] had been included in the previous version of our review [2]. Study authors confirmed or supplemented our data extraction for 53 of 65 included studies (82%) [16–20, 23, 25–33, 35–39, 42–45, 47–49, 53–55, 57, 58, 60–62, 65–72, 74–76, 78, 81, 83–86, 88]. Forty-seven included studies contribute outcomes to this review as well as other CCDSS interventions in the series; four studies [49, 56, 76, 80] to four reviews, 16 studies [16, 19, 21, 28, 40, 44, 45, 53, 55, 59, 62, 64, 68, 69, 74, 77–79, 82, 85, 89] to three reviews, and 27 studies [20, 22, 23, 26, 27, 29, 31, 32, 34, 35, 39, 41–43, 46–48, 50, 52, 54, 60, 63, 66, 70, 72, 75, 81, 86–88] to two reviews; but we focused here on drug prescribing-relevant outcomes.
Flow diagram of included and excluded studies for the update 1 January 2004 to 6 January 2010 with specifics for drug prescribing and management*. *Details provided in: Haynes RB et al. [9]. Two updating searches were performed, for 2004 to 2009 and to 6 January 2010 and the results of the search process are consolidated here.
Summary of trial quality is reported in Additional file 1, Table S1; system characteristics in Additional file 2, Table S2; study characteristics in Additional file 3, Table S3; outcome data in Additional file 4, Table S4 and Table 1, and other CCDSS-related outcomes in Additional file 5, Table S5.
Study characteristics
Thirty-six trials (55%) [17, 18, 20, 22–26, 28, 30, 32–39, 41–43, 46–48, 50, 51, 54, 58, 61, 62, 65, 67, 68, 73, 81, 83, 87–89] described systems classified as drug therapy-only with the remaining 29 (45%) [16, 19, 21, 27, 29, 31, 40, 44, 45, 49, 52, 53, 55–57, 59, 60, 63, 64, 66, 69–72, 74–80, 82, 84–86] describing multi-faceted CCDSSs. Forty-one of 65 included studies (63%) [16–59, 63, 68, 70] were published since the previous version of this review. Eleven trials (17%) were published prior to 2000 [77–89], 16 (25%) trials [58, 60–76] between 2000 and 2004 and 38 (58%) trials [16–34, 34–46, 46–57, 59] after 2004. Most studies (n = 41, 63%) [16–21, 23, 24, 26, 28, 31–33, 38, 40, 42–44, 47, 48, 55–57, 60, 61, 63, 66–68, 70, 72–80, 82–88] reported public funding; nine (14%) [29, 34, 35, 45, 46, 49, 50, 52, 53, 59, 71] reported private funding; six (9%) [22, 36, 37, 41, 54, 64, 65, 69] reported public and private funding, and 9 (14%) [25, 27, 30, 39, 51, 58, 62, 81, 89] did not disclose a funding source (see Additional file 3, Table S3). We were able to determine whether improvement occurred with a CCDSS for process of care outcomes in 59 studies [16–24, 26–38, 40–70, 72–76, 80–83, 85–87, 89]; 29 studies reported patient outcomes [19, 25, 26, 29, 33, 38, 39, 43, 45, 48, 49, 51–53, 56, 59–61, 64, 66–69, 71, 72, 75, 77–80, 84, 86, 88], and both patient and process outcomes were extracted from 23 (of 29, 79%) reports [19, 26, 29, 33, 38, 43, 45, 48, 49, 51–53, 56, 59–61, 64, 66–69, 72, 75, 80, 86] (Table 1 and see Additional file 4, Table S4). Twenty [32, 33, 38, 39, 43, 48, 51–53, 56, 60, 61, 66–69, 71–73, 75, 80, 84] of 29 (69%) studies reported a patient important outcome rather than an intermediate or surrogate outcome [90].
Study quality
Included trials had a median methodological quality score of 8 (interquartile range [IQR], 2) of a total possible score of 10. Quality assessments for each trial are presented in Additional file 1, Table S1. Most included studies were cluster randomized (n = 44/65, 68%) [16–26, 28, 30–32, 34, 41, 42, 44, 46–49, 53, 55, 56, 58, 60–67, 69, 70, 73–87], measured an objective outcome or blinded outcome assessments appropriately (n = 64/65, 98%) [16–56, 58–89], had 80% or greater follow-up of subjects (n = 56, 86%) [16–38, 40–50, 52–54, 56–59, 61–72, 74, 76–79, 81–87] and 41 (63%) [16, 18, 20–23, 30–34, 36–51, 53, 54, 56–59, 63, 64, 66, 68–70, 72, 74, 75, 77–82, 84] reported adequate allocation concealment. There was no change in quality score over time (R2 = 0.01, p = 0.53).
CCDSS and study characteristics
Additional file 2, Table S2 describes CCDSS users and Additional file 3, Table S3 describes study settings. A sum of 8,932 providers (median, 80; IQR, 193) used a CCDSS to assist with drug management for a total studied population of 1,246,686 patients (median, 2027; IQR 6960). Most CCDSSs were used by fully-trained physicians (61/65, 94%) [16–29, 31–35, 38–56, 58–89] and some by post-graduate medical trainees (19/65, 29%) [20, 23, 32, 35, 55, 56, 60, 66, 73, 74, 76, 80–82, 84, 85, 87–89]. After physicians, nurses in advanced practice roles (16/25 studies, 25%) [16, 18, 20–22, 25–27, 33, 35, 41, 42, 57, 58, 68, 73, 81, 87], physician assistants 8/65, 12%) [16, 18, 20, 21, 25, 42, 58, 63, 70, 77–79], and pharmacists (8/65, 12%) [30, 35–37, 54, 56, 60, 66] were the most common provider types interacting with CCDSSs. Many systems reported use by more than one type of provider. CCDSSs were studied in the United States (n = 44, 68%) [16, 18, 20–23, 26–30, 32, 33, 35–37, 40–43, 45, 47, 50–52, 54–56, 58–61, 66, 68, 72–74, 76, 80–85, 87–89], European Union or European Economic Area countries (n = 13, 20%) [31, 34, 38, 39, 44, 46, 49, 53, 62–64, 69–71, 75, 77–79], and Canada (n = 3, 5%) [17, 24, 65, 86], with the remaining five studies (8%) [19, 25, 48, 57, 67] occurring in multiple or other countries.
Outpatient settings were studied more often (n = 55, 85%) [16, 18–23, 26–41, 43–47, 49–67, 69–71, 73, 75–79, 81, 83, 85–89] than other settings of care. Studies were conducted in both academic settings (n = 34, 52%) [18, 23, 25, 26, 28, 33–35, 38, 39, 42, 46, 48, 51, 55–57, 60, 61, 66, 68, 71–74, 76, 80–85, 87–89] and outside academic centres (n = 31, 48%) [16, 17, 19–22, 24, 27, 29–32, 36, 37, 40, 41, 43–45, 47, 49, 50, 52–54, 58, 59, 62–65, 67, 69, 70, 75, 77–79, 86].
As presented in Additional file 2, Table S2, the majority of CCDSS systems in our sample were integrated with an EMR (n = 38/61, 62%) [17, 18, 20, 23–26, 28, 31, 32, 34, 40, 42, 44–47, 49, 55, 56, 58–66, 68–70, 73, 74, 77–85, 87, 89], delivered feedback via a computer display (n = 44/62, 71%) [17, 18, 20, 23–26, 28, 30–36, 38–40, 42, 44, 46–49, 51, 55–58, 60–66, 68–71, 73–76, 80, 82–84] at the time of care (n = 53/64, 83%) [16–21, 23–26, 28, 30–35, 38, 40, 42, 44, 46–51, 53, 55–58, 60–70, 72–85, 87–89]. A minority of authors reported testing a CCDSS with a graphical user interface (n = 22/25, 88%) [16–18, 20, 21, 23–25, 28, 30, 31, 34, 38, 40, 45, 46, 55, 56, 58–60, 63, 65, 70, 73, 75, 83], pilot-testing the system before the trial (n = 25/45, 56%) [17–19, 24, 28, 29, 31–33, 35, 38, 39, 43–45, 47, 48, 55, 59, 63, 66, 70–72, 74, 75, 77–79, 84], or training users on system use (n = 29/52, 56%) [16, 19, 21, 26, 28, 29, 31, 32, 34, 35, 38, 43–47, 53, 56–60, 62, 64–66, 69, 75–79, 83–85]. Data required by the CCDSS to produce recommendations were most commonly entered via EMR link (n = 32/61, 52%) [17, 18, 20, 24, 26, 28, 31, 32, 34, 40, 44–47, 49, 51, 55, 56, 58–60, 62–66, 68–70, 74, 80–82, 84, 85, 87, 89], followed by provider entry (n = 23/61, 38%) [16, 21, 23, 25, 30, 31, 34, 35, 38, 39, 46, 53, 56, 63, 64, 66, 67, 69–71, 73, 75, 76, 80, 83, 86, 88], study staff (n = 10/61, 16%) [19, 39, 42, 43, 48, 61, 63, 70, 74, 86, 89], and existing staff (n = 8/61, 13%) [36, 37, 68, 72–74, 88, 89], although multiple modes of entry were reported in some studies. Nineteen (29%) [17, 18, 20, 23–25, 31, 32, 34, 35, 42, 45–47, 56, 58–60, 80, 82–84] studies reported using systems that were integrated with CPOE.
Clinical characteristics
CCDSSs were grouped into one of three categories representing the primary pharmacotherapeutic purpose of the system. Systems designed to optimize drug therapy were tested in 47 (72%) trials [16, 19, 26, 27, 29–34, 38–40, 43–45, 48–53, 55–58, 60–62, 66–76, 78, 81, 82, 85–88]; systems to prevent adverse drug events accounted for 16 (25%) trials [17, 20, 23, 25, 28, 35–37, 41, 42, 47, 54, 65, 80, 84, 89];while the remaining two (3%) trials [18, 83] focused on drug cost management. Patient populations were identified (for each system) and consisted of seven (11%) systems for geriatric patients [17, 23, 25, 35, 37, 42, 65], three (5%) systems for paediatrics [32, 43, 73], four (6%) systems for women's health [36, 40, 70, 71], and 51 (78%) for adults or unspecified general populations. We attempted to identify the main disease state targeted by each system. Sixteen systems (25%) [17, 23, 25, 27, 34, 35, 37, 42, 52, 58, 65, 74, 76, 82–84] were employed for multiple conditions. Each of the following disease groupings included three or more systems: cardiovascular disease [26, 33, 51, 60, 61, 66, 69, 72, 81, 86, 88] (n = 11, 17%), diabetes mellitus [29, 30, 50, 55, 62, 78, 85] (n = 7, 11%), respiratory disease [43, 44, 53, 56, 75] (n = 5, 8%), dyslipidaemia [16, 19, 31, 45, 49] (n = 5, 8%) and infectious diseases [32, 48, 68, 70, 73] (n = 5, 8%). Nine of the remaining 16 systems [20, 28, 36, 41, 47, 54, 80, 87, 89] were designed to prevent or detect drug related problems via laboratory monitoring.
CCDSS effectiveness
Thirty-seven trials [16, 18, 19, 21–23, 26, 27, 29–33, 35–38, 40, 41, 43, 45, 48, 50, 52–54, 57–59, 62, 63, 65, 68, 70, 73–75, 80, 81, 87, 89] of 59 (63%) showed improvement in process of care outcomes due to CCDSS use. No significant difference was found between Rx-only (23/33) [18, 22, 23, 26, 30, 32, 33, 35–38, 41, 43, 48, 50, 54, 58, 62, 65, 68, 73, 81, 87, 89] and multi-faceted (14/26) [16, 19, 21, 27, 29, 31, 40, 45, 52, 53, 57, 59, 63, 70, 74, 75, 80] CCDSSs for process of care improvement.
Six trials [19, 29, 39, 52, 53, 75] (9% of all trials, 21% of trials measuring a patient outcome) demonstrated improved patient outcomes with CCDSS use compared to usual care without a CCDSS (see Table 1). Four [39, 52, 53, 75] of the six trials demonstrating improved patient outcomes measured patient-important outcomes. No significant difference in improvement was found between drug-only (1/12) [39] and multi-faceted (5/17) [19, 29, 52, 53, 75] CCDSSs.
Results did not significantly vary, for either process of care or patient outcomes, by the type of outcome (primary, pre-specified, or other) selected to determine improvement.
All studies demonstrating improved patient outcomes also showed improvement in measured process of care outcomes.
The proportion of successful trials was not significantly different between cluster trials where units of allocation were mismatched with units of analyses (7/15 for process and 1/5 for patient outcomes) compared with non-cluster trials or cluster trials with an appropriately adjusted analysis (29/43 for process (p = 0.22) and 5/24 for patient outcomes (p = 1)).
Predictors of success
This analysis was limited by incomplete data in many studies and by limited power for multivariate analysis. In univariate analysis, CCDSSs not integrated with an EMR were more likely to improve process of care outcomes, 16/20 (80%) non-integrated systems showed improvement versus 18/35 (51%) improved with EMR linkage (p = 0.03). The same trend was seen with integration of EMR and CCDSS for patient outcomes (6/15 (40%) improved outcomes without EMR link versus (0/13 (0%) with EMR link, p = 0.017). This association between EMR integration and CCDSS failure was not statistically significant via multivariate regression.
Improvement in process of care or patient outcomes was not affected by integration with CPOE, timing or method of decision support delivery, or method of data entry. Improvement in process or patient outcomes did not vary by country, provider type, and outpatient versus other settings of care. Systems trialed outside of academic settings were more likely to improve patient outcomes (5/12 (42%) outside academic settings versus 1/17 (6%) in academic settings, p = 0.03). This finding was not replicated in multivariate analyses. Investigators who developed the system under study were not significantly more likely to see improvement with a CCDSS than investigators studying systems developed by unrelated parties (p = 0.56 for process and p = 1 for patient outcomes). Patient important outcomes were as likely as surrogate outcomes to show improvement with a CCDSS. Post hoc, none of primary disease state, primary patient population, or pharmacotherapeutic purpose predicted success. We found no association between the presence of a sample size calculation and success or between number of trial participants and success. The proportion of studies added in this update demonstrating benefit with CCDSS for process of care and patient outcomes increased compared with studies included in the previous review version, although this trend was not statistically significant.
Costs and practical process related outcomes
Harms
Potential or actual harm resulting from CCDSS use was explicitly discussed in four (6%) [16, 21, 36, 68, 89] included studies (see Additional file 5, Table S5). Two studies reported quantitative data regarding harms. Raebel et al.[36] reported a trial stopped early due to a high rate (40%) of clinically inappropriate reminders generated by the CCDSS. Zanetti et al.[68] reported one inappropriate redose of intra-operative prophylactic antibiotic for every 137 appropriate redose reminders.
Costs
Some information on financial or economic costs associated with CCDSS was reported for 15 (23%) [17, 19, 24, 27, 43, 48, 49, 52, 53, 56, 57, 60, 66, 80, 83, 84] trials (see Additional file 5, Table S5). A formal cost-effectiveness analysis for a patient outcome was performed in only one case [53]. Twelve trials compared direct healthcare costs between CCDSSs and control groups with mixed results: significantly decreased costs were observed in six trials [27, 43, 48, 49, 52, 84], no significant change in five trials [53, 60, 66, 80, 83], and significantly increased costs in one trial [56].
User satisfaction
Fifteen authors reported on user satisfaction with the CCDSS studied (see Additional file 5, Table S5) [18, 19, 29, 30, 33, 39, 55, 57, 63, 64, 67, 69, 70, 75, 77–79, 83, 84]. All attempts to measure user satisfaction were conducted via surveys and the properties of the measure used were only discussed in a single trial [55]. Survey response rates ≥50% were found in eight studies. Of these eight, six reported [18, 29, 33, 55, 57, 84] that ≥70% of respondents thought the CCDSS improved care, was useful, or should be continued in use. Satisfaction data from the other two trials [77–79, 83] suggested users could not or would not use the CCDSS due to technical or user interface problems. Available data on user satisfaction were too sparse to determine if satisfaction impacted study results.
Discussion
We reviewed 65 RCTs of CCDSSs for drug therapy management reported over a 34-year span. Most trials measured process of care outcomes and results supported the use of CCDSSs to improve these outcomes in a majority of cases (improvement was based on at least 50% of the relevant study outcomes being statistically significantly positive). However, while nearly one-half of (29 studies) included studies measured a patient outcome, only a small proportion demonstrated any direct benefit to patients. While improvement in process outcomes could lead to benefits for patients, no consistent link was observed here. In the absence of data needed for an economic analysis, improved process of care measures alone are not sufficient to recommend adoption of these systems. The success rates we found for processs of care (64%) and clinical outcome measures (21%) are similar to those in our previous review [2] and also a recent umbrella review of systematic reviews of computerized decision support (57% and 30% respectively) by Jaspers et al.[91].
Several possible predictors of CCDSS success were examined. In most cases, these a priori factors did not explain success or failure across included studies. Our previous review [2] concluded that successful trials of CCDSS were more likely to have been conducted by the developers of the system under study. In our current review, no such association was noted. Previously, a significant trend towards increased study quality over time was noted, but not replicated in this update, and we attribute this to a more restrictive inclusion criterion (randomized controlled trials). Counter to our expectations, we found that integration of CCDSSs with EMRs and use in an academic setting was associated with CCDSS failure. This trend was not statistically significant when tested using multi-variate techniques and so we are unable to determine whether this finding represents a true association or is better explained by the lack of power in our multi-variate analysis. We report these findings as hypothesis generating only and suggest they be examined in future.
Compared with the review of Kawamoto et al.[92], we did not find that automatic provision of advice as part of the existing clinical workflow predicted CCDSS success. Because both the current analysis and that of Kawamoto were underpowered to detect such associations, we have refrained from drawing any conclusions in this regard.
Prospective data on the possible harms of CCDSSs are needed to facilitate informed adoption decisions. Only two trials quantitatively reported on harm from CCDSSs [36, 68] with one trial ending early due to increased risk of harm with the CCDSS. We suggest this absence of evidence of harm should not be taken as proof that CCDSSs are safe to employ for drug management in patient care.
Strengths and limitations of review
The results of our review should be interpreted with consideration of methodological strengths and limitations, including steps taken to mitigate the risk of bias. We based our review on the strongest studies available, RCTs. Reviews are necessarily retrospective and we employed multiple methods to limit the introduction of bias, including: duplicate study eligibility assessment, duplicate data abstraction, solicitation of study author feedback on abstracted data, and objective selection of outcomes used to determine improvement. We cannot exclude the possibility that a different method of selecting outcomes from each study to measure improvement could lead to different results, although sensitivity analyses did not suggest this to be the case. Several pre-specified analyses of possible predictors of system success were conducted. Several analyses demonstrated statistically significant results using univariate techniques that were not substantiated using a multi-variate model. Therefore, the few associations we reported between possible predictors of success and improved outcomes with CCDSS should be interpreted with caution.
We have relied upon vote counting as our method of obtaining an estimate of how often CCDSS for drug therapy management improve process or patient outcomes. Significant limitations to this approach as described by Hedges [93] include a tendency to inflate type II error and inadequate incorporation of the effect of unequal study sizes in overall results. The heterogeneity between studies included in our review precluded the use of more robust combination techniques. Formal assessment for publication bias using funnel plots was not possible with the vote-counting technique.
The effectiveness of any CCDSS will be determined in part by the efficacy of the underlying action suggested by the system. Where no benefit was detected with a particular CCDSS, we cannot exclude the possibility that the negative finding is due to a lack of efficacy of the intervention suggested by the system. Measurement of the concordance between decision advice given and followed would be a useful measure to address this issue. These outcomes were included in our analyses of process of care outcomes. It does not necessarily follow, however, that an effective CCDSS that recommends the appropriate prescription of an efficacious intervention will necessarily improve patient care. A multitude of intervening factors (e.g. patient non- or over-adherence or new errors introduced by CCDSS) may mitigate (or exaggerate) estimates of CCDSS effectiveness.
Finally, the systems reviewed constitute a heterogeneous group with differing functionality and clinical intent. While we have attempted to usefully divide the systems for the reader, we acknowledge other divisions were possible.
Implications for practice and research
Because CCDSSs have not been shown to reliably and positively impact patients, and in the absence of useful data on potential harms, costs, and clinician impacts, we cannot recommend the general adoption of CCDSSs for drug therapy management. It is possible that these systems are still evolving and success will improve with time. Clearly further innovation is needed if these systems are to be dependably useful in clinical practice. Rigorous trials of these innovations will be necessary, and we suggest that future research explicitly address patient outcomes, including potential harms, and costs and adverse clinician impacts of CCDSSs. Given the availability of effective non-computerized approaches for promoting safe and effective medication use [5, 94], future studies may wish to incorporate these interventions as active comparators to CCDSSs.
Conclusions
CCDSSs inconsistently improved process of care measures and seldom improved patient outcomes. Lack of clear patient benefit and lack of data on harms and costs preclude a recommendation to adopt CCDSSs for drug therapy management.
References
Sim I, Gorman P, Greenes RA, Haynes RB, Kaplan B, Lehmann H, Tang PC: Clinical decision support systems for the practice of evidence-based medicine. J Am Med Inform Assoc. 2001, 8 (6): 527-534. 10.1136/jamia.2001.0080527.
Garg AX, Adhikari NK, McDonald H, Rosas-Arellano MP, Devereaux PJ, Beyene J, Sam J, Haynes RB: Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: a systematic review. JAMA. 2005, 293 (10): 1223-1238. 10.1001/jama.293.10.1223.
Osheroff JA, Pifer EA, Teich JM, Sittig DF, Jenders RA, CDS Expert Review Panel: Improving outcomes with clinical decision support. 2005, Chicago, IL: Healthcare Information & Management Systems Society
Teich JM, Osheroff JA, Pifer EA, Sittig DF, Jenders RA, CDS Expert Review Panel: Clinical decision support in electronic prescribing: recommendations and an action plan: report of the joint clinical decision support workgroup. J Am Med Inform Assoc. 2005, 12 (4): 365-376. 10.1197/jamia.M1822.
Corrigan J, Donaldson MS, Kohn LT, Institute of Medicine. Committee on Quality of Healthcare in America: To err is human: building a safer health system. 2000, Washington, D.C.: National Academy Press
Berner ES: Clinical decision support systems: State of the Art. 2009, Rockville, Maryland: Agency for Healthcare Research and Quality
Touw DJ, Neef C, Thomson AH, Vinks AA, Cost-Effectiveness of Therapeutic Drug Monitoring Committee of the International Association for Therapeutic Drug Monitoring and Clinical Toxicology: Cost-effectiveness of therapeutic drug monitoring: a systematic review. Ther Drug Monit. 2005, 27 (1): 10-17. 10.1097/00007691-200502000-00004.
Hulley SB: Designing clinical research. 2007, Philadelphia, PA: Wolters Kluwer: Lippincott Williams & Wilkins, 3
Haynes RB, Wilczynski NL, Computerized Clinical Decision Support System (CCDSS) Systematic Review Team: Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: methods of a decision-maker-researcher partnership systematic review. Implement Sci. 2010, 5: 12-
Apkon M, Mattera JA, Lin Z, Herrin J, Bradley EH, Carbone M, Holmboe ES, Gross CP, Selter JG, Rich AS, Krumholz HM: A randomized outpatient trial of a decision-support information technology tool. Arch Intern Med. 2005, 165 (20): 2388-2394. 10.1001/archinte.165.20.2388.
Downs M, Turner S, Bryans M, Wilcock J, Keady J, Levin E, O'Carroll R, Howie K, Iliffe S: Effectiveness of educational interventions in improving detection and management of dementia in primary care: cluster randomised controlled study. BMJ. 2006, 332 (7543): 692-696. 10.1136/bmj.332.7543.692.
Holbrook A, Thabane L, Keshavjee K, Dolovich L, Bernstein B, Chan D, Troyan S, Foster G, Gerstein H, COMPETE II Investigators: Individualized electronic decision support and reminders to improve diabetes care in the community: COMPETE II randomized trial. CMAJ. 2009, 181 (1-2): 37-44. 10.1503/cmaj.081272.
Thomas JC, Moore A, Qualls PE: The effect on cost of medical care for patients treated with an automated clinical audit system. J Med Syst. 1983, 7 (3): 307-313. 10.1007/BF00993294.
Meigs JB, Cagliero E, Dubey A, Murphy-Sheehy P, Gildesgame C, Chueh H, Barry MJ, Singer DE, Nathan DM: A controlled trial of web-based diabetes disease management: the MGH diabetes primary care improvement project. Diabetes Care. 2003, 26 (3): 750-757. 10.2337/diacare.26.3.750.
Martin DC, Berger ML, Anstatt DT, Wofford J, Warfel D, Turpin RS, Cannuscio CC, Teutsch SM, Mansheim BJ: A randomized controlled open trial of population-based disease and case management in a Medicare Plus Choice health maintenance organization. Prev Chronic Dis. 2004, 1 (4): A05-
Bertoni AG, Bonds DE, Chen H, Hogan P, Crago L, Rosenberger E, Barham AH, Clinch CR, Goff DC: Impact of a multifaceted intervention on cholesterol management in primary care practices: guideline adherence for heart health randomized trial. Arch Intern Med. 2009, 169 (7): 678-686. 10.1001/archinternmed.2009.44.
Field TS, Rochon P, Lee M, Gavendo L, Baril JL, Gurwitz JH: Computerized clinical decision support during medication ordering for long-term care residents with renal insufficiency. J Am Med Inform Assoc. 2009, 16 (4): 480-485. 10.1197/jamia.M2981.
Fortuna RJ, Zhang F, Ross-Degnan D, Campion FX, Finkelstein JA, Kotch JB, Feldstein AC, Smith DH, Simon SR: Reducing the prescribing of heavily marketed medications: a randomized controlled trial. J Gen Intern Med. 2009, 24 (8): 897-903. 10.1007/s11606-009-1013-x.
Gilutz H, Novack L, Shvartzman P, Zelingher J, Bonneh DY, Henkin Y, Maislos M, Peleg R, Liss Z, Rabinowitz G, Vardy D, Zahger D, Ilia R, Leibermann N, Porath A: Computerized community cholesterol control (4C): meeting the challenge of secondary prevention. Israel Med Assoc J. 2009, 11 (1): 23-29.
Lo HG, Matheny ME, Seger DL, Bates DW, Gandhi TK: Impact of non-interruptive medication laboratory monitoring alerts in ambulatory care. J Am Med Inform Assoc. 2009, 16 (1): 66-71.
Rosenberger EL, Goff DC, Blackwell CS, Williams DT, Crago OL, Ellis SD, Bertoni AG, Bonds DE: Implementing a palm pilot intervention for primary care providers: lessons learned. Contemp Clin Trials. 2009, 30 (4): 321-325. 10.1016/j.cct.2009.03.009.
Smith DH, Feldstein AC, Perrin NA, Yang X, Rix MM, Raebel MA, Magid DJ, Simon SR, Soumerai SB: Improving laboratory monitoring of medications: an economic analysis alongside a clinical trial. Am J Manag Care. 2009, 15 (5): 281-289.
Terrell KM, Perkins AJ, Dexter PR, Hui SL, Callahan CM, Miller DK: Computerized decision support to reduce potentially inappropriate prescribing to older emergency department patients: a randomized, controlled trial. J Am Geriatr Soc. 2009, 57 (8): 1388-1394. 10.1111/j.1532-5415.2009.02352.x.
Field TS, Rochon P, Lee M, Gavendo L, Subramanian S, Hoover S, Baril J, Gurwitz J: Costs associated with developing and implementing a computerized clinical decision support system for medication dosing for patients with renal insufficiency in the long-term care setting. J Am Med Inform Assoc. 2008, 15 (4): 466-472. 10.1197/jamia.M2589.
Gurwitz JH, Field TS, Rochon P, Judge J, Harrold LR, Bell CM, Lee M, White K, LaPrino J, Erramuspe-Mainard J, DeFlorio M, Gavendo L, Baril JL, Reed G, Bates DW: Effect of computerized provider order entry with clinical decision support on adverse drug events in the long-term care setting. J Am Geriatr Soc. 2008, 56 (12): 2225-2233. 10.1111/j.1532-5415.2008.02004.x.
Hicks LS, Sequist TD, Ayanian JZ, Shaykevich S, Fairchild DG, Orav EJ, Bates DW: Impact of computerized decision support on blood pressure management and control: a randomized controlled trial. J Gen Intern Med. 2008, 23 (4): 429-441. 10.1007/s11606-007-0403-1.
Javitt JC, Rebitzer JB, Reisman L: Information technology and medical missteps: evidence from a randomized trial. J Health Econ. 2008, 27 (3): 585-602. 10.1016/j.jhealeco.2007.10.008.
Matheny ME, Sequist TD, Seger AC, Fiskio JM, Sperling M, Bugbee D, Bates DW, Gandhi TK: A randomized trial of electronic clinical reminders to improve medication laboratory monitoring. J Am Med Inform Assoc. 2008, 15 (4): 424-429. 10.1197/jamia.M2602.
Quinn CC, Clough SS, Minor JM, Lender D, Okafor MC, Gruber-Baldini A: WellDoc™ mobile diabetes management randomized controlled trial: change in clinical and behavioral outcomes and patient and physician satisfaction. Diabetes Technol Ther. 2008, 10 (3): 160-168. 10.1089/dia.2008.0283.
Reeve JF, Tenni PC, Peterson GM: An electronic prompt in dispensing software to promote clinical interventions by community pharmacists: a randomized controlled trial. Br J Clin Pharmacol. 2008, 65 (3): 377-385. 10.1111/j.1365-2125.2007.03012.x.
van Wyk JT, van Wijk MA, Sturkenboom MC, Mosseveld M, Moorman PW, van der Lei J: Electronic alerts versus on-demand decision support to improve dyslipidemia treatment: a cluster randomized controlled trial. Circulation. 2008, 117 (3): 371-378. 10.1161/CIRCULATIONAHA.107.697201.
Davis RL, Wright J, Chalmers F, Levenson L, Brown JC, Lozano P, Christakis DA: A cluster randomized clinical trial to improve prescribing patterns in ambulatory pediatrics. PLoS Clin Trials. 2007, 2 (5): e25-10.1371/journal.pctr.0020025.
Heidenreich PA, Gholami P, Sahay A, Massie B, Goldstein MK: Clinical reminders attached to echocardiography reports of patients with reduced left ventricular ejection fraction increase use of beta-blockers: a randomized trial. Circulation. 2007, 115 (22): 2829-2834. 10.1161/CIRCULATIONAHA.106.684753.
Martens JD, van der Weijden T, Severens JL, de Clercq PA, de Bruijn DP, Kester AD, Winkens RA: The effect of computer reminders on GPs' prescribing behaviour: a cluster-randomised trial. Int J Med Inform. 2007, 76 (Suppl 3): S403-S416.
Peterson JF, Rosenbaum BP, Waitman LR, Habermann R, Powers J, Harrell D, Miller RA: Physicians' response to guided geriatric dosing: initial results from a randomized trial. Stud Health Technol Inform. 2007, 129 (Pt 2): 1037-1040.
Raebel MA, Carroll NM, Kelleher JA, Chester EA, Berga S, Magid DJ: Randomized trial to improve prescribing safety during pregnancy. J Am Med Inform Assoc. 2007, 14 (4): 440-450. 10.1197/jamia.M2412.
Raebel MA, Charles J, Dugan J, Carroll NM, Korner EJ, Brand DW, Magid DJ: Randomized trial to improve prescribing safety in ambulatory elderly patients. J Am Geriatr Soc. 2007, 55 (7): 977-985. 10.1111/j.1532-5415.2007.01202.x.
Thomson RG, Eccles MP, Steen IN, Greenaway J, Stobbart L, Murtagh MJ, May CR: A patient decision aid to support shared decision-making on anti-thrombotic treatment of patients with atrial fibrillation: randomised controlled trial. Qual Saf Healthcare. 2007, 16 (3): 216-223. 10.1136/qshc.2006.018481.
Verstappen SMM, Jacobs JWG, Van der Veen MJ, Heurkens AHM, Schenk Y, Ter Borg EJ, Blaauw AAM, Bijlsma JWJ: Intensive treatment with methotrexate in early rheumatoid arthritis: aiming for remission. Computer Assisted Management in Early Rheumatoid Arthritis (CAMERA, an open-label strategy trial). Ann Rheum Dis. 2007, 66 (11): 1443-1449. 10.1136/ard.2007.071092.
Feldstein A, Elmer PJ, Smith DH, Herson M, Orwoll E, Chen C, Aickin M, Swain MC: Electronic medical record reminder improves osteoporosis management after a fracture: a randomized, controlled trial. J Am Geriatr Soc. 2006, 54 (3): 450-457. 10.1111/j.1532-5415.2005.00618.x.
Feldstein AC, Smith DH, Perrin N, Yang X, Rix M, Raebel MA, Magid DJ, Simon SR, Soumerai SB: Improved therapeutic monitoring with several interventions: a randomized trial. Arch Intern Med. 2006, 166 (17): 1848-1854. 10.1001/archinte.166.17.1848.
Judge J, Field TS, DeFlorio M, Laprino J, Auger J, Rochon P, Bates DW, Gurwitz JH: Prescribers' responses to alerts during medication ordering in the long term care setting. J Am Med Inform Assoc. 2006, 13 (4): 385-390. 10.1197/jamia.M1945.
Kattan M, Crain EF, Steinbach S, Visness CM, Walter M, Stout JW, Evans Iii R, Smartt E, Gruchalla RS, Morgan WJ: A randomized clinical trial of clinician feedback to improve quality of care for inner-city children with asthma. Pediatrics. 2006, 117 (6): e1095-e1103. 10.1542/peds.2005-2160.
Kuilboer MM, van Wijk MA, Mosseveld M, van der Does E, de Jongste JC, Overbeek SE, Ponsioen B, van der Lei J: Computed critiquing integrated into daily clinical practice affects physicians' behavior--a randomized clinical trial with AsthmaCritic. Methods Inf Med. 2006, 45 (4): 447-454.
Lester WT, Grant RW, Barnett GO, Chueh HC: Randomized controlled trial of an informatics-based intervention to increase statin prescription for secondary prevention of coronary disease. J Gen Intern Med. 2006, 21 (1): 22-29. 10.1111/j.1525-1497.2005.00268.x.
Martens JD, van der Aa A, Panis B, van der Weijden T, Winkens RA, Severens JL: Design and evaluation of a computer reminder system to improve prescribing behaviour of GPs. Stud Health Technol Inform. 2006, 124: 617-623.
Palen TE, Raebel M, Lyons E, Magid DM: Evaluation of laboratory monitoring alerts within a computerized physician order entry system for medication orders. Am J Manag Care. 2006, 12 (7): 389-395.
Paul M, Andreassen S, Tacconelli E, Nielsen AD, Almanasreh N, Frank U, Cauda R, Leibovici L, TREAT Study G: Improving empirical antibiotic treatment using TREAT, a computerized decision support system: cluster randomized trial. J Antimicrob Chemother. 2006, 58 (6): 1238-1245. 10.1093/jac/dkl372.
Cobos A, Vilaseca J, Asenjo C, Pedro-Botet J, Sanchez E, Val A, Torremade E, Espinosa C, Bergonon S: Cost effectiveness of a clinical decision support system based on the recommendations of the European Society of Cardiology and other societies for the management of hypercholesterolemia: Report of a cluster-randomized trial. Dis Manag Health Out. 2005, 13 (6): 421-432. 10.2165/00115677-200513060-00007.
Derose SF, Dudl JR, Benson VM, Contreras R, Nakahiro RK, Ziel FH: Point of service reminders for prescribing cardiovascular medications. Am J Manag Care. 2005, 11 (5): 298-304.
Heidenreich PA, Chacko M, Goldstein MK, Atwood JE: ACE inhibitor reminders attached to echocardiography reports of patients with reduced left ventricular ejection fraction. Am J Med. 2005, 118 (9): 1034-1037. 10.1016/j.amjmed.2004.12.028.
Javitt JC, Steinberg G, Locke T, Couch JB, Jacques J, Juster I, Reisman L: Using a claims data-based sentinel system to improve compliance with clinical guidelines: results of a randomized prospective study. Am J Manag Care. 2005, 11 (2): 93-102.
Plaza V, Cobos A, Ignacio-Garcia JM, Molina J, Bergonon S, Garcia-Alonso F, Espinosa C, Grupo Investigador A: [Cost-effectiveness of an intervention based on the Global INitiative for Asthma (GINA) recommendations using a computerized clinical decision support system: a physicians randomized trial]. Med Clin (Barc). 2005, 124 (6): 201-206. 10.1157/13071758.
Raebel MA, Lyons EE, Chester EA, Bodily MA, Kelleher JA, Long CL, Miller C, Magid DJ: Improving laboratory monitoring at initiation of drug therapy in ambulatory care: A randomized trial. Arch Intern Med. 2005, 165 (20): 2395-2401. 10.1001/archinte.165.20.2395.
Sequist TD, Gandhi TK, Karson AS, Fiskio JM, Bugbee D, Sperling M, Cook EF, Orav EJ, Fairchild DG, Bates DW: A randomized trial of electronic clinical reminders to improve quality of care for diabetes and coronary artery disease. J Am Med Inform Assoc. 2005, 12 (4): 431-437. 10.1197/jamia.M1788.
Tierney WM, Overhage JM, Murray MD, Harris LE, Zhou XH, Eckert GJ, Smith FE, Nienaber N, McDonald CJ, Wolinsky FD: Can computer-generated evidence-based care suggestions enhance evidence-based management of asthma and chronic obstructive pulmonary disease? A randomized, controlled trial. Health Serv Res. 2005, 40 (2): 477-497. 10.1111/j.1475-6773.2005.0t369.x.
Wolfenden L, Wiggers J, Knight J, Campbell E, Spigelman A, Kerridge R, Moore K: Increasing smoking cessation care in a preoperative clinic: a randomized controlled trial. Prev Med. 2005, 41 (1): 284-290. 10.1016/j.ypmed.2004.11.011.
Krall MA, Traunweiser K, Towery W: Effectiveness of an electronic medical record clinical quality alert prepared by off-line data analysis. Stud Health Technol Inform. 2004, 107 (Pt 1): 135-139.
Lester WT, Grant R, Barnett GO, Chueh H: Facilitated lipid management using interactive e-mail: preliminary results of a randomized controlled trial. Stud Health Technol Inform. 2004, 107 (Pt 1): 232-236.
Murray MD, Harris LE, Overhage JM, Zhou XH, Eckert GJ, Smith FE, Buchanan NN, Wolinsky FD, McDonald CJ, Tierney WM: Failure of computerized treatment suggestions to improve health outcomes of outpatients with uncomplicated hypertension: results of a randomized controlled trial. Pharmacotherapy. 2004, 24 (3): 324-337. 10.1592/phco.24.4.324.33173.
Ansari M, Shlipak MG, Heidenreich PA, Van Ostaeyen D, Pohl EC, Browner WS, Massie BM: Improving guideline adherence: a randomized trial evaluating strategies to increase beta-blocker use in heart failure. Circulation. 2003, 107 (22): 2799-2804. 10.1161/01.CIR.0000070952.08969.5B.
Filippi A, Sabatini A, Badioli L, Samani F, Mazzaglia G, Catapano A, Cricelli C: Effects of an automated electronic reminder in changing the antiplatelet drug-prescribing behavior among Italian general practitioners in diabetic patients: an intervention trial. Diabetes Care. 2003, 26 (5): 1497-1500. 10.2337/diacare.26.5.1497.
Flottorp S, Havelsrud K, Oxman AD: Process evaluation of a cluster randomized trial of tailored interventions to implement guidelines in primary care--why is it so hard to change practice?. Fam Pract. 2003, 20 (3): 333-339. 10.1093/fampra/cmg316.
Rousseau N, McColl E, Newton J, Grimshaw J, Eccles M: Practice based, longitudinal, qualitative interview study of computerised evidence based guidelines in primary care. BMJ. 2003, 326 (7384): 314-10.1136/bmj.326.7384.314.
Tamblyn R, Huang A, Perreault R, Jacques A, Roy D, Hanley J, McLeod P, Laprise R: The medical office of the 21st century (MOXXI): effectiveness of computerized decision-making support in reducing inappropriate prescribing in primary care. Can Med Assoc J. 2003, 169 (6): 549-556.
Tierney WM, Overhage JM, Murray MD, Harris LE, Zhou XH, Eckert GJ, Smith FE, Nienaber N, McDonald CJ, Wolinsky FD: Effects of computerized guidelines for managing heart disease in primary care. J Gen Intern Med. 2003, 18 (12): 967-976. 10.1111/j.1525-1497.2003.30635.x.
Weir CJ, Lees KR, MacWalter RS, Muir KW, Wallesch CW, McLelland EV, Hendry A, PRISM Study G: Cluster-randomized, controlled trial of computer-based decision support for selecting long-term anti-thrombotic therapy after acute ischaemic stroke. QJM. 2003, 96 (2): 143-153.
Zanetti G, Flanagan HL, Cohn LH, Giardina R, Platt R: Improvement of intraoperative antibiotic prophylaxis in prolonged cardiac surgery by automated alerts in the operating room. Infect Control Hosp Epidemiol. 2003, 24 (1): 13-16. 10.1086/502109.
Eccles M, McColl E, Steen N, Rousseau N, Grimshaw J, Parkin D, Purves I: Effect of computerised evidence based guidelines on management of asthma and angina in adults in primary care: cluster randomised controlled trial. BMJ. 2002, 325 (7370): 941-10.1136/bmj.325.7370.941.
Flottorp S, Oxman AD, Havelsrud K, Treweek S, Herrin J: Cluster randomised controlled trial of tailored interventions to improve the management of urinary tract infections in women and sore throat. BMJ. 2002, 325 (7360): 367-10.1136/bmj.325.7360.367.
Lesourd F, Avril C, Boujennah A, Parinaud J: A computerized decision support system for ovarian stimulation by gonadotropins. Fertil Steril. 2002, 77 (3): 456-460. 10.1016/S0015-0282(01)03231-9.
Selker HP, Beshansky JR, Griffith JL, TPI Trial Investigators: Use of the electrocardiograph-based thrombolytic predictive instrument to assist thrombolytic and reperfusion therapy for acute myocardial infarction. A multicenter, randomized, controlled, clinical effectiveness trial. Ann Intern Med. 2002, 137 (2): 87-95.
Christakis DA, Zimmerman FJ, Wright JA, Garrison MM, Rivara FP, Davis RL: A randomized controlled trial of point-of-care evidence to improve the antibiotic prescribing practices for otitis media in children. Pediatrics. 2001, 107 (2): E15-10.1542/peds.107.2.e15.
Dexter PR, Perkins S, Overhage JM, Maharry K, Kohler RB, McDonald CJ: A computerized reminder system to increase the use of preventive care for hospitalized patients. N Engl J Med. 2001, 345 (13): 965-970. 10.1056/NEJMsa010181.
McCowan C, Neville RG, Ricketts IW, Warner FC, Hoskins G, Thomas GE: Lessons from a randomized controlled trial designed to evaluate computer decision support software to improve the management of asthma. Med Inform Internet. 2001, 26 (3): 191-201. 10.1080/14639230110067890.
Demakis JG, Beauchamp C, Cull WL, Denwood R, Eisen SA, Lofgren R, Nichol K, Woolliscroft J, Henderson WG: Improving residents' compliance with standards of ambulatory care: results from the VA Cooperative Study on Computerized Reminders. JAMA. 2000, 284 (11): 1411-1416. 10.1001/jama.284.11.1411.
Hetlevik I, Holmen J, Krüger O, Kristensen P, Iversen H, Furuseth K: Implementing clinical guidelines in the treatment of diabetes mellitus in general practice. Evaluation of effort, process, and patient outcome related to implementation of a computer-based decision support system. Int J Technol Assess. 2000, 16 (1): 210-227. 10.1017/S0266462300161185.
Hetlevik I, Holmen J, Krüger O: Implementing clinical guidelines in the treatment of hypertension in general practice. Evaluation of patient outcome related to implementation of a computer-based clinical decision support system. Scand J Prim Healthcare. 1999, 17 (1): 35-40. 10.1080/028134399750002872.
Hetlevik I, Holmen J, Kruger O, Kristensen P, Iversen H: Implementing clinical guidelines in the treatment of hypertension in general practice. Blood Press. 1998, 7 (5-6): 270-276. 10.1080/080370598437114.
Overhage JM, Tierney WM, Zhou XH, McDonald CJ: A randomized trial of "corollary orders" to prevent errors of omission. J Am Med Inform Assoc. 1997, 4 (5): 364-375. 10.1136/jamia.1997.0040364.
Rossi RA, Every NR: A computerized intervention to decrease the use of calcium channel blockers in hypertension. J Gen Intern Med. 1997, 12 (11): 672-678. 10.1046/j.1525-1497.1997.07140.x.
Overhage JM, Tierney WM, McDonald CJ: Computer reminders to implement preventive care guidelines for hospitalized patients. Arch Intern Med. 1996, 156 (14): 1551-1556. 10.1001/archinte.156.14.1551.
Rotman BL, Sullivan AN, McDonald TW, Brown BW, DeSmedt P, Goodnature D, Higgins MC, Suermondt HJ, Young C, Owens DK: A randomized controlled trial of a computer-based physician workstation in an outpatient setting: implementation barriers to outcome evaluation. J Am Med Inform Assoc. 1996, 3 (5): 340-348. 10.1136/jamia.1996.97035025.
Tierney WM, Miller ME, Overhage JM, McDonald CJ: Physician inpatient order writing on microcomputer workstations. Effects on resource utilization. JAMA. 1993, 269 (3): 379-383. 10.1001/jama.269.3.379.
Mazzuca SA, Vinicor F, Einterz RM, Tierney WM, Norton JA, Kalasinski LA: Effects of the clinical environment on physicians' response to postgraduate medical education. Am Educ Res J. 1990, 27 (3): 473-488.
McAlister NH, Covvey HD, Tong C, Lee A, Wigle ED: Randomised controlled trial of computer assisted management of hypertension in primary care. Br Med J (Clin Res Ed). 1986, 293 (6548): 670-674. 10.1136/bmj.293.6548.670.
McDonald CJ, Wilson GA, McCabe GP: Physician response to computer reminders. JAMA. 1980, 244 (14): 1579-1581. 10.1001/jama.244.14.1579.
Coe FL, Norton E, Oparil S, Tatar A, Pullman TN: Treatment of hypertension by computer and physician-a prospective controlled study. J Chronic Dis. 1977, 30 (2): 81-92. 10.1016/0021-9681(77)90077-7.
McDonald CJ: Use of a computer to detect and respond to clinical events: its effect on clinician behavior. Ann Intern Med. 1976, 84 (2): 162-167.
Bucher HC, Kunz R, Cook DJ, Holbrook AM, Guyatt G: Surrogate Outcomes. Users' guides to the medical literature: a manual for evidence-based clinical practice. Edited by: Guyatt G, Rennie D. 2008, Evidence-Based Medicine Working Group. Chicago, IL: McGraw-Hill Professional, 442-2
Jaspers MW, Smeulers M, Vermeulen H, Peute LW: Effects of clinical decision-support systems on practitioner performance and patient outcomes: a synthesis of high-quality systematic review findings. J Am Med Inform Assoc. 2011, 18 (3): 327-334. 10.1136/amiajnl-2011-000094.
Kawamoto K, Houlihan CA, Balas EA, Lobach DF: Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ. 2005, 330 (7494): 765-10.1136/bmj.38398.500764.8F.
Hedges LV, Olkin I: Statistical methods for meta-analysis. 1985, Orlando: Academic Press
Grimshaw J, Eccles M, Thomas R, MacLennan G, Ramsay C, Fraser C, Vale L: Toward evidence-based quality improvement. Evidence (and its limitations) of the effectiveness of guideline dissemination and implementation strategies 1966-1998. J Gen Intern Med. 2006, 21 (Suppl 2): S14-S20.
Acknowledgements
The research was funded by a Canadian Institutes of Health Research Synthesis Grant: Knowledge Translation KRS 91791. The members of the Computerized Clinical Decision Support System (CCDSS) Systematic Review Team included the Principal Investigator, Co-Investigators, Co-Applicants/Senior Management Decision-makers, Co-Applicants/Clinical Service Decision-Makers, and Research Staff. The following were involved in collection and/or organization of data: Jeanette Prorok, MSc, McMaster University; Nathan Souza, MD, MMEd, McMaster University; Brian Hemens, BScPhm, MSc, McMaster University; Robby Nieuwlaat, PhD, McMaster University; Shikha Misra, BHSc, McMaster University; Jasmine Dhaliwal, BHSc, McMaster University; Navdeep Sahota, BHSc, University of Saskatchewan; Anita Ramakrishna, BHSc, McMaster University; Pavel Roshanov, BSc, McMaster University; Tahany Awad, MD, McMaster University. Nicholas Hobson, DiplT, Chris Cotoi, BEng, EMBA, and Rick Parrish, DiplT, at McMaster University provided programming and information technology support.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Competing interests
RBH, NLW, JAM, LWK, TN, BJH, AH, MT received support through the Canadian Institutes of Health Research Synthesis Grant: Knowledge Translation KRS 91791 for the submitted work. RBH is acquainted with several CCDSS developers and researchers, including authors of papers included in this review.
Authors' contributions
RBH was responsible for study conception and design; acquisition, analysis, and interpretation of data; drafting and critical revision of the manuscript; obtaining funding; study supervision. He is the guarantor. BJH acquired, analyzed and interpreted data; drafted the manuscript; and conducted statistical analysis. AH analyzed and interpreted data as well as critically revised the manuscript. MT critically revised the manuscript. JAM acquired, analyzed, and interpreted data; drafted the manuscript; and provided statistical analysis. LWK and TN acquired data and drafted the manuscript. NLW acquired, analyzed, and interpreted data; provided administrative, technical, or material support; and provided study supervision. All authors read and approved the final manuscript.
Electronic supplementary material
13012_2011_411_MOESM1_ESM.DOCX
Additional File 1: Study methods scores for trials of drug prescribing. Methods scores for the included studies. (DOCX 24 KB)
13012_2011_411_MOESM2_ESM.DOCX
Additional File 2: CCDSS characteristics for trials of drug prescribing. CCDSS characteristics of the included studies. (DOCX 47 KB)
13012_2011_411_MOESM3_ESM.DOCX
Additional File 3: Study characteristics for trials of drug prescribing. Study characteristics of the included studies. (DOCX 48 KB)
13012_2011_411_MOESM5_ESM.DOCX
Additional File 5: Costs and CCDSS process-related outcomes for drug prescribing. Cost and CCDSS process-related outcomes for the included studies. (DOCX 33 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Hemens, B.J., Holbrook, A., Tonkin, M. et al. Computerized clinical decision support systems for drug prescribing and management: A decision-maker-researcher partnership systematic review. Implementation Sci 6, 89 (2011). https://doi.org/10.1186/1748-5908-6-89
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1748-5908-6-89