Abstract
Background
Interspecific recombinant viruses R1ΔgC and R2ΔgI were isolated after in vitro co-infection with BoHV-1 and BoHV-5, two closely related alphaherpesviruses that infect cattle. The genetic characterization of R1ΔgC and R2ΔgI showed that they are composed of different sections of the parental genomes. The aim of this study was the characterization of the in vivo behavior of these recombinants in the natural host.
Results
Four groups of four 3-month-old calves of both genders were intranasally inoculated with either the recombinant or parental viruses. A control group of two animals was also included. Viral excretion and clinical signs were monitored after infection. Histopathological examination of the central nervous system (CNS) was performed and the establishment of latency in trigeminal ganglia was analyzed by PCR. The humoral response was also evaluated using ELISA tests.
Three out of four animals from the BoHV-5 infected group excreted virus for 4-10 days. Two calves shed R1ΔgC virus for one day. In R2ΔgI and BoHV-1.2ΔgCΔgI groups, infectious virus was isolated only after two or three blind passages. None of the infected animals developed neurological signs, although those infected with BoHV-5 showed histopathological evidence of viral infection. Latent viral DNA was detected in at least one calf from each infected group. Serum and/or mucosal antibodies were detected in all groups.
Conclusion
Both BoHV-1/-5 recombinants and the BoHV-1 parental strain are attenuated in calves, although they are able to replicate in animals at low rates and to establish latent infections.
Similar content being viewed by others
Background
Bovine herpesviruses 1 (BoHV-1) and 5 (BoHV-5) are two closely related alphaherpesviruses infecting cattle. BoHV-1 respiratory infection is usually associated with infectious bovine rhinotracheitis, abortion, and systemic infection in neonates [1]. BoHV-5 infection induces respiratory and nervous diseases, i.e. meningoencephalitis [2]. Both viruses are neurotropic and establish a life-long latent infection in neurons of sensory ganglia [2, 3].
BoHV-1 infection is present in all continents, although there are differences in prevalence and incidence [4]. In the USA, Canada, Australia and New Zealand the seroprevalence of BoHV-1 infection is variable but may be very high. BoHV-1 is also present in European countries, where a variety of control programs are carried out. Concerning South American countries, BoHV-1 infection is endemic and the seroprevalence is high [5]. On the other hand, BoHV-5 outbreaks are sporadic and appear to be very restricted in their geographical distribution, being mostly detected in the Southern hemisphere [2]. In Argentina and Brazil, BoHV-1 and BoHV-5 co-circulate and vaccination against BoHV-1 is not obligatory. In addition, cattle co-infection has been recently shown to exist [6]. In this context, in vivo genetic recombination between these two viruses may occur.
Recombination is a frequent event among alphaherpesviruses, enhancing their diversity and modifying virus replication properties in vivo [7]. Although the process of interspecific recombination appears inefficient relative to intraspecific recombination [8], a natural interspecies recombinant virus between equid herpesviruses 1 (EHV-1) and 4 (EHV-4) has been recently reported after analyzing EHV-1 field strains [9]. Both the recombinant virus and the parental EHV-4 were found to be non-neuropathogenic in the hamster model [9].
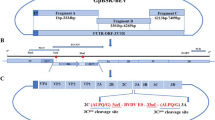
Regarding bovine alphaherpesviruses, two recombinant viruses have been isolated from in vitro interspecific recombination between a genetically modified strain of BoHV-1 (BoHV-1.2ΔgCΔgI) and BoHV-5 strain N569 [8]. The genetic composition of these recombinants, named R1ΔgC and R2ΔgI, has been published recently [10]. R1ΔgC is composed mainly of BoHV-5 (63%) whereas R2ΔgI is 86% homologous to BoHV-1 (Figure 1). Recombination could have a deep impact on BoHV-1 and BoHV-5 pathogenesis and epidemiology since it could lead to virulence restoration of attenuated strains or to an increase in pathogenicity by acquisition of virulence-associated genes. However, the consequences of recombination on R1ΔgC and R2ΔgI in vivo behavior have not been studied yet. The aim of this study was to assess the in vivo behavior of two BoHV-1/-5 interspecific recombinant viruses in calves. This study constitutes the first report on the evaluation of interspecific recombinants in the natural host.
Representation of the genetic background of the four viruses inoculated in this study. Parental strains: wild-type BoHV-5 (N569 strain) and mutant BoHV-1ΔgCΔgI; interspecific recombinants: R1ΔgC and R2ΔgI. The organization of the four genomes includes a unique long (UL) and a unique short (US) sequence. The latter is flanked by two repeated and inverted sequences (internal repeat, IR; terminal repeat, TR). The parental BoHV-1 is a mutated strain in which the genes encoding the glycoproteins gC (UL44) and gI (US7) were replaced by sequences encoding fluorescent proteins GFP and RFP respectively (labeled by large asterisks). The BoHV-5 and BoHV-1 sequences are represented by black or white lines and rectangles respectively. The site of recombination in each recombinant virus is represented by discontinuous lines.
Methods
Viruses and cell culture
The viruses used in this study are illustrated in Figure 1. Viral stocks -with high number of passages- were kindly provided by Virology and Immunology laboratory of University of Liege. The viruses were propagated in Madin Darby bovine kidney (MDBK, ATCC, USA) cells at a low multiplicity of infection as previously described [11]. Total DNA (viral and cell DNA) was prepared from infected cells using an extraction buffer 2× (Tris 200 mM pH 8, EDTA 20 mM pH 8, NaCl 200 mM, SDS 2%, 2-mercaptoethanol 20 mM) (Sigma-Aldrich, USA) and precipitated by alcohol.
Animal experiments
Eighteen 3-month-old calves of both genders were randomly separated in four groups of four animals each and one control group of two animals. Their naïve status for BoHV-1 and BoHV-5 exposure was verified by an enzyme-linked immunosorbent assay (ELISA) and seroneutralization assays (SN) [11] and virus isolation from nasal samples upon arrival and before experimental infection. After infection, all groups were strictly isolated from each other in order to prevent spread and contamination with the different virus strains used. Animal care and experimental procedures were performed in accordance with the requirements of the National Institute of Agricultural Technology Ethics Committee (INTA, Argentina).
Calves were infected intranasally (aerosolization) with 3 ml containing a total dose of 106.5 TCID50/ml (1.5 ml in each nostril) of the corresponding viral strain. The control group received 3 ml of cell culture medium. Animals were examined both before the start of the experience and daily after infection by a veterinarian who was not aware of the treatment of individual animals. Respiratory and nervous signs and rectal temperature were recorded. Between days 35-37 post-infection (pi) animals were sedated with acepromazine (Acedan, Holliday Scott S.A., Argentina) by the intramuscular route and then euthanized by barbiturate overdose (Euthanyle, Brouwer, Argentina). Necropsy was performed immediately and encephalon and trigeminal ganglia were dissected.
Sampling procedure, serological and virological analysis
Blood samples were taken on the day of inoculation and then weekly. Serum antibodies against BoHV-1 were detected by using an indirect ELISA and by SN assays [11].
Nasal samples were collected daily from day 0 to day 18 pi and then twice a week until the end of the experiment and inoculated onto MDBK cell monolayers immediately after collection. Titers were calculated by the Reed and Muench method. Three blind passages were performed on negative samples. Parental and recombinant viral strains were identified either by observation of infected cells with an epifluorescent microscope or by PCR assays using total DNA extracted from infected cells [10]. IgA antibodies in nasal secretions were determined by ELISA as previously described [11].
Histopathology and detection of latent BoHV DNA in trigeminal ganglia
Samples of CNS (olfactory, frontal, parietal and occipital areas, thalamus, midbrain, pons, obex and cerebellum) and trigeminal ganglion were fixed in 10% buffered formaline solution, embedded in paraffin, sectioned at 4 μm and stained with hematoxylin-eosin (H/E) for histopathological examination.
Pieces of approximately 100 mg of trigeminal ganglion stored at -70°C were digested and total DNA was extracted using the QIAamp DNA Mini kit (Qiagen, Tecnolab S.A., Argentina). Total DNA was subjected to two PCR reactions [12, 13] in order to examine the presence of viral DNA. PCR was also performed with a set of primers generating a 250 bp fragment from the bovine glyceraldehyde-3-phosphate dehydrogenase gene (GAPDH), selected as the bovine housekeeping gene. The primers were designated as GAPDH-For (5'-GCA TCG TGG AGG GAC TTA TGA-3') and GAPDH-Rev (5'-GGG CCA TCC ACA GTC TTC TG-3').
Results
Clinical and viral response after intranasal infection of cattle
In order to study the in vivo behavior of the BoHV-1/-5 recombinant viruses R1ΔgC and R2ΔgI (Figure 1), two groups of four animals were intranasally inoculated as described in Materials and Methods. Two other groups were infected accordingly with the parental strains, BoHV-5 and BoHV-1.2ΔgCΔgI. A mock infected control group of two animals was included.
Calves infected with R1ΔgC, R2ΔgI and parental BoHV-1.2ΔgCΔgI viruses did not show clinical signs or body temperature variations during the period studied (Table 1).
Viral excretion was observed in three out of four animals from the BoHV-5 infected group for 4-10 days, with peak titers of 4.3 to 5.3 log10 TCID50/ml. In contrast, the tested recombinants and parental BoHV-1.2ΔgCΔgI displayed reduced in vivo replication capacities (Table 1). Two calves shed R1ΔgC virus for one day with excretion titers of 2.3 and 2.5 log10 TCID50/ml, respectively. Concerning the R2ΔgI and BoHV-1.2ΔgCΔgI groups, infectious virus was isolated only after two or three blind passages on cell cultures infected with nasal swabs. The day of excretion onset for each group was variable and is detailed in Table 1. At least one positive isolate was observed in each group.
Post mortem analysis and establishment of latency
The histopathological examination performed in CNS samples revealed a severe non-suppurative meningoencephalitis in olfactory, frontal, parietal and occipital cortex, thalamus, midbrain and cerebellum in two of the BoHV-5-infected animals. Lesions were characterized by perivascular infiltration of mononuclear cells (mostly lymphocytes), focal gliosis and focal malacia (Figure 2). In addition, three animals showed slight vascular alterations in the trigeminal ganglion. In contrast, animals infected with R1ΔgC, R2ΔgI and parental BoHV-1.2ΔgCΔgI presented either slight or no histological alterations in all the sections examined.
To investigate the establishment of latency by the different viruses, trigeminal ganglia were dissected after euthanasia 35 days pi. PCR assays were performed to test for the presence of either BoHV-1 or BoHV-5 latent DNA. In addition, trigeminal ganglia were co-cultured with susceptible cells and monitored for BoHV replication. Since no cytopathic effect developed in the cultures, we concluded that the positive PCR results (Table 1) were due to the presence of latent viral genomes. The presence of viral DNA was observed in at least one calf from each of the infected groups, thus indicating that both the recombinant viruses and the parental strains were able to establish a latent infection in trigeminal ganglia.
Humoral immune response
The immune response specifically raised against BoHV-1 and BoHV-5 after the experimental infection was measured either by ELISA or by the SN assay. Seroconversion was detected in three out of the four BoHV-5-infected animals as well as in one animal from the R1ΔgC group (Table 1). Mucosal IgA was detected in all the infected groups but not in mock-infected calves (Table 1).
In summary, the data show that all the viruses were able to replicate in calves although R1ΔgC, R2ΔgI and parental BoHV-1.2ΔgCΔgI were attenuated in vivo. Detectable virus excretion by animals infected with these viruses was low and none of the infected animals developed clinical signs during the experimental infection.
Discussion
BoHV-1 and BoHV-5 have been shown to co-circulate in nature and cattle co-infection has been recently demonstrated [6]. Even if the process of interspecific recombination appears inefficient relative to intraspecific recombination, a natural interspecies recombinant virus between EHV-1 and EHV-4 has been reported by analyzing EHV-1 field strains [9]. Therefore, BoHV-1 and BoHV-5 may give rise to interspecific recombinants with unknown biological characteristics in co-infected cattle. Recombination may lead to virulence restoration of attenuated strains or to a decrease in the pathogenicity of parental viruses. Regarding this, there are several reports describing the generation of virulent HSV-1 and PrV intraspecific recombinants [14, 15] and avirulent HSV-1/-2 interspecific recombinants [16].
The in vivo behavior of the in vitro-generated interspecific recombinant viruses between BoHV-1 and BoHV-5 was investigated in the natural host. According to our experimental data, after nasal infection of calves, R1ΔgC and R2ΔgI are attenuated but able to replicate at low rates and establish latent infections.
According to the genomic characterization performed elsewhere [10] (Figure 1), most of the genomic background of R1ΔgC virus is homologous to BoHV-5 (63%), including some of the genes associated with the differential pathogenesis between BoHV-5 and BoHV-1 (US7-gI, US8-gE and US9) [2], whereas R2ΔgI virus is 86% homologous to BoHV-1. Despite their genomic content, both recombinant viruses appear to be attenuated in calves. This may suggest that the BoHV-5 genes enclosed in the US region, the internal and terminal repeats and part of the UL region are not able to confer BoHV-5 in vivo replication properties to R1ΔgC in the genetic background of the parental BoHV-1. However, since the recombinant viruses were obtained by in vitro co-infection between a highly attenuated BoHV-1 strain (tested in this study) and BoHV-5, the evaluation of the association between genetic composition and virulence of these recombinants is limited. Although BoHV-5-infected animals did not present neurological signs, viral excretion and histopathological lesions were observed. Animal age and individual susceptibility, among other factors, could account for the absence of clinical signs in the BoHV-5-infected group [2]. The histological alterations found in the CNS samples from BoHV-5-infected animals were similar to those reported after cattle infection with other BoHV-5 strains [17, 18]. The slight lesions observed in both the recombinants and parental BoHV-1 could be due to unspecific cellular alterations.
Serum and/or mucosal antibodies were detected in all groups, consistently with a previous report showing the induction of nasal IgA in calves after infection with different BoHV-1 strains [19]. However, humoral response was not detected in some animals. The low rate of viral replication could account for the lack of induction of immune response. In addition, the sensitivity of the ELISA tests available could be insufficient to detect a very low antibody response evoked by the recombinant viruses.
The reduced virulence observed in the R1ΔgC and R2ΔgI viruses could be attributed to the high attenuation that the parental BoHV-1ΔgCΔgI has per se. Indeed, different degrees of virulence have been reported among a variety of BoHV-1 wild type [20] and genetically modified [21] strains. A genetic characterization of the two BoHV-1/-5 recombinants and parental strains has been recently performed in the whole genome [10]. However, other genetic modifications introduced during the generation of the parental double deleted mutant or in the recombinant isolation process could also account for their in vivo attenuation. Moreover, small modifications in viral genomes can have a deep impact on the in vivo behavior. In this respect, variation of a single amino acid in the polymerase of neuropathogenic EHV-1 strains has been associated with their neurovirulence [22].
The high in vivo attenuation that the two BoHV-1/5 recombinants showed in cattle indicates that these recombinants might not constitute a major risk of persistence and spread in cattle population. However, in areas where co-infections with virulent strains of BoHV-1 and BoHV-5 exists (Southern hemisphere) the generation of unknown virulence recombinants could occur.
Conclusion
In this study we investigated the in vivo behavior of two interspecific recombinant viruses generated in vitro from closely related alphaherpesviruses infecting the same natural host. Although both recombinants showed reduced virulence following experimental cattle infection, our results demonstrate in vivo replication and establishment of latency of in vitro-generated BoHV-1/-5 recombinants.
References
Muylkens B, Thiry J, Kirten P, Schynts F, Thiry E: Bovine herpesvirus 1 infection and infectious bovine rhinotracheitis. Vet Res. 2007, 38: 181-209. 10.1051/vetres:2006059.
Del Medico Zajac MP, Ladelfa MF, Kotsias F, Muylkens B, Thiry J, Thiry E, Romera SA: Biology of bovine herpesvirus 5. Vet J. 2010, 184: 138-45. 10.1016/j.tvjl.2009.03.035.
Jones C: Herpes simplex virus type 1 and bovine herpesvirus 1 latency. Clin Microbiol Rev. 2003, 16: 79-95. 10.1128/CMR.16.1.79-95.2003.
Ackermann M, Engels M: Pro and contra IBR-eradication. Vet Microbiol. 2006, 113: 293-302. 10.1016/j.vetmic.2005.11.043.
Odeón AC, Spath EJ, Paloma EJ, Leunda MR, Fernandez Sainz IJ, Perez SE, Kaiser GG, Draghi MG, Cetrá BM, Cano A: Seroprevalencia de la diarrea viral bovina, herpesvirus bovino y virus sincicial respiratorio en Argentina. Revista de Medicina Veterinaria(Argentina). 2001, 82: 216-220.
Campos FS, Franco AC, Hubner SO, Oliveira MT, Silva AD, Esteves PA, Roehe PM, Rijsewijk FA: High prevalence of co-infections with bovine herpesvirus 1 and 5 found in cattle in southern Brazil. Vet Microbiol. 2009, 139: 67-73. 10.1016/j.vetmic.2009.05.015.
Thiry E, Meurens F, Muylkens B, McVoy M, Gogev S, Thiry J, Vanderplasschen A, Epstein A, Keil G, Schynts F: Recombination in alphaherpesviruses. Rev Med Virol. 2005, 15: 89-103. 10.1002/rmv.451.
Meurens F, Keil GM, Muylkens B, Gogev S, Schynts F, Negro S, Wiggers L, Thiry E: Interspecific recombination between two ruminant alphaherpesviruses, bovine herpesviruses 1 and 5. J Virol. 2004, 78: 9828-9836. 10.1128/JVI.78.18.9828-9836.2004.
Pagamjav O, Sakata T, Matsumura T, Yamaguchi T, Fukushi H: Natural recombinant between equine herpesviruses 1 and 4 in the ICP4 gene. Microbiol Immunol. 2005, 49: 167-179.
Del Medico Zajac MP, Romera SA, Ladelfa MF, Kotsias F, Thiry J, Ziant D, Meurens F, Keil GM, Thiry E, Muylkens B: Characterization of interspecific recombinants generated from closely related bovine herpesviruses 1 and 5 through multiple PCR sequencing assays. J Virol Methods. 2009, 161: 75-83. 10.1016/j.jviromet.2009.05.020.
Del Medico Zajac MP, Puntel M, Zamorano PI, Sadir AM, Romera SA: BHV-1 vaccine induces cross-protection against BHV-5 disease in cattle. Res Vet Sci. 2006, 81: 327-334. 10.1016/j.rvsc.2006.01.004.
Alegre M, Nanni M, Fondevila N: Development of a multiplex polymerase chain reaction for the differentiation of bovine herpesvirus-1 and -5. J Vet Med B Infect Dis Vet Public Health. 2001, 48: 613-621.
Schynts F, Baranowski E, Lemaire M, Thiry E: A specific PCR to differentiate between gE negative vaccine and wildtype bovine herpesvirus type 1 strains. Vet Microbiol. 1999, 66: 187-195. 10.1016/S0378-1135(99)00008-5.
Henderson LM, Levings RL, Davis AJ, Sturtz DR: Recombination of pseudorabies virus vaccine strains in swine. Am J Vet Res. 1991, 52: 820-825.
Javier RT, Sedarati F, Stevens JG: Two avirulent herpes simplex viruses generate lethal recombinants in vivo. Science. 1986, 234: 746-748. 10.1126/science.3022376.
Halliburton IW, Honess RW, Killington RA: Virulence is not conserved in recombinants between herpes simplex virus types 1 and 2. J Gen Virol. 1987, 68: 1435-1440. 10.1099/0022-1317-68-5-1435.
Hubner SO, Oliveira AP, Franco AC, Esteves PA, Silva AD, Spilki FR, Rijsewijk FA, Roehe P: Experimental infection of calves with a gI, gE, US9 negative bovine herpesvirus type 5. Comp Immunol Microbiol Infect Dis. 2005, 28: 187-96. 10.1016/j.cimid.2005.01.003.
Silva AD, Spilki FR, Franco AC, Esteves PA, Hubner SO, Driemeier D, Oliveira AP, Rijsewijk F, Roehe PM: Vaccination with a gE-negative bovine herpesvirus type 1 vaccine confers insufficient protection to a bovine herpesvirus type 5 challenge. Vaccine. 2006, 24: 3313-20. 10.1016/j.vaccine.2006.01.024.
Madic J, Magdalena J, Quak J, van Oirschot JT: Isotype-specific antibody responses in sera and mucosal secretions of calves experimentally infected with bovine herpesvirus 1. Vet Immunol Immunopathol. 1995, 46: 267-283. 10.1016/0165-2427(94)05363-W.
Kaashoek MJ, Straver PH, Van Rooij EM, Quak J, Van Oirschot JT: Virulence, immunogenicity and reactivation of seven bovine herpesvirus 1.1 strains: clinical and virological aspects. Vet Rec. 1996, 139: 416-21. 10.1136/vr.139.17.416.
Kaashoek MJ, Rijsewijk FA, Ruuls RC, Keil GM, Thiry E, Pastoret PP, Van Oirschot JT: Virulence, immunogenicity and reactivation of bovine herpesvirus 1 mutants with a deletion in the gC, gG, gI, gE, or in both the gI and gE gene. Vaccine. 1998, 16: 802-809. 10.1016/S0264-410X(97)00269-7.
Nugent J, Birch-Machin I, Smith KC, Mumford JA, Swann Z, Newton JR, Bowden RJ, Allen GP, Davis-Poynter NJ: Analysis of equid herpesvirus 1 strain variation reveals a point mutation of the DNA polymerase strongly associated with neuropathogenic versus nonneuropathogenic disease outbreaks. Virology. 2006, 80: 4047-4060. 10.1128/JVI.80.8.4047-4060.2006.
Acknowledgements and Funding
The authors are grateful to Diego Soraire and Javier Leiva for their dedication and care of the calves used in the experiments. This study was financially supported by the Agencia Nacional de Promoción Científica y Tecnológica (ANPyCT), Ministerio de Ciencia, Tecnología e Innovación Productiva, Argentina (BID-PICT N°1433).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
MPDMZ, SAR, JT, ET and BM designed the experiments, analysed the data and drafted the manuscript. MPDMZ, SAR, MFL and FK performed the experiments. FD carried out the histopathological analysis of the samples. FM and GK helped to draft the manuscript. All authors read and approved the final manuscript.
Maria P Del Medico Zajac, Sonia A Romera contributed equally to this work.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Del Medico Zajac, M.P., Romera, S.A., Ladelfa, M.F. et al. In vitro-generated interspecific recombinants between bovine herpesviruses 1 and 5 show attenuated replication characteristics and establish latency in the natural host. BMC Vet Res 7, 19 (2011). https://doi.org/10.1186/1746-6148-7-19
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1746-6148-7-19