Abstract
Background
Although several attempts have been made to control enzootic bovine leukosis (EBL) at the local level, a nationwide control program has not been implemented in Japan, except for passive surveillance. Effective control of EBL requires that the transmission routes of bovine leukemia virus (BLV) infection should be identified and intercepted based on scientific evidence. In this cross-sectional study, we examined the risk factors associated with within-herd transmission of BLV on infected dairy farms in Japan. Blood samples taken from 30 randomly selected adult cows at each of 139 dairy farms were tested by enzyme-linked immunosorbent assay (ELISA). Information on herd management was collected using a structured questionnaire.
Results
Infected farms were defined as those with more than one ELISA-positive animal and accounted for 110 (79.1%) of the 139 farms in the study. Completed questionnaires obtained from 90 of these 110 farms were used for statistical analysis. Seroprevalence, which was defined as the proportions of animals that tested positive out of all animals tested on the farm, was 17.1%, 48.1%, and 68.5% for the 25th, 50th, and 75th percentiles, respectively. A mixed logistic regression analysis implicated a loose housing system, dehorning, and a large number of horseflies in summer as risk factors (coefficient = 0.71, 1.11, and 0.82; p = 0.03, < 0.01, and 0.01, respectively) and feeding of colostrum to newborn calves from their dams as a protective factor (coefficient = -1.11, p = 0.03) against within-farm transmission of BLV on infected farms.
Conclusion
Control of EBL in infected dairy farms in Japan will be improved by focusing particularly on these risk and protective factors.
Similar content being viewed by others
Background
Bovine leukemia virus (BLV), a retrovirus of the family Retroviridae, is the causative agent of enzootic bovine leucosis (EBL). Approximately 30% of cattle infected with BLV have persistent lymphocytosis, and 1-5% develop B-cell lymphosarcoma [1]. With a worldwide distribution, EBL is listed by the World Organization for Animal Health as a disease of importance to international trade [2] and is included in the national eradication program in Australia and some member states of the European Union (EU), several of which have recently eliminated the disease [3, 4]. In contrast, 89% of dairy herds in the United States are reported to be infected with BLV [5], and the annual economic loss due to EBL is estimated at $525 million in decreased milk yield [6]. Although EBL causes serious economic damage in the dairy industry [7], thus far only regional voluntary control programs have been implemented in the United States [8].
In Japan, EBL is listed as a notifiable disease, but no nationwide control programs have been established. According to animal health statistics, EBL was reported in 159 cattle at 157 farms in 2000 and in 838 cattle at 677 farms in 2007. These data suggest that EBL has been gradually spreading in Japan.
BLV is present in the circulating peripheral blood lymphocytes of infected cattle, and horizontal transmission of the virus occurs via infected blood often as a result of unhygienic farm practices such as the use of plastic sleeves to perform rectal palpation and the same needle for vaccination of more than one animal [9–11] or unhygienic dehorning practices [11–13]. Furthermore, infected lymphocytes may also be transmitted mechanically by hematophagous insects such as horseflies [14–16]. BLV transmission has also been reported through physical contact between infected and uninfected cattle [17, 18]. In addition, since BLV and its antibodies are present in the colostrum and normal milk of infected cattle, BLV transmission from infected cows to calves via infected milk is negligible [19].
These transmission routes should be considered when designing preventive measures against EBL at the farm level. In addition, it may be important to determine which transmission routes are the most important for each farm. It is therefore essential to identify the risk factors associated with BLV transmission in the production systems targeted for disease control. However, no such epidemiological approach for BLV infection has been attempted in Japan. Therefore, the objective of this study was to clarify herd management factors associated with within-herd transmission of BLV and to facilitate a more rational and efficient approach for controlling BLV transmission on dairy farms in Japan.
Results
Results of serological tests showed that 110 (79.1%) of 139 dairy farms were infected with BLV. Statistical analyses were then performed for 90 of these farms (81.8%), for which complete data were obtained from the questionnaires.
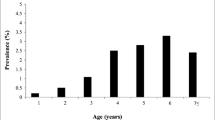
Seroprevalence at infected farms at the 25th, 50th (median), and 75th percentiles was 17.1%, 48.1%, and 68.5%, respectively (Figure 1 and Table 1). Visual inspection of Figure 1 and the Shapiro-Wilk test confirmed that seroprevalence was not normally distributed (p < 0.002). Univariate test results showed that cattle housing conditions, availability of own grazing area, the presence of horseflies in summer, dehorning, the use of a plastic sleeve for rectal palpation, not changing needles between animals during herd vaccination, and colostrum feeding were factors possibly associated with seroprevalence (p < 0.15, Table 1), whereas herd size (p = 0.51) and cattle replacement (p = 0.29) were not associated with it.
Distribution of BLV-infected farms classified by seroprevalence (n = 90). Seroprevalence on infected farms at the 25th, 50th (median), and 75th percentiles was 17.1%, 48.1%, and 68.5%, respectively. Visual inspection of this histogram and the Shapiro--Wilk test confirmed that seroprevalence was not normally distributed (p < 0.002).
Starting with the seven variables with p values < 0.15 in the univariate analyses, the final model was obtained with four variables with the smallest value of the Akaike information criterion (AIC) (Table 2). A loose housing system, dehorning, and the observable presence of horseflies were associated with increases in seroprevalence (coefficient = 0.71, 1.11, 0.82; p = 0.03, < 0.01, 0.01). These were considered as risk factors that facilitated the within-farm transmission of BLV. In contrast, colostrum feeding of calves from their dams was considered to be a protective factor that suppressed the within-farm transmission of the virus (coefficient = -1.11, p = 0.03). No biologically plausible two-way interactions between the remaining factors were observed in the final model.
Discussion
This is the first epidemiological study to assess herd management factors related to the seroprevalence of BLV on dairy farms in Japan. Based on our mixed logistic model with a random herd effect, the significant factors were found to be cattle housing conditions, the presence of horseflies in summer, dehorning practices, and colostrum feeding.
With regard to housing conditions, a loose housing system was found to be positively associated with seroprevalence compared with a tied housing system (p = 0.03). A possible explanation may be that direct contact between infected and uninfected cattle is facilitated in loose housing systems such as free stalls and barns. Furthermore, the chances of indirect contact between uninfected and infected cattle may be relatively high in a loose housing system because cattle randomly move about the barn or paddock and are not always handled in the same manner.
Dehorning is a practice in daily herd management that can reduce the risk of injury from horn-butting in farmers and in cattle during herd conflict. This practice was found to be positively associated with seroprevalence compared with farms without it (p < 0.01). A potential explanation for this result is the unhygienic procedure of dehorning, which is a risk factor for BLV transmission. It was previously reported that calves dehorned with contaminated dehorning apparatus were more likely to be infected than those that had not been dehorned [11–13]. Farmers often leave the dehorned heifers without hemostasis by cautery (personal communications with clinical veterinarians). Although we did not include a question related to the treatment of dehorned cattle in the questionnaire, the information obtained from clinical veterinarians supported our result.
With regard to the presence of horseflies in summer, a response of "very high" was positively associated with seroprevalence compared with a response of "never to seldom" (p = 0.01); however, there was no significant difference between "sometimes or often" and "never to seldom" (p = 0.49). BLV transmission by hematophagous insects has been reported [14–16]. In this study, we did not measure horsefly populations quantitatively on each farm. However, given the effect of the response of "very high" in increasing seroprevalence, our findings support the results of previous studies and suggest more quantitative study in future.
In the present study, colostrum feeding of calves from their dams resulted in a decrease in within-farm seroprevalence (coefficient = -1.11, p = 0.03). Because colostrum contains BLV and its antibodies, ingestion of colostrum from infected dams reduces the risk of BLV infection during the weaning period in calves [20–25]. Our result is consistent with previous studies, although there were only seven farms in our study without colostrum feeding, the reference category. In addition, despite marginal significance, pooled colostrum feeding on a farm had a negative impact on seroprevalence (coefficient = -0.90, p = 0.10). Therefore, colostrum feeding could be an effective way to reduce the prevalence of BLV in a herd. The effect of optimal conditions for the treatment of pooled colostrum, such as heating and freezing on the farm, should be evaluated in future studies.
Because this study had a cross-sectional design, it was difficult to elucidate definite relationships between seroprevalence and each risk or protective factor. However, in spite of this constraint, our results were consistent with previous studies and confirmed the important factors that should be controlled.
Conclusions
Controlling BLV infection at Japanese dairy farms should focus on these factors. Future research is required to compare the influences of each factor responsible for within-herd transmission and to facilitate more rational prioritization of control measures.
Methods
Target population and sampling criteria
This cross-sectional study examined dairy farms located in 7 of the 47 prefectures in Japan. Each prefectural government sought participation from approximately 20 dairy farmers, and a total of 139 agreed to enroll. At each farm, the owner was asked for the blood sampling by the sample collectors who gave the minimum possible pain to the cattle, and only those who understood the study objectives were involved in this study. Each animal was firmly restrained before sampling to prevent unnecessary apprehension and pain to the animal and unexpected accidents to the sample collectors. Samples were then collected from the jugular or caudal vein. Blood samples were taken from 30 randomly selected adult cows (or all cows on farms of herd size < 30) at each farm between June and December, 2007. Separated sera were transferred to the National Institute of Animal Health (NIAH, Tsukuba, Japan) and stored at -20°C for serological tests. Since this study was a field survey, no ethical approval for the study was required by the animal care and ethical committee of National Institute of Animal Health.
Questionnaire survey
Information regarding herd management was acquired at the time of blood sampling by local veterinary officers using a questionnaire prepared in advance [Additional file 1]. The questions inquired about cattle housing conditions, cow replacement, provision of own grazing area or free range on the farm, the presence of horseflies in summer, dehorning, the use of plastic sleeves for rectal palpation, change of needles used for herd vaccination, colostrum feeding, and general data on herd demography. Data from the completed questionnaires were stored and handled using commercially available spreadsheet software (Excel 2007; Microsoft Corp., Redmond, WA, USA).
Serological testing
Each serum sample was serologically tested at NIAH using a commercial enzyme-linked immunosorbent assay (ELISA) kit (Bovine Leukosis Serum Screening ELISA; Pourquier, Montpellier, France). The procedures were performed according to the manufacturer's instructions. If the ELISA results were inconclusive, confirmation was sought using an agar gel precipitation test reported previously [26].
Statistical analyses
In the present study, an infected farm was defined as a farm with more than one serologically positive cow. To analyze the risk factors associated with within-herd transmission of BLV, the subsequent modeling process targeted the seroprevalence of each infected farm as a response variable, which was defined as the proportion of cattle testing positive among all tested cattle on the farm.
To obtain a general overview of the association between seroprevalence and each herd management factor, the normality of the distribution of seroprevalence at infected farms was evaluated by visual inspection of the histogram and by the Shapiro-Wilk test, followed by univariate analysis. The Mann-Whitney U-test was used to analyze variables with two levels, and the Kruskal-Wallis test to analyze all other variables. Variables with a p value < 0.15 in these tests were used for multivariate model building. A generalized mixed linear model was used to evaluate the risk factors that influenced seroprevalence; the response variable being the logit of seroprevalence and the herd as a random effect. The model is described as follows:

p = number of positive animals/number of test animals,
where p represents the seroprevalence accounting for sample size, α is the model intercept, χ is fixed effects with p < 0.15 in the univariate analyses, β is its coefficient, RH is the random herd effect, and e is the binomially distributed residual term. The best model was constructed by a stepwise approach, observing the change in AIC of each model. The final model was obtained with the minimum AIC and p < 0.05 for the remaining fixed effects.
All statistical analyses were performed using R version 2.6.1 (R Development Core Team, 2007), and the glmmML package [27] was used for model building.
References
Burny A, Cleuter Y, Kettmann R, Mammerickx M, Marbaix G, Portetelle D, Broeke Van den A, Willems L, Thomas R: Bovine leukemia: facts and hypotheses derived from the study of an infectious cancer. Adv Vet Sci Comp Med. 1988, 32: 149-170.
OIE Terrestrial Animal Health Code. Article 1.11.11. [http://www.oie.int/eng/normes/mcode/en_chapitre_1.11.11.htm]
Nuotio L, Rusanen H, Sihvonen L, Neuvonen E: Eradication of enzootic bovine leukosis from Finland. Prev Vet Med. 2003, 59: 43-49. 10.1016/S0167-5877(03)00057-6.
Acaite J, Tamosiunas V, Lukauskas K, Milius J, Pieskus J: The eradication experience of enzootic bovine leukosis from Lithuania. Prev Vet Med. 2007, 82: 83-89. 10.1016/j.prevetmed.2007.05.010.
USDA-Veterinary Services: High prevalence of BLV in U.S. dairy herds. 1997, [http://nahms.aphis.usda.gov/dairy/dairy96/DR96blv.pdf]
Ott SL, Johnson R, Wells SJ: Association between bovine-leukosis virus seroprevalence and herd-level productivity on US dairy farms. Prev Vet Med. 2003, 61: 249-262. 10.1016/j.prevetmed.2003.08.003.
Miller JM, van der Maaten MJ: Bovine leukosis--its importance to the dairy industry in the United States. J Dairy Sci. 1982, 65: 2194-2203.
Brunner MA, Lein DH, Dubovi EJ: Experiences with the New York State bovine leukosis virus eradication and certification program. Vet Clin North Am Food Anim Pract. 1997, 13: 143-150.
Hopkins SG, Evermann JF, DiGiacomo RF, Parish SM, Ferrer JF, Smith S, Bangert RL: Experimental transmission of bovine leukosis virus by simulated rectal palpation. Vet Rec. 1988, 122: 389-391.
Hopkins SG, DiGiacomo RF, Evermann JF, Christensen JD, Deitelhoff DP, Mickelsen WD: Rectal palpation and transmission of bovine leukemia virus in dairy cattle. J Am Vet Med Assoc. 1991, 199: 1035-1038.
Lassauzet ML, Thurmond MC, Johnson WO, Stevens F, Picanso JP: Effect of brucellosis vaccination and dehorning on transmission of bovine leukemia virus in heifers on a California dairy. Can J Vet Res. 1990, 54: 184-189.
DiGiacomo RF, Darlington RL, Evermann JF: Natural transmission of bovine leukemia virus in dairy calves by dehorning. Can J Comp Med. 1985, 49: 340-342.
DiGiacomo RF, Hopkins SG, Darlington RL, Evermann JF: Control of bovine leukosis virus in a dairy herd by a change in dehorning. Can J Vet Res. 1987, 51: 542-544.
Bech-Nielsen S, Piper CE, Ferrer JF: Natural mode of transmission of the bovine leukemia virus: role of bloodsucking insects. Am J Vet Res. 1978, 39: 1089-1092.
Oshima K, Okada K, Numakunai S, Yoneyama Y, Sato B, Takahashi I: Evidence on horizontal transmission of bovine leukemia virus due to blood-sucking tabanid flies. Jpn J Vet Sci. 1981, 43: 79-81.
Manet G, Guilbert X, Roux A, Vuillaume A, Parodi AL: Natural mode of horizontal transmission of bovine leukemia virus (BLV): the potential role of tabanids (Tabanus spp.). Vet Immunol Immunopathol. 1989, 22: 255-263. 10.1016/0165-2427(89)90012-3.
Lassauzet ML, Thurmond MC, Johnson WO, Stevens F, Picanso JP: Factors associated with transmission of bovine leukemia virus by contact in cows on a California dairy. Am J Epidemiol. 1991, 133: 164-176.
Kono Y, Sentsui H, Arai K, Ishida H, Irishio W: Contact transmission of bovine leukemia virus under insect-free conditions. Jpn J Vet Sci. 1983, 45: 79-82.
Ferrer JF: Eradication of bovine leukemia virus infection from a high-prevalence herd, using radioimmunoassay for identification of infected animals. J Am Vet Med Assoc. 1982, 180: 890-893.
Ferrer JF, Kenyon SJ, Gupta P: Milk of dairy cows frequently contains a leukemogenic virus. Science. 1981, 213: 1014-1016. 10.1126/science.6267692.
Ferrer JF, Piper CE: Role of colostrum and milk in the natural transmission of the bovine leukemia virus. Cancer Res. 1981, 41: 4906-4909.
Nagy DW, Tyler JW, Kleiboeker SB: Decreased periparturient transmission of bovine leukosis virus in colostrum-fed calves. J Vet Intern Med. 2007, 21: 1104-1107. 10.1892/0891-6640(2007)21[1104:DPTOBL]2.0.CO;2.
van der Maaten MJ, Miller JM, Schmerr MJ: In utero transmission of bovine leukemia virus. Am J Vet Res. 1981, 42: 1052-1054.
van der Maaten MJ, Miller JM, Schmerr MJ: Effect of colostral antibody on bovine leukemia virus infection of neonatal calves. Am J Vet Res. 1981, 42: 1498-1500.
Lassauzet ML, Johnson WO, Thurmond MC, Stevens F: Protection of colostral antibodies against bovine leukemia virus infection in calves on a California dairy. Can J Vet Res. 1989, 53: 424-430.
Kono Y, Sentsui H, Miyamoto T, Morozumi K, Sakamoto Y: Changes in antibody titers in cattle infected clinically and subclinically with bovine leukemia virus. Int J Cancer. 1982, 30: 655-657. 10.1002/ijc.2910300517.
Generalized linear models with random intercepts. [http://rss.acs.unt.edu/Rdoc/library/glmmML/html/glmmML.html]
Acknowledgements
The authors thank the veterinary officers of the prefectural Livestock Hygiene Service Centers for their cooperation in blood sampling and epidemiological data collection, and Ms. Takako Shimada for technical support in the laboratory. This study was partly supported by the Ministry of Agriculture, Forestry and Fisheries, Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors' contributions
SK participated in the design of the study, performed all the data handling and statistical analyses, and drafted the manuscript. TT participated in the design of the study and helped to draft the manuscript. TY and YH investigated the data analyses performed by SK. KK and MK performed serological diagnoses and the related laboratory work. KM conceived the study, participated in its design and coordination, and helped to draft the manuscript. All authors read and approved the final manuscript.
Electronic supplementary material
12917_2009_181_MOESM1_ESM.DOC
Additional file 1: Questionnaire used for the sero-epidemiological survey of the enzootic bovine leukosis in Japan, 2007. The questions inquired about cattle housing conditions, cow replacement, provision of own grazing area or free range on the farm, the presence of horseflies in summer, dehorning, the use of plastic sleeves for rectal palpation, change of needles used for herd vaccination, colostrum feeding, and general data on herd demography. (DOC 34 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Kobayashi, S., Tsutsui, T., Yamamoto, T. et al. Risk factors associated with within-herd transmission of bovine leukemia virus on dairy farms in Japan. BMC Vet Res 6, 1 (2010). https://doi.org/10.1186/1746-6148-6-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1746-6148-6-1