Abstract
Background
Cutaneous mast cell tumours are one of the most common neoplasms in dogs and show a highly variable biologic behaviour. Several prognosis tools have been proposed for canine mast cell tumours, including histological grading and cell proliferation markers. CD117 is a receptor tyrosine kinase thought to play a key role in human and canine mast cell neoplasms. Normal (membrane-associated) and aberrant (cytoplasmic, focal or diffuse) CD117 immunoexpression patterns have been identified in canine mast cell tumours. Cytoplasmic CD117 expression has been found to correlate with higher histological grade and with a worsened post-surgical prognosis. This study addresses the role of CD117 in canine mast cell tumours by studying the correlations between CD117 immunoexpression patterns, two proliferation markers (Ki67 and AgNORs) histological grade, and several other pathological variables.
Results
Highly significant (p < 0,001) correlations were found between CD117 immunostaining patterns and histological grade, cell proliferation markers (Ki67, AgNORs) and tumoral necrosis. Highly significant (p < 0,001) correlations were also established between the two cellular proliferation markers and histological grade, tumour necrosis and epidermal ulceration. A significant correlation (p = 0.035) was observed between CD117 expression patterns and epidermal ulceration. No differences were observed between focal and diffuse cytoplasmic CD117 staining patterns concerning any of the variables studied.
Conclusion
These findings highlight the key role of CD117 in the biopathology of canine MCTs and confirm the relationship between aberrant CD117 expression and increased cell proliferation and higher histological grade. Further studies are needed to unravel the cellular mechanisms underlying focal and diffuse cytoplasmic CD117 staining patterns, and their respective biopathologic relevance.
Similar content being viewed by others
Background
While remaining infrequent in human beings, cutaneous mast cell tumours (MCTs) are one of the most common tumours in dogs, accounting for about 6% of all tumours and 13% of all skin tumours of the dog [1]. Thus, canine MCTs have attracted increasing attention in recent years, as spontaneous models for studying mast cell neoplastic disorders and developing new targeted chemotherapeutic drugs [2–4]. The biological behaviour of canine MCTs can vary widely and is often difficult to predict [1]. Histological grading [5] is widely used for prognosis analysis [6], but its adequacy remains debatable [7]. According to the internationally adopted system, MCTs are usually graded as well-differentiated (grade I), moderately differentiated (grade II) or poorly differentiated (grade III) tumours. Other prognostic factors have recently been proposed, most notably proliferation markers such as Ki67 (MIB-1) nuclear antigen labelling index and AgNORs mean counts [8–11]. Recent studies have demonstrated both normal (membrane associated) and abnormal (cytoplasmic, focal or diffuse) CD117 immunoexpression patterns in canine MCTs [12–14]. CD117 cytoplasmic expression patterns have been shown to correlate with reduced post-surgical survival [15]. Together with it's ligand (stem cell factor – SCF), also known as Steel factor or KIT ligand, CD117 (also referred as KIT or c-KIT) is a critical transmembrane receptor tyrosine kynase for a number of cell types, including some hematopoietic stem cells, mast cells, melanocytes, and germ cells. Binding of SCF by CD117 leads to receptor dimerization and activation of its tyrosine kynase activity [16]. A number of signal transduction pathways, such as the PI3-kinase and the RAS/Erk pathways have been implicated in mediating CD117 functions in mast cells, including cellular proliferation and differentiation, resistance to apoptosis, mobility and chemotaxis, adhesion to fibronectin and enhancement of serotonin and histamine release [17–19]. CD117 is encoded by the proto-oncogene c-kit [16]. Point mutations of this gene have been associated with human mastocytosis [20, 21] and other malignancies [22–26] while c-kit exon 11 deletions and duplications have been identified in canine MCTs [27–30]. This work aims to study the role of CD117 in canine MCTs by analysing the correlations between CD117 immunoexpression patterns, two proliferation markers (Ki67 and AgNORs), histological grading, and several pathological variables.
Results
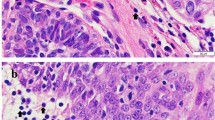
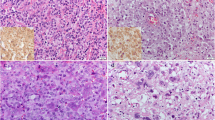
49 (47.6%) tumours presented a membrane-associated CD117 staining pattern (Figure 1), while 46 (44.7%) showed focal cytoplasmic staining (Figure 2) and 8 (7.8%) diffuse cytoplasmic staining (Figure 3). Ki67 labelling index ranged from 3.3 to 46.6, while mean nuclear AgNORs counts ranged from 1.1 to 4.2. Histologically, 35 (34.0%) tumours were grade I, 45 tumours (43.7%) were grade II and 22 tumours (22.3%) were grade III. Highly significant statistical correlations (p < 0,001) were found between CD117 staining patterns and histological grade, presence of necrosis and mitotic index (Table 1). However, no differences were observed between focal and diffuse staining patterns, concerning any of the variables studied. Highly significant statistical correlations (p < 0,001) were also established between Ki67 labelling index and CD117 staining patterns, histological grade, mitotic index and presence of necrosis and ulceration (Table 2) as well as between these variables and AgNORs counts (Table 3). Figure 4 shows a linear correlation between Ki67 labelling index and mean AgNOR counts (R Spearman correlation coefficient 0.639) and the distribution of CD117 staining patterns (membrane-associated versus cytoplasmic) according to these proliferation markers. AgNORs mean counts were significantly higher in MCTs showing cytoplasmic CD117 expression (p < 0,001). A significant correlation was found between CD117 cytoplasmic pattern and epidermal ulceration (p = 0.035). No significant statistical correlation was established between any pathologic variables and the sex, age or breed of the animals, nor with the number (single/multiple) or location (head and neck, trunk, limbs, perineal) of the lesions.
Discussion
The predominance of the Boxer breed among the animals studied reflects a well-known breed predisposition for canine MCTs. However, no significant correlations were found between the breed of the animals and any other of the variables now studied, and the significance of breed regarding the biopathology of MCTs remains unclear. The linear correlation observed between Ki67 labelling index and mean AgNOR counts validates the results of each technique and allows for confirmation of differences in cellular proliferation by two independent methods. The Ki67 labelling index increases in a step-wise way from histological grade I to III, but there is considerable overlapping of both AgNORs and Ki67 values between histological grades. Results have highlighted a strong correlation between cytoplasmic (altered) CD117 immunoexpression and increased cell proliferation and higher histological grade (itself partly based on mitotic index) when compared with the normal, membrane-associated expression pattern. Two distinct patterns for CD117 cytoplasmic staining (a focal, paranuclear, sometimes referred to as Golgi-like pattern and a diffuse pattern) have been described [12–15]. In this study, no significant differences were found between focal and diffuse cytoplasmic CD117 staining, concerning any of the variables studied. This suggests that focal and diffuse cytoplasmic staining may reflect similar cellular changes and, possibly, a progressive cytoplasmic accumulation of CD117. Additional studies are needed to elucidate the biopathologic relevance of these expression patterns as well as the corresponding underlying cellular mechanisms. C-kit mutations have been shown to induce ligand-independent (constitutive) CD117 phosphorylation and activation in human neoplasms, both by impairing the regulatory functions of the juxtamembrane domain and by directly targeting the kinase domain [31]. Such mutations are likely to be the cause of increased cell proliferation in MCTs showing cytoplasmic CD117 expression. It is interesting to speculate that mutations causing constitutive CD117 phosphorylation may also collide with the intracellular traffic of CD117 and cause the molecule to accumulate in cellular organelles, such as the Golgi apparatus or the endoplasmic reticulum. C-kit mutations have been shown to correlate with altered CD117 expression, though mutations weren't present in all MCTs with aberrant CD117 expression [32]. By elucidating the precise cellular location of cytoplasmic CD117 accumulations, especially in those lesions presenting a "Golgi-like" pattern, a deeper insight into the role of this molecule in tumorigenesis may perhaps be gained. Results suggest that translocation of CD117 into the cytoplasm correlates with activation of CD117-mediated cell proliferation. Although increased cell proliferation in itself is insufficient for neoplastic transformation, it may contribute to the malignant phenotype by increasing the risk of spontaneous mutations. Some human neoplasms have been demonstrated to present an autocrine loop in which neoplastic cells express both SCF and CD117 [33], thus securing permanent growth stimulation. This hypothesis has not been investigated in the case of canine MCTs and should be addressed in future studies. The correlation observed between tumoral necrosis and cytoplasmic CD117 expression (Table 1) may reflect increased cellular proliferation, insufficiently accompanied by angiogenesis, as suggested by the correlation observed between necrosis and higher Ki67 labelling index (Table 2). On the other hand, the correlation between the CD117 cytoplasmic pattern and epidermal ulceration may be due to a CD117-mediated enhancement of histamine and serotonine release and consequent pruritus and self-induced trauma. CD117 might also be thought to play a role in tumoral progression, as suggested by the correlation of cytoplasmic CD117 staining and higher histological grade, by facilitating neoplastic cell mobility and binding of fibronectin. The tumoral growth pattern (well circumscribed versus invasive) and the clinical variables studied (race, sex, age, tumour number and location) have shown no correlations with any of the pathological variables studied. The available survival data doesn't allow for conclusions as to which of the factors now studied is more suitable for prognostic analysis.
Conclusion
Cytoplasmic (altered) expression of CD117 correlates with increased cellular proliferation, as assessed by both Ki67 labelling index and by AgNORs mean counts. This is in accordance with the known functions of CD117 as a growth factor receptor and is probably associated with a c-kit mutation. Moreover, cytoplasmic CD117 expression also correlates with increased histological grade, tumour necrosis and epidermal ulceration. No differences were observed between focal and diffuse cytoplasmic staining patterns, suggesting that these represent similar cellular changes, or perhaps a progressive process of cytoplasmic CD117 accumulation. Our future aims are to look for possible mutations in the c-kit gene in canine MCCs.
Methods
Samples collection and processing
103 MCTs surgically removed from 67 dogs, between 2000 and 2006, were sent for examination at the ICBAS-UP Veterinary Pathology laboratory. The animals comprised 30 males and 37 females, with ages ranging between 2 and 20 years and a mean age of 7.3 years. 33 (49.25%) animals were of the Boxer breed, 18 (26.87%) of mixed breed and 16 (23.88%) of other breeds (with 3 or less animals of each breed). All nodules were considered as primary MCTs, even when occurring in a multiple pattern. Samples were fixed in 10% buffered formallin and paraffin-embedded. Thin serial sections were obtained for each sample and used for routine haematoxylin-eosin (H&E) staining, AgNOR staining and immunohistochemical detection of CD117 and Ki67. 3 μm-thick sections were used for all techniques except AgNORs (4 μm).
Histological evaluation
Histological grading was performed on H&E-stained slides, following Patnaik's system [5], by two independent pathologists (RMGC and FG). When grading differed, decision was taken by consensus. The number of mitotic figures per high power field and other pathologic changes, such as tumoral necrosis and epidermal ulceration were also noted.
AgNORs staining
AgNOR staining was performed as previously described [34]. The staining solution was freshly prepared for each experiment, from a 2% gelatin solution (Merck) and 50% silver nitrate (Merck), in a 1:2 ratio. Slides were immersed in the staining solution under conditions of reduced light for 45 minutes and washed in deionised water. Silver deposits were fixed in a 5% sodium thiosulfide (Merck) solution. Slides were then counterstained with light green (Merck) dehydrated, cleared and mounted in synthetic mounting medium (Entellan, Merck). Preparations were then examined on a light microscope, using a 100× objective, and the mean AgNOR count per nucleus was determined in 100 neoplastic mast cells, as previously described [35]. AgNORs were counted excluding areas of necrosis and inflammation.
Immunohistochemistry
Monoclonal antibodies against Ki67 (MIB-1, Dakopatts, Denmark) and polyclonal antibodies against CD117 (DakoCytomation, USA) were employed, using the avidin-biotin-peroxidase (ABC) method. Heat-induced antigen retrieval (HIER) was performed: for Ki67, slides were incubated for 30 minutes in a commercial antigen retrieval solution (Dako), at 100°C in a water bath. For CD117, slides were incubated in a 10 mM citrate buffer (pH = 6.0) in a steamer, for 2 minutes. Endogenous peroxidase activity was blocked by immersing slides in methanol containing 3% hydrogen peroxide for 10 minutes. Anti-Ki67 and anti-CD117 antibodies were diluted at 1:50 and 1:450 in 5% bovine serum albumin, respectively. Slides were incubated with antibodies overnight at 4°C. Human gastrointestinal stromal tumours (GISTs) were used as positive controls for CD117 staining. Detection was performed using 3,3'-diaminobenzidine substrate (Dako). Sections were then counterstained with Mayer's haematoxylin, dehydrated, cleared and mounted in Entellan mounting medium (Merck). Slides were evaluated under light microscopy. The KI67 index was determined in areas with high labelling immunoreactivity, excluding areas of necrosis and inflammation, per 1000 cells, as previously described [36]. For CD117, three staining patterns were recognized: a membrane-associated pattern with little to none cytoplasmic staining, a focal (paranuclear or Golgi-like) cytoplasmic pattern, with only occasional minor membrane staining and a diffuse cytoplasmic pattern [12–15].
Statistical analysis
Nonparametric analysis was conducted, using SPSS 14.0, with a significance level of 5% and bilateral tests. Pearson's independent chi-squared test was used to assess the correlations between CD117 immunostaining patterns and several clinical and pathological variables. Kruskal-Wallis test was used to assess differences between Ki67 median values and AgNORs mean counts of different variables. A Spearman's correlation was calculated between Ki67 labelling index and AgNORs mean counts.
References
Goldschmidt MH, Hendrick MJ: Tumours of the skin and soft tissues. Tumors in domestic animals 4th edition. Edited by: Meuten DJ. Ames: Iowa State Press; 2002:105-107.
Liao AT, Chien MB, Shenoy N, Mendel DB, McMahon G, Cherrington JM, London CA: Inhibition of constitutively active forms of mutant kit by multitargeted indolinone tyrosine kinase inhibitors. Blood. 2002, 100: 585-593. 10.1182/blood-2001-12-0350.
London CA, Hannh AL, Zadovoskaya R, Chien MB, Kollias-Baker C, Rosenberg M, Downing S, Post G, Boucher J, Shenoy N, Mendel DB, McMahon G, Cherrington JM: Phase I Dose-escalating study of SU1 a small molecule receptor tyrosine kinase inhibitor, in dogs with spontaneous malignancies. Clin Cancer Res. 1654, 9: 2755-2768.
Pryer NK, Lee LB, Zadovaskaya R, Yu X, Sukbuntherng J, Cherrington JM, London CA: Proof of target for SU11654: inhibition of KIT phosphorylation in canine mast cell tumors. Clin Cancer Res. 2003, 9: 5729-5734.
Patnaik A, Ehler WJ, MacEwen EG: Canine cutaneous mast cell tumor: morphologic grading and survival times in 83 dogs. Vet Pathol. 1984, 21: 469-474.
Hendrick MJ, Mahaffey EA, Moore FM, Vos JH, Walder EJ: Histological classification of mesenchimal tumours of the skin and soft tissues of domestic animals. WHO classification of tumours of domestic animals. 1998, Washington DC: AFIP, 28-29.
Kiupel M, Webster JD, Miller RA, Kaneene JB: Impact of tumour depth, tumour location and multiple synchronous masses on the prognosis of canine cutaneous mast cell tumours. J Vet Med A Physiol Pathol Clin Med. 2005, 52: 280-286.
Abadie JJ, Amardeilh MA, Delverdier ME: Immunohistochemical detection of proliferating cell nuclear antigen and ki67 in mast cell tumors from dogs. J Am Vet Med Assoc. 1999, 215: 1629-1634.
Bostock DE, Crocker J, Herris K, Smith P: Nucleolar organiser regions as indicators of post-surgical prognosis in canine spontaneous mast cell tumours. Br J Cancer. 1989, 59: 915-918.
Sakai H, Noda A, Shirai N, Iidaka T, Yanai T, Masegi T: Proliferative activity of canine mast cell tumours evaluated by bromodeoxyuridine incorporation and ki67 expression. J Comp Pathol. 2002, 127: 233-238. 10.1053/jcpa.2002.0586.
Simões JP, Schoning P, Butine M: Prognosis of canine mast cell tumors: a comparison of three methods. Vet Pathol. 1994, 31: 637-647.
London CA, Kisseberth WC, Galli SJ, Geissler EN, Helfand SC: Expression of stem cell factor receptor (c-kit) by the malignant mast cells from spontaneous canine mast cell tumours. J Comp Pathol. 1996, 115: 399-414. 10.1016/S0021-9975(96)80074-0.
Morini M, Bettini G, Preziosi R, Mandrioli L: C-kit gene product (CD117) immunoreactivity in canine and feline paraffin sections. J Histochem Cytochem. 2004, 52: 705-708.
Reguera MJ, Rabanal RM, Puigdemont A, Ferrer L: Canine mast cell tumors express stem cell factor receptor. Am J Dermatopathol. 2000, 22: 49-54. 10.1097/00000372-200002000-00010.
Kiupel M, Webster JD, Kaneene JB, Miller R, Yuzbasiyan-Gurkan V: The use of KIT and tryptase expression patterns as prognostic tools for canine mast cell tumors. Vet Pathol. 2004, 41: 371-377. 10.1354/vp.41-4-371.
Ronnstrand L: Signal transduction via the stem cell factor receptor/c-kit. Cell Mol Life Sci. 2004, 61: 2535-2548. 10.1007/s00018-004-4189-6.
Serve H, Yee NS, Stella G, Sepp-Lorenzino L, Tan JC, Besmer P: Differential roles of PI3-kinase and kit tyrosine 821 in Kit receptor-mediated proliferation, survival and cell adhesion in mast cells. The EMBO Journal. 1995, 14: 473-483.
Timokhina I, Kissel H, Stella G, Besmer P: Kit signalling through PI3-kinase and Src kinase pathways: an essential role for Rac1 and JNK activation in mast cell proliferation. EMBO J. 1998, 17: 6250-6262. 10.1093/emboj/17.21.6250.
Vosseller K, Stella G, Yee NS, Besmer P: C-Kit receptor signalling through it's phosphatidylinositide-3'-kinase-binding site and protein kinase C: role in mast cell enhancement of degranulation, adhesion and membrane ruffling. Mo Biol Cell. 1997, 8: 909-922.
Akin C, Fumo G, Yavuz AS, Lipsky PE, Neckers L, Metcalfe DD: A novel form of mastocytosis associated with a transmembrane c-kit mutation and response to imatinib. Blood. 2004, 103: 3222-3225. 10.1182/blood-2003-11-3816.
Metcalfe DD, Akin C: Mastocytosis: molecular mechanisms and clinical disease heterogeneity. Leuk Res. 2001, 25: 577-582. 10.1016/S0145-2126(01)00046-7.
Beghini A, Peterlongo P, Ripamonti CB, Larizza L, Cairoli R, Morra E, Mercucci C: C-kit mutations in core binding factor leukemias. Blood. 2000, 95: 726-727.
Hongyo T, Li T, Syaifudin M, Baskar R, Ikeda H, Kanakura Y, Aozasa K, Nomura T: Specific c-kit mutations in sinonasal natural killer/T-cell lymphoma in China and Japan. Cancer Res. 2000, 60: 2345-2347.
Isozaki K, Terris B, Belghiti J, Schiffmann S, Hirota S, Vanderwinden J: Germline-activating mutation in the kinase domain of KIT gene in familial gastrointestinal stromal tumors. Am J Pathol. 2000, 157: 1581-1585.
Kitamura Y, Hirota S, Nishida T: Gastrointestinal stromal tumors (GIST): a model for molecule-based diagnosis and treatment of solid tumors. Cancer Sci. 2003, 94: 315-320. 10.1111/j.1349-7006.2003.tb01439.x.
Tian Q, Frierson HF, Krystal GW, Moskaluk CA: Activating c-kit mutations in human germ cell tumors. Am J Pathol. 1999, 154: 1643-1647.
London CA, Galli SJ, Yuuki T, Hu Z-Q, Helfand SC, Geissler EN: Spontaneous canine mast cell tumours express tandem duplication in the proto-oncogene c-KIT. Exp Hematol. 1999, 27: 689-97. 10.1016/S0301-472X(98)00075-7.
Ma Y, Longley BJ, Wang X, Blount JL, Langley K, Caughey GH: Clustering of activating mutations in c-KIT' s, juxtamembrane coding region in canine mast cell neoplasms. J Invest Dermatol. 1999, 112: 165-70. 10.1046/j.1523-1747.1999.00488.x.
Riva F, Brizzola S, Stefanello D, Crema S, Turin L: A study of mutations in the c-kit gene of 32 dogs with mastocytoma. J Vet Diagn Invest. 2005, 17: 385-388.
Zemke D, Yamini B, Yuzbasiyan-Gurkan V: Mutations in the juxtamembrane domain of c-kit are associated with higher grade mast cell tumors in dogs. Veterinary Pathology. 2002, 39: 529-535. 10.1354/vp.39-5-529.
Longley BJ, Reguera MJ, Ma Y: Classes of c-KIT activating mutations: proposed mechanisms of action and implications for disease classification and therapy. Leukaemia Research. 2001, 25: 571-576. 10.1016/S0145-2126(01)00028-5.
Webster JD, Yuzbasyian-Gurkan V, Kaneene JB, Miller R, Resau JH, Kupel M: The role of c-KIT in tumorigenesis: evaluation in canine cutaneous mast cell tumors. Neoplasia. 2006, 8: 104-111. 10.1593/neo.05622.
Sheu LF, Lee WC, Lee HS, Kao WY, Chen A: Co-expression of c-kit and stem cell factor in primary and metastatic nasopharyngeal carcinomas and nasopharyngeal epithelium. J Pathol. 2005, 207: 216-223. 10.1002/path.1822.
Derenzini M, Ploton D: Interphase nucleolar organizer regions in cancer cells. Int Rev Exp Pathol. 1991, 32: 149-192.
Orrell JM, Evans AT, Grant A: A critical evaluation of AgNORs counting in benign naevi and malignant melanoma. J Pathol. 1991, 163: 239-244. 10.1002/path.1711630309.
Geraldes M, Gärtner Schmitt F: Hormonal receptors and cell proliferation in normal breast and spontaneous canine mammary tumours: an immunohistochemical study. Vet Rec. 2000, 146: 403-406.
Acknowledgements
RMGC is supported by an FCT grant (SFRH/BM/21610/2005) financed by the Portuguese Ministry of Science and Technology and the Social European Fund.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors' contributions
RMGC participated in conceiving and designing the study, diagnosed and graded the tumours, interpreted histochemical and immunohistochemical assays and drafted the manuscript; EM performed the statistical analysis, CL and AR carried out the histochemical and immunohistochemical assays; FG conceived and designed the study, participated in diagnosing and grading the tumours and in interpreting the results. MAP participated in designing the study and interpreting the results. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Gil da Costa, R.M., Matos, E., Rema, A. et al. CD117 immunoexpression in canine mast cell tumours: correlations with pathological variables and proliferation markers. BMC Vet Res 3, 19 (2007). https://doi.org/10.1186/1746-6148-3-19
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1746-6148-3-19