Abstract
The epidermal growth factor receptor (EGFR) family, consisting of four tyrosine kinase receptors, c-erb B1-4, seems to be influential in gliomagenesis. The aim of this study was to investigate EGFR gene amplification and expression of c-erb B1-4 receptor proteins in human anaplastic astrocytomas. Formalin-fixed and paraffin-embedded sections from 31 cases were investigated by standard immunohistochemical procedures for expression of c-erb B1-4 receptor proteins using commercial antibodies. EGFR gene amplification was studied by fluorescence in situ hybridization using paraffin-embedded tissues. Two monoclonal antibodies, NCL-EGFR-384 and NCL-EGFR, were used for EGFR detection and they displayed positive immunoreactivity in 97% and 71%, respectively. For c-erb B2 detection three monoclonal antibodies, CB11, 3B5, and 5A2, were applied and they displayed positive immunoreactivity in 45%, 100%, and 52%, respectively. Positive immunostaining for c-erb B3 and c-erb B4 was encountered in 97% and 74%, respectively. The EGFR gene was amplified in 9 out of 31 tumors (29%). After adjusting for age, Karnofsky performance status, and extent of surgical resection, Cox multiple regression analysis with overall survival as the dependent variable revealed that c-erb B2 overexpression detected by the monoclonal antibody clone CB11 was a statistically significant poor prognostic factor (P = 0.004). This study shows the convenience and feasibility of immunohistochemistry when determining the expression of receptor proteins in tissue sections of human astrocytomas. The synchronous overexpression of c-erb B1-4 proteins in anaplastic astrocytomas supports their role in the pathogenesis of these tumors. Further, c-erb B2 overexpression seems to predict aggressive behaviour.
Similar content being viewed by others
Background
Anaplastic astrocytomas constitute 4% of all malignant nervous system tumors [1]. Patients with anaplastic astrocytomas face a poor prognosis despite major efforts to improve radiation, chemotherapy, and surgical procedures. Median survival for patients with anaplastic astrocytoma is 3 to 5 years [2]. Age at diagnosis, extent of surgery and Karnofsky performance score (KPS) are established prognostic factors in high grade glioma patients [3].
The astrocytic tumors are prone to progress, and members of the epidermal growth factor receptor (EGFR) family have been linked to this malignant transformation. This receptor family consists of four tyrosine kinase receptors, c-erb B1-4, and seems to be influential and involved in tumor cell proliferation, differentiation, cell survival, and angiogenesis [4–9]. Coexpression of c-erb B1-4 renders the possibility of dimerization of these receptors, thereby recruiting and enhancing signal transducing pathways [4, 7, 10]. Due to overexpression of the c-erb B1-4 receptor proteins and their location on the surface of neoplastic astrocytes, they are attractive candidates for targeted therapy [11–13]. Current strategies include inhibition of the intrinsic kinase activity by monoclonal antibodies [14–16]. Such treatment, however, requires reliable detection systems for these receptor proteins in tumor tissue. Immunohistochemistry appears as the principal mean to detect these receptor proteins. Although this is a convenient and feasible technique, different staining results can be achieved due to varying sensitivity and specificity of commercial antibodies.
Several studies have to a varying degree shown amplification of the EGFR (c-erb B1) gene, located on chromosome 7, in glioblastoma multiforme [17–24]. EGFR gene amplification distinguishes small cell glioblastomas from anaplastic oligodendrogliomas, and it has been shown to be an indicator for resistance to radiotherapy [25, 26]. EGFR gene amplification can now simply be evaluated by means of fluorescence in situ hybdridization (FISH). However, there is limited knowledge concerning the occurrence of EGFR gene amplification and the expression of erb-receptors in anaplastic astrocytomas [27, 28].
This study was an extension of our research on erb receptor expression in glioblastomas [8, 29], and was designed to investigate the extent of EGFR gene amplification and overexpression in anaplastic astrocytomas. Further, we wanted to explore the expression of other members of the EGFR family in anaplastic astrocytomas and investigate their prognostic significance.
Patients and methods
All 31 supratentorial human anaplastic astrocytomas were operated at the Department of Neurosurgery, St. Olav University Hospital, Trondheim, Norway, and consecutively collected in the time period 1998 to 2006. Craniotomies were performed under general anesthesia, with the patient's head resting in a Mayfield frame system (OMI, Inc., Cincinnati, OH, USA) attached to a reference frame for neuronavigation. The preoperative data was imported into an ultrasound-based navigation system and used for surgical planning and resection guidance [30]. All patients underwent magnetic resonance imaging (MRI) a few days before and within 72 hours after surgery. The extent of tumor resection was determined by the postoperative MRI scans. Surgical resection was defined as gross total resection, partial resection, or biopsy. A chart review was performed to collect demographic and clinical data that included age, sex, and symptoms at presentation, tumor localization, treatment modalities, and postoperative survival. Preoperative Karnofsky performance status score was retrospectively determined from a routine neurological examination from patient admittance, one to three days before surgery.
Expression of c-erb B1-4 receptor proteins was determined by immunohistochemistry using various commercial monoclonal antibodies listed in Table 1. Formalin-fixed and paraffin-embedded sections, 4 μm thick, with representative tumor tissue, were incubated with primary antibodies after antigen retrieval by pressure cooking. An automatized histostainer was used for the immunohistochemcial procedures (Dako Autostainer, Glostrup, Denmark). For visualization of immunoreactivity, DAKO EnVision system was used with diaminobenzidin as chromogene. Sections were counterstained with haematoxylin. Positive controls were included in each staining run.
The immunoreactivity was assessed by means of intensity and percentage of immunoreactive tumor cells. Intensity was recorded as 0 (no reaction) to 3 (strong reaction). Fraction of immunoreactive tumor cells was recorded as 0 (no positive cells), 1 (<10% positive cells), 2 (10-50% positive cells), or 3 (>50% positive cells). A staining index was calculated as the product of intensity and fraction of positive tumor cells [8].
EGFR gene copy number was investigated on 4 μm thick paraffin sections by double FISH using the LSI EGFR Dual Color Probe- Hyb Set, Vysis no 32-191053 (Abbott Molecular Inc, IL, US). The first probe hybridized with the EGFR gene (chromosomal 7p12 region). Against the centromeric region a second probe was used which revealed the number of copies of chromosome 7. The sections were deparaffinised using xylene, rehydrated, and pretreated using DAKO solution kit (Histology FISH Accessory kit). The probes were added to the sections, coverslipped, sealed with rubber cement, and placed in a DAKO Hybridizer. The sections and probes were codenatured for 5 min at 73°C, followed by annealing at 37°C over night. After hybridization slides were washed in 0.4 × SSC (with detergent) at 73°C for two minutes followed by one minutes in 2 × SSC at room temperature. Then the sections underwent dehydrating in ethanol three times for 3 min. The slides were counterstained and embedded with a 4,6-diamidino-2-phenylindole/antifade solution (DAKO). FISH signals for each locus-specific FISH probe were assessed under a Nikon Eclipse 90i microscope (Nikon, Tokyo, Japan) using Cytovision software (Applied Imaging, Newcastle upon Tyne, England) equipped with a triple-pass filter (DAPI/Green/Orange). The entire area of tumour tissue was evaluated in each case, and all non-overlapping nuclei were assessed for orange (marker) and green (reference) signals by a pathologist (SHT) blinded to any information about the patients. Using the criteria given by Vysis Inc., for HER-2/neu FISH, EGFR signals to chromosome 7 centromere signals of 2 or greater were considered gene amplification [31].
Statistical analyses were made using SPSS version 16.0 (SPSS Inc., Chicago, IL). Survival time was calculated from date of surgery to date of death. Multiple Cox regression analyses were used to study the association between EGFR receptor protein expression (Staining Index, continuous variable) and survival, adjusting for age at diagnosis (continuous variable), Karnofsky performance status scores (continuous variable), and extent of surgical resection (categorical variable; gross total resection versus partial resection and biopsy). The association between results from the FISH investigations (categorical variables; positive versus negative) and survival were studied in the same manner. Two-sided P-values less than 0.05 were regarded as statistically significant.
The study was approved by the Regional Committee for Medical Research Ethics, and study protocols adhered to guidelines by the Helsinki Convention.
Results
Thirty-one consecutive patients (13 women and 18 men; mean age, 50.2 yr; age range, 28-78 yr) with anaplastic astrocytomas were included in the study. Clinical features of the study group are presented in Table 2. Gross total tumor resection or partial resection was achieved in 10 (32%) and 16 (52%) patients, respectively. In 5 (16%) patients only biopsies were obtained. Four patients had previously undergone surgical resection of diffuse WHO grade II astrocytomas. Chemotherapy and radiotherapy were provided in 7 and 24 cases, respectively.
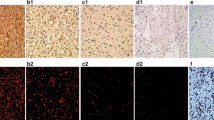
Immunohistochemical and FISH data are presented in Table 3. In general, the immunoreactivity was localized to either the cell membrane or the cytoplasm or both. The distribution of tumor cells overexpressing erb proteins was both diffuse and dispersed with intensity varying from weak to strong.
Two monoclonal antibodies were used for EGFR detection. NCL-EGFR (clone EGFR.113) is reactive against the external EGFR domain and rendered 22 positive tumors (71%). NCL-EGFR-384 is directed towards the internal domain, and 30 tumors (97%) were positive with this antibody.
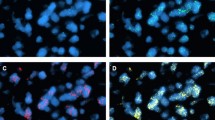
To determine c-erb B2 expression, three different monoclonal antibodies were applied. The antibody clones CB11, 3B5, and 5A2 were all reactive against the internal domain and displayed positive immunoreactivity in 45%, 100%, and 52%, respectively (Figure 1A).
C-erb B3 immunoreactivity occurred in 27 out of 31 anaplastic astrocytomas (97%), and c-erb B4 immunoreactivity was present in 23 out of 31 cases (74%).
The EGFR gene was amplified in 9 out of 31 tumors (29%) assessed by FISH (Figure 1B).
Multiple Cox regression analysis with overall survival as the dependent variable was performed for clinical disease features with expression of c-erb B1-4 and EGFR gene amplification (Table 4). After adjusting for age, Karnofsky performance status, and extent of surgical resection, the Cox analysis revealed that c-erb B2 overexpression detected by the monoclonal antibody clone CB11 was a statistically significant poor prognostic factor in patients with anaplastic astrocytoma (P = 0.004). Neither overexpression of EGFR, c-erb B3, and c-erb B4, nor EGFR gene amplification reached significance.
Discussion
In this study we have found abundant co-expression of c-erb B1-4 receptor proteins in anaplastic astrocytomas. Cox multiple regression analysis showed that c-erb B2 expression detected by the monoclonal antibody clone CB11 was a statistically significant poor prognostic factor. Additionally, the EGFR gene was amplified in 29% of the tumors.
The overexpression of EGFR in anaplastic astrocytomas is in line with our earlier report and other studies [24, 28, 32–34]. Interestingly, the antibody reactive against the cytoplasmic domain unveiled far more immunoreactive tumors than the antibody against the external domain (97% versus 71%). A similar observation was made in our recent report on glioblastomas [8]. A possible explanation might be that the extracellular part of the EGFR is more vulnerable to fixation and tissue processing. It may also be due to proteolytic cleavage and shedding of EGFR as is described for c-erb B2 [35, 36]. In any case, further studies must be undertaken to decide which antibody is best suited for standardized EGFR immunohistochemical detection. Such investigations require alternative assays for protein expression, such as protein blotting.
Amplification of the EGFR gene occurs in up to 50% of human glioblastomas, whereas the reported frequency in anaplastic astrocytomas varies considerably between none to about one third of the cases [23, 24, 28, 32, 37–40]. Different techniques and definitions of gene dosage may explain these conflicting data. In this regard, FISH analyses have turned out to be a simple and reliable method [28, 32]. In our series of anaplastic astrocytomas this approach revealed a relatively high frequency of EGFR gene amplification with 29% of the tumors exhibiting this genetic rearrangement. It seems this genetic event is not solely confined to glioblastomas, and it may be coupled to the malignant progression of anaplastic astrocytomas. It remains to be shown whether this gene amplification is a cause or a consequence of tumor progression. Nevertheless, it may be involved in the oncogenesis of both primary and secondary glioblastomas. Overexpression of EGFR in astrocytomas can also occur without gene amplification [8, 28, 32, 37, 39], suggesting other mechanisms at transcriptional and translational levels. An amplified EGFR gene may lead to expression of mutant EGFR (EGFRvIII) in high grade astrocytomas [27], providing a possible target for immunotherapy [41]. Even though there are several studies advocating the importance of EGFR in the oncogenesis of astrocytomas [5, 9], the prognostic role of EGFR overexpression and EGFR gene amplification is still not fully clarified [5, 8, 9, 27, 31, 39, 42–46]. Neither EGFR gene amplification nor EGFR overexpression was associated with survival in this study. Conflicting prognostic data may be related to various techniques and small series of tumors studied, so larger studies are required to explore the prognostic impact of EGFR.
C-erb B2 is already an established negative prognostic marker with an associated targeted therapy in human breast cancer [47]. It has been suggested that c-erb B2 is a preferred partner for heterodimerization with the other members of the EGFR family. The physiological ligand of c-erb B2 has not been identified, and it is possible that this receptor can undergo homo- and heterodimerization in the absence of ligand binding [48]. In our series of anaplastic astrocytomas, three different antibodies were applied for detection of the c-erb B2 receptor protein in tumor tissue. They were all reactive against the internal domain and provided satisfactory immunostainings. In line with divergent reports on the presence of this receptor in malignant astrocytomas [8, 21, 49–55], the number of immunoreactive tumors differed considerably between the antibodies used. This can partly be explained by the application of different antibodies, subjective assessments of immunostaining, differences in tissue processing, and small sample series. Accordingly, the reported prognostic power of this receptor protein is debatable as well. Even so, reports including the present study point to an unfavourable outcome in neuroepithelial tumors with c-erb B-2 overexpression [51, 52, 56, 57].
Unlike breast cancer, gene amplification is not a prerequisite for overexpression in gliomas [49, 58] and FISH analyses are therefore superfluous in this context.
The overexpression of c-erb B3 and c-erb B4 is in accordance with our previous study on glioblastomas as well as with others [8, 21, 54, 59, 60]. These findings suggest involvement of these two receptor proteins in the oncogenesis of astrocytomas. However, no association with survival was detected in our study. By forming heterodimers with other members of the EGFR family, c-erb B3 channels erb B signaling through the PI3K/Akt pathway which in turn promotes tumor growth and progression. This phenomenon may limit the impact of exclusively targeting EGFR and c-erb B2 proteins, and may explain the acquired resistance to anticancer drugs that inhibit receptor tyrosine kinases [61]. The close integration of different erb B signaling systems and their interactions with other tyrosine kinases such as IGF1-R and c-met, suggest that the members of the EGFR family should be dealt with as a group rather than individually.
The role of c-erb B4 in tumorigenesis has not been fully established. Cooperation between erb proteins may also be of clinical relevance as mutual expression of c-erb B2 and c-erb B4 serves as an independent negative prognostic factor in childhood medulloblastoma [56]. A unique feature for c-erb B4 is that pre-mRNA undergoes alternative splicing, generating structurally distinct isoforms. The c-erb B4 isoforms have different signalling capabilities and may lead to diverse cellular responses. This might explain the inconsistent reports evaluating the role of c-erb B4 in breast cancer [62].
This retrospective study has limitations typical of immunohistochemical approaches, including limited technical reproducibility and subjective interpretation. In summary, this study shows the convenience and feasibility of immunohistochemistry when determining the expression of receptor proteins in tumor tissue. Our findings indicate that c-erb B2 overexpression predicts aggressive behaviour in human anaplastic astrocytomas. The coexpression of members of the EGFR family underlines their role in the oncogenesis of anaplastic astrocytomas and supports their promising clinical relevance in regard to prognosis, diagnosis, and therapy.
Abbreviations
- EGFR:
-
Epidermal growth factor receptor
- FISH:
-
Fluorescence in situ hybridization
- KPS:
-
Karnofsky performance status
- GTR:
-
Gross total resection
- MRI:
-
Magnetic resonance imaging
- WHO:
-
World Health Organization
- N.D.:
-
No data.
References
Stupp R, Reni M, Gatta G, Mazza E, Vecht C: Anaplastic astrocytoma in adults. Crit Rev Oncol Hematol. 2007, 63: 72-80.
Chang SM, Parney IF, Huang W, Anderson FA, Asher AL, Bernstein M, Lillehei KO, Brem H, Berger MS, Laws ER, Glioma Outcomes Project I: Patterns of care for adults with newly diagnosed malignant glioma. JAMA. 2005, 293: 557-564.
Buckner JC: Factors influencing survival in high-grade gliomas. Semin Oncol. 2003, 30: 10-14.
Bazley LA, Gullick WJ: The epidermal growth factor receptor family. Endocr Relat Cancer. 2005, 12 (Suppl 1): S17-27.
Hamel W, Westphal M: Growth factors in gliomas revisited. Acta Neurochir (Wien). 2000, 142: 113-137. discussion 137-118
Kuan CT, Wikstrand CJ, Bigner DD: EGF mutant receptor vIII as a molecular target in cancer therapy. Endocr Relat Cancer. 2001, 8: 83-96.
Schlessinger J: Cell signaling by receptor tyrosine kinases. Cell. 2000, 103: 211-225.
Torp SH, Gulati S, Johannessen E, Dalen A: Coexpression of c-erbB 1-4 receptor proteins in human glioblastomas. An immunohistochemical study. J Exp Clin Cancer Res. 2007, 26: 353-359.
Weiner HL: The role of growth factor receptors in central nervous system development and neoplasia. Neurosurgery. 1995, 37: 179-193. discussion 193-174
Schlessinger J: Ligand-induced, receptor-mediated dimerization and activation of EGF receptor. Cell. 2002, 110: 669-672.
Halatsch ME, Schmidt U, Behnke-Mursch J, Unterberg A, Wirtz CR: Epidermal growth factor receptor inhibition for the treatment of glioblastoma multiforme and other malignant brain tumours. Cancer Treat Rev. 2006, 32: 74-89.
Herbst RS, Shin DM: Monoclonal antibodies to target epidermal growth factor receptor-positive tumors: a new paradigm for cancer therapy. Cancer. 2002, 94: 1593-1611.
Mischel PS, Cloughesy TF: Targeted molecular therapy of GBM. Brain Pathol. 2003, 13: 52-61.
Contessa JN, Hamstra DA: Revoking the privilege: targeting HER2 in the central nervous system. Mol Pharmacol. 2008, 73: 271-273.
Voelzke WR, Petty WJ, Lesser GJ: Targeting the epidermal growth factor receptor in high-grade astrocytomas. Curr Treat Options Oncol. 2008, 9: 23-31.
Emanuel SL, Hughes TV, Adams M, Rugg CA, Fuentes-Pesquera A, Connolly PJ, Pandey N, Moreno-Mazza S, Butler J, Borowski V, et al: Cellular and in vivo activity of JNJ-2887 a nonquinazoline pan-ErbB kinase inhibitor that crosses the blood-brain barrier and displays efficacy against intracranial tumors. Mol Pharmacol. 1063, 73: 338-348.
Libermann TA, Nusbaum HR, Razon N, Kris R, Lax I, Soreq H, Whittle N, Waterfield MD, Ullrich A, Schlessinger J: Amplification, enhanced expression and possible rearrangement of EGF receptor gene in primary human brain tumours of glial origin. Nature. 1985, 313: 144-147.
Liu L, Ichimura K, Pettersson EH, Collins VP: Chromosome 7 rearrangements in glioblastomas; loci adjacent to EGFR are independently amplified. J Neuropathol Exp Neurol. 1998, 57: 1138-1145.
Sauter G, Maeda T, Waldman FM, Davis RL, Feuerstein BG: Patterns of epidermal growth factor receptor amplification in malignant gliomas. Am J Pathol. 1996, 148: 1047-1053.
Schlegel J, Merdes A, Stumm G, Albert FK, Forsting M, Hynes N, Kiessling M: Amplification of the epidermal-growth-factor-receptor gene correlates with different growth behaviour in human glioblastoma. Int J Cancer. 1994, 56: 72-77.
Schlegel J, Stumm G, Brandle K, Merdes A, Mechtersheimer G, Hynes NE, Kiessling M: Amplification and differential expression of members of the erbB-gene family in human glioblastoma. J Neurooncol. 1994, 22: 201-207.
Schober R, Bilzer T, Waha A, Reifenberger G, Wechsler W, von Deimling A, Wiestler OD, Westphal M, Kemshead JT, Vega F, et al : The epidermal growth factor receptor in glioblastoma: genomic amplification, protein expression, and patient survival data in a therapeutic trial. Clin Neuropathol. 1995, 14: 169-174.
von Deimling A, Louis DN, von Ammon K, Petersen I, Hoell T, Chung RY, Martuza RL, Schoenfeld DA, Yasargil MG, Wiestler OD, et al: Association of epidermal growth factor receptor gene amplification with loss of chromosome 10 in human glioblastoma multiforme. J Neurosurg. 1992, 77: 295-301.
Wong AJ, Bigner SH, Bigner DD, Kinzler KW, Hamilton SR, Vogelstein B: Increased expression of the epidermal growth factor receptor gene in malignant gliomas is invariably associated with gene amplification. Proc Natl Acad Sci USA. 1987, 84: 6899-6903.
Barker FG, Simmons ML, Chang SM, Prados MD, Larson DA, Sneed PK, Wara WM, Berger MS, Chen P, Israel MA, Aldape KD: EGFR overexpression and radiation response in glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2001, 51: 410-418.
Krishnan S, Rao RD, James CD, Sarkaria JN: Combination of epidermal growth factor receptor targeted therapy with radiation therapy for malignant gliomas. Front Biosci. 2003, 8: e1-13.
Aldape KD, Ballman K, Furth A, Buckner JC, Giannini C, Burger PC, Scheithauer BW, Jenkins RB, James CD: Immunohistochemical detection of EGFRvIII in high malignancy grade astrocytomas and evaluation of prognostic significance. J Neuropathol Exp Neurol. 2004, 63: 700-707.
Guillaudeau A, Durand K, Pommepuy I, Robert S, Chaunavel A, Lacorre S, DeArmas R, Bourtoumieux S, El Demery M, Moreau JJ, Labrousse F: Determination of EGFR status in gliomas: usefulness of immunohistochemistry and fluorescent in situ hybridization. Appl Immunohistochem Mol Morphol. 2009, 17: 220-226.
Torp SH, Bringedal K, Dalen A: Immunohistochemical detection of epidermal growth factor receptor in human high-grade astrocytomas--a comparison between frozen- and paraffin sections. J Exp Clin Cancer Res. 2005, 24: 89-92.
Gulati S, Berntsen EM, Solheim O, Kvistad KA, Haberg A, Selbekk T, Torp SH, Unsgaard G: Surgical resection of high-grade gliomas in eloquent regions guided by blood oxygenation level dependent functional magnetic resonance imaging, diffusion tensor tractography, and intraoperative navigated 3D ultrasound. Minim Invasive Neurosurg. 2009, 52: 17-24.
Layfield LJ, Willmore C, Tripp S, Jones C, Jensen RL: Epidermal growth factor receptor gene amplification and protein expression in glioblastoma multiforme: prognostic significance and relationship to other prognostic factors. Appl Immunohistochem Mol Morphol. 2006, 14: 91-96.
Marquez A, Wu R, Zhao J, Tao J, Shi Z: Evaluation of epidermal growth factor receptor (EGFR) by chromogenic in situ hybridization (CISH) and immunohistochemistry (IHC) in archival gliomas using bright-field microscopy. Diagn Mol Pathol. 2004, 13: 1-8.
Reifenberger G, Prior R, Deckert M, Wechsler W: Epidermal growth factor receptor expression and growth fraction in human tumours of the nervous system. Virchows Arch A Pathol Anat Histopathol. 1989, 414: 147-155.
Torp SH, Helseth E, Dalen A, Unsgaard G: Epidermal growth factor receptor expression in human gliomas. Cancer Immunol Immunother. 1991, 33: 61-64.
Perez-Torres M, Valle BL, Maihle NJ, Negron-Vega L, Nieves-Alicea R, Cora EM: Shedding of epidermal growth factor receptor is a regulated process that occurs with overexpression in malignant cells. Exp Cell Res. 2008, 314: 2907-2918.
Zabrecky JR, Lam T, McKenzie SJ, Carney W: The extracellular domain of p185/neu is released from the surface of human breast carcinoma cells, SK-BR-3. J Biol Chem. 1991, 266: 1716-1720.
Ekstrand AJ, James CD, Cavenee WK, Seliger B, Pettersson RF, Collins VP: Genes for epidermal growth factor receptor, transforming growth factor alpha, and epidermal growth factor and their expression in human gliomas in vivo. Cancer Res. 1991, 51: 2164-2172.
Hurtt MR, Moossy J, Donovan-Peluso M, Locker J: Amplification of epidermal growth factor receptor gene in gliomas: histopathology and prognosis. J Neuropathol Exp Neurol. 1992, 51: 84-90.
Jarvela S, Helin H, Haapasalo J, Jarvela T, Junttila TT, Elenius K, Tanner M, Haapasalo H, Isola J: Amplification of the epidermal growth factor receptor in astrocytic tumours by chromogenic in situ hybridization: association with clinicopathological features and patient survival. Neuropathol Appl Neurobiol. 2006, 32: 441-450.
Smith JS, Tachibana I, Passe SM, Huntley BK, Borell TJ, Iturria N, O'Fallon JR, Schaefer PL, Scheithauer BW, James CD, et al: PTEN mutation, EGFR amplification, and outcome in patients with anaplastic astrocytoma and glioblastoma multiforme. J Natl Cancer Inst. 2001, 93: 1246-1256.
Choi BD, Archer GE, Mitchell DA, Heimberger AB, McLendon RE, Bigner DD, Sampson JH: EGFRvIII-targeted vaccination therapy of malignant glioma. Brain Pathol. 2009, 19: 713-723.
Bouvier-Labit C, Chinot O, Ochi C, Gambarelli D, Dufour H, Figarella-Branger D: Prognostic significance of Ki67, p53 and epidermal growth factor receptor immunostaining in human glioblastomas. Neuropathol Appl Neurobiol. 1998, 24: 381-388.
Newcomb EW, Cohen H, Lee SR, Bhalla SK, Bloom J, Hayes RL, Miller DC: Survival of patients with glioblastoma multiforme is not influenced by altered expression of p16, p53, EGFR, MDM2 or Bcl-2 genes. Brain Pathol. 1998, 8: 655-667.
Olson JJ, Barnett D, Yang J, Assietti R, Cotsonis G, James CD: Gene amplification as a prognostic factor in primary brain tumors. Clin Cancer Res. 1998, 4: 215-222.
Shinojima N, Tada K, Shiraishi S, Kamiryo T, Kochi M, Nakamura H, Makino K, Saya H, Hirano H, Kuratsu J, et al: Prognostic value of epidermal growth factor receptor in patients with glioblastoma multiforme. Cancer Res. 2003, 63: 6962-6970.
Torp SH, Helseth E, Dalen A, Unsgaard G: Relationships between Ki-67 labelling index, amplification of the epidermal growth factor receptor gene, and prognosis in human glioblastomas. Acta Neurochir (Wien). 1992, 117: 182-186.
Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, Watson M, Davies S, Bernard PS, Parker JS, et al: Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009, 101: 736-750.
Graus-Porta D, Beerli RR, Daly JM, Hynes NE: ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO J. 1997, 16: 1647-1655.
Haapasalo H, Hyytinen E, Sallinen P, Helin H, Kallioniemi OP, Isola J: c-erbB-2 in astrocytomas: infrequent overexpression by immunohistochemistry and absence of gene amplification by fluorescence in situ hybridization. Br J Cancer. 1996, 73: 620-623.
Haynik DM, Roma AA, Prayson RA: HER-2/neu expression in glioblastoma multiforme. Appl Immunohistochem Mol Morphol. 2007, 15: 56-58.
Mineo JF, Bordron A, Baroncini M, Maurage CA, Ramirez C, Siminski RM, Berthou C, Dam Hieu P: Low HER2-expressing glioblastomas are more often secondary to anaplastic transformation of low-grade glioma. J Neurooncol. 2007, 85: 281-287.
Potti A, Forseen SE, Koka VK, Pervez H, Koch M, Fraiman G, Mehdi SA, Levitt R: Determination of HER-2/neu overexpression and clinical predictors of survival in a cohort of 347 patients with primary malignant brain tumors. Cancer Invest. 2004, 22: 537-544.
Schwechheimer K, Laufle RM, Schmahl W, Knodlseder M, Fischer H, Hofler H: Expression of neu/c-erbB-2 in human brain tumors. Hum Pathol. 1994, 25: 772-780.
Andersson U, Guo D, Malmer B, Bergenheim AT, Brannstrom T, Hedman H, Henriksson R: Epidermal growth factor receptor family (EGFR, ErbB2-4) in gliomas and meningiomas. Acta Neuropathol. 2004, 108: 135-142.
Torp SH, Helseth E, Unsgaard G, Dalen A: C-erbB-2/HER-2 protein in human intracranial tumours. Eur J Cancer. 1993, 29A: 1604-1606.
Gilbertson RJ, Perry RH, Kelly PJ, Pearson AD, Lunec J: Prognostic significance of HER2 and HER4 coexpression in childhood medulloblastoma. Cancer Res. 1997, 57: 3272-3280.
Koka V, Potti A, Forseen SE, Pervez H, Fraiman GN, Koch M, Levitt R: Role of Her-2/neu overexpression and clinical determinants of early mortality in glioblastoma multiforme. Am J Clin Oncol. 2003, 26: 332-335.
Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A, et al: Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989, 244: 707-712.
Srinivasan R, Poulsom R, Hurst HC, Gullick WJ: Expression of the c-erbB-4/HER4 protein and mRNA in normal human fetal and adult tissues and in a survey of nine solid tumour types. J Pathol. 1998, 185: 236-245.
Westphal M, Meima L, Szonyi E, Lofgren J, Meissner H, Hamel W, Nikolics K, Sliwkowski MX: Heregulins and the ErbB-2/3/4 receptors in gliomas. J Neurooncol. 1997, 35: 335-346.
Stern DF: ERBB3/HER3 and ERBB2/HER2 duet in mammary development and breast cancer. J Mammary Gland Biol Neoplasia. 2008, 13: 215-223.
Sundvall M, Iljin K, Kilpinen S, Sara H, Kallioniemi OP, Elenius K: Role of ErbB4 in breast cancer. J Mammary Gland Biol Neoplasia. 2008, 13: 259-268.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
SG has received grants from Sparebanken Møre and The Family Blix Fund for medical research. The other authors have no competing interests.
Authors' contributions
SG carried out the experiments, collected and interpretated the data, and wrote the manuscript. BY carried out the experiments and wrote the manuscript. USG carried out the experiments and wrote the manuscript. MG collected data and wrote the manuscript. SL analyzed the data and wrote the manuscript. SHT contributed conception, designed the study, and wrote the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Gulati, S., Ytterhus, B., Granli, U.S. et al. Overexpression of c-erb B2 is a negative prognostic factor in anaplastic astrocytomas. Diagn Pathol 5, 18 (2010). https://doi.org/10.1186/1746-1596-5-18
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1746-1596-5-18