Abstract
Background
Gastroesophageal reflux disease (GERD) is a common cause of chronic cough. Both acid and nonacid reflux is thought to play a role in the initiation of coughing and cough hypersensitivity. The GABAB receptor agonist lesogaberan was developed as a peripherally restricted anti-reflux therapy that reduces the frequency of transient lower esophageal sphincter relaxations (TLESR; the major cause of reflux) in animals and in patients with GERD. GABAB receptor agonists have also been shown to possess antitussive effects in patients and in animals independent of their effects on TLESR, suggesting that lesogaberan may be a promising treatment for chronic cough.
Methods
We have assessed the direct antitussive effects of lesogaberan (AZD3355). The effects of other GABAB receptor agonists were also determined. Coughing was evoked in awake guinea pigs using aerosol challenges with citric acid.
Results
Lesogaberan dose-dependently inhibited citric acid evoked coughing in guinea pigs. Comparable effects of the GABAB receptor agonists baclofen and 3-aminopropylphosphinic acid (3-APPiA) on cough were also observed. Baclofen produced obvious signs of sedation and respiratory depression. By contrast, both lesogaberan and 3-APPiA (both inactivated centrally by GABA transporters) were devoid of sedative effects and did not alter respiratory rate.
Conclusions
Together, the data suggest that lesogaberan and related GABAB receptor agonists may hold promise as safe and effective antitussive agents largely devoid of CNS side effects.
Similar content being viewed by others
Cough is one of the most commonly reported symptoms amongst patients seeking medical advice. Acute cough is triggered primarily by viral infections, while the most common causes of chronic cough are asthma, upper airway inflammatory disorders, and gastroesophageal reflux disease (GERD). Therapeutics used specifically for the treatment of cough are either minimally effective or have unwanted side effects that limit their utility. In patients with chronic cough, treatment of their underlying disease can improve patient quality of life and reduce coughing. But for many patients with chronic, troublesome cough, even after aggressive medical treatment of their underlying illnesses, cough can remain a significant health problem that adversely impacts quality of life. New and more effective and selective treatments for cough thus represent an unmet need in respiratory medicine [1, 2].
Agonists of the metabotropic GABAB receptor such as baclofen have been evaluated for their utility in targeting a number of peripheral disorders thought to involve aberrant reflexes and sensations including pain, overactive bladder, hiccups, tetanus/spasticity, and headache [3–10]. GABAB receptor agonists have also been evaluated for their effects on airways hyperresponsiveness, GERD and cough [11–20]. Although clinical benefit has been reported in these latter studies, a sedative effect of baclofen has also been noted [21, 22]. Ideally, an effective treatment for cough would prevent cough through direct effects on sensory nerves innervating the airways and independent of any significant CNS-dependent side effects. A therapy that targets both cough and GERD would be especially desirable, given the association between these conditions.
Key to the side effect profile of systemically administered GABAB receptor agonists is their CNS penetrance and susceptibility to inactivation by uptake [23, 24]. GABA and analogs of GABA are subject to uptake in the central nervous system by the 4 identified GABA transporters (GAT1-GAT4). Baclofen is not a substrate for uptake and can act centrally when administered peripherally [25]. By contrast, 3-APPiA is a GABAB receptor agonist that is inactivated centrally by transport [24–26]. There are reports of antitussive effects of 3-APPiA in guinea pigs and cats [27, 28]. It is thus possible that GABAB receptor agonists work peripherally to prevent vagal reflexes.
Lesogaberan (AZD3355) is a GABAB receptor agonist with limited CNS side effects that was developed for the treatment of GERD [20, 25, 29–31]. Like 3-APPiA, lesogaberan is inactivated centrally by GAT-dependent transport [25]. The purpose of this study was to evaluate the effects of lesogaberan and other GABAB receptor agonists on citric acid induced coughing in guinea pigs.
Methods
The institutional animal care and use committee approved all of the studies described below. Male Hartley guinea pigs (200-400 g, Charles River) were placed in a flow through chamber filled with air by an air pump. A pressure transducer was connected to the outflow of the chamber to monitor respiratory efforts and coughing in response to citric acid challenge. Data was recorded digitally using a Biopac data acquisition system.
Guinea pigs were pretreated 30 minutes prior to citric acid challenge with vehicle, lesogaberan (0.3-10 mg/ kg), baclofen (0.3 and 3 mg/ kg), 3-APPiA (0.3 and 3 mg/ kg) or SKF97541 (0.1 and 0.3 mg/ kg), administered by subcutaneous injection. After a 10 minute equilibration period in the exposure chamber when basal respiratory rate was monitored, guinea pigs were then challenged with increasing concentrations of citric acid (0.01, 0.1, 0.3 and 1 M), delivered by nebulizer (particle size: <5 μm) connected in series with the air pump. Each dose was delivered for 5 minutes, with a 5 minute interval in between doses. The total number of coughs evoked by each concentration of citric acid both during the 5 minute challenge and during the 5 minutes following the challenge was determined. The results of these studies are presented as the cumulative number of coughs evoked.
Cough was defined visually and by the characteristic pressure changes in the chamber, reflecting an enhanced inspiratory effort followed by a forceful expiratory effort, with expiratory pressure changes ≥ 500% of the pressures associated with expiration during eupnea, all occurring in less than 1 second. Coughing is readily differentiated from enhanced breaths (sighs), often associated with stimuli inducing bronchospasm, which have a roughly symmetric pressure signature (with equally enhanced inspiratory and expiratory pressures) and slower cycle (Figure 1). In these healthy, young animals and with the cough evoked by citric acid aerosols, the tussive responses evoked were presumed and indeed likely to be the result of a direct effect of the citric acid on airway sensory nerves. By extension, any antitussive effects of the compounds studied were likely to be the result of a direct effect on cough, and not the consequence of any effects on underlying pathology (e.g. GERD).
A representative trace of coughing evoked by a citric acid challenge to an awake guinea pig is depicted. Inspiratory (I) efforts produce a negative pressure in the chamber, with expiratory (E) efforts producing positive pressures. These traces were used to measure respiratory rate at the outset of each experiment (breaths/ min), the time to first cough following initiation of the citric acid challenges, the Peak to Peak (P-P) pressures associated with cough (measured by comparing the P-P pressures associated with coughing, expressed as a percentage of the P-P pressures measured at eupnea), the total number of coughs evoked by each dose of citric acid and the total number of coughs evoked cumulatively by all doses of citric acid studied.
The results are presented as a mean ± sem of n experiments, where n is the number of guinea pigs studied. Guinea pigs were challenged only once (to each dose of citric acid) in this unpaired experimental design. Differences amongst group means were evaluated by 1 way analysis of variance. A p-value of less than 0.05 was considered significant.
Reagents
Baclofen and citric acid were purchased from Sigma (St. Louis, MO). AstraZeneca (Mölndal, Sweden) provided lesogaberan, 3-APPiA and SKF97541. All reagents were dissolved in 0.9% NaCl solution.
Results
Baseline respiratory rate in control animals averaged 96 ± 1 breaths/ min (n = 15). At a dose of 3 mg/ kg, both baclofen and SKF97541 reduced baseline respiratory rate (78 ± 7 and 69 ± 3 breaths/ min, respectively; n = 5-6; p < 0.05) and produced obvious signs of sedation and lethargy. By contrast, 3 mg/ kg 3-APPiA and lesogaberan, even up to a dose of 10 mg/ kg, were without effect on respiratory rate (98 ± 2 and 97 ± 1 breaths/min, respectively; n = 7-8; p > 0.1) and produced no other overt signs of sedation or lethargy (Figure 2).
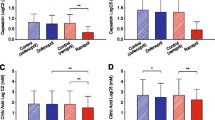
Citric acid inhalation evoked concentration-dependent coughing in the awake guinea pigs. No animals in any of the treatment groups coughed in response to the citric acid vehicle (water) inhalation, while 56/61 animals coughed at least once to 0.3 M citric acid challenge. The highest concentration of citric acid was not always well-tolerated. Five-minute challenges with 1 M citric acid were interrupted in 2/15 control experiments due to labored breathing. All but 3 of 41 animals pretreated with baclofen, 3-APPiA or lesogaberan completed the 1 M citric acid challenges. The coughing evoked by citric acid had a characteristic pattern of single, powerful coughs, occasionally 2 on consecutive breaths, but never the paroxysmal coughing we reported previously in studies of bradykinin evoked coughing [32, 33]. No signs of tachyphylaxis were apparent in these studies. Thus, if a robust cough response was evoked by a lower concentration of citric acid, subsequent challenge with a higher dose of citric acid still evoked coughing.
Baclofen, 3-APPiA and lesogaberan all inhibited citric acid induced coughing (Figure 3). The effects of all of these drugs were found to be dose-dependent. Lesogaberan was equipotent to baclofen in these studies (but, as mentioned above, without coincident sedative effects and respiratory depression). The sedating effects of SKF97541 were so profound that its antitussive actions were not extensively studied. Baclofen, 3-APPiA and lesogaberan all slightly reduced the percentage of animals coughing to any given dose of citric acid, and reduced the number of coughs evoked by given doses of citric acid. None of the drugs studied had any pronounced effects on the time to onset of coughing, nor on the peak pressures produced during the cough (Table 1).
Discussion
Lesogaberan is a GABAB receptor agonist with a limited CNS side effect profile. This compound has been evaluated as a treatment for GERD, a primary cause of chronic cough [20, 25, 29–31]. In the present study, lesogaberan was found to be an effective antitussive agent, preventing citric acid evoked coughing in conscious guinea pigs in a dose-dependent manner but without coincident sedative effects or respiratory depression. We found this compound was as potent and effective as baclofen, a GABAB receptor agonist used clinically for the treatment of several disorders. Unlike lesogaberan, however, the antitussive effects of baclofen were accompanied by undesirable side effects, including sedation, respiratory depression and a trend towards a decrease in peak cough pressures. Based on these results, we conclude that lesogaberan may hold promise for the treatment of acute and chronic cough and may have a better side effect profile than baclofen.
The four GABAB receptor agonists used in this study are essentially identical in receptor pharmacological profile. All are selective GABAB receptor agonists, with SKF97541, 3-APPiA and lesogaberan sharing structural similarities. Key to their differential side effect profile, however, is their susceptibility to uptake by GABA transporters [23–25]. Baclofen and SKF97541 are not transported, whereas both 3-APPiA and lesogaberan are substrates for GAT. Thus, the site of action for the latter 2 compounds is thought to be limited to peripheral locations and perhaps central locations with limited GAT expression [19, 25]. The marked respiratory depression and lethargy induced by SKF97541 and baclofen but not by lesogaberan and 3-APPiA we observed are consistent with the known susceptibility to uptake of these compounds.
Our results confirm and extend the studies by Bolser et al. [27, 28, 34], who reported that both baclofen and 3-APPiA prevented coughing in guinea pigs and cats. Because 3-APPiA was more effective administered peripherally than centrally, the authors concluded that GABAB receptor agonists prevent cough at least in part through peripheral sites of action. But a central site of action of these drugs cannot be entirely dismissed. We found that intraperitoneally administered baclofen inhibits cough [33], but we also reported that baclofen microinjected into the nTS inhibits cough in anesthetized guinea pigs [35]. Bolser et al. concluded that baclofen works primarily via central effects [28, 34]. Similarly, Callaway and King [36] found that baclofen inhalation prevented citric acid induced alterations in respiratory pattern (possibly by inhibiting bronchospasm) but was without effect on citric acid induced cough. Perhaps cell groupings in the brainstem, accessible to peripherally administered agonists and on the fringe of the blood brain barrier, are targeted by these drugs. For a central site of action to be viable, agonists such as 3-APPiA and lesogaberan must penetrate brainstem regions relevant to cough and attain sufficient concentrations to prevent or blunt synaptic transmission. There is conflicting evidence relating to blood-borne access and GABA uptake mechanisms in nTS [37–43].
GABAB receptor activation might prevent coughing through peripheral inhibitory effects on bronchopulmonary vagal afferent nerves. The vagal afferent nerves regulating cough in guinea pigs are C-fibers arising from the jugular ganglia, and cough receptors, terminating in the larynx, trachea and mainstem bronchi and with cell bodies in the nodose ganglia [32, 44]. Both of these vagal afferent nerve subtypes are responsive to acid but largely insensitive to changes in airway luminal pressure, stretch or airway smooth muscle contraction. The cough receptors are, however, insensitive to capsaicin. Given that Bolser et al. studied the antitussive effects of baclofen and 3-APPiA with capsaicin as the tussive stimulus, the available data would suggest that if GABAB receptor agonists are working peripherally, they act at least in part through effects on airway vagal C-fiber terminals [27, 28]. In fact, there is functional evidence to suggest that the vagal C-fibers regulating cough express GABAB receptors on their peripheral terminals [45, 46], but as yet, there is no evidence to suggest their activation would prevent action potential discharge. Interestingly, however, there are several studies showing direct inhibitory effects of GABAB receptor agonists on the excitability of vagal afferent nerves, including those innervating the stomach and esophagus [47–50].
Non-neuronal sites of action for GABAB receptor agonists are also possible. Acid evoked cough may be mast cell dependent, and mast cell activation may trigger or at least modulate subsequently evoked cough [51, 52]. GABAB receptor agonists can attenuate allergen-induced inflammation, perhaps through effects on mast cell activation [53, 54]. GABAB receptors on non-neural airway cells has also been documented, including airway smooth muscle and epithelium [55, 56], and we have described a transduction pathway dependent upon activation of chemosensory epithelial cells [57]. Endogenous GABA in the lung may also regulate cough [58]. Finally, GABAB receptor agonists have also been shown to attenuate parasympathetic-cholinergic responses in the airways, which might also indirectly attenuate cough responses [59–61]. In general, however, these non-neural influences on cough would seem more likely to be modulatory than essential to evoked cough. Bronchospasm is not an effective stimulus for cough, and bronchodilators have modest, variable effects on evoked cough [44].
GABAB receptor agonists may hold promise in the treatment of GERD due to their ability to prevent TLESR, the primary physiologic process responsible for reflux [15, 17, 18, 62, 63]. Compounds such as lesogaberan may offer a distinct advantage over conventional therapeutic approaches to GERD inasmuch as proton pump inhibitors and histamine H2 receptor antagonists do not prevent reflux, but rather, reduce the acid content of gastric fluid. While acid is thought to be the major trigger of symptoms and pathology in GERD, other components of refluxate such as pepsin likely contribute to the pathophysiology of this disease [64, 65]. GERD is also a major cause of chronic cough [1, 2]. Indeed, cough may be the only presenting symptom of GERD in some patients. The ability of compounds such as lesogaberan to prevent coughing evoked directly from the airways is another potential therapeutic advantage of GABAB receptor agonists in the treatment of GERD. These attributes along with the limited side effect profile of lesogaberan relative to that of baclofen provides impetus for continued study of this compound as a treatment for both GERD and chronic cough.
Funding
This study was funded by AstraZeneca and by a grant from the National Institutes of Health (HL083192).
References
Morice AH, Fontana GA, Sovijarvi AR, Pistolesi M, Chung KF, Widdicombe J, O'Connell F, Geppetti P, Gronke L, De Jongste J, Belvisi M, Dicpinigaitis P, Fischer A, McGarvey L, Fokkens WJ, Kastelik J: ERS Task Force. The diagnosis and management of chronic cough. Eur Respir J. 2004, 24 (3): 481-492. 10.1183/09031936.04.00027804.
Irwin RS, Baumann MH, Bolser DC, Boulet LP, Braman SS, Brightling CE, Brown KK, Canning BJ, Chang AB, Dicpinigaitis PV, Eccles R, Glomb WB, Goldstein LB, Graham LM, Hargreave FE, Kvale PA, Lewis SZ, McCool FD, McCrory DC, Prakash UB, Pratter MR, Rosen MJ, Schulman E, Shannon JJ, Smith Hammond C, Tarlo SM: American College of Chest Physicians (ACCP). Diagnosis and management of cough executive summary: ACCP evidence-based clinical practice guidelines. Chest. 2006, 129 (1 Suppl): 1S-23S.
Müller H, Börner U, Zierski J, Hempelmann G: Intrathecal baclofen in tetanus. Lancet. 1986, 1 (8476): 317-318.
Launois S, Bizec JL, Whitelaw WA, Cabane J, Derenne JP: Hiccup in adults: an overview. Eur Respir J. 1993, 6 (4): 563-575.
Hering-Hanit R, Gadoth N: Baclofen in cluster headache. Headache. 2000, 40 (1): 48-51. 10.1046/j.1526-4610.2000.00009.x.
Meythaler JM, Guin-Renfroe S, Brunner RC, Hadley MN: Intrathecal baclofen for spastic hypertonia from stroke. Stroke. 2001, 32 (9): 2099-2109. 10.1161/hs0901.095682.
Pehrson R, Lehmann A, Andersson KE: Effects of gamma-aminobutyrate B receptor modulation on normal micturition and oxyhemoglobin induced detrusor overactivity in female rats. J Urol. 2002, 168 (6): 2700-2705. 10.1016/S0022-5347(05)64247-4.
Enna SJ, McCarson KE: The role of GABA in the mediation and perception of pain. Adv Pharmacol. 2006, 54: 1-27.
Brennan PM, Whittle IR: Intrathecal baclofen therapy for neurological disorders: a sound knowledge base but many challenges remain. Br J Neurosurg. 2008, 22 (4): 508-519. 10.1080/02688690802233364.
Miyazato M, Sasatomi K, Hiragata S, Sugaya K, Chancellor MB, de Groat WC, Yoshimura N: GABA receptor activation in the lumbosacral spinal cord decreases detrusor overactivity in spinal cord injured rats. J Urol. 2008, 179 (3): 1178-1183. 10.1016/j.juro.2007.10.030.
Dicpinigaitis PV, Spungen AM, Bauman WA, Absgarten A, Almenoff PL: Inhibition of bronchial hyperresponsiveness by the GABA-agonist baclofen. Chest. 1994, 106 (3): 758-761. 10.1378/chest.106.3.758.
Dicpinigaitis PV: Use of baclofen to suppress cough induced by angiotensin-converting enzyme inhibitors. Ann Pharmacother. 1996, 30 (11): 1242-1245.
Dicpinigaitis PV, Dobkin JB: Antitussive effect of the GABA-agonist baclofen. Chest. 1997, 111 (4): 996-999. 10.1378/chest.111.4.996.
Dicpinigaitis PV, Dobkin JB, Rauf K, Aldrich TK: Inhibition of capsaicin-induced cough by the gamma-aminobutyric acid agonist baclofen. J Clin Pharmacol. 1998, 38 (4): 364-367.
Lehmann A, Antonsson M, Bremner-Danielsen M, Flärdh M, Hansson-Brändén L, Kärrberg L: Activation of the GABA(B) receptor inhibits transient lower esophageal sphincter relaxations in dogs. Gastroenterology. 1999, 117 (5): 1147-1154. 10.1016/S0016-5085(99)70400-2.
Dicpinigaitis PV, Grimm DR, Lesser M: Baclofen-induced cough suppression in cervical spinal cord injury. Arch Phys Med Rehabil. 2000, 81 (7): 921-923. 10.1053/apmr.2000.5612.
Lidums I, Lehmann A, Checklin H, Dent J, Holloway RH: Control of transient lower esophageal sphincter relaxations and reflux by the GABA(B) agonist baclofen in normal subjects. Gastroenterology. 2000, 118 (1): 7-13. 10.1016/S0016-5085(00)70408-2.
Zhang Q, Lehmann A, Rigda R, Dent J, Holloway RH: Control of transient lower oesophageal sphincter relaxations and reflux by the GABA(B) agonist baclofen in patients with gastro-oesophageal reflux disease. Gut. 2002, 50 (1): 19-24. 10.1136/gut.50.1.19.
Lehmann A: GABAB receptors as drug targets to treat gastroesophageal reflux disease. Pharmacol Ther. 2009, 122 (3): 239-245. 10.1016/j.pharmthera.2009.02.008.
Boeckxstaens GE, Rydholm H, Lei A, Adler J, Ruth M: Effect of lesogaberan, a novel GABA-receptor agonist, on transient lower esophageal sphincter relaxations in male subjects. Aliment Pharmacol Ther. 2010, 31 (11): 1208-1217. 10.1111/j.1365-2036.2010.04283.x.
Choo YM, Kim GB, Choi JY, Park JH, Park JH, Yang CW, Kim YS, Bang BK: Severe respiratory depression by low-dose baclofen in the treatment of chronic hiccups in a patient undergoing CAPD. Nephron. 2000, 86 (4): 546-547. 10.1159/000045866.
Marshall FH: Is the GABA B heterodimer a good drug target?. J Mol Neurosci. 2005, 26 (2–3): 169-176.
Clausen RP, Madsen K, Larsson OM, Frølund B, Krogsgaard-Larsen P, Schousboe A: Structure-activity relationship and pharmacology of gamma-aminobutyric acid (GABA) transport inhibitors. Adv Pharmacol. 2006, 54: 265-284.
Lehmann A, Antonsson M, Aurell-Holmberg A, Blackshaw L, Brändén L, Elebring T, Jensen J, Kärrberg L, Mattsson J, Nilsson K, Oja S, Saransaari P, von Unge S: Different in vitro and in vivo profiles of substituted 3-aminopropylphosphinate and 3-aminopropyl(methyl)phosphinate GABA(B) receptor agonists as inhibitors of transient lower oesophageal sphincter relaxation. Br J Pharmacol. 2012, 165 (6): 1757-1772. 10.1111/j.1476-5381.2011.01682.x.
Lehmann A, Antonsson M, Aurell Holmberg A, Blackshaw LA, Branden L, Brauner-Osborne H, Christiansen B, Dent J, Elebring T, Jacobson BM, Jensen J, Mattsson JP, Nilsson K, Oja SS, Page AJ, Saransaari P, von Unge S: AZD3355, a novel GABAB receptor agonist, inhibits transient lower esophageal sphincter relaxation through a peripheral mode of action. J Pharmacol Exp Ther. 2009, 331 (2): 504-512. 10.1124/jpet.109.153593.
Ong J, Kerr DI: The gamma-aminobutyric acid uptake inhibitor NO-711 potentiates 3-aminopropylphosphinic acid-induced actions in rat neocortical slices. Eur J Pharmacol. 1998, 347 (2–3): 197-200.
Bolser DC, Aziz SM, DeGennaro FC, Kreutner W, Egan RW, Siegel MI, Chapman RW: Antitussive effects of GABAB agonists in the cat and guinea-pig. Br J Pharmacol. 1993, 110 (1): 491-495. 10.1111/j.1476-5381.1993.tb13837.x.
Bolser DC, DeGennaro FC, O'Reilly S, Chapman RW, Kreutner W, Egan RW, Hey JA: Peripheral and central sites of action of GABA-B agonists to inhibit the cough reflex in the cat and guinea pig. Br J Pharmacol. 1994, 113 (4): 1344-1348. 10.1111/j.1476-5381.1994.tb17145.x.
Alstermark C, Amin K, Dinn SR, Elebring T, Fjellström O, Fitzpatrick K, Geiss WB, Gottfries J, Guzzo PR, Harding JP, Holmén A, Kothare M, Lehmann A, Mattsson JP, Nilsson K, Sundén G, Swanson M, von Unge S, Woo AM, Wyle MJ, Zheng X: Synthesis and pharmacological evaluation of novel gamma-aminobutyric acid type B (GABAB) receptor agonists as gastroesophageal reflux inhibitors. J Med Chem. 2008, 51 (14): 4315-4320. 10.1021/jm701425k.
Brändén L, Fredriksson A, Harring E, Jensen J, Lehmann A: The novel, peripherally restricted GABAB receptor agonist lesogaberan (AZD3355) inhibits acid reflux and reduces esophageal acid exposure as measured with 24-h pHmetry in dogs. Eur J Pharmacol. 2010, 634 (1–3): 138-141.
Boeckxstaens GE, Denison H, Jensen JM, Lehmann A, Ruth M: Translational gastrointestinal pharmacology in the 21st century: 'the lesogaberan story'. Curr Opin Pharmacol. 2011, 11 (6): 630-633. 10.1016/j.coph.2011.10.011.
Canning BJ, Mazzone SB, Meeker SN, Mori N, Reynolds SM, Undem BJ: Identification of the tracheal and laryngeal afferent neurones mediating cough in anaesthetized guinea-pigs. J Physiol. 2004, 557 (Pt 2): 543-558.
Smith JA, Young EC, Saulsberry L, Canning BJ: Anti-tussive effects of memantine in guinea pigs. Chest. 141 (4): 996-1002.
Bolser DC, Hey JA, Chapman RW: Influence of central antitussive drugs on the cough motor pattern. J Appl Physiol. 1999, 86 (3): 1017-1024.
Canning BJ, Mori N: Encoding of the cough reflex in anesthetized guinea pigs. Am J Physiol Regul Integr Comp Physiol. 2011, 300 (2): R369-R377. 10.1152/ajpregu.00044.2010.
Callaway JK, King RG: Effects of inhaled alpha 2-adrenoceptor and GABAB receptor agonists on citric acid-induced cough and tidal volume changes in guinea pigs. Eur J Pharmacol. 1992, 220 (2–3): 187-195.
Simon JR, DiMicco SK, Aprison MH: Neurochemical studies of the nucleus of the solitary tract, dorsal motor nucleus of the vagus and the hypoglossal nucleus in rat: topographical distribution of glutamate uptake, GABA uptake and glutamic acid decarboxylase activity. Brain Res Bull. 1985, 14 (1): 49-53. 10.1016/0361-9230(85)90176-5.
Gross PM, Wall KM, Pang JJ, Shaver SW, Wainman DS: Microvascular specializations promoting rapid interstitial solute dispersion in nucleus tractus solitarius. Am J Physiol. 1990, 259 (6 Pt 2): R1131-R1138.
Ikegaki N, Saito N, Hashima M, Tanaka C: Production of specific antibodies against GABA transporter subtypes (GAT1, GAT2, GAT3) and their application to immunocytochemistry. Brain Res Mol Brain Res. 1994, 26 (1–2): 47-54.
Evans JE, Frostholm A, Rotter A: Embryonic and postnatal expression of four gamma-aminobutyric acid transporter mRNAs in the mouse brain and leptomeninges. J Comp Neurol. 1996, 376 (3): 431-446. 10.1002/(SICI)1096-9861(19961216)376:3<431::AID-CNE6>3.0.CO;2-3.
Wang QP, Guan JL, Pan W, Kastin AJ, Shioda S: A diffusion barrier between the area postrema and nucleus tractus solitarius. Neurochem Res. 2008, 33 (10): 2035-2043. 10.1007/s11064-008-9676-y.
Maolood N, Meister B: Protein components of the blood–brain barrier (BBB) in the brainstem area postrema-nucleus tractus solitarius region. J Chem Neuroanat. 2009, 37 (3): 182-195. 10.1016/j.jchemneu.2008.12.007.
Batten TF, Pow DV, Saha S: Co-localisation of markers for glycinergic and GABAergic neurones in rat nucleus of the solitary tract: implications for co-transmission. J Chem Neuroanat. 2010, 40 (2): 160-176. 10.1016/j.jchemneu.2010.04.001.
Canning BJ, Mori N, Mazzone SB: Vagal afferent nerves regulating the cough reflex. Respir Physiol Neurobiol. 2006, 152 (3): 223-242. 10.1016/j.resp.2006.03.001.
Belvisi MG, Ichinose M, Barnes PJ: Modulation of non-adrenergic, non-cholinergic neural bronchoconstriction in guinea-pig airways via GABAB-receptors. Br J Pharmacol. 1989, 97 (4): 1225-1231. 10.1111/j.1476-5381.1989.tb12582.x.
Ray NJ, Jones AJ, Keen P: GABAB receptor modulation of the release of substance P from capsaicin-sensitive neurones in the rat trachea in vitro. Br J Pharmacol. 1991, 102 (4): 801-804. 10.1111/j.1476-5381.1991.tb12255.x.
Page AJ, Blackshaw LA: GABA(B) receptors inhibit mechanosensitivity of primary afferent endings. J Neurosci. 1999, 19 (19): 8597-8602.
Blackshaw LA, Smid SD, O'Donnell TA, Dent J: GABA(B) receptor-mediated effects on vagal pathways to the lower oesophageal sphincter and heart. Br J Pharmacol. 2000, 130 (2): 279-288. 10.1038/sj.bjp.0703244.
Partosoedarso ER, Young RL, Blackshaw LA: GABA(B) receptors on vagal afferent pathways: peripheral and central inhibition. Am J Physiol Gastrointest Liver Physiol. 2001, 280 (4): G658-G668.
Smid SD, Young RL, Cooper NJ, Blackshaw LA: GABA(B)R expressed on vagal afferent neurones inhibit gastric mechanosensitivity in ferret proximal stomach. Am J Physiol Gastrointest Liver Physiol. 2001, 281 (6): G1494-G1501.
Lai YL, Lin TY: Mast cells in citric acid-induced cough of guinea pigs. Toxicol Appl Pharmacol. 2005, 202 (1): 18-24. 10.1016/j.taap.2004.05.012.
Mazzone SB, Undem BJ: Cough sensors. V. Pharmacological modulation of cough sensors. Handb Exp Pharmacol. 2009, 187: 99-127. 10.1007/978-3-540-79842-2_6.
Gentilini G, Franchi-Micheli S, Mugnai S, Bindi D, Zilletti L: GABA-mediated inhibition of the anaphylactic response in the guinea-pig trachea. Br J Pharmacol. 1995, 115 (3): 389-394. 10.1111/j.1476-5381.1995.tb16345.x.
Tohda Y, Ohkawa K, Kubo H, Muraki M, Fukuoka M, Nakajima S: Role of GABA receptors in the bronchial response: studies in sensitized guinea-pigs. Clin Exp Allergy. 1998, 28 (6): 772-777. 10.1046/j.1365-2222.1998.00289.x.
Osawa Y, Xu D, Sternberg D, Sonett JR, D'Armiento J, Panettieri RA, Emala CW: Functional expression of the GABAB receptor in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2006, 291 (5): L923-L931. 10.1152/ajplung.00185.2006.
Mizuta K, Osawa Y, Mizuta F, Xu D, Emala CW: Functional expression of GABAB receptors in airway epithelium. Am J Respir Cell Mol Biol. 2008, 39 (3): 296-304. 10.1165/rcmb.2007-0414OC.
Krasteva G, Canning BJ, Hartmann P, Veres TZ, Papadakis T, Mühlfeld C, Schliecker K, Tallini YN, Braun A, Hackstein H, Baal N, Weihe E, Schütz B, Kotlikoff M, Ibanez-Tallon I, Kummer W: Cholinergic chemosensory cells in the trachea regulate breathing. Proc Natl Acad Sci USA. 2011, 108 (23): 9478-9483. 10.1073/pnas.1019418108.
Xiang YY, Wang S, Liu M, Hirota JA, Li J, Ju W, Fan Y, Kelly MM, Ye B, Orser B, O'Byrne PM, Inman MD, Yang X, Lu WY: A GABAergic system in airway epithelium is essential for mucus overproduction in asthma. Nat Med. 2007, 13 (7): 862-867. 10.1038/nm1604.
Tamaoki J, Graf PD, Nadel JA: Effect of gamma-aminobutyric acid on neurally mediated contraction of guinea pig trachealis smooth muscle. J Pharmacol Exp Ther. 1987, 243 (1): 86-90.
Chapman RW, Danko G, Rizzo C, Egan RW, Mauser PJ, Kreutner W: Prejunctional GABA-B inhibition of cholinergic, neurally-mediated airway contractions in guinea-pigs. Pulm Pharmacol. 1991, 4 (4): 218-224. 10.1016/0952-0600(91)90014-T.
Chapman RW, Hey JA, Rizzo CA, Bolser DC: GABAB receptors in the lung. Trends Pharmacol Sci. 1993, 14 (1): 26-29. 10.1016/0165-6147(93)90110-6.
Blackshaw LA, Staunton E, Lehmann A, Dent J: Inhibition of transient LES relaxations and reflux in ferrets by GABA receptor agonists. Am J Physiol. 1999, 277 (4 Pt 1): G867-G874.
Staunton E, Smid SD, Dent J, Blackshaw LA: Triggering of transient LES relaxations in ferrets: role of sympathetic pathways and effects of baclofen. Am J Physiol Gastrointest Liver Physiol. 2000, 279 (1): G157-G162.
Fock KM, Talley NJ, Fass R, Goh KL, Katelaris P, Hunt R, Hongo M, Ang TL, Holtmann G, Nandurkar S, Lin SR, Wong BC, Chan FK, Rani AA, Bak YT, Sollano J, Ho KY, Manatsathit S: Asia-Pacific consensus on the management of gastroesophageal reflux disease: update. J Gastroenterol Hepatol. 2008, 23 (1): 8-22.
Kahrilas PJ: Clinical practice. Gastroesophageal reflux disease. N Engl J Med. 2008, 359 (16): 1700-1707. 10.1056/NEJMcp0804684.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
AL is an employee of AstraZeneca.
Authors’ contributions
BC designed and conducted many of the experiments, interpreted the results and wrote multiple sections of the manuscript. NM conducted many of the experiments, analyzed the data and created the graphic summaries of the results. AL designed the experiments, interpreted the results and wrote multiple sections of the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Canning, B.J., Mori, N. & Lehmann, A. Antitussive effects of the peripherally restricted GABAB receptor agonist lesogaberan in guinea pigs: comparison to baclofen and other GABABreceptor-selective agonists. Cough 8, 7 (2012). https://doi.org/10.1186/1745-9974-8-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1745-9974-8-7