Abstract
Introduction
Using French cut-offs for the Tuberculin Skin Test (TST), results of the TST were compared with the results of an Interferon-γ Release Assay (IGRA) in Healthcare Workers (HCW) after contact to AFB-positive TB patients.
Methods
Between May 2006 and May 2007, a total of 148 HCWs of the University Hospital in Nantes, France were tested simultaneously with IGRA und TST. A TST was considered to indicate recent latent TB infection (LTBI) if an increase of >10 mm or if TST ≥ 15 mm for those with no previous TST result was observed. For those with a positive TST, chest X-ray was performed and preventive chemotherapy was offered.
Results
All HCWs were BCG-vaccinated. The IGRA was positive in 18.9% and TST ≥ 10 mm was observed in 65.5%. A recent LTBI was believed to be highly probable in 30.4% following TST. Agreement between IGRA and TST was low (kappa 0.041). In 10 (16.7%) out of 60 HCWs who needed chest X-ray following TST the IGRA was positive. In 9 (20%) out of 45 HCWs to whom preventive chemotherapy was offered following TST the IGRA was positive. Of those considered TST-negative following the French guidelines, 20.5% were IGRA-positive. In a two-step strategy - positive TST verified by IGRA - 18 out of 28 (64.3%) IGRA-positive HCWs would not have been detected using French guidelines for TST interpretation.
Conclusion
The introduction of IGRA in contact tracings of BCG-vaccinated HCWs reduces X-rays and preventive chemotherapies. Increasing the cut-off for a positive TST does not seem to be helpful to overcome the effect of BCG vaccination on TST.
Similar content being viewed by others
Introduction
The increased risk of healthcare workers (HCWs) for tuberculosis is well established [1, 2]. Therefore screening healthcare workers (HCWs) for latent tuberculosis infection (LTBI) and active tuberculosis (TB) is fundamental in infection control programs in hospitals [3]. For about a century, the Tuberculin Skin Test (TST) has been used to detect LTBI. However the TST has its known limitations, including cross-reactivity with BCG and non-tubercular mycobacteria (NTM) infections [4]. Advances in molecular biology have led to the development of new in-vitro assays that measure interferon (INF)-γ released by sensitized T-cells after stimulation with M. tuberculosis antigens. These tests are more specific than the TST because they use antigens not shared by any of the BCG vaccine strains nor by the more common species of NTM (e.g. M. avium) [5]. Besides the higher specificity and at least equal sensitivity as the TST, IGRAs correlate better with surrogate measures of exposure to M. tuberculosis [6–8] and have a higher predictive value for LTBI progression to active TB in close contacts in low-incidence settings [9].
So far several systematic investigations of LTBI in HCWs using TST and IGRA have been published [10–18] showing a high proportion of TST-positive/IGRA-negative HCWs which is most likely explained by BCG vaccination. In order to reduce the effect of BCG vaccination on TST, the French guidelines for TST interpretation propose high cut points for the TST - increase >10 mm or ≥ 15 mm if no earlier TST is available [19]. Alternatively to high cut-offs for TST, a number of European Guidelines on the use of IGRAs suggest use of a two-step strategy - performing an IGRA in those initially positive by the TST and excluding LTBI in those IGRA-negative [20–22]. In our study we compared the performance of the TST and IGRA in French HCWs when using a high cut-off for the TST.
Materials and methods
Study setting and study subjects
In France the TB incidence in the general population has been declining for several years and was as low as 5.2 cases per 100,000 inhabitants in 2006 [23]. However, increasing differences regarding region and risk groups are seen. Most cases are observed in urban areas and the incidence rate in foreign-born inhabitants was 38.8/100,000).
The population of this cross-sectional study comprises all workers of the University Hospital of Nantes, France, who participated in TB screening from May 2006 through May 2007 because of contact to infectious TB patients or materials. The University Hospital of Nantes is the largest hospital in the Nantes region and serves as a referral center for TB patients throughout the region. Unprotected contact of the HCWs to acid-fast bacillus (AFB)-positive patients occurred in the emergency department and lasted between 1 and 2 hours. Screening was performed 8 to 10 weeks after exposure.
Screening was performed using TST and IGRA simultaneously. Following French guidelines chest X-ray in order to exclude active TB was performed when TST was ≤ 10 mm if no previous TST was available for comparison [19]. If a previous TST was available, X-ray was performed when TST increased by >10 mm (Figure 1). Preventive chemotherapy is proposed if recent LTBI is very probable (TST ≥ 15 mm and no previous TST available or increase >10 mm in TST). For the purpose of this study agreement between IGRA and TST was also analyzed using ≥ 10 mm as cut-off for the TST.
Decision tree for TST interpretation in contact tracing for contacts of AFB-positive TB patients following [19]. The French guidelines for TST interpretation in HCWs are presented.
BCG vaccination was assessed through the individual vaccination record or by scars. Following the national vaccination plan, BCG vaccination for newborns is mandatory in France and until 2008 was repeated if TST was <5 mm [23]. Therefore every HCW has been vaccinated at least once. All participating HCWs were French-born.
TST was performed by trained personnel following standard procedures. In brief, 0.1 mL (2 TU) of purified protein derivate (Tubertest from SanofiPasteur) was injected intradermally at the volar side of the forearm and the transverse diameter of the induration was read after 72 to 96 hours [19]. A diameter ≥ 10 mm was considered positive.
Before TST application, the interview was performed and blood for the IGRA was drawn. The interview covered age, gender, BCG vaccination history and employment in healthcare. As IGRA, the QuantiFERON® -TB Gold In-Tube Assay (Cellestis Limited, Carnegie, Australia) was administrated following the manufacturer's protocol. Observers were blinded to the results of the TST and vice versa.
Statistical analysis
Chi-square tests were used for categorical data. Kappa was calculated for the agreement between IGRA and TST. Adjusted odds ratios (OR) and 95% confidence intervals (CI) were calculated for putative predictive variables using conditional logistic regression. Model building was performed backwards using the chance criteria for variable selection [24].
All persons gave their informed consent prior to their inclusion in the study. No ethics approval of the study was needed because no examinations in addition to those needed for contact tracing were performed.
Results
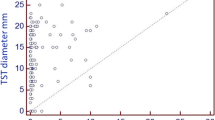
The study population comprises 148 HCWs. The characteristics of the study population are described in Table 1. Repeated BCG vaccination had 62.2%. For 83.1% the last vaccination was performed more than 20 years before. No undetermined result of the QFT was observed. A positive QFT was observed in 18.9% and a TST ≥ 10 mm in 65.5% (Table 2). The QFT was positive in 9.8% of those with a TST 0-9 mm and in 21.1% of those with a TST ≥ 20 mm. The association between TST diameter and QFT positivity was weak (p for test for trend: 0.081).
In 8 persons an increase >10 mm of the TST was observed but only one out of these 8 HCWs (12.5%) had a positive IGRA (Table 3). Most persons (35.1%) had an increase of the TST ≥ 10 mm. 25.0% of the HCWs pertaining to this category were IGRA-positive. No statistically significant association was found between the different TST results and QFT positivity (p = 0.58). Of the 28 HCWs positive in QFT 10 (35.7%) were considered for X-ray or preventive chemotherapy following French interpretation of the TST. Again, following French Guidelines for TST interpretation a recent LTBI was very probable in 45 (30.4%) HCWs (Table 4). Of these, 20% were positive in the QFT which is a proportion similar to the one of those for whom a recent LTBI was not suspected (18.4%). Compared to the French definition for very probable recent LTBI a cut-off of ≥ 10 mm increased the kappa value for agreement between TST and QFT slightly from 0.02 to 0.11. But in both strategies agreement between TST and QFT was weak or non existent.
Using ≥ 10 mm as cut-off for the TST regardless of an earlier TST, TST+/QFT- discordance was observed in 74 (50%) HCWs and TST-/QFT+ discordance in 5 (3.4%) out of 148 exposed HCWs. In those with TST ≥ 10 mm 23.7% were positive in the QFT.
X-ray was performed in 60 HCWs and no active TB was found. Chemoprevention was proposed to 45 HCWs (Table 3). Logistic regression did not reveal any association between positive QFT or positive TST and age, gender, BCG vaccination, or years spent in healthcare. This was also true when instead of the French definitions the cut-off for TST was reduced to ≥ 10 mm regardless of earlier TST results (data not shown).
In a two-step strategy - performing QFT in HCWs with suspected recent LTBI following TST - 10 (1+8+1) instead of 60 (16.7%) would have been proposed for X-ray. On the other side, using this two-step approach with a high cut-off for the TST would allow to detect 10 out of 28 (35.7%) HCWs positive in the QFT only (Table 3). Using a cut-off of ≥ 10 mm for the TST in a two-step strategy would decrease the number of QFTs needed in this population from 100% to 65.5%, again with the drawback that 5 out of 28 (17.9%) HCWs positive in the QFT would be missed (Table 4).
Discussion
To our knowledge this is the first study that compared the performance of TST and QFT when screening French HCWs. In those in which recent LTBI was suspected following French guidelines [19], the confirmation rate of the QFT was not higher than in those in whom recent LTBI was not probable. Only about one third (35.7%) of those positive in QFT were also considered positive in TST, which is a rate lower than that reported in other studies [10, 16, 18]. Therefore, our data suggest that the French guidelines for the interpretation of TST in exposed HCWs should be reconsidered. Agreement between TST and QFT was better with ≥ 10 mm as cut-off for TST but still remained weak (kappa = 0.11). Following our data neither increasing the cut-off for TST nor a two-step strategy (IGRA only in TST-positive HCWs) seemed to reduce the influence of BCG vaccination on TST results in a satisfying way.
Positive in QFT were 18.9% of the HCWs with recent contact to an AFB-positive TB case were positive in the QFT. The rate of positive QFT is lower than the one (33%) observed in Portuguese HCWs [25] but lower than the one (10%) observed in German HCWs [16, 18].
As reported in another study [26] we observed an association between the diameter of the TST and the probability of a positive QFT even though the test for trend was of borderline statistical significance. This might be due to the small sample size (n = 148) of our study. The number of TST-/QFT+ HCWs in our population is much higher than in two meta-analyses [8, 26]. But surprisingly, even though the criteria for a positive TST following French guidelines are high compared to other countries, the proportion of TST+/QFT- HCWs was high, too. The risk of progression to active TB in TST+/QFT- HCWs is unknown. A number of publications suggest it is low [9, 27–29]. Because of the high sensitivity of the IGRA and the low specificity of the TST, none of the several national guidelines recommend X-ray or chemoprevention in HCWs with a positive TST and a negative IGRA [20–22]. Therefore it seems reasonable to assume that most TST+/QFT- results do not indicate infection with Mycobacteria tuberculosis.
Introducing IGRA for TB screening in France would reduce the number of X-rays and the preventive chemotherapies by a high proportion (from 65.5% for TST ≥ 10 mm or from 40.5% for TST interpretation following French guidelines to 18.9%). We did not conduct a cost-effectiveness analysis. But nevertheless our analysis corroborates the findings of other cost-benefit analyses. A German cost-effectiveness analysis based on German data on TST and QFT positivity and costs of treatment showed that using the QFT assay, but especially combining the QFT assay following the TST screening of close contacts at a cut-off induration size of 5 mm before LTBI treatment, is highly cost-effective in reducing the disease burden of TB [30]. Similar results were observed in an analysis of a hypothetical cohort based on data from Switzerland [31] and Canada [32]. There is still an ongoing debate as to whether the introduction of IGRA in TB screening reduces the costs for BCG-vaccinated contacts only [33] or for both vaccinated and non-vaccinated contacts [34]. It is still unknown for how long the QFT remains positive after elimination of Mycobacterium tuberculosis from the body either spontaneously or after chemoprevention [35, 36].
So far the risk of progression towards active tuberculosis for those with TST-/QFT+ combinations is unknown. The well-established high specificity of the QFT and the known limitations of TST sensitivity [8] suggest that this combination indicates infections with Mycobacterium tuberculosis. Following our data a two-step strategy using ≥ 10 mm as cut-off for a positive TST helps to reduce unwarranted X-rays but risks to miss a high proportion (17.9%) of the HCWs positive in QFT and therefore likely infected with Mycobacterium tuberculosis. Further reducing the cut-off for a positive TST to >5 mm would not be useful in our population because already 65% had a TST ≥ 10 mm.
Conclusion
Our data show that the increase criteria (increase >10 mm) for TST interpretation lead to a low sensitivity of the TST without reducing the specificity problems of the TST in a meaningful way. Therefore the use of the increase criteria should be reconsidered. Furthermore it could be shown that a two-step strategy - IGRA if TST ≥ 10 mm - might also lead to a low sensitivity of a TB screening. Therefore our data suggest that IGRA should replace TST when screening HCWs for tuberculosis.
References
Seidler A, Nienhaus A, Diel R: Review of epidemiological studies on the occupational risk of tuberculosis in low-incidence areas. Respiration 2005,72(4):431–446. 10.1159/000086261
Diel R, Seidler A, Nienhaus A, Rusch-Gerdes S, Niemann S: Occupational risk of tuberculosis transmission in a low-incidence area. Respir Res 2005,6(1):35–45. 10.1186/1465-9921-6-35
CDC - Center for Disease Control and Prevention: Guidelines for Preventing the Transmission of Mycobacterium tuberculosis in Healthcare Settings, 2005. MMWR 2005,54(No RR-17):1–141.
Menzies D: What does tuberculin reactivity after Bacille Calmette-Guerin vaccination tell us? Clin infect Dis 2000,31(Suppl 3):S71-S74. 10.1086/314075
Andersen P, Munk ME, Pollock JM, Doherty TM: Specific immune-based diagnosis of tuberculosis. Lancet 2000,356(9235):1099–1104. 10.1016/S0140-6736(00)02742-2
Nahid P, Pai M, Hopewell PC: Advances in the diagnosis and treatment of tuberculosis. Proc Am Thorac Soc 2006, 3: 103–110. 10.1513/pats.200511-119JH
Pai M, Riley LW, Colford JM: Interferon-gamma assays in the immunodiagnosis of tuberculosis: a systematic review. Lancet Infect Dis 2004, 4: 761–776. 10.1016/S1473-3099(04)01206-X
Menzies D, Pai M, Comstock G: Meta-analysis: New tests for the diagnosis of latent tuberculosis infection: Areas of uncertainty and recommendations for research. Ann Intern Med 2007, 146: 340–352.
Diel R, Loddenkemper R, Meywald-Walter K, Niemann S, Nienhaus A: Predictive value of a whole-blood IFN-{gamma} assay for the development of active TB disease. Am J Respir Crit Care Med 2008, 177: 1164–1170. 10.1164/rccm.200711-1613OC
Harada N, Nakajima Y, Higuchi K, Sekiya Y, Rothel J, Mori T: Screening for tuberculosis infection using whole-blood interferon-γ and Mantoux testing among Japanese healthcare workers. Infection control and hospital epidemiology 2006,27(5):442–448. 10.1086/504358
Soberg B, Andersen AB, Larsen HK, Weldingh K, Andersen P, Kofoerd K, Ravn P: Detecting a low prevalence of latent tuberculosis among health care workers in Denmark detected by M: tuberculosis specific INF-γ whole-blood test. Scandinavian Journal of Infectious Diseases 2007, 39: 554–559. 10.1080/00365540601148483
Kobashi Y, Obase Y, Fukuda M, Yoshida K, Miyashita N, Fujii M, Oka M: Usefulness of QuantiFERON TB-2G, a diagnostic method for latent tuberculosis infection, in a contact investigation of health care workers. Intern Med 2007,46(18):1543–9. 10.2169/internalmedicine.46.0088
Mirtskhulava V, Kempker R, Shields KL, Leonard MK, Tsertsvadze T, del Rio C, Salakaia A, Blumberg HM: Prevalence and risk factors for latent tuberculosis infection among health care workers in Georgia. Int J Tuberc Lung Dis 2008,12(5):513–519.
Nienhaus A, Schablon A, Siano B, le Bacle C, Diel R: Evaluation of the interferon-gamma release assay in healthcare workers. Int Arch Occup Environ Health 2008, 81: 295–300. 10.1007/s00420-007-0212-1
Lee SS-J, Liu Y-C, Huang T-S, Chen Y-S, Tsai H-C, Wann S-R, Lin H-H: Comparison of the interferon-γ release assay and the tuberculin skin test for contact investigation of tuberculosis in BCG-vaccinated health care workers. Scandinavian Journal of Infectious Disease 2008,40(5):373–80. 10.1080/00365540701730743
Schablon A, Beckmann G, Harling M, Diel R, Nienhaus A: Prevalence of latent tuberculosis infection among healthcare workers in a hospital for pulmonary diseases. J Occup Med Toxicol 2009, 4: 1. 10.1186/1745-6673-4-1
Vinton P, Mihrshahi S, Johnson P, Jenkin GA, Jolley D, Biggs BA: Comparison of QuantiFERON-TB Gold In-Tube test and tuberculin skin test for identification of latent Mycobacterium tuberculosis infection in healthcare staff and association between positive test results and known risk factors for infection. Infection Control and Hospital Epidemiology 2009.,30(3): online first
Ringshausen FC, Schlösser S, Nienhaus A, Schablon A, Schultze-Werninghaus G, Rohde G: In-hospital contact investigation among health care workers after exposure to smear-negative tuberculosis. J Occup Med Tox 2009, 4: 11. 10.1186/1745-6673-4-11
French guidelines for TB screening in HCWs: Investigations à conduire autour d'un cas de tuberculose-maladie ou tuberculose-infection récente. Revue des Maladies Infectieuses 2004, 34: 391–396.
National Institute for Health and Clinical Excellence: Tuberculosis: clinical diagnosis and management of tuberculosis, and measures for its prevention and control. London 2006.
Zellweger JP, Zellweger A, Ansermet S, de Senarclens B, Wrighton-Smith P: Contact tracing using a new T-cell-based test: better correlation with tuberculosis exposure than the tuberculin skin test. Int J Tuberc Lung Dis 2005, 9: 1242–1247.
Diel R, Forssbohm M, Loytved G, Haas W, Hauer B, Maffei D, Magdorf K, Nienhaus A, Rieder HL, Schaberg T, Zellweger JP, Loddenkemper R: Recommendations for environmental contact tracing in tuberculosis. German Central Committee against Tuberculosis. Gesundheitswesen 2007, 69: 488–503. 10.1055/s-2007-980089
Che D, Lefebvre N, Antoun F, Fraisse P, Depinoy M, Antoine D, Farge D, Paty MC: Tuberculosis in France: New Challenges for the practitioners. La Revue de Médecine Interne 2009, 30: 142–9. 10.1016/j.revmed.2008.07.014
Hosmer D, Lemeshow S: Applied Logistic Regression. New York, NY: John Wiley & Sons; 2000.
Torres Costa J, Sá R, Cardoso MJ, Silva R, Ferreira J, Ribeiro C, Miranda M, Plácido JL: Nienhaus. Tuberculosis screening in Portuguese healthcare workers using the Tuberculin Skin Test and the Interferon-γ release assay. Eur Resp J 2009,34(6):1423–1428. 10.1183/09031936.00053809
Nienhaus A, Schablon A, Diel R: Interferon-γ release assay for the diagnosis of latent TB infection - analysis of discordant results, when compared to the tuberculin skin test. PLoS ONE 2008,3(7):e2665. 10.1371/journal.pone.0002665
Higuchi K, Harada N, Mori T, Sekiya Y: Use of QuantiFERON-TB Gold to investigate tuberculosis contacts in a high school. Respirology 2007,12(1):88–92. 10.1111/j.1440-1843.2006.01000.x
Higuchi K, Kondo S, Wada M, Hayashi S, Ootsuka G, Sakamoto N, Harada N: Contact investigation in a primary school using a whole-blood interferon-gamma assay. I Infect 2009,58(5):352–7. 10.1016/j.jinf.2009.02.019
Aichelburg MC, Rieger A, Breitenecker F, Pfistershammer K, Tittes J, Eltz S, Aichelburg AC, Stingl G, Makristathis A, Kohrgruber N: Detection and Prediction of Active Tuberculosis Disease by a Whole-Blood Interferon-gamma Release Assay in HIV-1-Infected Individuals. Clin Infect Dis 2009,48(7):954–62. 10.1086/597351
Diel R, Nienhaus A, Loddenkemper R: Cost-effectiveness of interferon-gamma release assay screening for latent tuberculosis infection treatment in Germany. Chest 2007, 131: 1424–1434. 10.1378/chest.06-2728
Diel R, Wrighton-Smith P, Zellweger JP: Cost-effectiveness of interferon-gamma release assay testing for the treatment of latent tuberculosis. Eur Respir J 2007, 30: 321–332. 10.1183/09031936.00145906
Oxlade O, Schwartzman K, Menzies D: Interferon-gamma release assays and TB screening in high-income countries: a cost-effectiveness analysis. Int J Tuberc Lung Dis 2007,11(1):16–26.
Marra F, Marra CA, Sadatsafavi M: Cost-effectiveness of a new interferon-based blood assay, QuantiFERON-TB Gold, in screening tuberculosis contacts. Int J Tuberc Lung Dis 2008, 12: 1414–1424.
De Perio MA, Tsevat J, Roselle GA: Cost-effectiveness of interferon gamma release assays vs tuberculin skin tests in health care workers. Arch Intern Med 2009, 169: 179–187. 10.1001/archinternmed.2008.524
Mack U, Migliori GB, Sester M, Rieder HL, Ehlers S, Goletti D, Bossink A, Magdorf K, Hölscher C, Kampmann B, Arend SM, Detjen A, Bothamley G, Zellweger JP, Milburn H, Diel R, Ravn P, Cobelens F, Cardona PJ, Kann B, Solovic I, Duarte R, Cirillo DM, Lange C: LTBI: latent tuberculosis infection or lasting immune responses to M. tuberculosis? - A TBNET consensus statement. Eur Respir J 2009, 33: 956–73. 10.1183/09031936.00120908
Pai M, Joshi R, Dogra S, Mendiratta DK, Narang P, Dheda K, Kalantri S: Persistently elevated T cell interferon-γ response after treatment for latent tuberculosis infection among healthcare workers in India: a preliminary report. Journal of Occupational Medicine and Toxicology 2006, 1: 7. 10.1186/1745-6673-1-7
Acknowledgements
We wish to thank all HCWs who participated in the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
DT designed the study and was involved in data collection and writing of the paper.
BBC was involved in data collection and gave critical comments for manuscript writing.
VN was involved in data collection and gave critical comments for manuscript writing.
MA was involved in data collection and gave critical comments for manuscript writing.
EC was involved in data collection and gave critical comments for manuscript writing.
PG was involved in data collection and gave critical comments for manuscript writing.
FN was involved in data collection and gave critical comments for manuscript writing.
JYM was involved in data collection and gave critical comments for manuscript writing.
MBL was involved in data collection and gave critical comments for manuscript writing.
MHDP was involved in data collection and gave critical comments for manuscript writing.
CG was involved in data collection and gave critical comments for manuscript writing.
GG was involved in data collection and gave critical comments for manuscript writing.
MTH was involved in data collection and gave critical comments for manuscript writing.
FR was involved in data collection and gave critical comments for manuscript writing.
DB was involved in data collection and gave critical comments for manuscript writing.
CB was involved in data collection and gave critical comments for manuscript writing.
GP was involved in data collection and gave critical comments for manuscript writing.
CR was involved in data collection and gave critical comments for manuscript writing.
CG was involved in data collection and gave critical comments for manuscript writing.
AN analysed the data and drafted the manuscript.
All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Tripodi, D., Brunet-Courtois, B., Nael, V. et al. Evaluation of the tuberculin skin test and the interferon-γ release assay for TB screening in French healthcare workers. J Occup Med Toxicol 4, 30 (2009). https://doi.org/10.1186/1745-6673-4-30
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1745-6673-4-30