Abstract
Background
Several studies had demonstrated the involvement of the dorsolateral portion of periaqueductal grey matter (dlPAG) in defensive responses. This region contains a significant number of neurons containing the enzyme nitric oxide synthase (NOS) and previous studies showed that non-selective NOS inhibition or glutamate NMDA-receptor antagonism in the dlPAG caused anxiolytic-like effects in the elevated plus maze.
Methods
In the present study we verified if the NMDA/NO pathway in the dlPAG would also involve in the behavioral suppression observed in rats submitted to the Vogel conflict test. In addition, the involvement of this pathway was investigated by using a selective nNOS inhibitor, Nω-propyl-L-arginine (N-Propyl, 0.08 nmol/200 nL), a NO scavenger, carboxy-PTIO (c-PTIO, 2 nmol/200 nL) and a specific NMDA receptor antagonist, LY235959 (4 nmol/200 nL).
Results
Intra-dlPAG microinjection of these drugs increased the number of punished licks without changing the number of unpunished licks or nociceptive threshold, as measure by the tail flick test.
Conclusion
The results indicate that activation of NMDA receptors and increased production of NO in the dlPAG are involved in the anxiety behavior displayed by rats in the VCT.
Similar content being viewed by others
Background
The periaqueductal gray matter (PAG) is structure closely related to nociceptive and defensive responses which can be divided into four different columns along its rostro-caudal axis: dorsomedial, dorsolateral (dlPAG), lateral and ventrolateral columns [1–3]. Microinjection of NMDA receptor agonists into the dlPAG evokes flight reactions [4–6] and glutamate receptors are widely expressed in the dlPAG [7]. Moreover, antagonism of NMDA receptors AP7 in the dlPAG causes an anxiolytic-like effect in rats submitted to the elevated plus maze and Vogel conflict test [8–10]. These data indicate that glutamate receptors in the dlPAG play an important role in defensive responses.
Glutamate-NMDA receptor activation can induce the production of nitric oxide (NO) by activation of a calmodulin-dependent enzyme, the neuronal isoform of the nitric oxide synthase (nNOS), present in several corticolimbic structures [11, 12]. nNOS inhibition promotes effects similar to those observed after NMDA antagonism [13, 14]. Likewise, injections of non-selective NOS inhibitors into the dlPAG induced an anxiolytic-like effect in the elevated plus maze (EPM) model [15]. This model measures the conflict generated by the drive to explore a safe (closed arms) versus unsafe (open arms) place and is based on the innate fear of open spaces displayed by rodents. However, several pieces of evidence indicate that different aversive contingencies may engage distinct neurobiological systems [16]. It is important, though, to investigate drug effects in a specific brain site using different tests of anxiety.
The Vogel conflict test (VCT) is another animal model of anxiety that measures suppression of punished responses [16–22]. Different from the EPM, however, it is based on conflict induced by a learned contingency (electrical shock on a drinking spout) in thirsty animals [16, 22].
Since the possible anxiolytic effects of NOS inhibition in the dlPAG have only been tested so far using the EPM, the aim of this study was to verify if the NMDA/NO pathway in the dlPAG is also involved in the behavioral suppression observed in rats submitted to the VCT. In addition, instead of the non-specific NOS inhibitors used in a previous work [15] we investigated the effects of a selective nNOS inhibitor, Nω-propyl-L-arginine (N-Propyl), and a NO scavenger, carboxy-PTIO (c-PTIO) microinjected into the dlPAG. These effects were compared to those produced by a specific NMDA receptor antagonist LY235959.
Methods
Subjects
Male Wistar rats weighing 230-250 g were used. Animals were kept in the Animal Care Unit of the Department of Pharmacology, School of Medicine of Ribeirão Preto, University of São Paulo. Rats were housed four per cage in plastic cages (18 x 32 x 40 cm) under standard laboratory conditions, with free access to food and water and under a 12 h light/dark cycle (lights on at 06:30 h). The Institution's housing conditions and the experimental procedures were previously approved by the local Animal Ethics Committee (process number: 067-2009).
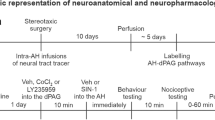
Seven days before the experiments the rats were anesthetized with tribromoethanol (10 ml/kg i.p.) and fixed on a stereotaxic apparatus (Stoelting, Wood Dale, Illinois, USA). Immediately before the surgery they received antibiotic (0.2 ml/animal, I.M., Pentabiotico®, Fort Dodge) and anti-inflammatory (2.5 mg/Kg, s.c., Banamine®, Schering-Plough) treatment. After local anesthesia of the scalp with 2% lidocaine chloridrate with norepinephrine (Xylestesin®, Cristália), the skull was surgically exposed and stainless steel guide cannulae (0.6 mm OD) were unilaterally implanted on the right side aimed at the dorsolateral periaqueductal gray (coordinates: AP = 0 from lambda, L = 1.9 mm at an angle of 16°, D = 4.0 mm) [23] with the help of a stereotaxic apparatus. The cannula tip was located 1 mm dorsal to the final injection site. The cannulae were fixed to the skull with dental cement and one metal screw. An obturator inside the guide cannula prevented obstruction.
Drugs
LY235959 (Tocris), Nω-propyl-L-arginine (Tocris, Ellisvalle, MO, USA) and carboxy-PTIO (S)-3-Carboxy-4-hydroxyphenylglicine (c-PTIO, RBI, St. Louis, MO, USA); were dissolved in sterile artificial cerebrospinal fluid (composition: NaCl 100 mM, Na3PO4 2 mM, KCl 2.5 mM, MgCl2 1 mM, NaHCO3 27 mM, CaCl2 2.5 mM; pH = 7.4).); tribromoethanol (Aldrich, St. Louis, MO, USA), and urethane (Sigma, St. Louis, MO, USA) were dissolved in saline.
Apparatus
Vogel conflict test
The Vogel conflict test was performed in a Plexiglas box (42 × 50 × 25 cm) with a stainless steel grid floor. The metallic spout of a drinking bottle containing water projected into the box. The contact of the animal with the spout and the grid floor closed an electrical circuit controlled by a sensor (Anxio-Meter model 102, Columbus, USA), which produced 7 pulses/s whenever the animal was in contact with both components. Each pulse was considered as a lick and at every 20 licks the animal received a 0.5 mA electrical shock on the metallic drinking spout for 2 s. The sensor recorded the total number of licks and shocks delivered during the test period. The whole apparatus was located inside a sound-attenuated cage [24, 25].
Water consummatory evaluation
The apparatus was the same used in the test above except that the electrical shock delivering system was render inoperative. The number of unpunished licks was measured after 24 h (pre-drug treatment) and 48 h of water deprivation. In the latter situation the measurement of water consumption was performed 10 min after intra-dlPAG microinjections of vehicle, LY235959, N-Propyl or c-PTIO.
Tail-flick test
The apparatus consisted of an acrylic platform with a nichrome wire coil (Insight Instruments. Brazil) maintained at room temperature (24-26°C). The rats were gently handled and had their tails laid across the coil. The coil temperature was then raised at 9°C/s by the passage of electric current. The system had a cut-off time of 6 s to prevent tissue damage when the coil temperature approached 80°C. The time to withdraw the tail was recorded as tail-flick latency. The electric current was calibrated to provoke this reflex within 2.5-3.5 s in non-treated animals [15, 25].
Experimental design
In the Vogel conflict test the animals received microinjection of 200 nL of vehicle, 4 nmol of LY235959, 0.08 nmol of N-Propyl or 2 nmol of c-PTIO. Each animal was used only once and the doses were based on those employed in previous studies using intra-cerebral administration [14, 26, 27]. Intracerebral injections were performed with a thin dental 33G needle (Small Parts, Miami Lakes, FL, USA) introduced through the guide cannula until its tip was 1.0 mm below the cannula end. A volume of 0.2 μl was injected in 30 s using a syringe (Hamilton, USA) connected to an infusion pump (Kd Scientific, USA). In order to prevent reflux the guide cannula was left in place for 30 s after the end of each injection. A polyethylene catheter (PE 10) was interposed between the upper end of the dental needle and the syringe. Morphine hydrochloride [9, 24] was used as a positive control in the tail-flick test (see bellow).
Procedure
The Vogel conflict test
The animals were water deprived for 48 h before the test. After the first 24 h of deprivation they were allowed to freely drink for 3 min in the test cage in order to find the drinking bottle spout. Some animals did not find the spout and were not included in the experiment. Twenty-four h later the animals received the microinjections and after 10 min were placed into the test box. The test period lasted for 3 min and the animals received a 0.5 mA shock every 20 licks. During this period the number of licks and shocks delivered were registered.
Water consumption test
This test was performed in independent groups of animals and the procedure was the same used in the Vogel conflict test except that the electric shock delivering system was render inoperative. After the first 24 h of deprivation they were allowed to freely drink for 3 min in the test cage. Twenty four h later the animals received the microinjections and were again allowed to drink for 3 min. The number of licks during these two periods was registered.
Tail-flick test
The tail-flick test was conducted in independent groups of animals receiving vehicle, morphine, LY235959, N-Propyl or c-PTIO. The heating was applied to a portion of the ventral surface of the tail located between 4 and 6 cm from its end. The tail-flick latency was measured at 5-min intervals until a stable baseline (BL) was obtained over three consecutive trials. The latency was measured again 30 s after drug administration and then at 10-min intervals for up to 40 min [22]. Morphine was administered systemically (i.p.) 10 min before the test. Vehicle, LY235959, N-Propyl or c-PTIO was microinjected into the dlPAG as described above.
Histology
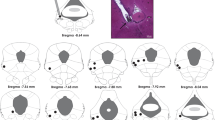
After the behavioral tests the rats were sacrificed under deep urethane anesthesia and perfused through the left ventricle of the heart with isotonic saline followed by 10% formalin solution. After that, the brains were removed and, after a minimum period of 5 days immersed in a 10% formalin solution, 50 μm sections were obtained in a Cryostat (Cryocut 1800). The injection sites were identified in diagrams from the Paxinos and Watson's atlas [23] and are illustrated in Figure 1. Rats receiving drug injections outside the dlPAG, dorsomedial PAG, lateral PAG and superior colliculus, were included in an additional (OUT) group.
Microinjections sites. Photomicrograph of a coronal brain section showing an unilateral microinjection site in the dorsal periaqueductal gray and a histological localization of injection sites (200 nL) in diagrams based on the atlas of Paxinos and Watson [23]. The solid and the open circles represent injection sites inside of the dlPAG. Numbers represent distances from interaural level (mm). dPAG: dorsal periaqueductal gray; dlPAG: dorsolateral periaqueductal gray.
Statistical analysis
The data were expressed as mean ± S.E.M. The number of punished licks was analyzed by one-way ANOVA. The latency of tail withdrawal and water consumption were analyzed by a two-way repeated-measure ANOVA with treatment as the independent factor and time or day as the repeated measure. In case of significant interactions post-hoc comparisons were performed using the Dunnet's test. Results of statistical tests with P < 0.05 were considered significant.
Results
The Vogel conflict test
In the first experiment, the animals that had received LY235959 (n = 8), N-propyl (n = 7) or c-PTIO (n = 9) into the dlPAG showed an increased the number of punished licks in the Vogel conflict test as compared to animals which had received vehicle (n = 10, F6,57= 6.71; P < 0.01, Figure 2). Moreover, drug injection outside this region (LY: n = 11, N-Propyl: 6 and c-PTIO: 8, Figure 2) did not modify the number of licks (P > 0.05).
Vogel conflict test. Effects of LY235959 (4 nmol/200 nL), Nω-propyl-L-arginine (0.04 nmol/200 nL) and c-PTIO (1 nmol/200 nL) injected into the dlPAG of rats submitted to the Vogel test. Bars represent the mean ± SEM total number of punished licks in the 3 min session. Animals that received drugs outside the dlPAG were included as OUT groups. Asterisk indicates significant difference from vehicle (P < 0.05, ANOVA followed by the Dunnet's test; vehicle (veh, n = 10), LY235959 (n = 8), Nω-propyl-L-arginine (n = 7), c-PTIO (n = 9); OUT-LY235959 (n = 11), OUT-Nω-propyl-L-arginine (n = 5) and OUT-c-PTIO (n = 8).
Drug effects on water consumption and tail-flick test
These additional experiments were performed to discard changes in nociceptive responses and water consumption as confounding factors in the Vogel test. In the water consumption test (Figure 3) neither drug (LY235959: n = 6, N-propyl: n = 6 and c-PTIO: n = 6) changed the number of unpunished lick compared to vehicle (n = 6, F3,40 = 1.380, P > 0.05). There was no difference for days (F1,40 = 2.8, P > 0.05), and no interaction treatment x drug (F3,40 = 0.63, P > 0.05).
Water consume test. Effect of LY235959 (4 nmol/200 nL), Nω-propyl-L-arginine (0.04 nmol/200 nL) and c-PTIO (1 nmol/200 nL) injected into the dlPAG in the water consumption test during training (Day 1) and test (Day 2). Vehicle (veh, n = 6) LY235959 (n = 6), Nω-propyl-L-arginine (n = 6), c-PTIO (n = 6).
Finally, the tail-flick test results can be seen in Figure 4. There were a significant drug effect (LY235959: n = 6, N-propyl: n = 6 and c-PTIO: n = 6) (F4,161 = 14.59, P < 0.001), time (F6,161 = 9.65, P < 0.001) and drug × time interaction (F24,161 = 4.05, P < 0.001). Withdrawal latencies were significantly greater than vehicle at 20, 30 and 40 min after morphine injection (n = 5). No other difference against vehicle was found.
Tail flick nociception test. Time course of the effects of vehicle (veh, n = 6), LY235959 (n = 6), Nω-propyl-L-arginine (n = 6), c-PTIO (n = 6) or Morphine 5 mg/kg (n = 4) in the tail flick test. Each point represents the mean ± S.E.M. for the latency of tail withdrawal. Asterisk indicates significant difference from vehicle (P < 0.05, ANOVA followed by the Dunnet's test).
Discussion
The present study showed that a selective nNOS inhibitor and a NO scavenger microinjected into the dlPAG produce effects similar to a glutamate NMDA receptor antagonist, increasing the number of punished licks in rats submitted to the VCT. The drugs did not interfere with the number of unpunished licks or nociceptive threshold measured in the tail flick test, indicating an anxiolytic-like effect.
The results confirm, using the highly selective NMDA-antagonist LY235959 [26, 28, 29], those previously obtained with AP7, another antagonist of these receptors, in the VCT and elevated plus maze [9]. They also agree with previous findings obtained after systemic injection of NMDA antagonists. For example, using systemic administration of noncompetitive or competitive NMDA receptor antagonists, Plaztnik et al. (1994) described increased punished responses induced by these drugs in the VCT. These antagonists also attenuated the anxious-like behavior observed in rats which had been previously exposed to a cat, suggesting that NMDA receptors are involved in the neural changes mediating the anxiogenic effect of severe stress [30]. Moreover, after repeated systemic administration in rats the NMDA receptor antagonist CGP 37849 not only retained its anxiolytic-like potency in the VCT but even enhanced rat exploratory behavior in a new environment, independently of changes in animal motor activity [31].
The present results reinforce the proposal that the dlPAG could be one of the brains sites where systemically administered NMDA-receptor antagonists exert their anxiolytic effects. The dlPAG is recognized as a key structure in the organization of defensive responses [3] and receives important projections from different cortical and limbic regions, including the prefrontal cortex and medial hypothalamus [1], that are intensively activated by anxiogenic drugs or by aversive stimuli [32, 33]. Moreover, in addition to glutamate-receptors antagonists, anxiolytic effects have been observed after intra-dlPAG microinjection of drugs with different mechanisms of action, including benzodiazepines [34] and cannabinoids [24]. These latter drugs could be acting by inhibiting glutamate release [24, 35].
In the central nervous system NO production by nNOS correlates with activation of NMDA receptors [36]. Moreover, NOS immunoreactive neurons are highly localized in the dlPAG [37]. Previous studies have shown that inhibition of NO formation or its effects in this region produces anxiolytic-like effects [13, 15]. However, these studies employed non-selective NOS inhibitors and therefore could not exclude the involvement of other NOS isoforms. N-propyl is a NOS inhibitor that has a much higher potency to inhibit nNOS compared to eNOS or iNOS [38–40] whereas c-PTIO is a cell membrane-impermeable NO scavenger [41]. Together, the results indicate that that both nNOS activation and extracellular release of NO in the dlPAG are involved in anxiety modulation.
It has been showed by Aguiar et al [6] that that NO may have a facilitatory role in defensive reactions mediated by dlPAG. Corroborating our data, previous results had described that local NOS inhibition into the dlPAG, using an non-specific NOS inhibitor, induced anxiolytic-like effects in the elevated plus maze [15]. Our results using C-PTIO are in agreement with previous finds where C-PTIO injected into the dlPAG of rats exposed to the EPM evoked anxiolytic-like effect in this model [13, 42]. Finally, the systemic administration the NOS inhibitor evoked anxiolytic like effects [43] and the benzodiazepine anxiolytic-like effect is NOS dependent in EPM [44].
Another caveat of the previous studies, as mentioned above, is that they were performed using only the EPM. It is important, however, to investigate the presence of anxiolytic drug effects in several animal models, since different aversive contingencies may engage distinct neurobiological systems [16]. In this way, although both the EPM and VCT are based on approach-avoidance conflict, the former relates to innate fear of open spaces against the drive to explore new environments whereas the latter involves fear of a learned contingency (electrical shock on a drinking spout) in thirsty animals [16, 22, 42].
Conclusion
In summary, the present study provide evidence indicating that activation of NMDA receptors and increased production of NO in the dlPAG are involved in the anxiety behavior displayed by rats in the VCT.
References
Bandler R, Keay KA, Floyd N, Price J: Central circuits mediating patterned autonomic activity during active vs. passive emotional coping. Brain Res Bull. 2000, 53 (1): 95-104. 10.1016/S0361-9230(00)00313-0.
Carrive P: The periaqueductal gray and defensive behavior: functional representation and neuronal organization. Behav Brain Res. 1993, 58 (1-2): 27-47. 10.1016/0166-4328(93)90088-8.
Graeff FG: Serotonin, the periaqueductal gray and panic. Neurosci Biobehav Rev. 2004, 28 (3): 239-259. 10.1016/j.neubiorev.2003.12.004.
Krieger JE, Graeff FG: Defensive behavior and hypertension induced by glutamate in the midbrain central gray of the rat. Braz J Med Biol Res. 1985, 18 (1): 61-67.
Bittencourt AS, Carobrez AP, Zamprogno LP, Tufik S, Schenberg LC: Organization of single components of defensive behaviors within distinct columns of periaqueductal gray matter of the rat: role of N-methyl-D-aspartic acid glutamate receptors. Neuroscience. 2004, 125 (1): 71-89. 10.1016/j.neuroscience.2004.01.026.
Aguiar DC, Moreira FA, Guimaraes FS: Flight reactions induced by injection of glutamate N-methyl-d-aspartate receptor agonist into the rat dorsolateral periaqueductal gray are not dependent on endogenous nitric oxide. Pharmacol Biochem Behav. 2006, 83 (2): 296-301. 10.1016/j.pbb.2006.02.010.
Albin RL, Makowiec RL, Hollingsworth Z, Dure LSt, Penney JB, Young AB: Excitatory amino acid binding sites in the periaqueductal gray of the rat. Neurosci Lett. 1990, 118 (1): 112-115. 10.1016/0304-3940(90)90261-7.
Guimaraes FS, Carobrez AP, De Aguiar JC, Graeff FG: Anxiolytic effect in the elevated plus-maze of the NMDA receptor antagonist AP7 microinjected into the dorsal periaqueductal grey. Psychopharmacology (Berl). 1991, 103 (1): 91-94. 10.1007/BF02244080.
Molchanov ML, Guimaraes FS: Anxiolytic-like effects of AP7 injected into the dorsolateral or ventrolateral columns of the periaqueductal gray of rats. Psychopharmacology (Berl). 2002, 160 (1): 30-38. 10.1007/s00213-001-0941-x.
Matheus MG, Guimaraes FS: Antagonism of non-NMDA receptors in the dorsal periaqueductal grey induces anxiolytic effect in the elevated plus maze. Psychopharmacology (Berl). 1997, 132 (1): 14-18. 10.1007/s002130050314.
Contestabile A: Roles of NMDA receptor activity and nitric oxide production in brain development. Brain Res Brain Res Rev. 2000, 32 (2-3): 476-509. 10.1016/S0165-0173(00)00018-7.
Garthwaite J: Glutamate, nitric oxide and cell-cell signalling in the nervous system. Trends Neurosci. 1991, 14 (2): 60-67. 10.1016/0166-2236(91)90022-M.
Guimaraes FS, Beijamini V, Moreira FA, Aguiar DC, de Lucca AC: Role of nitric oxide in brain regions related to defensive reactions. Neurosci Biobehav Rev. 2005, 29 (8): 1313-1322. 10.1016/j.neubiorev.2005.03.026.
Resstel LB, Correa FM, Guimaraes FS: The expression of contextual fear conditioning involves activation of an NMDA receptor-nitric oxide pathway in the medial prefrontal cortex. Cereb Cortex. 2008, 18 (9): 2027-2035. 10.1093/cercor/bhm232.
Guimaraes FS, de Aguiar JC, Del Bel EA, Ballejo G: Anxiolytic effect of nitric oxide synthase inhibitors microinjected into the dorsal central grey. Neuroreport. 1994, 5 (15): 1929-1932.
Menard J, Treit D: Effects of centrally administered anxiolytic compounds in animal models of anxiety. Neurosci Biobehav Rev. 1999, 23 (4): 591-613. 10.1016/S0149-7634(98)00056-6.
Carlson JN, Haskew R, Wacker J, Maisonneuve IM, Glick SD, Jerussi TP: Sedative and anxiolytic effects of zopiclone's enantiomers and metabolite. Eur J Pharmacol. 2001, 415 (2-3): 181-189. 10.1016/S0014-2999(01)00851-2.
Flores P, Pellon R: Antipunishment effects of diazepam on two levels of suppression of schedule-induced drinking in rats. Pharmacol Biochem Behav. 2000, 67 (2): 207-214. 10.1016/S0091-3057(00)00313-0.
Griebel G, Perrault G, Letang V, Granger P, Avenet P, Schoemaker H, Sanger DJ: New evidence that the pharmacological effects of benzodiazepine receptor ligands can be associated with activities at different BZ (omega) receptor subtypes. Psychopharmacology (Berl). 1999, 146 (2): 205-213. 10.1007/s002130051108.
Griebel G, Perrault G, Tan S, Schoemaker H, Sanger DJ: Comparison of the pharmacological properties of classical and novel BZ-omega receptor ligands. Behav Pharmacol. 1999, 10 (5): 483-495.
Vogel JR, Beer B, Clody DE: A simple and reliable conflict procedure for testing anti-anxiety agents. Psychopharmacologia. 1971, 21 (1): 1-7. 10.1007/BF00403989.
Millan MJ, Brocco M: The Vogel conflict test: procedural aspects, gamma-aminobutyric acid, glutamate and monoamines. Eur J Pharmacol. 2003, 463 (1-3): 67-96. 10.1016/S0014-2999(03)01275-5.
Paxinos G, Watson C: The rat brain in stereotaxic coordinates. 1997, San Diego, CA.: Academic press inc, Fourth
Lisboa SF, Resstel LB, Aguiar DC, Guimaraes FS: Activation of cannabinoid CB1 receptors in the dorsolateral periaqueductal gray induces anxiolytic effects in rats submitted to the Vogel conflict test. Eur J Pharmacol. 2008, 593 (1-3): 73-78. 10.1016/j.ejphar.2008.07.032.
Resstel LB, Souza RF, Guimaraes FS: Anxiolytic-like effects induced by medial prefrontal cortex inhibition in rats submitted to the Vogel conflict test. Physiol Behav. 2008, 93 (1-2): 200-205. 10.1016/j.physbeh.2007.08.009.
Pajolla GP, Pelosi GG, Correa FM: Involvement of NMDA receptors in the hypotensive response to the injection of L-glutamate into the lateral hypothalamus of unanesthetized rats. Brain Res. 2005, 1053 (1-2): 19-26. 10.1016/j.brainres.2005.05.056.
Resstel LB, Correa FM: Injection of l-glutamate into medial prefrontal cortex induces cardiovascular responses through NMDA receptor - nitric oxide in rat. Neuropharmacology. 2006, 51 (1): 160-167. 10.1016/j.neuropharm.2006.03.010.
Benveniste M, Mayer ML: Kinetic analysis of antagonist action at N-methyl-D-aspartic acid receptors. Two binding sites each for glutamate and glycine. Biophys J. 1991, 59 (3): 560-573. 10.1016/S0006-3495(91)82272-X.
Dhruva A, Bhatnagar T, Sapru HN: Cardiovascular responses to microinjections of glutamate into the nucleus tractus solitarii of unanesthetized supracollicular decerebrate rats. Brain Res. 1998, 801 (1-2): 88-100. 10.1016/S0006-8993(98)00550-2.
Adamec RE, Burton P, Shallow T, Budgell J: NMDA receptors mediate lasting increases in anxiety-like behavior produced by the stress of predator exposure--implications for anxiety associated with posttraumatic stress disorder. Physiol Behav. 1999, 65 (4-5): 723-737. 10.1016/S0031-9384(98)00226-1.
Jessa M, Nazar M, Bidzinski A, Plaznik A: The effects of repeated administration of diazepam, MK-801 and CGP 37849 on rat behavior in two models of anxiety. Eur Neuropsychopharmacol. 1996, 6 (1): 55-61. 10.1016/0924-977X(95)00068-Z.
Singewald N, Salchner P, Sharp T: Induction of c-Fos expression in specific areas of the fear circuitry in rat forebrain by anxiogenic drugs. Biol Psychiatry. 2003, 53 (4): 275-283. 10.1016/S0006-3223(02)01574-3.
Singewald N: Altered brain activity processing in high-anxiety rodents revealed by challenge paradigms and functional mapping. Neurosci Biobehav Rev. 2007, 31 (1): 18-40. 10.1016/j.neubiorev.2006.02.003.
Russo AS, Guimaraes FS, De Aguiar JC, Graeff FG: Role of benzodiazepine receptors located in the dorsal periaqueductal grey of rats in anxiety. Psychopharmacology (Berl). 1993, 110 (1-2): 198-202. 10.1007/BF02246973.
Terzian AL, Aguiar DC, Guimaraes FS, Moreira FA: Modulation of anxiety-like behaviour by Transient Receptor Potential Vanilloid Type 1 (TRPV1) channels located in the dorsolateral periaqueductal gray. Eur Neuropsychopharmacol. 2008
Garthwaite J, Boulton CL: Nitric oxide signaling in the central nervous system. Annu Rev Physiol. 1995, 57: 683-706. 10.1146/annurev.ph.57.030195.003343.
Onstott D, Mayer B, Beitz AJ: Nitric oxide synthase immunoreactive neurons anatomically define a longitudinal dorsolateral column within the midbrain periaqueductal gray of the rat: analysis using laser confocal microscopy. Brain Res. 1993, 610 (2): 317-324. 10.1016/0006-8993(93)91416-P.
Hevel JM, White KA, Marletta MA: Purification of the inducible murine macrophage nitric oxide synthase. Identification as a flavoprotein. J Biol Chem. 1991, 266 (34): 22789-22791.
Martasek P, Liu Q, Liu J, Roman LJ, Gross SS, Sessa WC, Masters BS: Characterization of bovine endothelial nitric oxide synthase expressed in E. coli. Biochem Biophys Res Commun. 1996, 219 (2): 359-365. 10.1006/bbrc.1996.0238.
Zhang HQ, Fast W, Marletta MA, Martasek P, Silverman RB: Potent and selective inhibition of neuronal nitric oxide synthase by N omega-propyl-L-arginine. J Med Chem. 1997, 40 (24): 3869-3870. 10.1021/jm970550g.
Ko GY, Kelly PT: Nitric oxide acts as a postsynaptic signaling molecule in calcium/calmodulin-induced synaptic potentiation in hippocampal CA1 pyramidal neurons. J Neurosci. 1999, 19 (16): 6784-6794.
File SE: Behavioural detection of anxiolytic action. Experimental Approaches to Anxiety and Depression. Edited by: Elliott JM. 1992, DJHaCAM. New York, 25-44.
Volke V, Koks S, Vasar E, Bourin M, Bradwejn J, Mannisto PT: Inhibition of nitric oxide synthase causes anxiolytic-like behaviour in an elevated plus-maze. Neuroreport. 1995, 6 (10): 1413-1416. 10.1097/00001756-199507100-00013.
Volke V, Soosaar A, Koks S, Vasar E, Mannisto PT: L-Arginine abolishes the anxiolytic-like effect of diazepam in the elevated plus-maze test in rats. Eur J Pharmacol. 1998, 351 (3): 287-290. 10.1016/S0014-2999(98)00364-1.
Acknowledgements
This research was supported by grants from FAEPA, CNPq (305996/2008-8 and 470042/2009-5) and FAPESP. We thank to I.A.C. Fortunato and J.C. de Aguiar for the excellent technical support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Financial competing interests
The authors declare that they have no competing interests.
Authors' contributions
EADB, FSG, and LBMR contributed to the conception, design and critically revised the manuscript of the study. Moreover, LBMR was responsible by analysis and interpretation of data, drafted the manuscript and continuously supervised the study. LLMTand ALT contributed were responsible for data collection and helped to draft the manuscript. All authors read and approved the final manuscript.
Lucas LM Tonetto, Ana L Terzian contributed equally to this work.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Tonetto, L.L., Terzian, A.L., Del Bel, E.A. et al. Inhibition of the NMDA receptor/Nitric Oxide pathway in the dorsolateral periaqueductal gray causes anxiolytic-like effects in rats submitted to the Vogel conflict test. Behav Brain Funct 5, 40 (2009). https://doi.org/10.1186/1744-9081-5-40
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1744-9081-5-40