Abstract
Background
The occurrence of aberrant functional connectivity in the neuronal circuit is one of the integrative theories of the etiology of schizophrenia. Previous studies have reported that the protein and mRNA levels of the synapsin 2 (SYN2) and complexin 2 (CPLX2) genes were decreased in patients with schizophrenia. Synapsin 2 and complexin 2 are involved in synaptogenesis and the modulation of neurotransmitter release. This report presents a study of the association of polymorphisms of SYN2 and CPLX2 with schizophrenia in the Korean population.
Methods
Six single nucleotide polymorphisms (SNPs) and one 5-bp insertion/deletion in SYN2 and five SNPs in CPLX2 were genotyped in 154 Korean patients with schizophrenia and 133 control patients using direct sequencing or restriction fragment length polymorphism analysis. An intermarker linkage disequilibrium map was constructed for each gene.
Results
Although there was no significant difference in the genotypic distributions and allelic frequencies of either SYN2 or CPLX2 polymorphisms between the schizophrenia and control groups, the two-way haplotype analyses revealed significant associations with the disease (P < 0.05 after Bonferroni correction). The three-way haplotype analyses also revealed a significant association of SYN2 with schizophrenia (P < 0.001 after Bonferroni correction).
Conclusion
These results suggest that both SYN2 and CPLX2 may confer susceptibility to schizophrenia in the Korean population.
Similar content being viewed by others
Background
Schizophrenia is a severe, chronic mental illness affecting 0.5–1.5% of the general population worldwide [1]. The contribution of genetic factors to the vulnerability to schizophrenia has been well established by family, twin, and adoption studies that have suggested a significant heritability of approximately 50–70% [2]. Many studies have attempted to identify the allelic variants that confer susceptibility to the illness, but no single genes have been identified that produce a major effect on the vulnerability [3].
Recently the synaptic hypothesis of schizophrenia has gained attention by attributing the fundamental pathology of schizophrenia to the dysfunction of synaptic transmission involving various molecules [4]. Synapsins, a family of synaptic vesicle-associated phosphoproteins, play a crucial role in the regulation of neurotransmission, synaptogenesis, and neuronal plasticity [5]. Three human synapsin genes have been identified (SYN1, 2, and 3; OMIM 313440, 600755, and 602705) [6]. Complexin 1 and complexin 2, which are encoded by CPLX1 (OMIM 605032) and CPLX2 (OMIM 605033), respectively, and are also called synaphins, are pre-synaptic membrane proteins that preferentially bind to syntaxin within the SNARE (soluble N-ethylmaleimide-sensitive fusion attachment protein receptors) complex. These proteins are important regulators of transmitter release immediately preceding vesicle fusion [7]. Previous studies have demonstrated that the concentrations of synapsins and complexins are reduced in the brains of schizophrenics [8, 9]. The expression levels of both synapsins were significantly decreased in the hippocampal tissue of schizophrenic patients [10]. The levels of synapsin 2 and complexin 2 mRNA were also significantly reduced in the prefrontal cortex, cerebellum, and hippocampus of schizophrenics [11–14].
SYN2 was mapped to chromosome 3p25 [15], and CPLX2 is located on chromosome 5q35.3 (OMIM 605033). These loci were identified as potential regions conferring susceptibility to schizophrenia in diverse populations [16–18]. Based on their localization, well-established neurobiological roles, and expression patterns in schizophrenic patients, we selected SYN2 and CPLX2 as candidate genes for conferring susceptibility to schizophrenia. In this report, we present an association study of SYN2 and CPLX2 with schizophrenia using 12 polymorphisms in the Korean population.
Results
SYN2 polymorphisms in the schizophrenia and control groups
Of the seven polymorphisms in SYN2, rs2623873 (SYN2-1) is located in the promoter region, whereas the others are all located in the intronic regions (SYN2-2–7) (Fig. 1, Table 1). The genotypic distributions and allelic frequencies of polymorphisms in SYN2 were determined in 113 schizophrenic patients and 114 normal healthy controls by direct sequencing or Dde I RFLP. The genotypic distributions and allelic frequencies of polymorphisms in SYN2 are shown in Table 2. The average allelic frequency of the SNPs was 0.312. Given the equivalent frequency for the susceptible allele, the expected detection power for SYN2 was 0.9538 to 0.9929 under the multiplicative model with a genotype relative risk = 1.8 to 2.0 [22]. None of the SNPs showed any significant deviation from Hardy-Weinberg equilibrium (P > 0.05). We observed no significant difference in the genotypic distributions and allelic frequencies between the schizophrenics and control groups (Table 2).
Genomic organization of SYN2 and CPLX2 and locations of SNPs. a; SYN2 spans over 140 kb and is composed of 14 exons. Seven markers are indicated with the dbSNP reference ID http://www.ncbi.nlm.nih.gov/SNP. b; CPLX2 spans over 83 kb and is composed 3 exons. Five markers are indicated with the dbSNP reference ID http://www.ncbi.nlm.nih.gov/SNP.
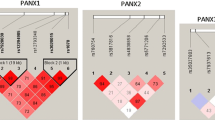
We compared the LD for all possible two-way comparisons of the SNPs in the controls (Table 3). The pairwise D' values for the seven SNPs were consistently high, except in one instance (SYN2-2 vs. SYN2-6; D' = 0.300, r2 = 0.200). Out of the 21 possible pairs of SNPs, significant haplotype associations with schizophrenia were observed for 4 pairs: SYN2-1 – SYN2-2 (χ2 = 27.58, df = 3, P = 4.45 × 10-6), SYN2-2 – SYN2-4 (χ2 = 16.46, df = 3, P = 9.12 × 10-4), SYN2-2 – SYN2-7 (χ2 = 8.08, df = 3, P = 0.044), and SYN2-3 – SYN2-4 (χ2 = 10.66, df = 3, P = 0.014) (Table 3). Even after the Bonferroni correction (number of haplotypes, n = 21), the associations of the SYN2-1 – SYN2-2 and SYN2-2 – SYN2-4 haplotypes with schizophrenia remained significant (P corr = 9.35 × 10-5 and P corr = 0.019) (Table 3). The T allele-the deletion allele haplotype for the SYN2-1 – SYN2-2 combination and the deletion allele-the G allele haplotype for the SYN2-2 – SYN2-4 combination were observed more frequently in schizophrenia than the controls (Table 4).
We also investigated the association of three-way haplotypes formed by SYN2-1, SYN2-2, and SYN2-4 with schizophrenia. A significant difference in the haplotype frequencies between the schizophrenia and control groups was observed (χ2 = 35.0, df = 7, P corr = 1.1 × 10-5). For the combination of SYN2-1, SYN2-2, and SYN2-4, the estimated frequencies of the T-deletion-G haplotype differed between the schizophrenia (0.570) and controls groups (0.440).
CPLX2 polymorphisms in schizophrenia and control groups
Of the five SNPs in CPLX2, rs2247916 (CPLX2-1) is located in the promoter region, rs2243404 (CPLX2-2) is located in the 5'UTR, and the others are located in the intronic regions (Fig. 1, Table 1). We determined the genotypic distributions and allelic frequencies of the SNPs in 154 schizophrenic patients and 133 normal healthy controls by direct sequencing or RFLP analysis. The genotypic distributions and allelic frequencies for CPLX2 SNPs are shown in Table 2. The average allelic frequency of the SNPs was 0.126. Given the equivalent frequency for the susceptible allele, the expected detection power for CPLX2 was 0.7445 to 0.8802 based on the multiplicative model with the genotype relative risk = 1.8 to 2.0 [22]. None of the five SNPs showed any significant deviations from Hardy-Weinberg equilibrium. We observed no significant differences in genotypic distributions or allelic frequencies between the schizophrenia and control groups (Table 2).
We compared LD for all possible two-way comparisons of the SNPs in controls (Table 5). The pairwise D' values for the five SNPs were consistently low, except in one instance (CPLX2-2 vs. CPLX2-4; D' = 0.715, r2 = 0.011). Only one pair of SNPs (CPLX2-1 vs. CPLX2-2) showed a significant haplotype association with schizophrenia (χ2 = 16.28, df = 3, P = 0.0009), even after the Bonferroni correction (n = 10, P corr = 0.009, Table 5). For the combination of CPLX2-1 – CPLX2-2, the G allele-the C allele haplotype was observed more frequently in the schizophrenia group than the control group (Table 6).
Discussion
In this study, we observed significant, pairwise haplotype associations with schizophrenia for two pairs of SNPs in SYN2 (SYN2-1 – SYN2-2 and SYN2-2 – SYN2-4; P corr = 9.35 × 10-5 and P corr = 0.019, respectively) and one pair of SNPs in CPLX2 (CPLX2-1 – CPLX2-2, P corr = 0.009) (Table 3, 5). The three-way haplotype (SYN2-1, SYN2-2, and SYN2-4) also showed a significant association with schizophrenia (P corr = 1.1 × 10-5). The SYN2-1 and CPLX2-1 SNPs are located in the respective promoter regions, -98 and -156. SYN2-1 was located within the GC box motif and CPLX2-1 within the C/EBP motif in a database search http://www.cbrc.jp/research/db/TFSEARCH. The positive haplotype associations seem to be based on an increase of LD in the schizophrenia group compared to the control group because the D' values of the schizophrenia group were higher than those of the controls [(SYN2-1 – SYN2-2, 0.935 vs. 0.531 (schizophrenics vs. controls)), (SYN2-2 – SYN2-4, 0.750 vs. 0.453)] (Table 3). A similar situation was also observed with the positive association of the haplotype in CPLX2 with schizophrenia [CPLX2-1 – CPLX2-2, 0.852 vs. 0.412 (schizophrenics vs. controls)] (Table 5).
Chen et al. [23] recently reported an association study of four SNPs in SYN2 using Han Chinese samples. They found significant associations of SNP rs795009 and a haplotype constructed by the four SNPs with schizophrenia. Chen et al. [23] and our study examined two SNPs (rs2308169 and rs308963) in common, and their genotypic and allelic frequencies were similar in both studies. Although Chen et al. [23] did not mention the pairwise haplotype association study that we performed, they did report a significant difference in the overall four-way haplotype frequencies between schizophrenics and controls. Since two independent studies have reported a significant haplotype association of SYN2 with schizophrenia, this gene is probably involved in the pathogenesis of schizophrenia.
Several studies have suggested that the decreased expression of synaptic genes is characteristic of schizophrenia. In the hippocampus of schizophrenic patients, several studies have shown a consistent pattern of decreases in presynaptic proteins and their encoding mRNAs, such as synapsin 2, synaptophysin, and synaptosomal-associated protein-25 (SNAP-25) [8–10, 24]. Furthermore, a reduction in the synapsin 2 mRNA levels was observed in the prefrontal cortex of schizophrenic patients [14], but controvertible results have also been reported [25]. The altered expression levels of other presynaptic proteins, complexin 1 and complexin 2, have been reported in schizophrenic patients [11–13]. Interestingly, complexin 1 is enriched in axosomatic regions, inhibitor neurons, and their synapses, while complexin 2 is enriched in the axodendritic terminals [9, 26]. The differential expression of complexins 1 and 2 implies their involvement in the excitatory synapse in the hippocampus of schizophrenic patients [11]. These observations suggest that abnormal expression of SYN2 and CPLX2 may cause the vulnerability to schizophrenia by altering neurotransmitter release and neuroplasticity.
Conclusion
We found significant differences in the haplotype frequencies in both SYN2 and CPLX2 polymorphisms between schizophrenia and control groups. In addition, the haplotype constructed from three polymorphisms (SYN2-1, SYN2-2, and SYN2-4) showed a significant association with schizophrenia. Our results suggest that both SYN2 and CPLX2 polymorphisms may contribute susceptibility to schizophrenia in the Korean population.
Methods
Subjects
A total of 154 unrelated Korean schizophrenia patients (80 male and 74 female with a mean ± SD age of 43.8 ± 11.4 yr) and 133 unrelated Korean controls (64 male and 69 female; age 50.6 ± 11.7 yr) were recruited. For the SYN2 analysis, 113 unrelated Korean schizophrenia patients (60 male and 53 female with a mean ± SD age of 42.2 ± 11.3 yr) and 114 unrelated Korean controls (60 male and 54 female; age 51.7 ± 10.9 yr) were participated. The schizophrenia patients were diagnosed using the Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV criteria. The control subjects were recruited after they had been designated as mentally healthy in a general health check-up program. The average age of the controls exceeded 50 years because we tried to avoid misincorporation of patients with late onset schizophrenia in the control group, while it may produce statistical bias potentially. Written informed consent was obtained from all subjects. This study was approved by the Ethics Committee of Kyung Hee University, Faculty of Medicine. Genomic DNA was extracted from whole blood cells using a NucleoSpin® Blood kit (Macherey-Nagel, Easton, PA).
SNP Selection and PCR-based Genotyping
Since the genomic sizes of SYN2 and CPLX2 are about 187 and 89 kb, respectively, we initially intended to select common polymorphisms at intervals of approximately 20–50 kb from the dbSNP http://www.ncbi.nlm.nih.gov/SNP/. After validating the frequency of each polymorphism in 24 healthy Korean individuals using direct sequencing, we selected seven common polymorphisms from SYN2 and five from CPLX2 for further analyses (Fig. 1, Table 1). We amplified the fragments containing polymorphisms individually and genotyped DNA samples for each SNP with either PCR-based restriction fragment length polymorphism (RFLP) assays or direct sequencing performed with an ABI PRISM® Dye Terminator Cycle Sequencing kit (Applied Biosystems, Foster City, CA) on an ABI PRISM® 3100 DNA sequencer (Applied Biosystems) (Table 1). In case of unclear sequence data, we repeated direct sequencing under various conditions until the genotype was determined correctly.
Statistics
The deviation of the genotypic frequencies from Hardy-Weinberg equilibrium was examined using the chi-square test (df = 1). Statistical differences in the genotypic distributions and allelic frequencies between the schizophrenia and control groups were examined using the Fisher's exact probability test. We calculated D' and r2 to evaluate the magnitude of linkage disequilibrium (LD) [19]. We estimated haplotype frequencies using the EH program, version 1.14 [20]. The statistical analysis of haplotype association was done as previously described [21]. We applied the Bonferroni correction to multiple testing based on the number of haplotypes. The significance level for all the statistical tests was 0.05.
References
Lewis DA, Lieberman JA: Catching up on schizophrenia: natural history and neurobiology. Neuron. 2002, 28: 325-334. 10.1016/S0896-6273(00)00111-2.
Owen MJ, O'Donovan MC, Gottesman II: Schizophrenia. Psychiatric Genetics & Genomics. Edited by: McGuffin P, Owen MJ, Gottesman II. 2002, Oxford, 247-266.
Owen MJ, Williams NM, O'Donovan MC: The molecular genetics of schizophrenia: new findings promise new insights. Mol Psychiatry. 2004, 9: 14-27. 10.1038/sj.mp.4001444.
Frankle WG, Lerma J, Laruelle M: The synaptic hypothesis of schizophrenia. Neuron. 2003, 39: 205-215. 10.1016/S0896-6273(03)00423-9.
Hilfiker S, Pieribone VA, Czernik AJ, Kao H-T, Augustine GJ, Greengard P: Synapsins as regulators of neurotransmitter release. Phil Trans R Soc Lond B. 1999, 354: 269-279. 10.1098/rstb.1999.0378.
Kao H-T, Porton B, Hilfiker S, Stefani G, Pieribone VA, DeSalle R: Molecular evolution of the synapsin gene family. J Exp Zool (Mol Dev Evo). 1999, 285: 360-377. 10.1002/(SICI)1097-010X(19991215)285:4<360::AID-JEZ4>3.0.CO;2-3.
Tokumaru H, Umayahara K, Pellegrini LL, Ishizuka T, Saisu H, Betz H: SNARE complex oligomerization by synaphin/complexin is essential for synaptic vesicle exocytosis. Cell. 2001, 104: 421-432. 10.1016/S0092-8674(01)00229-X.
Browning MD, Dudek EM, Rapier JL, Leonard S, Freedman R: Significant reductions in synapsin but not synaptophysin specific activity in the brains of some schizophrenics. Biol Psychiatry. 1993, 34: 529-535. 10.1016/0006-3223(93)90195-J.
Harrison PJ, Eastwood SL: Preferential involvement of excitatory neurons in medial temporal lobe in schizophrenia. Lancet. 1998, 352: 1669-1673. 10.1016/S0140-6736(98)03341-8.
Vawter MP, Thatcher L, Usen N, Hyde TM, Kleinman JE, Freed WJ: Reduction of synapsin in the hippocampus of patients with bipolar disorder and schizophrenia. Mol Psychiatry. 2002, 7: 571-578. 10.1038/sj.mp.4001158.
Eastwood SL, Harrison PJ: Hippocampal synaptic pathology in schizophrenia, bipolar disorder and major depression: a study of complexin mRNAs. Mol Psychiatry. 2000, 5: 425-432. 10.1038/sj.mp.4000741.
Eastwood SL, Cotter D, Harrison PJ: Cerebellar synaptic protein expression in schizophrenia. Neuroscience. 2001, 105: 219-229. 10.1016/S0306-4522(01)00141-5.
Knable MB, Barci BM, Webster MJ, Meador-Woodruff L, Torrey EF: Molecular abnormalities of hippocampus in severe psychiatry illness: postmortem findings from the Stanley Neuropathology Consortium. Mol Psychiatry. 2004, 9: 609-620. 10.1038/sj.mp.4001471.
Mirnics K, Middleton FA, Marquez A, Lewis DA, Levitt P: Molecular characterization of schizophrenia viewed by microarray analysis of gene expression in prefrontal cortex. Neuron. 2000, 28: 53-67. 10.1016/S0896-6273(00)00085-4.
Karlin S, Chen C, Gentles AJ, Cleary M: Associations between human disease genes and overlapping gene groups and multiple amino acid runs. Proc Nat Acad Sci. 2002, 99: 17008-17013. 10.1073/pnas.262658799.
Lewis CM, Levinson DF, Wise LH, DeLisi LE, Straub RE, Hovatta I: Genome scan meta-analysis of schizophrenia and bipolar disorder, Part II: Schizophrenia. Am J Hum Genet. 2003, 73: 34-48. 10.1086/376549.
Pulver AE, Lasseter K, Kasch L, Wolyniec P, Nestadt G, Blouin JL: Schizophrenia: a genome scan targets chromosomes 3p and 8p as potential sites of susceptibility genes. Am J Med Genet. 1995, 60: 252-260. 10.1002/ajmg.1320600316.
Sklar P, Pato MT, Kirby A, Kirby A, Petryshen TL, Medeiros H, Carvalho C: Genome-wide scan in Portuguese island families identifies 5q31-5q35 as a susceptibility locus for schizophrenia. Mol Psychiatry. 2004, 9: 213-218. 10.1038/sj.mp.4001418.
Taillon-Miller P, Bauer-Sardina I, Saccone NL, Putzel J, Laitinen T, Cao A, Kere J, Pilia G, Rice JP, Kwok PY: Juxtaposed regions of extensive and minimal linkage disequilibrium in human Xq25 and Xq28. Nat Genet. 2000, 25: 324-328. 10.1038/77100.
Xie X, Ott J: Testing linkage disequilibrium between a disease gene and marker loci. Am J Hum Genet. 1993, 53: 1170-
Sham P: The analysis of allelic association. Statistics in Human Genetics. Edited by: Sham P. 1997, London: Arnold, 145-185.
Ohashi J, Tokunaga K: The power of genome-wide association studies of complex disease genes: statistical limitations of indirect approaches using SNP markers. J Hum Genet. 2001, 46: 478-482. 10.1007/s100380170048.
Chen Q, He G, Wang XY, Chen QY, Liu XM, Gu ZZ: Positive association between synapsin II and schizophrenia. Biol Psychiatry. 2004, 56: 177-181. 10.1016/j.biopsych.2004.05.010.
Thompson PM, Egbufoama S, Vawter MP: SNAP-25 reduction in the hippocampus of patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2003, 27: 411-417. 10.1016/S0278-5846(03)00027-7.
Imai C, Sugai T, Iritani S, Niizato K, Nakamura R, Makifuchi T: A quantitative study on the expression of synapsin II and N-ethylmaleimide-sensitive fusion protein in schizophrenic patients. NeuroSci Lett. 2001, 305: 185-188. 10.1016/S0304-3940(01)01844-4.
Takahashi S, Yamamoto H, Matsuda Z, Ogawa M, Yagyu K, Taniguchi T: Identification of two highly homologous presynaptic proteins distinctly localized at the dendritic and somatic synapses. FEBS Lett. 1995, 368: 455-460. 10.1016/0014-5793(95)00713-J.
Acknowledgements
This work was supported by JPSS and KOSEF, and in part by a grant of the Korean Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea. (0405-NS01-0704-0001).
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors' contributions
HJ Lee conceived of the study, carried out sequencing, participated in the interpretation of the data and drafted manuscript, JY Song, JW Kim and JK Park recruited the samples of schizophrenia patients, SY Jin, MS Hong, and J-H Chung recruited the samples of normal control, H Shibata and Y Fukumaki participated in its design, carried out the statistical analyses, and participated in the interpretation of data. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Lee, H.J., Song, J.Y., Kim, J.W. et al. Association study of polymorphisms in synaptic vesicle-associated genes, SYN2 and CPLX2, with schizophrenia. Behav Brain Funct 1, 15 (2005). https://doi.org/10.1186/1744-9081-1-15
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1744-9081-1-15