Abstract
Background
Since 1958 many, but not all studies have demonstrated that paternal age is a risk factor for schizophrenia. There may be many different explanations for differences between studies, including study design, sample size, collection criteria, heterogeneity and the confounding effects of environmental factors that can for example perturb epigenetic programming and lead to an increase in disease risk. The small number of children in Western families makes risk comparisons between siblings born at different paternal ages difficult. In contrast, more Eastern families have children both at early and later periods of life. In the present study, a cross-sectional population study in an Iranian population was performed to compare frequency of schizophrenia in younger offspring (that is, older paternal age) versus older offspring.
Methods
A total of 220 patients with the diagnosis of schizophrenia (cases) from both psychiatric hospitals and private clinics and 220 individuals from other hospital wards (controls), matched for sex and age were recruited for this study. Patients with neurological problem, substance abuse, mental retardation and mood disorder were excluded from both groups.
Results
Birth rank comparisons revealed that 35% vs 24% of the cases vs the controls were in the third or upper birth rank (P = 0.01). Also, the mean age of fathers at birth in case group (30 ± 6.26 years) was significantly more than the control group (26.45 ± 5.64 years; P = 0.0001). The age of 76 fathers at birth in case group was over 32 versus 33 fathers in control group. Individuals whose fathers' age was more than 32 (at birth) were at higher risk (2.77 times) for schizophrenia versus others (P < 0.0001, 95% CI 1.80 to 4.27). The maternal age at parturition of the case versus controls groups was 26.1 ± 5.41 vs 25.07 ± 4.47 (P = 0.02). Logistic regression analysis suggests that maternal age is less likely to be involved in the higher risk of schizophrenia than advanced parental age.
Discussion
This study demonstrates a relationship between paternal age and schizophrenia in large families of an Iranian population. Arguments have been put forth that DNA bases changes or epigenetic changes in sperm account for the increased risk associated with older fathers. However, it would not be surprising that both de novo germline mutations and epigenetic changes contribute to disease occurrence because DNA replication and DNA methylation are closely linked at both the macromolecular level (that is, methylation closely follows replication), and at the metabolic level (both processes require folate), and susceptible to modulation by the environment. Further research on samples such as those collected here are needed to sort out the contributions of de novo mutations versus epigenetic changes to schizophrenia.
Similar content being viewed by others
Introduction
Schizophrenia is a chronic and disabling disorder with an approximate 1% incidence in the population, and 120 million afflicted individuals worldwide [1]. Hallucinations, delusions, and emotional, cognitive, and motor deficits are common symptoms that appear usually in late adolescence or early adulthood [2, 3]. Many studies detected a genetic link to the disorder, although no single or small number of genes accounts for the majority of cases [4, 5]. Systematic analyses of different pedigrees segregating schizophrenia suggested multiple genes with a complex inheritance pattern [6, 7]. Child adoption studies have supported a polygenic and multifactorial aetiology [8].
Environmental factors linked to schizophrenia include winter parturition [9], maternal stress during pregnancy [10, 11], second trimester and postnatal infection, labour and perinatal traumas, immigration, residency in urban areas and so on [12, 13], and paternal occupation and age [14]. Paternal age has been linked to schizophrenia since 1958 [15]. Most studies including a recent meta-analysis have confirmed paternal age as a risk factor for schizophrenia [16–24], however negative results also exist in the literature [25]. The link between paternal age and schizophrenia, argues that de novo changes to DNA can lead to schizophrenia, because the sole biological contribution of fathers to progeny is DNA. De novo mutations are linked to neurodevelopmental disorders and cancers [26, 27] and may explain the high prevalence of schizophrenia despite the decreased fertility of schizophrenia patient [14]
Malaspina suggested that single base pair mutations, trinucleotide repeat expansion, or genetic imprinting changes might be responsible for the observed effect [28]. However, DNA replication and epigenetic changes are closely linked at both the macromolecules level (for example, DNA replication and global DNA methylation), and the metabolic level (for example, synthesis of purines and deoxythymidine triphosphate (dTTP) and S-adenosyl methionine, the cofactor that donates a methyl group). Hence, it would not be surprising that paternal age influences both of these as well as other overlapping processes.
In 2002, Malaspina et al. reported that the paternal age at birth of sporadic cases averaged 4.7 years older than familial cases of schizophrenia, and attributed approximately 27% of the sporadic cases to paternal age, and likely due to de novo mutations [14]. However, Pulver et al. observed that paternal age is not different in (1) familial versus sporadic cases, and (2) first-degree and second-degree paternal relatives of probands, and argued that these results did not support the linkage of paternal age to spontaneous forms of schizophrenia [29]. It is quite clear that studies on large families and pre/post-index generations are important to distinguish genetic from sporadic cases. However, it should be noted that, if advanced paternal age is a risk factor for schizophrenia, the frequency and coincidence of disease would be more in children of fathers with higher age and could be misinterpreted as genetic inheritance. This may obscure the detection of sporadic versus familial cases that could be overcome in part by the examining the effects of higher birth rank, accompanied with advanced paternal age, on schizophrenia pathogenesis in large families.
This study examined the relationship between paternal age and schizophrenia in an Iranian population, a distinct cultural group not previously studied. Typical Iranian families are large with a wide variation in paternal age not typically seen in Western families. The study of large families provides us with an opportunity to examine the relationship between birth order, paternal age and schizophrenia. If advanced paternal age is an important risk factor for schizophrenia, disease frequency would be greater in children with higher birth rank as well (that is, higher paternal age). Hence, we examined the effects of higher birth rank and advanced paternal age on schizophrenia.
Methods
This case-control study was performed in Tehran in 2005 and 2006 recruiting 220 patients (case group) with a diagnosis of schizophrenia from both psychiatric hospitals and private clinics. The diagnosis was made based on Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) criteria in a semistructured interview by two psychiatrists. The control group was made up of 220 individuals from non-psychiatric hospital wards of the same district, matched for sex (P = 0.84, χ2 test) and age (P = 0.37, t test) versus the case group. Individuals with any neurological problem, substance abuse, mental retardation or mood disorder were excluded from the case and control groups. Patients in an acute psychotic phase were excluded because of their inability to give consent. No organic problems were identified in any patients with schizophrenia by medical examinations. All cases and controls completed consent forms and data was collected on each subjected by trained medical students completing two instruments. The demographic questionnaire recorded sex, employment, education, paternal age, maternal age, paternal and maternal age at parturition, family history of psychotic disorders, number of children, birth rank within siblings, and age of disease onset. Both the patients and their carers were asked about the presence of similar disorders in first-degree and second-degree relatives. Those with evidence for positive family history underwent for further evolution by contacting their psychiatrist, inspecting their medical records or structured interview of the subjects and their carers in order to confirm or rule out the presence of mental diseases. The second diagnostic instrument was the Structured Clinical Interview for DSM Disorders (SCID), previously validated for diagnosis of schizophrenia in the Iranian population.
Differences between study groups were examined using the χ2 test for categorical variables, and an independent sample t test (or Mann-Whitney U test) for continuous variables. Probability values of P < 0.05 were considered statistically significant. Multiple logistic regression analysis was performed with adjustment for independent association of all factors with schizophrenia as a dependent variable. One model (included all independent variables at one stage) was developed to adjust for covariates. The analysis was performed using SPSS software (V.11.5; SPSS, Chicago, IL, USA).
Results
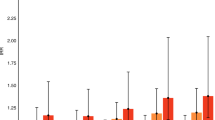
A greater number of cases (Table 1) had a birth rank of ≥3 versus controls (35% versus 24%, P = 0.01, OD = 1.7, 95% CI 1.12 to 2.5). The difference in the occurrence of psychiatric disorders, including schizophrenia, schizoaffective disorder and major mood disorders (unipolar and bipolar disorder) in first-degree relatives was significant in case versus control groups (P = 0.04): 20.5% versus 13.2%, respectively (Table 1).
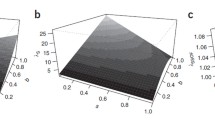
Paternal age at parturition of the proband was significantly different between the case and control groups. For instance, the mean paternal age at birth was 30 ± 6.26 versus 26.45 ± 5.64, in case versus control groups, respectively (P = 0.0001) (Tables 1 and 2). A significantly higher number of fathers with an age of ≥32 were in the case group versus the control group (Table 3). Analysis of the paternal age distribution (Figure 1) revealed that the fathers of the case group tended to have children at an earlier age, but that a greater number (76, 35%) of cases' fathers age were ≥32, than those in control group (n = 33, 15%). Individuals with fathers' ages at birth ≥32 were 2.77 times at higher risk for schizophrenia (P < 0.0001), OR = 3.7 (95% CI 1.9 to 7.3). The mean age of mothers at birth was marginally different (P = 0.02) between the case and control groups (26 versus 25.07, respectively). Logistic regression analysis (Table 4), adjusting for the effect of maternal age, birth rank and family history, indicated that paternal age is an independent predisposing factor to schizophrenia; its OR was 3.78 (95% CI 1.9 to 7.3).
This case-control study providing data on a previously unexplored Iranian population supports the hypothesis that advanced paternal age and a birth rank of higher than 3 (that is, higher age of father at birth) are linked to schizophrenia pathogenesis.
Discussion
Our results linking advanced paternal age to schizophrenia are comparable to observations in other populations. Further, logistic regression analysis has suggested that higher birth rank along with advanced paternal age is more of a risk factor for schizophrenia than maternal age. Birth rank and short birth interval were previously found to be risk factors for schizophrenia [30–32]. The effect of higher birth order/rank may reflect the costs of childbearing on a mother's long-term health, as Christensen et al. analysing twin data found support for the proverb 'a tooth, a child' [33]. Higher birth rank effects may also be a function of advanced paternal and/or maternal age. Along these lines, our study of large families provides evidence for the first time that the relationship between birth order/rank and schizophrenia may be due to paternal age. In fact, as advanced paternal age was a risk factor for schizophrenia, the disease frequency was greater in children with higher birth rank as well (that is, higher paternal age). Higher birth rank (and advanced paternal age) may account for the coincidence of disease in multiple siblings, whereas such coincidences are taken as evidence for genetic inheritance. However, paternal age effects on schizophrenia pathogenesis may be mediated through both genetic and/or epigenetic mechanisms [34, 35].
Epigenetics refers to heritable but potentially reversible changes in DNA methylation, RNA (editing and interference), and protein (for example, histone) modification and provide mechanisms for the environment to interact with the genome [36]. The multiplicity of targets and modifications linked to epigenetic programming suggests that perturbations to such pathways are likely to account for a large amount of phenotypic variations. Advanced paternal age is associated with an elevated incidence of environmental exposures that may affect the spermatozoid epigenetic memory. These epigenetic changes could be the underlying mechanisms of paternal age effects on schizophrenia pathogenesis.
About 50% of monozygotic (aka identical) twins afflicted by disease and starting life with identical genomes are discordant for schizophrenia, and when concordant they do not have the same psychiatric phenotypes [1]. Additionally, all children of monozygotic twins concordant or discordant for schizophrenia have the same elevated probability of becoming ill [37, 38]. These observations suggest that genetic susceptibility is transmitted to offspring, but environmental factors also contribute in disease occurrence and presentation. An elevated somatic mutation rate in twins discordant for schizophrenia was detected around simple trinucleotide repeat sequences, for example, (CAG)n [39]. It is of note that this study was limited to (CAG)n repeat occurrences, and we do not know whether the increased mutation rate extends to other sequences. However, other studies have also detected an increase in neuronal aneuploidies in schizophrenic brains [40, 41]. Several groups have suggested that the genetic-environmental linkage reflects the need for 'two-hits' (genetic plus somatic changes) to DNA. Other studies have shown that genetic and epigenetic changes occur with age [42, 43], and epigenetic differences exist between monozygotic twins [44, 45].
In sperm, dominant and codominant base change mutations will impact progeny and subsequent generations in a simple Mendelian fashion. A recessive mutation will not be detected in progeny (except occasionally when a second mutated allele is present) unless the target gene is maternally imprinted and silenced. Epigenetic changes at imprinted genes could lead to inappropriate expression or lack of expression. The effects on subsequent generations will be dependent on the parental origin of the change. Further, epigenetic DNA methylation is reset during passage through the germline and during embryonic and post natal development; hence, it is not clear what changes will be passed to subsequent generations [36].
The dissection of the paternal age effect in large families is advantageous given the plethora of potential DNA changes and the consequences for subsequent generations. The paternal age effect may be due to inadequate nutrition and/or exposure to environmental toxins during spermatogenesis. For instance, it is estimated that 30% of American diets are deficient in folate [46, 47], a nutrient required for the biosynthesis of TTP and DNA synthesis as well as purine nucleotides that are necessary for DNA and RNA synthesis and other cellular processes (for example, epigenetic changes to RNA and proteins, energy transduction, and signally). The success of genetic studies on rare human disease has prompted research on complex common disease to focus on genetic approaches while largely ignoring environmental factors that have the potential to change not only DNA but also cause epigenetic changes to DNA, RNA and proteins. For instance, paternal age and occupations linked to schizophrenia in progeny may involve exposures that induce oxidative stress and impact folate metabolism [48]. A comprehensive understanding of schizophrenia and other common complex diseases requires studies on the interaction of the environment with specific genotypes, some predisposed, to severe illness. Such studies are required as several preventable known environmental and nutritional factors (for example, folic acid deficiency) can induce epigenetic alterations that may impact the quality of human life in general.
References
Saddock B, Saddock V: Kaplan and Saddock's Comprehensive Textbook of Psychiatry. 2005, Philadelphia, PA: Lippincott Williams & Wilkins, 1330-1395.
Murray CJ, Lopez AD: The Global Burden of Disease. 1996, Cambridge, MA: Harvard University Press
McLeen VA: Healthy People 2000. 2000, Washington, DC: US Department of Health and Human Services/International Medical Publishing
Kato C, Petronis A, Okazaki Y, Tochigi M, Umekage T, Sasaki T: Molecular genetic studies of schizophrenia: challenges and insights. Neurosci Res. 2002, 43: 295-304. 10.1016/S0168-0102(02)00064-0.
Abdolmaleky HM, Thiagalingam S, Wilcox M: Genetics and epigenetics in major psychiatric disorders: dilemmas, achievements, applications, and future scope. Am J Pharmacogenomics. 2005, 5: 149-160.
Faraone SV, Brown CH, Glatt SJ, Tsuang MT: Preventing schizophrenia and psychotic behaviour: definitions and methodological issues. Can J Psychiatry. 2002, 47: 527-537.
Ban TA: Neuropsychopharmacology and the genetics of schizophrenia: a history of the diagnosis of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2004, 28: 753-762. 10.1016/j.pnpbp.2004.05.021.
Beckmann H, Franzek E: The genetic heterogeneity of "schizophrenia". World J Biol Psychiatry. 2000, 1: 35-41. 10.3109/15622970009150564.
Torrey EF, Miller J, Rawlings R, Yolken RH: Seasonality of births in schizophrenia and bipolar disorder: a review of the literature. Schizophr Res. 1997, 28: 1-38. 10.1016/S0920-9964(97)00092-3.
Khashan AS, Abel KM, McNamee R, Pedersen MG, Webb RT, Baker PN, Kenny LC, Mortensen PB: Higher risk of offspring schizophrenia following antenatal maternal exposure to severe adverse life events. Arch Gen Psychiatry. 2008, 65: 146-152. 10.1001/archgenpsychiatry.2007.20.
Malaspina D, Corcoran C, Kleinhaus KR, Perrin MC, Fennig S, Nahon D, Friedlander Y, Harlap S: Acute maternal stress in pregnancy and schizophrenia in offspring: a cohort prospective study. BMC Psychiatry. 2008, 8: 71-10.1186/1471-244X-8-71.
Compton MT: Considering schizophrenia from a prevention perspective. Am J Prev Med. 2004, 26: 178-185. 10.1016/j.amepre.2003.10.003.
McGrath JJ: The surprisingly rich contours of schizophrenia epidemiology. Arch Gen Psychiatry. 2007, 64: 14-16. 10.1001/archpsyc.64.1.14.
Malaspina D, Corcoran C, Fahim C, Berman A, Harkavy-Friedman J, Yale S, Goetz D, Goetz R, Harlap S, Gorman J: Paternal age and sporadic schizophrenia: evidence for de novo mutations. Am J Med Genet. 2002, 114: 299-303. 10.1002/ajmg.1701.
Johanson E: A study of schizophrenia in the male. Acta Psychiatrica Neurological Scandinavica. 1958, 33 (Suppl 125): 7-107.
Hare EH, Moran PA: Raised parental age in psychiatric patients: evidence for the constitutional hypothesis. Br J Psychiatry. 1979, 134: 169-177. 10.1192/bjp.134.2.169.
Brown AS, Schaefer CA, Wyatt RJ, Begg MD, Goetz R, Bresnahan MA, Harkavy-Friedman J, Gorman JM, Malaspina D, Susser ES: Paternal age and risk of schizophrenia in adult offspring. Am J Psychiatry. 2002, 159: 1528-1533. 10.1176/appi.ajp.159.9.1528.
Dalman C, Allebeck P: Paternal age and schizophrenia: further support for an association. Am J Psychiatry. 2002, 159: 1591-1592. 10.1176/appi.ajp.159.9.1591.
Zammit S, Allebeck P, Dalman C, Lundberg I, Hemmingson T, Owen MJ, Lewis G: Paternal age and risk for schizophrenia. Br J Psychiatry. 2003, 183: 405-408. 10.1192/bjp.183.5.405.
Byrne M, Agerbo E, Ewald H, Eaton WW, Mortensen PB: Parental age and risk of schizophrenia: a case-control study. Arch Gen Psychiatry. 2003, 60: 673-678. 10.1001/archpsyc.60.7.673.
Sipos A, Rasmussen F, Harrison G, Tynelius P, Lewis G, Leon DA, Gunnell D: Paternal age and schizophrenia: a population based cohort study. BMJ. 2004, 329: 1070-10.1136/bmj.38243.672396.55.
El-Saadi O, Pedersen CB, McNeil TF, Saha S, Welham J, O'Callaghan E, Cantor-Graae E, Chant D, Mortensen PB, McGrath J: Paternal and maternal age as risk factors for psychosis: findings from Denmark, Sweden and Australia. Schizophr Res. 2004, 67: 227-236. 10.1016/S0920-9964(03)00100-2.
Tsuchiya KJ, Takagai S, Kawai M, Matsumoto H, Nakamura K, Minabe Y, Mori N, Takei N: Advanced paternal age associated with an elevated risk for schizophrenia in offspring in a Japanese population. Schizophr Res. 2005, 76: 337-342. 10.1016/j.schres.2005.03.004.
Wohl M, Gorwood P: Paternal ages below or above 35 years old are associated with a different risk of schizophrenia in the offspring. Eur Psychiatry. 2007, 22: 22-26. 10.1016/j.eurpsy.2006.08.007.
Malama IM, Papaioannou DJ, Kaklamani EP, Katsouyanni KM, Koumantaki IG, Trichopoulos DV: Birth order sibship size and socio-economic factors in risk of schizophrenia in Greece. Br J Psychiatry. 1988, 152: 482-486. 10.1192/bjp.152.4.482.
Zhang Y, Kreger BE, Dorgan JF, Cupples LA, Myers RH, Splansky GL, Schatzkin A, Ellison RC: Parental age at child's birth and son's risk of prostate cancer. The Framingham Study. Am J Epidemiol. 1999, 150: 1208-1212.
Hemminki K, Kyyrönen P: Parental age and risk of sporadic and familial cancer in offspring: implications for germ cell mutagenesis. Epidemiology. 1999, 10: 747-751. 10.1097/00001648-199911000-00016.
Malaspina D: Paternal factors and schizophrenia risk: de novo mutations and imprinting. Schizophr Bull. 2001, 27: 379-393.
Pulver AE, McGrath JA, Liang KY, Lasseter VK, Nestadt G, Wolyniec PS: An indirect test of the new mutation hypothesis associating advanced paternal age with the etiology of schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2004, 124B: 6-9. 10.1002/ajmg.b.20066.
Bathaee H, Djalili A, Azardagan F: Birth order and family size in schizophrenia. Acta Med Iran. 1977, 19 (2): 155-66.
Gaughran F, Blizard R, Mohan R, Zammit S, Owen M: Birth order and the severity of illness in schizophrenia. Psychiatry Res. 2007, 150: 205-210. 10.1016/j.psychres.2006.05.012.
Smits L, Pedersen C, Mortensen P, van Os J: Association between short birth intervals and schizophrenia in the offspring. Schizophr Res. 2004, 70: 49-56. 10.1016/j.schres.2003.10.002.
Christensen K, Gaist D, Jeune B, Vaupel JW: A tooth per child?. Lancet. 1998, 352: 204-10.1016/S0140-6736(05)77810-7.
Perrin MC, Brown AS, Malaspina D: Aberrant epigenetic regulation could explain the relationship of paternal age to schizophrenia. Schizophr Bull. 2007, 33: 1270-1273.
Malaspina D, Perrin M, Kleinhaus KR, Opler M, Harlap S: Growth and schizophrenia: aetiology, epidemiology and epigenetics. Novartis Found Symp. 2008, 289: 196-203.
Abdolmaleky HM, Smith CL, Faraone SV, Shafa R, Stone W, Glatt SJ, Tsuang MT: Methylomics in psychiatry: Modulation of gene-environment interactions may be through DNA methylation. Am J Med Genet B Neuropsychiatr Genet. 2004, 15: 51-59.
Gottesman II, Bertelsen A: Confirming unexpressed genotypes for schizophrenia. Risks in the offspring of Fischer's Danish identical and fraternal discordant twins. Arch Gen Psychiatry. 1989, 46: 867-872.
Kringlen E, Cramer G: Offspring of monozygotic twins discordant for schizophrenia. Arch Gen Psychiatry. 1989, 46: 873-877.
Nguyen GH, Bouchard J, Boselli MG, Tolstoi LG, Keith L, Baldwin C, Nguyen NC, Schultz M, Herrera VL, Smith CL: DNA stability and schizophrenia in twins. Am J Med Genet B Neuropsychiatr Genet. 2003, 120B: 1-10. 10.1002/ajmg.b.20010.
Kunugi H, Lee KB, Nanko S: Cytogenetic findings in 250 schizophrenics: evidence confirming an excess of the X chromosome aneuploidies and pericentric inversion of chromosome 9. Schizophr Res. 1999, 40: 43-47. 10.1016/S0920-9964(99)00035-3.
Yurov YB, Vostrikov VM, Vorsanova SG, Monakhov VV, Iourov IY: Multicolor fluorescent in situ hybridization on post-mortem brain in schizophrenia as an approach for identification of low-level chromosomal aneuploidy in neuropsychiatric diseases. Brain Dev. 2001, 23 (Suppl 1): S186-190.
Tamura Y, Kunugi H, Ohashi J, Hohjoh H: Epigenetic aberration of the human REELIN gene in psychiatric disorders. Mol Psychiatry. 2007, 12: 519-10.1038/sj.mp.4002014. 593-600
Abdolmaleky HM, Smith CL, Zhou JR, Thiagalingam S: Epigenetic modulation of reelin function in schizophrenia and bipolar disorders. Reelin Glycoprotein, Biology, Structure and Roles in Health and Disease. 2008, London, UK: Springer
Petronis A, Gottesman II, Kan P, Kennedy JL, Basile VS, Paterson AD, Popendikyte V: Monozygotic twins exhibit numerous epigenetic differences: clues to twin discordance?. Schizophr Bull. 2003, 29: 169-178.
Petronis A: The origin of schizophrenia: genetic thesis, epigenetic antithesis, and resolving synthesis. Biol Psychiatry. 2004, 15: 965-970.
Nath SD, Koutoubi S, Huffman FG: Folate and vitamin B12 status of a multiethnic adult population. J Natl Med Assoc. 2006, 98: 67-72.
Affenito SG, Thompson DR, Franko DL, Striegel-Moore RH, Daniels SR, Barton BA, Schreiber GB, Schmidt M, Crawford PB: Longitudinal assessment of micronutrient intake among African-American and white girls: The National Heart, Lung, and Blood Institute Growth and Health Study. J Am Diet Assoc. 2007, 107: 1113-1123. 10.1016/j.jada.2007.04.014.
Waly M, Olteanu H, Banerjee R, Choi SW, Mason JB, Parker BS, Sukumar S, Shim S, Sharma A, Benzecry JM, Power-Charnitsky VA, Deth RC: Activation of methionine synthase by insulin-like growth factor-1 and dopamine: a target for neurodevelopmental toxins and thimerosal. Mol Psychiatry. 2004, 4: 358-70.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
H-RA designed the study, collected clinical sample data and drafted the manuscript. BM participated in sample collection. MN performed data analysis and CLS contributed to the editing and writing of the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Naserbakht, M., Ahmadkhaniha, HR., Mokri, B. et al. Advanced paternal age is a risk factor for schizophrenia in Iranians. Ann Gen Psychiatry 10, 15 (2011). https://doi.org/10.1186/1744-859X-10-15
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1744-859X-10-15