Abstract
Background
Fibromyalgia (FM) is characterized by chronic widespread pain, which is often refractory to conventional painkillers. Numerous clinical studies have demonstrated that antidepressants are effective in treating FM pain. We previously established a mouse model of FM-like pain, induced by intermittent cold stress (ICS).
Results
In this study, we find that ICS exposure causes a transient increase in plasma corticosterone concentration, but not in anxiety or depression-like behaviors. A single intrathecal injection of an antidepressant, such as milnacipran, amitriptyline, mianserin or paroxetine, had an acute analgesic effect on ICS-induced thermal hyperalgesia at post-stress day 1 in a dose-dependent manner. In addition, repeated daily antidepressant treatments during post-stress days 1-5 gradually reversed the reduction in thermal pain threshold, and this recovery was maintained for at least 7 days after the final treatment. In addition, relief from mechanical allodynia, induced by ICS exposure, was also observed at day 9 after the cessation of antidepressant treatment. In contrast, the intravenous administration of these antidepressants at conventional doses failed to provide relief.
Conclusions
These results suggest that the repetitive intrathecal administration of antidepressants permanently cures ICS-induced FM pain in mice.
Similar content being viewed by others
2. Background
Fibromyalgia (FM) is characterized by generalized tenderness and chronic widespread pain that affects 2-4% of the population in industrialized nations and primarily affects females [1]. Although its etiology and pathogenesis are largely unknown, emerging evidence indicates that pain amplification within the central nervous system (CNS) plays a critical role in the pathology of FM pain [2]. Recent studies, including functional imaging, have revealed that this central amplification process depends, in part, on deficits in endogenous descending pain inhibitory pathways [3, 4] and abnormal pain processing [5]. In addition, FM pain is often refractory to treatment using conventional painkillers, such as non-steroidal anti-inflammatory drugs and opioids [6]. However, numerous studies have demonstrated the effectiveness of antidepressants and antiepileptics, such as gabapentin and pregabalin, in the treatment of FM pain [7, 8].
There are several animal models of FM pain, induced by either intramuscular injection of acidic saline [9], vagotomy [10], sound stress [11] or depletion of biogenic amines [12]. However, in order to better understand the molecular basis of the underlying pain mechanisms, it is necessary to establish an animal model which accurately reflects the pathological and pharmacotherapeutic features of the disease.
Recently, we established a mouse model of FM using intermittent cold stress (ICS), which produces long-lasting thermal hyperalgesia and mechanical allodynia, predominantly in females [13]. We found that gabapentin, particularly when injected intracerebroventricularly, had potent anti-hyperalgesic and anti-allodynic effects in this model [13]. In addition, systemically and intracerebroventricularly-administered morphine was found to have no analgesic effect in ICS-exposed mice, due to a failure to activate descending pain inhibitory pathways [14]. These findings indicate that our ICS model might accurately reflect the pathological and pharmacotherapeutic features of FM pain. In this study, we examine whether various antidepressants can ameliorate the abnormal pain sensations in this model.
3. Materials and methods
3.1. Animals
Male C57BL/6J mice weighing 18-22 g were used. They were kept in a room with an ambient temperature of 21 ± 2°C, with free access to a standard laboratory diet and tap water. All procedures were approved by the Nagasaki University Animal Care Committee and complied with the recommendations of the International Association for the Study of Pain [15].
3.2. Drug treatments
Antidepressants were obtained from Sigma (St. Louis, MO, USA). Milnacipran, paroxetine, and amitriptyline were dissolved in artificial cerebrospinal fluid (aCSF; 125 mM NaCl, 3.8 mM KCl, 2.0 mM CaCl2, 1.0 mM MgCl2, 1.2 mM KH2PO4, 26 mM NaHCO3, 10 mM glucose, pH 7.4). Mianserin was dissolved in physiological saline. For vehicle treatments, aCSF or saline was injected. Intrathecal (i.t.) injections were administered according to Hylden and Wilcox [16] using a 30-gauge needle. The site of injection was chosen to be between spinal L5 and L6--near where the spinal cord ends and the cauda equina begins. This allowed us to maximize inter-vertebral accessibility and to minimize the possibility of spinal damage. After sufficient training, the experimenters were able to perform the technique without causing injury to the animals.
3.3. Experimental model of fibromyalgia
ICS exposure and constant cold stress (CCS) were performed as previously reported [13]. Briefly, for the ICS model, mice were placed on stainless mesh plate in a cold room at 4°C overnight (from 4:30 pm to 10:00 am), followed by ICS with environmental temperatures alternating between 24 and 4°C every 30 min, from 10:00 am to 4:30 pm. These procedures were repeated twice. On day 3, the mice were adapted to 24°C for 1 h before behavior testing. We designated day 3 following the onset of stress exposure as day 1 post-stress exposure (P1). For the CCS model, mice were placed in the cold room from 4:30 pm on day 1 to 10:00 am on day 3, followed by adaptation at 24°C for 1 h. Mice in the control group were kept at 24°C for all 3 days (from 4:30 pm on day 1 to 10:00 am on day 3). During the stress period, two mice were kept in each cage (12 × 15 × 10.5 cm), with free access to food and agar as alternate drink water in place of fluid. Although the body weight of mice was decreased during and after the ICS stress, it attained to the control mice level as early as 4 day after the stress (Figure 1).
3.4. Measurement of plasma corticosterone
Plasma corticosterone levels were measured as described previously [17]. Briefly, plasma was separated by centrifugation at 3 000 g for 15 min at 4°C and collected into ice-chilled tubes containing 0.1% EDTA and stored at -80°C until use. Blood samples were collected at 9:00 pm in order to exclude the effect of circadian rhythms on circulating plasma corticosterone. The plasma corticosterone level was estimated fluorometrically, according to the method of Zenker and Bernstein [18].
3.5. Assessment of stress-related behaviors
Spontaneous locomotor activity was measured in the open filed (22 × 33 cm) for 3 min, using SCANET apparatus (Melquest, Japan). In the elevated plus-maze test used to estimate anxiety, the time spent in the open arm was recorded during a 6-min period. To assess depression-like behaviors, the tail-suspension test was performed [19, 20]. Mice were suspended 30 cm above the floor using adhesive tape, and the total duration of immobility during a 6-min period was measured.
3.6. Nociception tests
In the thermal paw withdrawal test, the nociception threshold was assessed using the latency of paw withdrawal upon a thermal stimulus [21, 22]. Unanesthetized animals were placed in plexiglass cage on top of a glass sheet and acclimated for 1 h. A thermal stimulator (IITC Inc., Woodland Hills, CA, USA) was positioned under the glass sheet and the focus of the projection bulb was aimed exactly at the middle of the plantar surface of the animal. A mirror attached to the stimulator permitted visualization of the plantar surface. A cut-off time of 20 s was set to prevent tissue damage.
The mechanical paw pressure test was performed as described previously [22]. Briefly, mice were placed in a plexiglass chamber on a 6 × 6 mm wire mesh grid floor and allowed to acclimate for a period of 1 h. A mechanical stimulus was then delivered to the middle of the plantar surface of the right-hind paw using a Transducer Indicator (Model 1601; IITC Inc., Woodland Hills, CA, USA). The pressure needed to induce a flexor response was defined as the pain threshold. A cut-off pressure of 20 g was set to avoid tissue damage. In these experiments using thermal and mechanical tests, the thresholds were determined from three repeated challenges at 10 min intervals, and the averages were used for statistical analysis. For the time-course experiments, we measured the paw-withdrawal latencies (PWL) at 30, 60, and 180 min after intrathecal injection of antidepressant. In the area under the curve (AUC) analysis of antidepressant-induced analgesia, we calculated the AUC generated by plotting analgesic threshold (after deducting the control threshold from each threshold point) against time, from 30 to 180 min after antidepressant treatment, using a trapezoidal method.
3.7. Statistical analysis
In Figure 2 and Table 1, data were analyzed using Student's t-test. In Figures 1, 3, 4, 5 and 6, Tables 2 and 3, the differences between multiple groups were analyzed using a one-way ANOVA with the Tukey-Kramer multiple comparison post-hoc analysis. Significance was set at p < 0.05. All results are expressed as means ± S.E.M.
Effects of stress exposure on plasma corticosterone levels, spontaneous locomotor activity, and anxiety and depression-like behaviors. (A) Time-course of plasma corticosterone levels after ICS or CCS exposure. (B-D) Lack of changes in spontaneous locomotor activity in the SCANET apparatus (B), duration of time spent in the open arm of the plus-maze test (C), and total duration of immobility in the tail suspension test (D) at P1. *p < 0.05, vs. control group. Data are expressed as the means ± S.E.M; 3-5 mice per group.
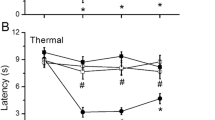
Antidepressant-induced acute analgesic effects in ICS treated mice. Thermal pain threshold was assessed at P1 after control or ICS treatment, using the thermal paw withdrawal test. Results represent the time course of thermal paw withdrawal latencies (PWL, in seconds) after a single intrathecal injection of antidepressants. (A-D) Each data point in [control + vehicle] and [ICS + vehicle] groups is common. *p < 0.05, vs. vehicle-treated control group; #p < 0.05, vs. vehicle-treated and ICS-exposed groups. Data are expressed as the means ± S.E.M.; 4-8 mice per group.
Permanent relief from ICS-induced thermal hyperalgesia by repeated intrathecal administration of milnacipran. Intrathecal injections of milnacipran (0.1 μg) were given once daily at 11:30 a.m. from P1-P5 after assessment of nociceptive thresholds at 11:00 a.m. Results represent the basal threshold as the latency to paw-withdrawal from thermal stimuli (PWL, in seconds), just before the daily injection of vehicle or milnacipran. *p < 0.05, vs. vehicle-treated control group; #p < 0.05, vs. vehicle-treated and ICS-exposed groups. Data are expressed as the means ± S.E.M.;4-8 mice per group.
Complete relief from ICS-induced mechanical allodynia. Basal mechanical paw-withdrawal threshold (PWT, in grams) was assessed at P14, using paw pressure tests. Intrathecal injection of milnacipran (0.1 μg), amitriptyline (15 μg), mianserin (20 μg), or paroxetine (5 μg), was given once daily from P1-P5, as described in Figure 4. *p < 0.05, vs. vehicle-treated control group; #p < 0.05, vs. vehicle-treated and ICS-exposed groups. Data are expressed as the means ± S.E.M.; 3-6 mice per group.
Lack of anti-hyperalgesic effects by systemic administration of antidepressants. Thermal pain threshold was assessed at P1 after control or ICS-treatment, using thermal paw withdrawal tests. (A-D) Results represent the time-course of thermal paw-withdrawal latencies (PWL, in seconds) after a single i.v. injection of antidepressants. (A-D) Each data point in [control + vehicle] and [ICS + vehicle] groups is common. (E) Milnacipran was given i.v. once daily for 5 days, as described in Figure 4. Results represent the basal threshold as the latency to paw-withdrawal from thermal stimuli (PWL, in seconds), just before the daily injection of vehicle or milnacipran. *p < 0.05, vs. vehicle-treated control group; #p < 0.05, vs. vehicle-treated and ICS-exposed groups. Data are expressed as the means ± S.E.M.; 3-6 mice per group.
4. Results
4.1. Effects of ICS stress exposure on plasma corticosterone levels and anxiety and depression-like behaviors
We previously designed an improved mouse model for dysautonomia, also referred to as the specific alternation of rhythm in temperature (SART) model [23], and found that ICS, but not CCS, caused long-lasting abnormal pain sensations [13]. In the present study, we used plasma corticosterone levels as a biomarker for stress. As shown in Figure 2A, we found that ICS exposure caused a transient increase in plasma corticosterone levels at P1. In contrast, CCS exposure had no effect on plasma corticosterone levels between P1 and P12 (Figure 2A). ICS had no effect on spontaneous locomotor activity at P1 (Figure 2B). Furthermore there was no significant change in the duration of time spent in the open arm in the elevated plus-maze test or in the total duration of immobility in the tail-suspension test at P1 (Figures 2C, D). In addition, there were no gross behavioral changes in mice as early as 1 h after the transfer from 4°C to 24°C room.
4.2. Antidepressant-induced acute analgesic effects on thermal hyperalgesia in ICS-exposed mice
Previous reports demonstrated that thermal hyperalgesia is elicited at P1 after ICS exposure and lasts for at least 12 days [13, 14]. As shown in Figure 3, the nociceptive thermal threshold was significantly reduced and stable throughout experiments for 180 min. A single intrathecal injection of milnacipran (0.1 μg) had no effect on the nociceptive threshold in control mice (Figure 3A), but produced significant anti-hyperalgesic effects that persisted for at least 180 min post-injection at P1 (Figure 3A). This effect of milnacipran was dose-dependent in the range of 0.03-0.1 μg, but declined at 0.3 μg (Table 1). Statistical significance was observed at 0.1 and 0.3 μg. Similar results were observed with other antidepressants, such as amitriptyline (5-30 μg), mianserin (10 and 20 μg), and paroxetine (2-10 μg), as shown in Figures 3B-D and Table 1. However, with 20 μg of mianserin, a significant analgesic effect was observed at 60 min in the control mice, and anti-hyperalgesic effects were observed until 180 min (Figure 3C). Both amitriptyline and paroxetine showed significant anti-hyperalgesic effects, but no significance was observed at 180 min (Figures 3B, D).
4.3. Permanent relief of abnormal pain by repeated central administration
As the anti-hyperalgesic effect of milnacipran remained 180 min after intrathecal administration at day P1 after ICS stress (threshold: ~ 7.46 ± 0.2 s), we measured the nociceptive threshold at 11:00 a.m. on day P2. As seen in Figure 4, a significant anti-hyperalgesic effect still remained (threshold: ~ 7.67 ± 0.6 s). The second administration of milnacipran was performed at 11:30 a.m. The basal nociceptive threshold at 11:00 a.m. on day P3 further increased to 8.56 ± 0.8 s. The increase in basal threshold was maintained by daily administration of milnacipran. Complete recovery to the normal pain threshold was observed on P6, the day following the last administration, and lasted until P12. Similar complete reversals of hyperalgesia on P5 and P12 were observed after 5-day administrations of amitriptyline (15 μg), mianserin (20 μg), and paroxetine (5 μg), as seen in Table 2. Complete recovery was also observed with ICS-induced mechanical allodynia, even on P14, following a 5-day administration of the antidepressants (Figure 5).
4.4. Lack of beneficial effects by repeated systemic administration
When milnacipran was given by intravenous (i.v.) injection (10 mg/kg), there was a significant analgesic effect in the thermal nociception test at 30 min in control mice. However, there was no significant suppression in the ICS mouse model using this dose of antidepressant up to 180 min on P1 (Figure 6A). The absence of an ameliorative effect on ICS-induced hyperalgesia was also observed with amitriptyline (3 mg/kg, i.v.), mianserin (10 mg/kg, i.v.), and paroxetine (1 mg/kg, i.v.), despite producing significant acute analgesia at 30 min in control mice (Figures 6B-D). In addition, the repeated systemic administration of milnacipran for 5 days did not affect the basal threshold throughout the experiment (Figure 6E). Repeated administrations of amitriptyline, mianserin or paroxetine also did not provide relief from ICS-induced hyperalgesia (Table 3).
5. Discussion
Patients with FM exhibit widespread pain, with diverse symptoms, such as fatigue, depression, and sleep disturbance. Although the pathogenesis of FM is not clearly understood, certain biological stressors, such as autonomic nervous system disorder and psychological distress seem to be closely related to the development of FM [24]. An important role for such stressors is supported by studies using animal models in which rats or mice are subjected to stressors, such as chemical, sound, or surgery stress, which induce long-lasting abnormal pain [9–11, 25]. Recently, we reported that ICS produces long-lasting thermal hyperalgesia and mechanical allodynia in mice [13, 14]. The ICS-induced pain is bilateral and female-predominant (after gonadectomy) [13], which are also features found in FM patients [26].
In this study, mice subjected to ICS exhibited a transient increase in plasma corticosterone levels on P1. In contrast, there was no significant change in corticosterone levels in mice subjected to CCS. Considering that the abnormal pain in CCS mice was only transient, and not long-lasting [13, 14], the rise in corticosterone levels in ICS mice likely played a role in the appearance of abnormal pain. A recent report suggests that the stress-induced increase in corticosterone concentration may be related to abnormal pain behavior in an FM-like animal model, possibly through a mechanism involving epinephrine release [27].
In our ICS model, the mice did not show significant changes in the tail-suspension test, a behavioral test designed to assess depression-like behavior [28]. This is in contrast to a study using less frequent temperature alternation (the SART model), in which mice exhibited hyperalgesia for only a week [29], and there was a transient reduction of immobility duration in forced swimming test, followed by gradual recovery in 5-6 days [30]. As the forced swimming causes a facilitation of immobility in an antidepressant-reversible manner [31], it is not clear whether the transient reduction of immobility duration reflects depression. From this point of view, the tail suspension test seems to be a better method for evaluation of depression-related despair behavior. Gabapentin and pregabalin are widely used to treat FM patients in the clinic [32, 33]. These medicines alleviate abnormal pain and the accompanying fatigue and insomnia, without affecting depressive symptoms [33, 34]. Therefore, the presence of depression-like behavior is unlikely to be necessary in animal models of FM. Consequently, the ICS model may be more clinically relevant than the SART model for evaluating long-term pain.
Various antidepressants have been used for FM in the clinic [35, 36]. Recently milnacipran and duloxetine, serotonin/norepinephrine reuptake inhibitors, and serotonin-specific reuptake inhibitors have been approved for treating FM pain by the United States Food and Drug Administration. As the antinociceptive activities of these compounds are largely independent of their effects on mood, making them potentially efficacious for patients with or without depressive [37], it appears to reflect the importance of central descending monoaminergic pathways in pain regulation [38, 39]. Recent studies revealed that polymorphisms in the 5-HT receptor, transporter, and metabolic enzyme can contribute to the etiology of FM [40–42]. The fMRI study also demonstrates that brain regions involved in descending pain inhibitory pathways appear to have decreased activity in FM patients [43]. Although serotonergic and/or noradrenergic pathways are well documented as descending pain inhibitory pathways [39], there is no report that the abnormality of such descending monoaminergic systems is observed in FM patients. However, it would be challenging to examine the effects of representative antidepressants on ICS-induced abnormal pain by introducing the drugs into the intrathecal space, very close to target regions.
Our study shows that the repeated intrathecal administration of different antidepressants gradually suppressed ICS-induced pain. The gradual reversal of abnormal pain may be related to the down-regulation of β-adrenoceptors or abnormal monoaminergic metabolism [44–46]. Alternative mechanisms may include the altered expression of multiple receptors and ion channels, such as the NMDA receptor, opioid receptors, and sodium channels [47–49]. It should be noted that the reversal of abnormal pain continued after the cessation of drug treatment, for each of the antidepressants tested. Although further investigation is required to clarify the molecular mechanisms of antidepressant action and to provide a permanent cure for ICS-induced abnormal pain, it is interesting to speculate that the chronic pain may be due to a vicious cycle of pain elicited by reduced inhibitory input from monoaminergic pathways. Thus, the rescue of pain-inhibitory mechanisms by repeated antidepressant treatment should halt chronic pain. Similar observations were made in our previous study using central administration of gabapentin [13, 14]. In that study, using the ICS model, a single intracerebroventricular administration of gabapentin produced a 4-day period of anti-hyperalgesia. As the injection had no effect on peripheral nerve injury-induced neuropathic pain [13, 14], and the gabapentin was unlikely to have remained in the brain for 4 days, it is interesting to speculate that the observed effect is due to the inhibition of the pain cycle, possibly through enhancement of inhibitory transmission. However, the present study demonstrates that systemic administration of various antidepressants had no significant beneficial effect on ICS-induced hyperalgesia, though they had a significant acute analgesic effect in control mice. As the clinically beneficial effects of oral antidepressants to FM patients were evident when they are treated for more than several weeks [50], the lack of effects of intravenous antidepressants in the present study may be attributed to the shortage of treatments (5 days). In this meaning it is surprising that only 5 days repetitive intrathecal treatments abolishes abnormal pain even after the cession of treatments. Furthermore, although the mechanisms underlying the lack of antihyperalgesic effect remain elusive, it may be worthwhile to investigate possible involvements of interference of spinal effects by peripheral pain facilitating serotonergic actions or by descending pain facilitating monoaminergic systems [39]. Thus, we expect that repetitive intrathecal administration of antidepressants are likely to be more effective at treating FM-like pain in mouse models.
Finally, this study demonstrates that the ICS model has similarities to clinical features of FM in terms of the sensitivity to analgesics or adjuvant analgesics. In our previous findings, we observed that the effective dose of gabapentin was 3 mg/kg for ICS-induced pain, but was over 30 mg/kg for nerve injury-induced neuropathic pain in mice [13, 14], consistent with the fact that the clinically-effective dose of gabapentin for FM patients is lower than that for neuropathic pain [51]. In addition, we observed that ICS-induced thermal hyperalgesia was resistant to morphine treatment [13, 14], consistent with the clinical evidence [52]. Considering that other experimental animal models of FM-like pain exhibit morphine analgesia (albeit with low potency) [53–56], the ICS model may be pharmacologically distinct from the others.
6. Conclusion
This study demonstrates that repeated intrathecal antidepressant treatment provides a complete cure of ICS-induced FM-like abnormal pain. Based on the pharmacological similarity of ICS-induced pain to clinical FM, the ICS model appears to be suitable for investigating the pathogenesis of FM and for evaluating therapeutic strategies for this debilitating illness.
Abbreviations
- aCSF:
-
artificial cerebrospinal fluid
- AUC:
-
area under the curve: CCS: constant cold stress
- FM:
-
fibromyalgia
- ICS:
-
intermittent cold stress
- PWL:
-
paw withdrawal latency
- PWT:
-
paw withdrawal threshold.
References
Wolfe F, Ross K, Anderson J, Russell IJ, Hebert L: The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum 1995,38(1):19–28.
Schmidt-Wilcke T, Clauw DJ: Pharmacotherapy in fibromyalgia (FM)--implications for the underlying pathophysiology. Pharmacol Ther 127(3):283–294.
Jensen KB, Kosek E, Petzke F, Carville S, Fransson P, Marcus H, Williams SC, Choy E, Giesecke T, Mainguy Y, et al.: Evidence of dysfunctional pain inhibition in Fibromyalgia reflected in rACC during provoked pain. Pain 2009,144(1–2):95–100.
Julien N, Goffaux P, Arsenault P, Marchand S: Widespread pain in fibromyalgia is related to a deficit of endogenous pain inhibition. Pain 2005,114(1–2):295–302.
Clauw DJ: Fibromyalgia: an overview. Am J Med 2009,122(12 Suppl):S3-S13.
Arnold LM, Bradley LA, Clauw DJ, Glass JM, Goldenberg DL: Multidisciplinary care and stepwise treatment for fibromyalgia. J Clin Psychiatry 2008,69(12):e35.
Arnold LM, Goldenberg DL, Stanford SB, Lalonde JK, Sandhu HS, Keck PE Jr, Welge JA, Bishop F, Stanford KE, Hess EV, et al.: Gabapentin in the treatment of fibromyalgia: a randomized, double-blind, placebo-controlled, multicenter trial. Arthritis Rheum 2007,56(4):1336–1344.
Arnold LM, Russell IJ, Diri EW, Duan WR, Young JP Jr, Sharma U, Martin SA, Barrett JA, Haig G: A 14-week, randomized, double-blinded, placebo-controlled monotherapy trial of pregabalin in patients with fibromyalgia. J Pain 2008,9(9):792–805.
Sluka KA, Kalra A, Moore SA: Unilateral intramuscular injections of acidic saline produce a bilateral, long-lasting hyperalgesia. Muscle Nerve 2001,24(1):37–46.
Khasar SG, Miao JP, Janig W, Levine JD: Modulation of bradykinin-induced mechanical hyperalgesia in the rat by activity in abdominal vagal afferents. Eur J Neurosci 1998,10(2):435–444.
Khasar SG, Dina OA, Green PG, Levine JD: Sound Stress-Induced Long-Term Enhancement of Mechanical Hyperalgesia in Rats Is Maintained by Sympathoadrenal Catecholamines. J Pain 2009,10(10):1073–1077.
Nagakura Y, Oe T, Aoki T, Matsuoka N: Biogenic amine depletion causes chronic muscular pain and tactile allodynia accompanied by depression: A putative animal model of fibromyalgia. Pain 2009,146(1–2):26–33.
Nishiyori M, Ueda H: Prolonged gabapentin analgesia in an experimental mouse model of fibromyalgia. Mol Pain 2008, 4: 52.
Nishiyori M, Nagai J, Nakazawa T, Ueda H: Absence of morphine analgesia and its underlying descending serotonergic activation in an experimental mouse model of fibromyalgia. Neurosci Lett 2010,472(3):184–187.
Zimmermann M: Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983,16(2):109–110.
Hylden JL, Wilcox GL: Intrathecal morphine in mice: a new technique. Eur J Pharmacol 1980,67(2–3):313–316.
Inoue M, Mishina M, Ueda H: Locus-specific rescue of GluRepsilon1 NMDA receptors in mutant mice identifies the brain regions important for morphine tolerance and dependence. J Neurosci 2003,23(16):6529–6536.
Zenker N, Bernstein DE: The estimation of small amounts of corticosterone in rat plasma. J Biol Chem 1958,231(2):695–701.
Pellow S: Anxiolytic and anxiogenic drug effects in a novel test of anxiety: are exploratory models of anxiety in rodents valid? Methods Find Exp Clin Pharmacol 1986,8(9):557–565.
Steru L, Chermat R, Thierry B, Simon P: The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl) 1985,85(3):367–370.
Hargreaves K, Dubner R, Brown F, Flores C, Joris J: A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988,32(1):77–88.
Inoue M, Rashid MH, Fujita R, Contos JJ, Chun J, Ueda H: Initiation of neuropathic pain requires lysophosphatidic acid receptor signaling. Nat Med 2004,10(7):712–718.
Kita T, Hata T, Iida J, Yoneda R, Isida S: Decrease in pain threshold in SART stressed mice. Jpn J Pharmacol 1979,29(3):479–482.
Smith HS, Harris R, Clauw D: Fibromyalgia: an afferent processing disorder leading to a complex pain generalized syndrome. Pain Physician 14(2):E217–245.
Khasar SG, Green PG, Levine JD: Repeated sound stress enhances inflammatory pain in the rat. Pain 2005,116(1–2):79–86.
Yunus MB: The role of gender in fibromyalgia syndrome. Curr Rheumatol Rep 2001,3(2):128–134.
Khasar SG, Burkham J, Dina OA, Brown AS, Bogen O, Alessandri-Haber N, Green PG, Reichling DB, Levine JD: Stress induces a switch of intracellular signaling in sensory neurons in a model of generalized pain. J Neurosci 2008,28(22):5721–5730.
Imbe H, Iwai-Liao Y, Senba E: Stress-induced hyperalgesia: animal models and putative mechanisms. Front Biosci 2006, 11: 2179–2192.
Ohara H, Kawamura M, Namimatsu A, Miura T, Yoneda R, Hata T: Mechanism of hyperalgesia in SART stressed (repeated cold stress) mice: antinociceptive effect of neurotropin. Jpn J Pharmacol 1991,57(2):243–250.
Porsolt RD, Bertin A, Jalfre M: Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther 1977,229(2):327–336.
Hata T, Nishikawa H, Itoh E, Watanabe A: Depressive state with anxiety in repeated cold-stressed mice in forced swimming tests. Jpn J Pharmacol 1999,79(2):243–249.
Tzellos TG, Toulis KA, Goulis DG, Papazisis G, Zampeli VA, Vakfari A, Kouvelas D: Gabapentin and pregabalin in the treatment of fibromyalgia: a systematic review and a meta-analysis. J Clin Pharm Ther 35(6):639–656.
Hauser W, Bernardy K, Uceyler N, Sommer C: Treatment of fibromyalgia syndrome with gabapentin and pregabalin - A meta-analysis of randomized controlled trials. Pain 2009,145(1–2):69–81.
Crofford LJ, Rowbotham MC, Mease PJ, Russell IJ, Dworkin RH, Corbin AE, Young JP Jr, LaMoreaux LK, Martin SA, Sharma U: Pregabalin for the treatment of fibromyalgia syndrome: results of a randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2005,52(4):1264–1273.
Rao SG, Bennett RM: Pharmacological therapies in fibromyalgia. Best Pract Res Clin Rheumatol 2003,17(4):611–627.
Gendreau RM, Thorn MD, Gendreau JF, Kranzler JD, Ribeiro S, Gracely RH, Williams DA, Mease PJ, McLean SA, Clauw DJ: Efficacy of milnacipran in patients with fibromyalgia. J Rheumatol 2005,32(10):1975–1985.
Arnold LM, Hudson JI, Wang F, Wohlreich MM, Prakash A, Kajdasz DK, Chappell AS: Comparisons of the efficacy and safety of duloxetine for the treatment of fibromyalgia in patients with versus without major depressive disorder. Clin J Pain 2009,25(6):461–468.
Fishbain DA, Cutler R, Rosomoff HL, Rosomoff RS: Evidence-based data from animal and human experimental studies on pain relief with antidepressants: a structured review. Pain Med 2000,1(4):310–316.
Millan MJ: Descending control of pain. Prog Neurobiol 2002,66(6):355–474.
Offenbaecher M, Bondy B, de Jonge S, Glatzeder K, Kruger M, Schoeps P, Ackenheil M: Possible association of fibromyalgia with a polymorphism in the serotonin transporter gene regulatory region. Arthritis Rheum 1999,42(11):2482–2488.
Gursoy S: Absence of association of the serotonin transporter gene polymorphism with the mentally healthy subset of fibromyalgia patients. Clin Rheumatol 2002,21(3):194–197.
Tander B, Gunes S, Boke O, Alayli G, Kara N, Bagci H, Canturk F: Polymorphisms of the serotonin-2A receptor and catechol-O-methyltransferase genes: a study on fibromyalgia susceptibility. Rheumatol Int 2008,28(7):685–691.
Mainguy Y: Functional magnetic resonance imagery (fMRI) in fibromyalgia and the response to milnacipran. Hum Psychopharmacol 2009,24(Suppl 1):S19–23.
Banerjee SP, Kung LS, Riggi SJ, Chanda SK: Development of beta-adrenergic receptor subsensitivity by antidepressants. Nature 1977,268(5619):455–456.
Wolfe BB, Harden TK, Sporn JR, Molinoff PB: Presynaptic modulation of beta adrenergic receptors in rat cerebral cortex after treatment with antidepressants. J Pharmacol Exp Ther 1978,207(2):446–457.
Antkiewicz-Michaluk L, Romanska I, Michaluk J, Vetulani J: Role of calcium channels in effects of antidepressant drugs on responsiveness to pain. Psychopharmacology (Berl) 1991,105(2):269–274.
Yaron I, Shirazi I, Judovich R, Levartovsky D, Caspi D, Yaron M: Fluoxetine and amitriptyline inhibit nitric oxide, prostaglandin E2, and hyaluronic acid production in human synovial cells and synovial tissue cultures. Arthritis Rheum 1999,42(12):2561–2568.
Petrie RX, Reid IC, Stewart CA: The N-methyl-D-aspartate receptor, synaptic plasticity, and depressive disorder. A critical review. Pharmacol Ther 2000,87(1):11–25.
Wattiez AS, Libert F, Privat AM, Loiodice S, Fialip J, Eschalier A, Courteix C: Evidence for a differential opioidergic involvement in the analgesic effect of antidepressants: prediction for efficacy in animal models of neuropathic pain? Br J Pharmacol 163(4):792–803.
Arnold LM: Biology and therapy of fibromyalgia. New therapies in fibromyalgia. Arthritis Res Ther 2006,8(4):212.
Tzellos TG, Papazisis G, Toulis KA, Sardeli C, Kouvelas D: A2delta ligands gabapentin and pregabalin: future implications in daily clinical practice. Hippokratia 14(2):71–75.
Sorensen J, Bengtsson A, Backman E, Henriksson KG, Bengtsson M: Pain analysis in patients with fibromyalgia. Effects of intravenous morphine, lidocaine, and ketamine. Scand J Rheumatol 1995,24(6):360–365.
Harris RE, Clauw DJ, Scott DJ, McLean SA, Gracely RH, Zubieta JK: Decreased central mu-opioid receptor availability in fibromyalgia. J Neurosci 2007,27(37):10000–10006.
Nielsen AN, Mathiesen C, Blackburn-Munro G: Pharmacological characterisation of acid-induced muscle allodynia in rats. Eur J Pharmacol 2004,487(1–3):93–103.
Sluka KA, Rohlwing JJ, Bussey RA, Eikenberry SA, Wilken JM: Chronic muscle pain induced by repeated acid Injection is reversed by spinally administered mu- and delta-, but not kappa-, opioid receptor agonists. J Pharmacol Exp Ther 2002,302(3):1146–1150.
Furuta S, Shimizu T, Narita M, Matsumoto K, Kuzumaki N, Horie S, Suzuki T: Subdiaphragmatic vagotomy promotes nociceptive sensitivity of deep tissue in rats. Neuroscience 2009,164(3):1252–1262.
Acknowledgements
The authors thank Drs. L Ma and W Xie for technical assistance. This study was supported in part by MEXT KAKENHI (17109015 to Hiroshi Ueda) and Health Labor Sciences Research Grants from the Ministry of Health, Labor and Welfare of Japan (to Hiroshi Ueda): Research on Allergic disease and Immunology and Third Term Comprehensive Control Research for Cancer (398-49).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
MN participated in the experimental designing, collection and analyses of data, and drafted the manuscript in equal contribution. HU and JN performed the statistical analyses and carried out surgical manipulation, data collection, and drafted the manuscript. KA and TM performed stress exposing and participated nociceptive behavior assay. SK measured plasma corticosterone levels. HU conceived of the study, participated in its design and coordination. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Nishiyori, M., Uchida, H., Nagai, J. et al. Permanent relief from intermittent cold stress-induced fibromyalgia-like abnormal pain by repeated intrathecal administration of antidepressants. Mol Pain 7, 69 (2011). https://doi.org/10.1186/1744-8069-7-69
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1744-8069-7-69