Abstract
The basal ganglia (BG) are composed of several nuclei involved in neural processing related to the execution of motor, cognitive and emotional activities. Preclinical and clinical data have implicated a role for these structures in pain processing. Recently neuroimaging has added important information on BG activation in conditions of acute pain, chronic pain and as a result of drug effects. Our current understanding of alterations in cortical and sub-cortical regions in pain suggests that the BG are uniquely involved in thalamo-cortico-BG loops to integrate many aspects of pain. These include the integration of motor, emotional, autonomic and cognitive responses to pain.
Similar content being viewed by others
Introduction
The basal ganglia (BG) consist of the striatum (caudate (C), putamen (Pu) and the core of the nucleus accumbens), the external segment of the globus pallidus (GP), the internal segment of the globus pallidus (GPi), the subthalamic nucleus (STh), and the substantia nigra (SN) [1–3] (Figure 1). Although best known for their role in motor systems, these regions are a major site for adaptive plasticity in the brain, affecting a broad range of normal behaviors [4] and neurological and psychiatric conditions [5].
Basal Ganglia. Horizontal (A), Coronal (B-D) and (E) 3-D representation of the Basal Ganglia in the Human Brain. Sub-regions are noted in the color-coded key. Brain sections from FMRIB Software Library http://www.fmrib.ox.ac.uk/fsl/.
The first section of this review, Basal Ganglia: Pain Processing, summarizes the role of the BG in pain processing as has been reported in specific research and review papers. In the section titled Basal Ganglia: Functional Imaging Studies of Pain in Humans, we describe functional imaging studies in humans reporting activation of the BG in acute pain, chronic pain, and in response to analgesics. The third section titled Basal Ganglia: Lessons Learned from Functional Imaging attempts to integrate the information about lessons learned from functional imaging. The final section, Basal Ganglia: Imaging and Improved understanding of Clinical Applications, describes how imaging may provide insight to how and why certain therapies may be useful. Future measures of BG function may contribute significantly to our understanding about the brain changes associated with chronic pain and specific therapies that may change the brain in a manner that corresponds with therapeutic effects.
Basal Ganglia: Pain Processing
Both preclinical and clinical data support a role for the BG in pain processing [6, 7].
Data supporting a role for the BG in pain and analgesia processing have been derived from numerous preclinical studies. These studies include electrophysiology [8, 9], analgesic effects of microinjections into these regions [10], electrolytic lesion studies [11], chemical lesions of dopaminergic terminals [12, 13], activation of striatal dopamine systems producing analgesia in rats [14] and imaging drug effects in neuropathic rat models [15]. In addition, novel pain pathways, for example those projecting from the spinal cord to the globus pallidus [16], have been discovered. Since the major review on the involvement of the basal ganglia in pain [6, 7], the examples of preclinical studies noted above are not exhaustive and more recent studies have included numerous other contributions (e.g., [9, 10, 17–19]).
In the clinical domain, two disease patterns epitomize the role of the BG in pain - Parkinson's disease (PD) and Complex Regional Pain Syndrome (CRPS). In PD, the initial pathology involves the BG (i.e., loss of dopaminergic neurons in the substantia nigra [20]) that results in movement disorders, and affected subjects frequently have chronic pain [21–24]. In CRPS the initial event is most often relatively minor damage to a peripheral nerve [25, 26], but with time expression of frequently associated movement disorders may become evident, thereby implicating BG involvement [27–30]. Both conditions are associated with movement disorders and both are associated with pain. In chronic pain, alterations of multiple sub-cortical and cortical processing, including sensory, emotional/affective, cognitive and modulatory systems, are present. Independent of specific somatosensory regions and pain modulation (e.g., SI and pain intensity, PAG and pain facilitation/inhibition), recent functional neuroimaging data suggest that the BG appear to be intimately involved in these processes.
Lesions of the BG in patients have offered further insights into the potential role of BG in pain and analgesia. Infarction of the lenticular nucleus (composed of the putamen and globus pallidus) may result in sensory deficits including pain in some patients [31]. Both unilateral and bilateral deep brain stimulation of the globus pallidus have been reported to improve pain by approximately 70%, and this improvement may persist for a significant period of time [32]. In Parkinson's disease, bilateral pallidotomy produces pain relief [33]. Taken together, these data provide insights into the potential contributions of the BG in chronic pain in humans.
Multisensory integration takes place in the BG [34, 35] that serves an important role in behavioral actions including motor responses [36]. The BG receives inputs from all cortical areas (including medial and orbital, prefrontal, dorsolateral, premotor and motor cortex, sensorimotor and parietal cortex) and the thalamus. Multiple cortical areas receive afferents from a single thalamic nucleus and send back information to different thalamic nuclei [37]. Efferent pathways from the BG project to frontal lobe areas including prefrontal, premotor and supplementary motor areas (used in motor planning). Thus the BG are well positioned to have influences on cortical regions involved in motor responses, in behavior relating to predicting events, and involved in attention and learning [38]. Using probabilistic tractography, a recent diffusion tensor imaging (DTI) study on connectivity patterns of the BG in humans [39] showed segregated and overlapping loops that include prefrontal, premotor, and motor networks to BG sub-regions (see Figure 2). In the cat for example, electrophysiological recordings have shown that neurons in the BG respond to visual, auditory and somatosensory information [35]. How this integration takes place is not well understood, but BG circuits are in a unique position to integrate cortical information to increase the speed of data processing tasks [40–42]. Similar processing may take place with emotional and cognitive information. Such processing is relevant to pain because the response to acute or chronic pain involves sensory, motor, autonomic, cognitive and emotional integration.
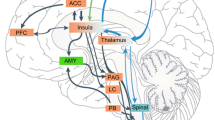
Cortical Connectivity and Basal Ganglia. The BG receive multiple inputs from cortical and subcortical regions as noted in the figure. Many of these regions are involved in pain processing (see text). (From [175], Nature Neuroscience, Nature Publishing Group, with permission).
Figure 3 shows the connectivity patterns of the BG with respect to possible pain processing. Pain inputs into the BG may be considered to be from two major sources: (1) Afferent inputs from pain sensing systems via direct (e.g., spino-BG) and indirect (e.g., spino-thalamic-BG) pathways; and (2) from cortical and sub-cortical brain regions that contribute to the BG-Thalamic-Cortical loops. Cortical regions involved in these feedback loops are also known to have important roles in pain processing. These areas include the ACC, regions of the frontal lobe (e.g., dorsolateral prefrontal cortex (DLPF), orbitofrontal cortex (Gob)), parietal, insular, and hippocampal regions. Chudler and Dong have also reported putative nociceptive pathways into and out of the basal ganglia [6, 7].
Basal Ganglia and Pain Systems. Afferent inputs from spinal cord and brainstem have direct and indirect inputs into the BG, most are into the striatum but some input into the globus pallidus and substantia nigra. Thalamic inputs into various cortical regions are then processed and complete the cortico-BG-thalamic loop. Cortical inputs include those from a number of regions known to be involved in pain processing. Key: (1) Spino-accumbens pathway [57]; (2) Spino-parabrachial-amygdala pathways [176]; (3) Spino-thalamic pathway [177]; (4) Thalamo-Striatal pathway [38].
Basal Ganglia: Functional Imaging Studies of Pain in Humans
Functional imaging studies in healthy volunteers and patients with chronic pain have supported a growing role of the BG in pain processing. Previous work has suggested that the BG may be involved in most aspects of pain processing including sensory-discriminative, emotional/affective, cognitive dimension of pain and pain modulation [7]. Given our current understanding of these brain regions in the pain brain phenotype (i.e., functional brain changes in healthy and disease (see [43]) and our understanding of the basic integrative role the BG may play neural processing, it is suggested that the BG play a pivotal role in the behavioral manifestations of chronic pain. Although a large literature exists on the role of the BG in non-motor activities [44], brain imaging studies of acute and chronic pain have contributed to supporting preclinical and clinical work as playing an increasingly important role in acute and chronic pain processing and in the effects of some analgesics on brain function (Tables 1, 2 and 3).
Since there are multiple sub-regions within the BG, preclinical studies using specific methods cannot easily define how these interact at the same time to integrate such information, including pain. Understanding the functional processing of pain and analgesia in these regions might afford insights into complex behaviors of brain systems in acute and chronic pain and help us understand how analgesics might affect pain processing. Brain imaging in humans includes a number of methods such as functional MRI (fMRI), pharmacological MRI (phMRI), morphometric/anatomical measures (diffusion tensor imaging and gray matter volumetric analysis), and positron emission tomography (functional PET (fPET) and ligand binding/displacement (phPET)) that allow whole brain evaluation of specific circuits.
Basal Ganglia Activation in Acute Pain (Figure 4, Tables 1, 2 and 3)
Noxious Stimuli
Early studies of pain using fMRI indicated activation patterns in the BG including the putamen and globus pallidus. These early observations have now been replicated in a number of functional imaging studies of experimental pain in humans, examples of which are shown in Tables 1 and 2. As noted in the tables, a considerable similarity exists in the activation patterns from multiple stimuli, including thermal (heat and cold), mechanical (in a hyperalgesic capsaicin model), painful electrical and visceral pain. Subsequent studies showed what seemed to be specific regional activation in other BG structures including the nucleus accumbens [45] and putamen [46, 47]. However, most studies have evaluated or reported activation patterns and observed striatal involvement in a general context.
Tables 1 and 2 show positive activation is present in the caudate nucleus across multiple studies and across multiple modalities. In addition to those mentioned in the tables, activation in the caudate is present in "prickle" sensation evoked by cold stimuli [48]. In our own studies, cold has previously been shown to activate basal ganglia including the caudate [49]. Activation in the caudate nucleus to noxious stimulation has been suggested to be part of a pain modulatory system [50, 51]. In support of this, electrical stimulation of the caudate in non-human primates diminished pain reactivity [52]. The authors suggested that the results indicate that the effect of caudate stimulation is to reduce the affective components of pain elicited by noxious electrocutaneous stimuli.
A few studies have evaluated specific activation in the putamen and nucleus accumbens. As noted in Tables 1 and 2, the putamen is commonly activated across most acute pain imaging studies. Pain activated the putamen bilaterally and a somatotopic organization for hand- and foot-related responses was only present in the contralateral putamen [53]. In healthy women, pain-evoked putaminal activation occurred during their follicular phase [54].
Activation of globus pallidus following painful stimulation has been shown in healthy subjects (Tables 1 and 2). Unlike the caudate and putamen, fewer studies have specifically reported activation in this BG structure. However, like the caudate and putamen, the globus pallidus has neurons that respond to noxious stimulation [9].
The nucleus accumbens (NAc), a brain substrate known to be involved in reward-aversion processing, has been shown to respond with opposite BOLD signal valence to rewarding or aversive stimuli [45, 55]. Using a pain onset (aversive) and pain offset (rewarding) prolonged stimulus, a negative signal change with pain onset and a positive signal change with pain offset was observed in the NAc contralateral to the stimulus. The study supports the idea that the NAc fMRI signal may provide a useful marker for the effects of pain and analgesia in healthy volunteers. A parallel study on NAc activation in response to the direct effects of the analgesic (pain offset) morphine, showed the same increased (rewarding) BOLD signal in the structure [56]. Direct afferents from the trigeminal nucleus to the nucleus accumbens have been demonstrated in the rat; these have contralateral projections from lamina I but bilateral with contralateral predominance from lamina V [57]. These anatomical studies seem to agree with other studies showing contralateral activation in the NAc [45, 55]. Subsequent studies indicated two components of activation within the NAc - an anterior, superior, and lateral component and another component that was posterior, inferior, and medial within the structure. The anatomical segregation may correlate with the functional components of the NAc (i.e., shell and core) that have been defined in other species (see [58]). The results support heterogeneity of function within the NAc and have implications for understanding the contribution of NAc function in processing of pain and analgesia [58]. The NAc has a pivotal role in aversive and rewarding (hedonic) processing [59].
Given that the reward system is part of the pain network [45], and that the placebo response is clearly involved in analgesia [60, 61], it is not surprising that functional imaging has been the ideal technology to better understand neural networks involved in placebo in humans [62, 63]. Recent reviews suggest that multiple brain regions (e.g., anterior cingulate cortex, anterior insula, prefrontal cortex and periaqueductal grey) are important in the placebo response [64]. Other studies strongly implicate the NAc and ventral BG in the placebo response [65].
Basal Ganglia Activation in Chronic Pain (Figure 4, Tables 1, 2 and 3)
An increase in gray matter in the BG has been reported in chronic back pain patients [66], in fibromyalgia [67], and in chronic vulval pain [68], suggesting some underlying functional alterations that drive these changes. In other chronic pain conditions, alterations in activation in BG regions have been demonstrated [55, 69] where stimulation to the affected neuropathic regions produces significant activation in the BG. Specifically, contrast analysis between the affected and unaffected regions resulted in increased activation in the globus pallidus and putamen to mechanical and cold allodynia, as well as decreased activation in the caudate nucleus to mechanical, cold and heat allodynia [55]. Taken together these data appear to show a consistent decrease in the caudate nucleus to pain produced across multiple painful stimuli. Similarly, functional imaging of pain in a group of pediatric patients with CRPS has shown significant activation in the BG with cold and brush stimuli, and also displays decreased activation in the caudate nucleus [70].
Chronic pain has also been shown to alter BG structure with measures of gray matter volume and white matter tract integrity. In a more recent report, measures of gray matter morphometry and white matter anisotropy were measured in patients with complex regional pain syndrome (CRPS) [71]. Alterations in morphometry indicated atrophy in a single cluster encompassing the right insula, right ventromedial prefrontal cortex (VMPFC), and right NAc. Additionally, decreased connectivity was reported between the ventromedial prefrontal cortices to the BG [71].
Alterations in dopaminergic and opioidergic function in the BG have been reported in clinical pain conditions. In patients with burning mouth syndrome, decreases in dopamine in the putamen suggest reduced dopaminergic inhibition may contribute to the chronic pain condition [72]. In addition, both atypical facial pain [73] and fibromyalgia patients [74] show an abnormal dopamine response. Such data are consistent with other conditions associated with decreased dopamine, including Parkinson's disease [75, 76]. In addition to the alterations in dopamine, decreased μ binding in chronic pain conditions such as fibromyalgia may contribute to altered pain processing [77]. In psychiatric disorders, pain produces increased activation in putaminal regions in post traumatic stress disorder, or PTSD, [78] that when considered with the diminished pain sensitivity reported in this patient group may also be caused in part to abnormalities within the BG.
Basal Ganglia Activation and Analgesics (Figure 4, Tables 1, 2 and 3)
Functional imaging of drug effects (also known as pharmacological MRI or phMRI) has been done in the context of BG function in preclinical [79] and clinical [80, 81] conditions. The evaluation of direct brain changes in functional activity in response to known analgesic drugs has been limited to a small group that include morphine [55], naloxone [82], remifentanil [83] and buprenorphine [84]. Direct effects of μ opioid agonist or antagonist infusions both show activation in the BG, including the NAc. In these experiments, the direction of BOLD signal is opposite with increased activation following morphine infusion [55] and a decreased activation following infusion of naloxone [82] in healthy volunteers. The opposite direct pharmacological effects indicate some specificity of response but may also underlie NAc drive with respect to pain and analgesia. An increased activation may be part of an endogenous analgesic drive or emotional drive since increased activation patterns within the NAc are also seen in other fMRI experiments on reward [85–87]. The increase (hyperalgesic) pain response (inhibition of tonic opioid drive) to pain was observed in brain regions following naloxone that included concurrent activation in the globus pallidus, caudate and putamen. Opioids may produce changes in the BG including increases in D2 receptor binding [88]. However not all imaging studies report BG activation in response to opioids, either because they were not specifically focused on these regions or they used less sensitive methods (e.g., alfentanil [89]; remifentanil [90]).
Few authors have evaluated the effects of non-opioid analgesics on BG responsiveness. In one example, although not specifically referred to in their paper, gabapentin reduced activation in the BG [91]; specifically, for gabapentin vs. placebo comparison in a hyperalgesic paradigm, deactivation in the caudate was present in the single dose study in healthy volunteers.
Other drugs, considered to be analgesics, have been evaluated using imaging in a similar manner to that noted above, and these studies report alterations in the human striatum. For example tetrahydrocannabinol (known to be an analgesic [92]) produces dopamine release in the striatum [93]. Similar results have been shown for the NMDA antagonist ketamine (reviewed by [94]).
Lessons Learned from Imaging
Based on imaging data of pain as well as a growing literature on pain in conditions with abnormalities of BG function, it is clear that a constellation of brain regions plays an important role in acute and chronic pain (see Tables 1, 2 and 3). With the exception of studies on the NAc and the pallidum (see above), few functional imaging studies in humans have attempted to evaluate other regions of the BG function in pain. Nevertheless there is increasing evidence for the important roles of the region in neural systems' adaptation to pain.
Putamen (P) shows consistent increase in activation across multiple pain imaging studies
The putamen, a structure that shows somatotopic activation to pain [53], is consistently activated during acute and chronic pain conditions and is affected by analgesic administration. Chronic pain has also been shown to increase putamen volume (see [67]) suggesting, perhaps, that a continuous drive producing increased activation may result in processes that enhance gray matter volume in the structure. This region may receive direct inputs from sensory systems, but also from cortical inputs. Some studies (e.g., [83]) have suggested delayed functional activation in BG circuits (e.g., caudate) following remifentanil, implicating that activation in these regions occurs as a consequence or subsequent to initial activation of thalamo-cortical circuits. Activation seems to be present with painful stimuli but missing with non-painful stimuli [95].
Opposite Signal Changes in Caudate and NAc in Acute vs. Chronic Pain
In contrast to increases in putaminal activation for acute pain, BOLD signals are opposite in chronic pain across multiple pain studies. The reason for this observation is not known although the nature of reward processing in acute vs. chronic pain is clearly different [45, 59, 71].
Smaller Nuclei (ST, SN) are not clearly defined in many fMRI studies
Only a few brain imaging studies have reported activation in the smaller nuclei of the BG even though they may be present. Although there are studies in animals investigating SN mechanisms of pain [96], future brain imaging studies in humans will contribute to our understanding of the role of these nuclei in pain processing. Developments such as accurate automated identification of sub-nuclei within the basal ganglia should be helpful in measures of functional brain changes [97].
Feed-forward and Feedback Loops - Integrating, Modifying, and Modulating the Pain Experience
The BG are involved in the integration of information between cortical and thalamic regions and in particular the three domains of pain processing - sensory, emotional/cognitive and endogenous/modulatory. Some investigators have suggested that these regions have evolved as a "centralized selection device to resolve conflicts over access to limited motor and cognitive resources" [98]. Phylogenetically older, sub-cortical connections exist between the BG and brainstem regions involved in sensorimotor processing [99], and more recent evidence points to BG involvement through direct connections from sensory inputs not involving cortical loops [100]. Dysfunctional cortico-BG-thalamic loops may contribute to the maintenance of chronic pain and the evolution of altered neural processing that may be a basis for co-morbid behaviors. Whether brain processing of pain is dependent on external neural drive (i.e., peripheral/feed-forward inputs) or internal neural drive (i.e., subcortical or cortical drive/feedback), the BG are central processors that may play a role in integrating these divergent inputs that may modify pain over time.
Basal Ganglia: Imaging and Improved Understanding of Clinical Applications
Cortical Stimulation and Chronic Pain - Acting via Cortico-Striatal-Thalamic Loops
Cortical stimulation has been used either directly with electrodes placed on the motor cortex [101] or through the use of repetitive transcranial stimulation (rTMS) [102]. Some investigators have postulated that motor cortex neurostimulation produces an analgesic effect by modulation of the affective components of pain and not of the sensory components [103]. In support of this hypothesis, stimulation of the secondary somatosensory cortex (SII), but not motor cortex, results in increased pain thresholds and altered discriminative capacity to pain [104]. A combination of motor cortex stimulation (MCS) and postoperative fMRI showed an inhibiting effect on the primary sensorimotor cortex as well as on the contralateral primary motor and sensitive cortices [105] without changes in BG. Others have argued that MCS may act through activation of perigenual cingulate and orbitofrontal areas to alter the emotional appraisal of pain or cortical-brainstem (e.g., PAG) activation that enhances inhibition of pain [106] or alterations in endogenous opioid systems [107]. Given the cortico-striatal loops, stimulation of a number of cortical regions may thus be involved in pain reduction. Stimulation of dorsolateral prefrontal cortex, motor cortex or sensory cortices may have different effects based on the specificity of cortico-striatal loops. In addition, connectivity between the BG and modulatory regions including the PAG/brainstem or through the NAc [108–110] may also contribute to the potential mechanism of cortical activation resulting in analgesia.
Anterior Cingulate Lesions and Pain Control
Both clinical [111] and preclinical [112] studies have suggested that the ACC results in alterations of pain processing. The interesting issue seems to be the dissociation of pain intensity from pain affect (caring about the pain) [113, 114]. The ACC in humans may be involved with linking reward-related information [115] and with alternative actions, since destruction of the ACC results in errors related to planning [116]. The ACC has projections to the caudate nucleus and the NAc [117], and ACC lesions may produce an attenuation of a negative pain affect [118] through a combination of neural networks that include these circuits.
BG and Learned Behaviors
Pain is clearly a complex process that affects multiple brain systems. It debatable if pain is a learned behavior, but the BG may have a role in learning due to its involvement in habit and stimulus-response learning [119]. Such learning may be derived from pain related regions involved in sensory (e.g., pain intensity coding regions such as SI), affective (e.g., cingulate or anterior insula) or cognitive functioning (medial and lateral prefrontal cortices). Similar to the notion of "chunking action repertoires" for motor action, it may be that pain related repertoires include motor related changes.
BG and Emergence of Central Pain
Aside from CRPS, other conditions including depression and Parkinson's disease have BG pathology that may be important in their clinical presentation of centralized pain [120]. In Parkinson's disease with central pain, electrophysiological measures of pain pathways are normal, but hyperalgesia to repetitive stimuli is present; this is attenuated by L-dopa [121], and altered central processing in these conditions results in generalized pain symptoms. Although the basis for these changes is unknown, it may be the result of altered chemicals in the BG, such as central dopamine, or related to aberrant networks that induce a kindling-like pain [122]. Changes in neural connectivity that produce changes in increased synaptic strength [123] and could include pain facilitatory circuits [124] may also underlie these changes. Although the basis for these changes is unknown, alterations may then lead to other changes such as abnormal gating of sensory-motor function involving parallel changes thalamic regions [125]. Imaging patients with depression in whom non-dermatomal sensory deficits were present shows that hypometabolic patterns (FDG-PET) of activation in the putamen was observed [126].
Regions of the thalamus including the paramedian and anterolateral are known to be involved with central pain (e.g., post stroke pain) [38, 127]. The striatum receives excitatory input from the thalamus. The centromedian (CM) and parafascicular (Pf) thalamic nuclei are important sources of thalamostriatal projections [128] and send connections to the putamen and caudate [128, 129]. Moreover, the topography of these connections corresponds with cortical sensorimotor territory observed following cortical injections [130].
Basal Ganglia and Opioid Systems
The BG has high levels of endogenous opioids, and high binding of opioid receptors are present within the BG [131]. In a number of chronic pain conditions, receptor-binding studies indicate a decreased opioidergic tone. Many analgesics act at the level of the basal ganglia and may contribute to both analgesic and addictive processes. An understanding of how these functional processes are differentiated by different endogenous chemicals (e.g., opioidergic vs. dopaminergic (see [65])) may contribute to better analgesics since opioidergic tone might be abnormal [77].
Dopaminergic Drugs and Pain
Given that pain now has a functional basis in terms of regions activated including those that are classically involved in reward [45], and that chronic pain may be a reward deficit syndrome [132], modulating dopamine may have important possibilities for pain treatment. Parkinson's disease patients have improved pain control when treated with L-dopa [21, 121]. Changes in dopamine are a critical element in Parkinson's disease where abnormal pain processing is present in both conditions. Recent imaging data have reported a direct correlation between striatal dopamine D2/D3 receptors and sensory thresholds as being selective for the modality of pain but not for non-painful stimuli [133]. Striatal dopamine D2/D3 receptors may control a modulatory pathway producing a parallel shift in the stimulus-response function for sensory signals [134]. Others have suggested differential processing of pain within the BG - a more dorsal DA D2 receptor-mediated neurotransmission in the caudate and putamen that correlates with subjective ratings of sensory and affective qualities of the pain, along with a more ventral system involving the NAc, is associated with emotional processing [135]. Such differences may be important in drug effects on pain. The use of antipsychotic medications for pain is not new [136], and the number needed to treat (NNT; this is the number of patients to be treated for the first subject to show a 50% analgesic effect) of 2.6 is very competitive with the best drugs available for chronic pain [137]. However, these drugs have extrapyramidal and sedative effects, and atypical antipsychotics that have fewer side effects may have analgesic properties as assessed in a limited number of studies [138]. Newer drugs that target specific dopamine receptors may prove to be more useful.
BG Deep Brain Stimulation and Strategies for Pain Relief
Brain imaging affords the possibility to measure changes in brain circuits that may be altered as a result of deep brain stimulation. Some of these have shown specific changes in BG circuitry [139]. In the latter, stimulation of the ventroposterior medial thalamus (VPM) resulted in decreases in activation of the substantia nigra when activation prior to stimulation vs. post stimulation that provided pain relief was measured (pain, no stimulation vs. no pain, no stimulation). Thalamic stimulation may activate thalamo-cortical-BG loops that contribute to the analgesic response [140].
Conclusions
Functional imaging of pain has shown clear and consistent changes in the BG in pain conditions. Table 4 summarizes the salient features of each sub-region of the BG as it pertains to overall putative function and specific functions in pain, and Figure 5 summarizes potential alterations in BG outputs affecting behaviors in acute and chronic pain. Future work should help contribute to further understanding functional and anatomical connectivity of inputs and circuits that show how the BG may be involved in acute and chronic pain. Such findings may present an increasing and important role of these brain regions in the centralization of chronic pain and the contribution to the altered brain in chronic pain conditions. Future studies, using a combination of imaging approaches, will help define the specificity of BG in pain processing. For example, functional connectivity analysis can demonstrate probable correlations between BG subdivisions and other brain regions [141].
Basal Ganglia Function in Acute and Chronic Pain. Top: Acute Pain Processing is a normal response where pain produces responses in brain circuits that usually revert to normal. Some of these processes are integrated in the BG and result in escape responses: components of memory and learning of pain and affective responses to pain. Bottom: In chronic pain both inputs from peripheral systems and cortical and subcortical regions are abnormal. The result is that BG functions as well as cortico-BG-thalamic loop functions are altered. The result may be altered integration of sensori-motor responses, cognitive impairment and emotional processing.
References
Graybiel AM: The basal ganglia: learning new tricks and loving it. Curr Opin Neurobiol 2005, 15: 638–644. 10.1016/j.conb.2005.10.006
Kreitzer AC, Malenka RC: Striatal plasticity and basal ganglia circuit function. Neuron 2008, 60: 543–554. 10.1016/j.neuron.2008.11.005
Yelnik J: Modeling the organization of the basal ganglia. Rev Neurol (Paris) 2008, 164: 969–976.
Graybiel AM: Network-level neuroplasticity in cortico-basal ganglia pathways. Parkinsonism Relat Disord 2004, 10: 293–296. 10.1016/j.parkreldis.2004.03.007
Obeso JA, Rodriguez MC, DeLong MR: Basal ganglia pathophysiology. A critical review. Adv Neurol 1997, 74: 3–18.
Barker RA: The basal ganglia and pain. Int J Neurosci 1988, 41: 29–34. 10.3109/00207458808985739
Chudler EH, Dong WK: The role of the basal ganglia in nociception and pain. Pain 1995, 60: 3–38. 10.1016/0304-3959(94)00172-B
Bernard JF, Huang GF, Besson JM: Nucleus centralis of the amygdala and the globus pallidus ventralis: electrophysiological evidence for an involvement in pain processes. J Neurophysiol 1992, 68: 551–569.
Chudler EH: Response properties of neurons in the caudate-putamen and globus pallidus to noxious and non-noxious thermal stimulation in anesthetized rats. Brain Res 1998, 812: 283–288. 10.1016/S0006-8993(98)00971-8
Tashev R, Belcheva S, Milenov K, Belcheva I: Antinociceptive effect of somatostatin microinjected into caudate putamen. Peptides 2001, 22: 1079–1083. 10.1016/S0196-9781(01)00431-4
Saade NE, Shbeir SA, Atweh SF, Jabbur SJ: Effects of cerebral cortical and striatal lesions on autotomy following peripheral neurectomy in rats. Physiol Behav 1996, 60: 559–566. 10.1016/S0031-9384(96)80032-1
Saade NE, Atweh SF, Bahuth NB, Jabbur SJ: Augmentation of nociceptive reflexes and chronic deafferentation pain by chemical lesions of either dopaminergic terminals or midbrain dopaminergic neurons. Brain Res 1997, 751: 1–12. 10.1016/S0006-8993(96)01164-X
Takeda R, Ikeda T, Tsuda F, Abe H, Hashiguchi H, Ishida Y, Nishimori T: Unilateral lesions of mesostriatal dopaminergic pathway alters the withdrawal response of the rat hindpaw to mechanical stimulation. Neurosci Res 2005, 52: 31–36. 10.1016/j.neures.2005.01.005
Lin MT, Wu JJ, Chandra A, Tsay BL: Activation of striatal dopamine receptors induces pain inhibition in rats. J Neural Transm 1981, 51: 213–222. 10.1007/BF01248953
Porro CA, Cavazzuti M, Baraldi P, Giuliani D, Panerai AE, Corazza R: CNS pattern of metabolic activity during tonic pain: evidence for modulation by beta-endorphin. Eur J Neurosci 1999, 11: 874–888. 10.1046/j.1460-9568.1999.00494.x
Braz JM, Nassar MA, Wood JN, Basbaum AI: Parallel "pain" pathways arise from subpopulations of primary afferent nociceptor. Neuron 2005, 47: 787–793. 10.1016/j.neuron.2005.08.015
Chudler EH, Lu Y: Nociceptive behavioral responses to chemical, thermal and mechanical stimulation after unilateral, intrastriatal administration of 6-hydroxydopamine. Brain Res 2008, 1213: 41–47. 10.1016/j.brainres.2008.03.053
Greco R, Tassorelli C, Armentero MT, Sandrini G, Nappi G, Blandini F: Role of central dopaminergic circuitry in pain processing and nitroglycerin-induced hyperalgesia. Brain Res 2008, 1238: 215–223. 10.1016/j.brainres.2008.08.022
Heindl-Erdmann C, Axmann R, Kreitz S, Zwerina J, Penninger J, Schett G, Brune K, Hess A: Combining functional magnetic resonance imaging with mouse genomics: new options in pain research. Neuroreport 2009.
Schapira AH: Neurobiology and treatment of Parkinson's disease. Trends Pharmacol Sci 2009, 30: 41–47. 10.1016/j.tips.2008.10.005
Cheon SM, Park MJ, Kim WJ, Kim JW: Non-motor off symptoms in Parkinson's disease. J Korean Med Sci 2009, 24: 311–314. 10.3346/jkms.2009.24.2.311
Defazio G, Berardelli A, Fabbrini G, Martino D, Fincati E, Fiaschi A, Moretto G, Abbruzzese G, Marchese R, Bonuccelli U, et al.: Pain as a nonmotor symptom of Parkinson disease: evidence from a case-control study. Arch Neurol 2008, 65: 1191–1194. 10.1001/archneurol.2008.2
Ford B: Parkinson disease: Pain in Parkinson disease: the hidden epidemic. Nat Rev Neurol 2009, 5: 242–243. 10.1038/nrneurol.2009.50
Djaldetti R, Shifrin A, Rogowski Z, Sprecher E, Melamed E, Yarnitsky D: Quantitative measurement of pain sensation in patients with Parkinson disease. Neurology 2004, 62: 2171–2175.
Birklein F: Complex regional pain syndrome. J Neurol 2005, 252: 131–138. 10.1007/s00415-005-0737-8
Schwartzman RJ, Alexander GM, Grothusen J: Pathophysiology of complex regional pain syndrome. Expert Rev Neurother 2006, 6: 669–681. 10.1586/14737175.6.5.669
Agrawal SK, Rittey CD, Harrower NA, Goddard JM, Mordekar SR: Movement disorders associated with complex regional pain syndrome in children. Dev Med Child Neurol 2009, 51: 557–562. 10.1111/j.1469-8749.2008.03181.x
Lang AE, Angel M, Bhatia K, Chen R, Fahn S, Hallett M, Schrag A, Thompson P: Myoclonus in complex regional pain syndrome. Mov Disord 2009, 24: 314–316. author reply 316 10.1002/mds.22355
van Rijn MA, Marinus J, Putter H, van Hilten JJ: Onset and progression of dystonia in complex regional pain syndrome. Pain 2007, 130: 287–293. 10.1016/j.pain.2007.03.027
Schott GD: Peripherally-triggered CRPS and dystonia. Pain 2007, 130: 203–207. 10.1016/j.pain.2007.04.013
Russmann H, Vingerhoets F, Ghika J, Maeder P, Bogousslavsky J: Acute infarction limited to the lenticular nucleus: clinical, etiologic, and topographic features. Arch Neurol 2003, 60: 351–355. 10.1001/archneur.60.3.351
Loher TJ, Burgunder JM, Weber S, Sommerhalder R, Krauss JK: Effect of chronic pallidal deep brain stimulation on off period dystonia and sensory symptoms in advanced Parkinson's disease. J Neurol Neurosurg Psychiatry 2002, 73: 395–399. 10.1136/jnnp.73.4.395
Favre J, Burchiel KJ, Taha JM, Hammerstad J: Outcome of unilateral and bilateral pallidotomy for Parkinson's disease: patient assessment. Neurosurgery 2000, 46: 344–353. discussion 353–345 10.1097/00006123-200002000-00017
Chudler EH, Sugiyama K, Dong WK: Multisensory convergence and integration in the neostriatum and globus pallidus of the rat. Brain Res 1995, 674: 33–45. 10.1016/0006-8993(94)01427-J
Nagy A, Eordegh G, Paroczy Z, Markus Z, Benedek G: Multisensory integration in the basal ganglia. Eur J Neurosci 2006, 24: 917–924. 10.1111/j.1460-9568.2006.04942.x
Markus Z, Eordegh G, Paroczy Z, Benedek G, Nagy A: Modality distribution of sensory neurons in the feline caudate nucleus and the substantia nigra. Acta Biol Hung 2008, 59: 269–279. 10.1556/ABiol.59.2008.3.1
Haber SN, Calzavara R: The cortico-basal ganglia integrative network: the role of the thalamus. Brain Res Bull 2009, 78: 69–74. 10.1016/j.brainresbull.2008.09.013
Herrero MT, Barcia C, Navarro JM: Functional anatomy of thalamus and basal ganglia. Childs Nerv Syst 2002, 18: 386–404. 10.1007/s00381-002-0604-1
Draganski B, Kherif F, Kloppel S, Cook PA, Alexander DC, Parker GJ, Deichmann R, Ashburner J, Frackowiak RS: Evidence for segregated and integrative connectivity patterns in the human Basal Ganglia. J Neurosci 2008, 28: 7143–7152. 10.1523/JNEUROSCI.1486-08.2008
Baev KV: Disturbances of learning processes in the basal ganglia in the pathogenesis of Parkinson's disease: a novel theory. Neurol Res 1995, 17: 38–48.
Leyden J, Kleinig T: The role of the basal ganglia in data processing. Med Hypotheses 2008, 71: 61–64. 10.1016/j.mehy.2008.02.013
Prodoehl J, Yu H, Wasson P, Corcos DM, Vaillancourt DE: Effects of visual and auditory feedback on sensorimotor circuits in the basal ganglia. J Neurophysiol 2008, 99: 3042–3051. 10.1152/jn.01108.2007
Borsook D, Becerra L: Phenotyping central nervous system circuitry in chronic pain using functional MRI: considerations and potential implications in the clinic. Curr Pain Headache Rep 2007, 11: 201–207. 10.1007/s11916-007-0191-7
Graybiel AM: Habits, rituals, and the evaluative brain. Annu Rev Neurosci 2008, 31: 359–387. 10.1146/annurev.neuro.29.051605.112851
Becerra L, Breiter HC, Wise R, Gonzalez RG, Borsook D: Reward circuitry activation by noxious thermal stimuli. Neuron 2001, 32: 927–946. 10.1016/S0896-6273(01)00533-5
Bingel U, Quante M, Knab R, Bromm B, Weiller C, Buchel C: Subcortical structures involved in pain processing: evidence from single-trial fMRI. Pain 2002, 99: 313–321. 10.1016/S0304-3959(02)00157-4
Sung EJ, Yoo SS, Yoon HW, Oh SS, Han Y, Park HW: Brain activation related to affective dimension during thermal stimulation in humans: a functional magnetic resonance imaging study. Int J Neurosci 2007, 117: 1011–1027. 10.1080/00207450600934432
Davis KD, Pope GE, Crawley AP, Mikulis DJ: Neural correlates of prickle sensation: a percept-related fMRI study. Nat Neurosci 2002, 5: 1121–1122. 10.1038/nn955
Tracey I, Becerra L, Chang I, Breiter H, Jenkins L, Borsook D, Gonzalez RG: Noxious hot and cold stimulation produce common patterns of brain activation in humans: a functional magnetic resonance imaging study. Neurosci Lett 2000, 288: 159–162. 10.1016/S0304-3940(00)01224-6
Freund W, Klug R, Weber F, Stuber G, Schmitz B, Wunderlich AP: Perception and suppression of thermally induced pain: a fMRI study. Somatosens Mot Res 2009, 26: 1–10. 10.1080/08990220902738243
Freund W, Stuber G, Wunderlich AP, Schmitz B: Cortical correlates of perception and suppression of electrically induced pain. Somatosens Mot Res 2007, 24: 203–212. 10.1080/08990220701723636
Lineberry CG, Vierck CJ: Attenuation of pain reactivity by caudate nucleus stimulation in monkeys. Brain Res 1975, 98: 119–134. 10.1016/0006-8993(75)90513-2
Bingel U, Lorenz J, Glauche V, Knab R, Glascher J, Weiller C, Buchel C: Somatotopic organization of human somatosensory cortices for pain: a single trial fMRI study. Neuroimage 2004, 23: 224–232. 10.1016/j.neuroimage.2004.05.021
Choi JC, Park SK, Kim YH, Shin YW, Kwon JS, Kim JS, Kim JW, Kim SY, Lee SG, Lee MS: Different brain activation patterns to pain and pain-related unpleasantness during the menstrual cycle. Anesthesiology 2006, 105: 120–127. 10.1097/00000542-200607000-00021
Becerra L, Harter K, Gonzalez RG, Borsook D: Functional magnetic resonance imaging measures of the effects of morphine on central nervous system circuitry in opioid-naive healthy volunteers. Anesth Analg 2006, 103: 208–216. table of contents 10.1213/01.ane.0000221457.71536.e0
Becerra L, Morris S, Bazes S, Gostic R, Sherman S, Gostic J, Pendse G, Moulton E, Scrivani S, Keith D, et al.: Trigeminal neuropathic pain alters responses in CNS circuits to mechanical (brush) and thermal (cold and heat) stimuli. J Neurosci 2006, 26: 10646–10657. 10.1523/JNEUROSCI.2305-06.2006
Burstein R, Giesler GJ Jr: Retrograde labeling of neurons in spinal cord that project directly to nucleus accumbens or the septal nuclei in the rat. Brain Res 1989, 497: 149–154. 10.1016/0006-8993(89)90981-5
Aharon I, Becerra L, Chabris CF, Borsook D: Noxious heat induces fMRI activation in two anatomically distinct clusters within the nucleus accumbens. Neurosci Lett 2006, 392: 159–164. 10.1016/j.neulet.2005.09.054
Borsook D, Becerra L, Carlezon WA Jr, Shaw M, Renshaw P, Elman I, Levine J: Reward-aversion circuitry in analgesia and pain: implications for psychiatric disorders. Eur J Pain 2007, 11: 7–20. 10.1016/j.ejpain.2005.12.005
Enck P, Benedetti F, Schedlowski M: New insights into the placebo and nocebo responses. Neuron 2008, 59: 195–206. 10.1016/j.neuron.2008.06.030
Hoffman GA, Harrington A, Fields HL: Pain and the placebo: what we have learned. Perspect Biol Med 2005, 48: 248–265. 10.1353/pbm.2005.0054
Colloca L, Benedetti F, Porro CA: Experimental designs and brain mapping approaches for studying the placebo analgesic effect. Eur J Appl Physiol 2008, 102: 371–380. 10.1007/s00421-007-0593-6
Zubieta JK, Stohler CS: Neurobiological mechanisms of placebo responses. Ann N Y Acad Sci 2009, 1156: 198–210. 10.1111/j.1749-6632.2009.04424.x
Kong J, Kaptchuk TJ, Polich G, Kirsch I, Gollub RL: Placebo analgesia: findings from brain imaging studies and emerging hypotheses. Rev Neurosci 2007, 18: 173–190.
Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK: Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Arch Gen Psychiatry 2008, 65: 220–231. 10.1001/archgenpsychiatry.2007.34
Schmidt-Wilcke T, Leinisch E, Ganssbauer S, Draganski B, Bogdahn U, Altmeppen J, May A: Affective components and intensity of pain correlate with structural differences in gray matter in chronic back pain patients. Pain 2006, 125: 89–97. 10.1016/j.pain.2006.05.004
Schmidt-Wilcke T, Luerding R, Weigand T, Jurgens T, Schuierer G, Leinisch E, Bogdahn U: Striatal grey matter increase in patients suffering from fibromyalgia--a voxel-based morphometry study. Pain 2007,132(Suppl 1):S109–116. 10.1016/j.pain.2007.05.010
Schweinhardt P, Kuchinad A, Pukall CF, Bushnell MC: Increased gray matter density in young women with chronic vulvar pain. Pain 2008, 140: 411–419. 10.1016/j.pain.2008.09.014
Becerra L, Schwartzman RJ, Kiefer RT, Rohr P, Moulton EA, Wallin D, Pendse G, Morris S, Borsook D: CNS Measures of Pain Responses Pre- and Post-Anesthetic Ketamine in a Patient with Complex Regional Pain Syndrome. Pain Med 2009.
Lebel A, Becerra L, Wallin D, Moulton EA, Morris S, Pendse G, Jasciewicz J, Stein M, Aiello-Lammens M, Grant E, et al.: fMRI reveals distinct CNS processing during symptomatic and recovered complex regional pain syndrome in children. Brain 2008, 131: 1854–1879. 10.1093/brain/awn123
Geha PY, Baliki MN, Harden RN, Bauer WR, Parrish TB, Apkarian AV: The brain in chronic CRPS pain: abnormal gray-white matter interactions in emotional and autonomic regions. Neuron 2008, 60: 570–581. 10.1016/j.neuron.2008.08.022
Jaaskelainen SK, Rinne JO, Forssell H, Tenovuo O, Kaasinen V, Sonninen P, Bergman J: Role of the dopaminergic system in chronic pain -- a fluorodopa-PET study. Pain 2001, 90: 257–260. 10.1016/S0304-3959(00)00409-7
Hagelberg N, Forssell H, Aalto S, Rinne JO, Scheinin H, Taiminen T, Nagren K, Eskola O, Jaaskelainen SK: Altered dopamine D2 receptor binding in atypical facial pain. Pain 2003, 106: 43–48. 10.1016/S0304-3959(03)00275-6
Wood PB, Schweinhardt P, Jaeger E, Dagher A, Hakyemez H, Rabiner EA, Bushnell MC, Chizh BA: Fibromyalgia patients show an abnormal dopamine response to pain. Eur J Neurosci 2007, 25: 3576–3582. 10.1111/j.1460-9568.2007.05623.x
Hagelberg N, Jaaskelainen SK, Martikainen IK, Mansikka H, Forssell H, Scheinin H, Hietala J, Pertovaara A: Striatal dopamine D2 receptors in modulation of pain in humans: a review. Eur J Pharmacol 2004, 500: 187–192. 10.1016/j.ejphar.2004.07.024
Wood PB: Role of central dopamine in pain and analgesia. Expert Rev Neurother 2008, 8: 781–797. 10.1586/14737175.8.5.781
Harris RE, Clauw DJ, Scott DJ, McLean SA, Gracely RH, Zubieta JK: Decreased central mu-opioid receptor availability in fibromyalgia. J Neurosci 2007, 27: 10000–10006. 10.1523/JNEUROSCI.2849-07.2007
Geuze E, Westenberg HG, Jochims A, de Kloet CS, Bohus M, Vermetten E, Schmahl C: Altered pain processing in veterans with posttraumatic stress disorder. Arch Gen Psychiatry 2007, 64: 76–85. 10.1001/archpsyc.64.1.76
Sanchez-Pernaute R, Jenkins BG, Choi JK, Iris Chen YC, Isacson O: In vivo evidence of D3 dopamine receptor sensitization in parkinsonian primates and rodents with l-DOPA-induced dyskinesias. Neurobiol Dis 2007, 27: 220–227. 10.1016/j.nbd.2007.04.016
Bhattacharyya S, Fusar-Poli P, Borgwardt S, Martin-Santos R, Nosarti C, O'Carroll C, Allen P, Seal ML, Fletcher PC, Crippa JA, et al.: Modulation of mediotemporal and ventrostriatal function in humans by Delta9-tetrahydrocannabinol: a neural basis for the effects of Cannabis sativa on learning and psychosis. Arch Gen Psychiatry 2009, 66: 442–451. 10.1001/archgenpsychiatry.2009.17
Kelly C, de Zubicaray G, Di Martino A, Copland DA, Reiss PT, Klein DF, Castellanos FX, Milham MP, McMahon K: L-dopa modulates functional connectivity in striatal cognitive and motor networks: a double-blind placebo-controlled study. J Neurosci 2009, 29: 7364–7378. 10.1523/JNEUROSCI.0810-09.2009
Borras MC, Becerra L, Ploghaus A, Gostic JM, DaSilva A, Gonzalez RG, Borsook D: fMRI measurement of CNS responses to naloxone infusion and subsequent mild noxious thermal stimuli in healthy volunteers. J Neurophysiol 2004, 91: 2723–2733. 10.1152/jn.00249.2003
Leppa M, Korvenoja A, Carlson S, Timonen P, Martinkauppi S, Ahonen J, Rosenberg PH, Aronen HJ, Kalso E: Acute opioid effects on human brain as revealed by functional magnetic resonance imaging. Neuroimage 2006, 31: 661–669. 10.1016/j.neuroimage.2005.12.019
Upadhyay J, Pendse G, Anderson J, Schwarz AJ, Baumgartner R, Coimbra A, Bishop J, Knudsen J, George E, Grachev I, et al.: Improved characterization of BOLD responses for evoked sensory stimuli. Neuroimage 2009.
Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Goodman JM, Kantor HL, Gastfriend DR, Riorden JP, et al.: Acute effects of cocaine on human brain activity and emotion. Neuron 1997, 19: 591–611. 10.1016/S0896-6273(00)80374-8
Schott BH, Minuzzi L, Krebs RM, Elmenhorst D, Lang M, Winz OH, Seidenbecher CI, Coenen HH, Heinze HJ, Zilles K, et al.: Mesolimbic functional magnetic resonance imaging activations during reward anticipation correlate with reward-related ventral striatal dopamine release. J Neurosci 2008, 28: 14311–14319. 10.1523/JNEUROSCI.2058-08.2008
Filbey FM, Schacht JP, Myers US, Chavez RS, Hutchison KE: Marijuana craving in the brain. Proc Natl Acad Sci USA 2009, 106: 13016–13021. 10.1073/pnas.0903863106
Hagelberg N, Kajander JK, Nagren K, Hinkka S, Hietala J, Scheinin H: Mu-receptor agonism with alfentanil increases striatal dopamine D2 receptor binding in man. Synapse 2002, 45: 25–30. 10.1002/syn.10078
Adler LJ, Gyulai FE, Diehl DJ, Mintun MA, Winter PM, Firestone LL: Regional brain activity changes associated with fentanyl analgesia elucidated by positron emission tomography. Anesth Analg 1997, 84: 120–126. 10.1097/00000539-199701000-00023
Wagner KJ, Sprenger T, Kochs EF, Tolle TR, Valet M, Willoch F: Imaging human cerebral pain modulation by dose-dependent opioid analgesia: a positron emission tomography activation study using remifentanil. Anesthesiology 2007, 106: 548–556. 10.1097/00000542-200703000-00020
Iannetti GD, Zambreanu L, Wise RG, Buchanan TJ, Huggins JP, Smart TS, Vennart W, Tracey I: Pharmacological modulation of pain-related brain activity during normal and central sensitization states in humans. Proc Natl Acad Sci USA 2005, 102: 18195–18200. 10.1073/pnas.0506624102
Guindon J, Hohmann AG: The Endocannabinoid System and Pain. CNS Neurol Disord Drug Targets 2009, 8: 403–421.
Bossong MG, van Berckel BN, Boellaard R, Zuurman L, Schuit RC, Windhorst AD, van Gerven JM, Ramsey NF, Lammertsma AA, Kahn RS: Delta 9-tetrahydrocannabinol induces dopamine release in the human striatum. Neuropsychopharmacology 2009, 34: 759–766. 10.1038/npp.2008.138
Rabiner EA: Imaging of striatal dopamine release elicited with NMDA antagonists: is there anything there to be seen? J Psychopharmacol 2007, 21: 253–258. 10.1177/0269881107077767
Downar J, Mikulis DJ, Davis KD: Neural correlates of the prolonged salience of painful stimulation. Neuroimage 2003, 20: 1540–1551. 10.1016/S1053-8119(03)00407-5
Brown MT, Henny P, Bolam JP, Magill PJ: Activity of neurochemically heterogeneous dopaminergic neurons in the substantia nigra during spontaneous and driven changes in brain state. J Neurosci 2009, 29: 2915–2925. 10.1523/JNEUROSCI.4423-08.2009
Pinzon-Morales RD, Garces-Arboleda M, Orozco-Gutierrez AA: Automatic identification of various nuclei in the basal ganglia for Parkinson's disease neurosurgery. Conf Proc IEEE Eng Med Biol Soc 2009, 1: 3473–3476.
Redgrave P, Prescott TJ, Gurney K: The basal ganglia: a vertebrate solution to the selection problem? Neuroscience 1999, 89: 1009–1023. 10.1016/S0306-4522(98)00319-4
McHaffie JG, Stanford TR, Stein BE, Coizet V, Redgrave P: Subcortical loops through the basal ganglia. Trends Neurosci 2005, 28: 401–407. 10.1016/j.tins.2005.06.006
Schulz JM, Redgrave P, Mehring C, Aertsen A, Clements KM, Wickens JR, Reynolds JN: Short-latency activation of striatal spiny neurons via subcortical visual pathways. J Neurosci 2009, 29: 6336–6347. 10.1523/JNEUROSCI.4815-08.2009
Lima MC, Fregni F: Motor cortex stimulation for chronic pain: systematic review and meta-analysis of the literature. Neurology 2008, 70: 2329–2337. 10.1212/01.wnl.0000314649.38527.93
Lefaucheur JP: Use of repetitive transcranial magnetic stimulation in pain relief. Expert Rev Neurother 2008, 8: 799–808. 10.1586/14737175.8.5.799
Andre-Obadia N, Mertens P, Gueguen A, Peyron R, Garcia-Larrea L: Pain relief by rTMS: differential effect of current flow but no specific action on pain subtypes. Neurology 2008, 71: 833–840. 10.1212/01.wnl.0000325481.61471.f0
Valmunen T, Pertovaara A, Taiminen T, Virtanen A, Parkkola R, Jaaskelainen SK: Modulation of facial sensitivity by navigated rTMS in healthy subjects. Pain 2009, 142: 149–158. 10.1016/j.pain.2008.12.031
Roux FE, Ibarrola D, Lazorthes Y, Berry I: Chronic motor cortex stimulation for phantom limb pain: a functional magnetic resonance imaging study: technical case report. Neurosurgery 2008, 62: 978–985. 10.1227/01.neu.0000333765.28198.18
Garcia-Larrea L, Peyron R: Motor cortex stimulation for neuropathic pain: From phenomenology to mechanisms. Neuroimage 2007,37(Suppl 1):S71–79. 10.1016/j.neuroimage.2007.05.062
Maarrawi J, Peyron R, Mertens P, Costes N, Magnin M, Sindou M, Laurent B, Garcia-Larrea L: Motor cortex stimulation for pain control induces changes in the endogenous opioid system. Neurology 2007, 69: 827–834. 10.1212/01.wnl.0000269783.86997.37
Ma QP, Shi YS, Han JS: Further studies on interactions between periaqueductal gray, nucleus accumbens and habenula in antinociception. Brain Res 1992, 583: 292–295. 10.1016/S0006-8993(10)80036-8
Altier N, Stewart J: The role of dopamine in the nucleus accumbens in analgesia. Life Sci 1999, 65: 2269–2287. 10.1016/S0024-3205(99)00298-2
Gear RW, Aley KO, Levine JD: Pain-induced analgesia mediated by mesolimbic reward circuits. J Neurosci 1999, 19: 7175–7181.
Wilkinson HA: Bilateral anterior cingulotomy for chronic noncancer pain. Neurosurgery 2000, 46: 1535–1536. 10.1097/00006123-200006000-00051
Ren LY, Lu ZM, Liu MG, Yu YQ, Li Z, Shang GW, Chen J: Distinct roles of the anterior cingulate cortex in spinal and supraspinal bee venom-induced pain behaviors. Neuroscience 2008, 153: 268–278. 10.1016/j.neuroscience.2008.01.067
LaGraize SC, Borzan J, Peng YB, Fuchs PN: Selective regulation of pain affect following activation of the opioid anterior cingulate cortex system. Exp Neurol 2006, 197: 22–30. 10.1016/j.expneurol.2005.05.008
LaGraize SC, Labuda CJ, Rutledge MA, Jackson RL, Fuchs PN: Differential effect of anterior cingulate cortex lesion on mechanical hypersensitivity and escape/avoidance behavior in an animal model of neuropathic pain. Exp Neurol 2004, 188: 139–148. 10.1016/j.expneurol.2004.04.003
Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, Rosen BR: Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc Natl Acad Sci USA 2002, 99: 523–528. 10.1073/pnas.012470999
Williams ZM, Bush G, Rauch SL, Cosgrove GR, Eskandar EN: Human anterior cingulate neurons and the integration of monetary reward with motor responses. Nat Neurosci 2004, 7: 1370–1375. 10.1038/nn1354
Baleydier C, Mauguiere F: The duality of the cingulate gyrus in monkey. Neuroanatomical study and functional hypothesis. Brain 1980, 103: 525–554. 10.1093/brain/103.3.525
LaBuda CJ, Fuchs PN: Attenuation of negative pain affect produced by unilateral spinal nerve injury in the rat following anterior cingulate cortex activation. Neuroscience 2005, 136: 311–322. 10.1016/j.neuroscience.2005.07.010
Graybiel AM: The basal ganglia and chunking of action repertoires. Neurobiol Learn Mem 1998, 70: 119–136. 10.1006/nlme.1998.3843
Ford B: Pain in Parkinson's disease. Clin Neurosci 1998, 5: 63–72. 10.1016/S0967-5868(98)90204-1
Schestatsky P, Kumru H, Valls-Sole J, Valldeoriola F, Marti MJ, Tolosa E, Chaves ML: Neurophysiologic study of central pain in patients with Parkinson disease. Neurology 2007, 69: 2162–2169. 10.1212/01.wnl.0000295669.12443.d3
Post RM: Do the epilepsies, pain syndromes, and affective disorders share common kindling-like mechanisms? Epilepsy Res 2002, 50: 203–219. 10.1016/S0920-1211(02)00081-5
Ji RR, Kohno T, Moore KA, Woolf CJ: Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci 2003, 26: 696–705. 10.1016/j.tins.2003.09.017
McNally GP: Pain facilitatory circuits in the mammalian central nervous system: their behavioral significance and role in morphine analgesic tolerance. Neurosci Biobehav Rev 1999, 23: 1059–1078. 10.1016/S0149-7634(99)00040-8
Kaji R: [Sensory-motor disintegration in the basal ganglia disorders]. Rinsho Shinkeigaku 2001, 41: 1076–1078.
Egloff N, Sabbioni ME, Salathe C, Wiest R, Juengling FD: Nondermatomal somatosensory deficits in patients with chronic pain disorder: Clinical findings and hypometabolic pattern in FDG-PET. Pain 2009.
Wessel K, Vieregge P, Kessler C, Kompf D: Thalamic stroke: correlation of clinical symptoms, somatosensory evoked potentials, and CT findings. Acta Neurol Scand 1994, 90: 167–173. 10.1111/j.1600-0447.1994.tb01573.x
Sadikot AF, Rymar VV: The primate centromedian-parafascicular complex: anatomical organization with a note on neuromodulation. Brain Res Bull 2009, 78: 122–130. 10.1016/j.brainresbull.2008.09.016
Sidibe M, Pare JF, Smith Y: Nigral and pallidal inputs to functionally segregated thalamostriatal neurons in the centromedian/parafascicular intralaminar nuclear complex in monkey. J Comp Neurol 2002, 447: 286–299. 10.1002/cne.10247
Francois C, Sintonen H, Sulkava R, Rive B: Cost effectiveness of memantine in moderately severe to severe Alzheimer's disease: a markov model in Finland. Clin Drug Investig 2004, 24: 373–384. 10.2165/00044011-200424070-00001
Baumgartner U, Buchholz HG, Bellosevich A, Magerl W, Siessmeier T, Rolke R, Hohnemann S, Piel M, Rosch F, Wester HJ, et al.: High opiate receptor binding potential in the human lateral pain system. Neuroimage 2006, 30: 692–699. 10.1016/j.neuroimage.2005.10.033
Comings DE, Blum K: Reward deficiency syndrome: genetic aspects of behavioral disorders. Prog Brain Res 2000, 126: 325–341. full_text
Martikainen IK, Hagelberg N, Mansikka H, Hietala J, Nagren K, Scheinin H, Pertovaara A: Association of striatal dopamine D2/D3 receptor binding potential with pain but not tactile sensitivity or placebo analgesia. Neurosci Lett 2005, 376: 149–153. 10.1016/j.neulet.2004.11.045
Pertovaara A, Martikainen IK, Hagelberg N, Mansikka H, Nagren K, Hietala J, Scheinin H: Striatal dopamine D2/D3 receptor availability correlates with individual response characteristics to pain. Eur J Neurosci 2004, 20: 1587–1592. 10.1111/j.1460-9568.2004.03622.x
Scott DJ, Heitzeg MM, Koeppe RA, Stohler CS, Zubieta JK: Variations in the human pain stress experience mediated by ventral and dorsal basal ganglia dopamine activity. J Neurosci 2006, 26: 10789–10795. 10.1523/JNEUROSCI.2577-06.2006
Patt RB, Proper G, Reddy S: The neuroleptics as adjuvant analgesics. J Pain Symptom Manage 1994, 9: 446–453. 10.1016/0885-3924(94)90201-1
Seidel S, Aigner M, Ossege M, Pernicka E, Wildner B, Sycha T: Antipsychotics for acute and chronic pain in adults. Cochrane Database Syst Rev 2008, CD004844.
Fishbain DA, Cutler RB, Lewis J, Cole B, Rosomoff RS, Rosomoff HL: Do the second-generation "atypical neuroleptics" have analgesic properties? A structured evidence-based review. Pain Med 2004, 5: 359–365. 10.1111/j.1526-4637.2004.04054.x
Kupers RC, Gybels JM, Gjedde A: Positron emission tomography study of a chronic pain patient successfully treated with somatosensory thalamic stimulation. Pain 2000, 87: 295–302. 10.1016/S0304-3959(00)00295-5
Duncan GH, Kupers RC, Marchand S, Villemure JG, Gybels JM, Bushnell MC: Stimulation of human thalamus for pain relief: possible modulatory circuits revealed by positron emission tomography. J Neurophysiol 1998, 80: 3326–3330.
Peyron R, Faillenot I, Mertens P, Laurent B, Garcia-Larrea L: Motor cortex stimulation in neuropathic pain. Correlations between analgesic effect and hemodynamic changes in the brain. A PET study. Neuroimage 2007, 34: 310–321. 10.1016/j.neuroimage.2006.08.037
Becerra L, Iadarola M, Borsook D: CNS activation by noxious heat to the hand or foot: site-dependent delay in sensory but not emotion circuitry. J Neurophysiol 2004, 91: 533–541. 10.1152/jn.00326.2003
Strigo IA, Duncan GH, Boivin M, Bushnell MC: Differentiation of visceral and cutaneous pain in the human brain. J Neurophysiol 2003, 89: 3294–3303. 10.1152/jn.01048.2002
Oshiro Y, Quevedo AS, McHaffie JG, Kraft RA, Coghill RC: Brain mechanisms supporting spatial discrimination of pain. J Neurosci 2007, 27: 3388–3394. 10.1523/JNEUROSCI.5128-06.2007
Keltner JR, Furst A, Fan C, Redfern R, Inglis B, Fields HL: Isolating the modulatory effect of expectation on pain transmission: a functional magnetic resonance imaging study. J Neurosci 2006, 26: 4437–4443. 10.1523/JNEUROSCI.4463-05.2006
Brooks JC, Zambreanu L, Godinez A, Craig AD, Tracey I: Somatotopic organisation of the human insula to painful heat studied with high resolution functional imaging. Neuroimage 2005, 27: 201–209. 10.1016/j.neuroimage.2005.03.041
Dunckley P, Wise RG, Fairhurst M, Hobden P, Aziz Q, Chang L, Tracey I: A comparison of visceral and somatic pain processing in the human brainstem using functional magnetic resonance imaging. J Neurosci 2005, 25: 7333–7341. 10.1523/JNEUROSCI.1100-05.2005
Bingel U, Glascher J, Weiller C, Buchel C: Somatotopic representation of nociceptive information in the putamen: an event-related fMRI study. Cereb Cortex 2004, 14: 1340–1345. 10.1093/cercor/bhh094
Zhang WT, Jin Z, Cui GH, Zhang KL, Zhang L, Zeng YW, Luo F, Chen AC, Han JS: Relations between brain network activation and analgesic effect induced by low vs. high frequency electrical acupoint stimulation in different subjects: a functional magnetic resonance imaging study. Brain Res 2003, 982: 168–178. 10.1016/S0006-8993(03)02983-4
Gracely RH, Petzke F, Wolf JM, Clauw DJ: Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum 2002, 46: 1333–1343. 10.1002/art.10225
Lee MC, Zambreanu L, Menon DK, Tracey I: Identifying brain activity specifically related to the maintenance and perceptual consequence of central sensitization in humans. J Neurosci 2008, 28: 11642–11649. 10.1523/JNEUROSCI.2638-08.2008
Lu CL, Wu YT, Yeh TC, Chen LF, Chang FY, Lee SD, Ho LT, Hsieh JC: Neuronal correlates of gastric pain induced by fundus distension: a 3T-fMRI study. Neurogastroenterol Motil 2004, 16: 575–587. 10.1111/j.1365-2982.2004.00562.x
Geha PY, Baliki MN, Wang X, Harden RN, Paice JA, Apkarian AV: Brain dynamics for perception of tactile allodynia (touch-induced pain) in postherpetic neuralgia. Pain 2008, 138: 641–656. 10.1016/j.pain.2008.02.021
Burgmer M, Pogatzki-Zahn E, Gaubitz M, Wessoleck E, Heuft G, Pfleiderer B: Altered brain activity during pain processing in fibromyalgia. Neuroimage 2009, 44: 502–508. 10.1016/j.neuroimage.2008.09.008
Gracely RH, Geisser ME, Giesecke T, Grant MA, Petzke F, Williams DA, Clauw DJ: Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain 2004, 127: 835–843. 10.1093/brain/awh098
Baliki MN, Geha PY, Jabakhanji R, Harden N, Schnitzer TJ, Apkarian AV: A preliminary fMRI study of analgesic treatment in chronic back pain and knee osteoarthritis. Mol Pain 2008, 4: 47. 10.1186/1744-8069-4-47
Song GH, Venkatraman V, Ho KY, Chee MW, Yeoh KG, Wilder-Smith CH: Cortical effects of anticipation and endogenous modulation of visceral pain assessed by functional brain MRI in irritable bowel syndrome patients and healthy controls. Pain 2006, 126: 79–90. 10.1016/j.pain.2006.06.017
Gu X, Han S: Attention and reality constraints on the neural processes of empathy for pain. Neuroimage 2007, 36: 256–267. 10.1016/j.neuroimage.2007.02.025
Botvinick M, Jha AP, Bylsma LM, Fabian SA, Solomon PE, Prkachin KM: Viewing facial expressions of pain engages cortical areas involved in the direct experience of pain. Neuroimage 2005, 25: 312–319. 10.1016/j.neuroimage.2004.11.043
Ushida T, Ikemoto T, Tanaka S, Shinozaki J, Taniguchi S, Murata Y, McLaughlin M, Arai YC, Tamura Y: Virtual needle pain stimuli activates cortical representation of emotions in normal volunteers. Neurosci Lett 2008, 439: 7–12. 10.1016/j.neulet.2008.04.085
Jones AK, Brown WD, Friston KJ, Qi LY, Frackowiak RS: Cortical and subcortical localization of response to pain in man using positron emission tomography. Proc Biol Sci 1991, 244: 39–44. 10.1098/rspb.1991.0048
Coghill RC, Gilron I, Iadarola MJ: Hemispheric lateralization of somatosensory processing. J Neurophysiol 2001, 85: 2602–2612.
Coghill RC, Sang CN, Maisog JM, Iadarola MJ: Pain intensity processing within the human brain: a bilateral, distributed mechanism. J Neurophysiol 1999, 82: 1934–1943.
Korotkov A, Ljubisavljevic M, Thunberg J, Kataeva G, Roudas M, Pakhomov S, Radovanovic S, Lyskov E, Medvedev S, Johansson H: Changes in human regional cerebral blood flow following hypertonic saline induced experimental muscle pain: a positron emission tomography study. Neurosci Lett 2002, 335: 119–123. 10.1016/S0304-3940(02)01181-3
Ladabaum U, Minoshima S, Hasler WL, Cross D, Chey WD, Owyang C: Gastric distention correlates with activation of multiple cortical and subcortical regions. Gastroenterology 2001, 120: 369–376. 10.1053/gast.2001.21201
Schmidt B, Mayr J, Fasching G, Nores H: [Equestrian accidents in children and adolescents]. Unfallchirurg 1994, 97: 661–662.
Firestone LL, Gyulai F, Mintun M, Adler LJ, Urso K, Winter PM: Human brain activity response to fentanyl imaged by positron emission tomography. Anesth Analg 1996, 82: 1247–1251. 10.1097/00000539-199606000-00025
Hartvig P, Valtysson J, Lindner KJ, Kristensen J, Karlsten R, Gustafsson LL, Persson J, Svensson JO, Oye I, Antoni G, et al.: Central nervous system effects of subdissociative doses of (S)-ketamine are related to plasma and brain concentrations measured with positron emission tomography in healthy volunteers. Clin Pharmacol Ther 1995, 58: 165–173. 10.1016/0009-9236(95)90194-9
Koyama T, Kato K, Mikami A: During pain-avoidance neurons activated in the macaque anterior cingulate and caudate. Neurosci Lett 2000, 283: 17–20. 10.1016/S0304-3940(00)00894-6
Borsook D, Becerra L, Hargreaves R: A role for fMRI in optimizing CNS drug development. Nat Rev Drug Discov 2006, 5: 411–424. 10.1038/nrd2027
Stancanello J, Romanelli P, Pantelis E, Sebastiano F, Modugno N: Atlas-based functional radiosurgery: early results. Med Phys 2009, 36: 457–463. 10.1118/1.3056460
Anagnostakis Y, Zis V, Spyraki C: Analgesia induced by morphine injected into the pallidum. Behav Brain Res 1992, 48: 135–143. 10.1016/S0166-4328(05)80149-4
Benabid AL, Wallace B, Mitrofanis J, Xia C, Piallat B, Fraix V, Batir A, Krack P, Pollak P, Berger F: Therapeutic electrical stimulation of the central nervous system. C R Biol 2005, 328: 177–186. 10.1016/j.crvi.2004.10.011
Eagle DM, Baunez C: Is there an inhibitory-response-control system in the rat? Evidence from anatomical and pharmacological studies of behavioral inhibition. Neurosci Biobehav Rev 2010, 34: 50–72. 10.1016/j.neubiorev.2009.07.003
Cohen MX, Schoene-Bake JC, Elger CE, Weber B: Connectivity-based segregation of the human striatum predicts personality characteristics. Nat Neurosci 2009, 12: 32–34. 10.1038/nn.2228
Bernard JF, Carroue J, Besson JM: Efferent projections from the external parabrachial area to the forebrain: a Phaseolus vulgaris leucoagglutinin study in the rat. Neurosci Lett 1991, 122: 257–260. 10.1016/0304-3940(91)90872-Q
Hodge CJ Jr, Apkarian AV: The spinothalamic tract. Crit Rev Neurobiol 1990, 5: 363–397.
Acknowledgements
This work was supported by NINDS K24 NS064050 (DB) and the L Herlands Grant for Pain Research (DB and LB). We would like to thank N. Maleki for help with some of the brain images.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
DB and JU conceptualized the paper. All authors contributed to the drafting of the paper. All authors have read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Borsook, D., Upadhyay, J., Chudler, E.H. et al. A key role of the basal ganglia in pain and analgesia - insights gained through human functional imaging. Mol Pain 6, 27 (2010). https://doi.org/10.1186/1744-8069-6-27
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1744-8069-6-27