Abstract
Pain is comprised of the sensory and affective components. Compared to the well-investigated mechanisms of the sensory pain, much less is known about the mechanisms underlying the affective pain. In recent years, accumulating evidence suggests that the anterior cingulate cortex (ACC) is a key structure for pain affection. To identify the molecules that may be involved in the affective component of pain, we have searched the Allen Brain Atlas expression database for genes whose expression is enriched in the ACC, and found that P311, an 8-kDa peptide, showed the strong expression in the ACC. P311 is also expressed in other areas associated with pain affection including the amygdala, insular cortex and thalamus. To understand the role of P311 in pain perception, we have examined the pain behaviors of the mice lacking P311. P311-/- mice showed normal heat and mechanical sensitivity, as well as normal formalin-induced inflammatory pain. In contrast, the formalin-induced avoidance behavior, which reflects pain-related negative emotion, was significantly attenuated in P311-/- mice relative to the control mice. These results suggest that P311 is involved in the affective, but not in the sensory component of pain. Our study thus provides the first evidence suggesting that the affective and sensory pain may be regulated by distinct molecular mechanisms.
Similar content being viewed by others
Introduction
The pain experience includes a sensory-discriminative dimension, such as its location, intensity and quality, and an affective dimension, such as unpleasantness and emotions associated with future implications (secondary affect) [1–3]. Recent studies in humans and experimental animals have established that the sensory and affective dimensions of pain are processed by partially dissociable brain networks [2, 3]. Human imaging studies showed that the activity of the somatosensory (SS) cortex is correlated with the intensity of the noxious heat, whereas that of the anterior cingulate cortex (ACC) with subjective unpleasantness [4, 5], suggesting a differentially functional requirement for the SS cortex and ACC in pain perception [3]. Animal behavioral studies using the formalin-induced conditioned place avoidance (F-CPA) or place escape/avoidance paradigm also support the hypothesis that the ACC mediates the affective-like responses of tonic pain [6–9].
Electrophysiological studies on neuroplasticity in the ACC have also suggested an involvement of the ACC in pain-related information processing [10, 11]. It has been shown that the ACC neurons respond to noxious stimuli [12]. Importantly, peripheral injury caused long-term potentiation (LTP) in the ACC [13]. LTP may be the mechanisms shared by both pain and memory [14]. LTP can be induced in the ACC, and numerous molecular pathways underlying the LTP in the ACC have been identified by genetic and pharmacological approaches [10, 15, 16]. In contrast to molecular mechanisms of the sensory component of pain which have been subjected to intensive studies [17], the molecular basis for pain affection remains elusive. To elucidate the molecular mechanisms underlying the affective component of pain, we identified several genes that are highly expressed in the ACC compared with the SS cortex, and examined one of the genes, P311, in pain affection.
P311 (also called PTZ17) was first identified because of its high expression in the embryonic brain and does not belong to any known family [18]. P311 encodes an 8-kDa polypeptide and contains three PEST-like domains subjected to rapid degradation by multiple proteolytic pathways [19]. As a non-secreted peptide, P311 is highly conserved across the species and is localized in both the cytoplasm and nucleus [19, 20]. Previous studies have implicated P311 in the induction of ameboid-like migration [21], glioblastoma cell migration [22], and in the myogenesis of smooth muscle myogenesis [19]. In the brain, P311 has been associated with seizures because its expression altered when seizures was induced [23]. Moreover, P311 expression was found to be up-regulated in the axotomized motoneurons during axonal elongation, and its overexpression can further enhance axon elongation of neurons in vivo and in vitro [20]. In this report, we examined the role of P311 not only in the sensory but also in the affective component of pain processing in mice.
Methods
Identification of ACC higher genes
The ACC-enriched genes were identified by searching the Allen Brain Atlas (ABA) [24]. Total of 2265 genes expressed in the cortex, of which 763 have coronal section expression data. The expression images of each gene (0–1.0 mm anterior to Bregma) containing both the ACC and SS were retrieved from ABA. And the expression level of each gene in the ACC and the SS measured by the density (ACC: VACC, SS: VSS.) was analyzed using a NIH ImageJ software by manually defining the ACC and SS region in every section (0 represents the lowest expression, 255 represents the highest expression). The difference (D) is calculated by D = VACC-VSS, and the genes are sorted by descending of D value. The higher D value means the higher expression in the ACC. The top 10 genes with higher expression in the ACC were listed in Table 1.
Animals
P311-/- and wild-type littermate mice were used in behavior experiments (Taylor et al, manuscript submitted). Male mice aged between 8 and 12 weeks were acclimatized to the experimental room and used for behavioral tests by observers blinded to the genotype of the animals. All the experiments were performed in accordance with the guidelines of the National Institutes of Health and the International Association for the Study of Pain and were approved by the Animal Studies Committee at Washington University School of Medicine.
Pain behavioral experiments
Pain behavior tests were performed as described [25, 26]. Briefly, thermal sensitivity was determined using hot-plate (48, 52, 56°C), paw-flick (method of Hargreaves) or water immersion tail-flick methods (48, 50, 52°C). For the hot plate, the latency for the mouse to lick its hindpaw or jump was recorded. For the Hargreaves test, thermal sensitivity was measured using a Hargreaves-type apparatus (IITC Inc., Woodland Hills, CA), and the latency for the mouse to withdraw from the heat source was recorded. For water immersion tail-flick, tails dipped beneath the water in a temperature-controlled water bath (IITC Inc., Woodland Hills, CA), the latency to withdrawal was measured with a 10-s cutoff. Mechanical sensitivity: Mechanical sensitivity was assessed using a set of calibrated von Frey filaments (Touch-Test kit, Stoelting, Chicago, IL). Each filament was applied 5 consecutive times and the smallest filament that evoked reflexive flinches of the paw on 3 of the 5 trials was taken as paw withdrawal threshold. The formalin test was performed by intraplantar injection of formalin (Sigma, 15 μl of 5% formalin in saline) into the plantar surface of the right hindpaw. The total time spent in licking and flinching of the injected paw was monitored for 60 min at 5-min intervals.
Assessment of motor function
A rotarod system of accelerating treadmills (Ugo Basile, Italy) was used to assess coordinate motor activity and general motor disability as described [27]. The mice were tested for 3 trials with 15-min intervals.
Formalin-induced conditioned place avoidance (F-CPA)
This procedure was modified from F-CPA in rats [6, 7]. Animals were trained in a shuttle box (47.5 × 20 × 20 cm3), containing three compartments separated by guillotine doors. Two large conditioning compartments (A and B, 20 × 20 × 20 cm3) were separated by a small gray center choice compartment (C, 7.5 × 20 × 20 cm3). The A compartment had white walls and stiff metal mesh flooring with an odor of 1.0% acetic acid, and the B compartment had black walls and parallel metal bars flooring with cinnamon scent. The C compartment had gray walls and a plain floor without distinctive odor. The experimental procedures were recorded by a video camera connected to a computer. The training procedure lasted for 5 days. The pre-conditioning test was performed on Day 1. Mice were placed into the central compartment and allowed to freely explore the entire apparatus for 20 min. Time spent in each compartment and the travel distance in three compartments was recorded. Any animal that spent more than 600 sec or less than 300 sec in any large compartment will be discarded from the experiment. Only animals that did not show a baseline preference were admitted into the study (>90% mice met this criteria). Then, mice were conditioned for 3 consecutive days with 2 pairing sessions each day. In the first session, the animals were restricted to one of the conditioning compartments (50% of the mice in compartment A, 50% of them in compartment B) for 35 min in the morning. In the second session in the afternoon, about 4 hr later, the animals received hindpaw injection of 5% formalin (15 μl) or normal saline (15 μl), and restricted to the opposite conditioning compartment for 35 min. For post-conditioning test on day 5, the animals were tested as day 1. On both day 1 and day 5, the animals' activities were recorded by a wide-angle video camera (Creative Technology Ltd.) connected with a computer, and the travel distance and time in each compartment were analyzed by ANYMAZE software (Stoelting Co., Wood Dale, Illinois USA).
LiCl-induced conditioned place aversion (LiCl-CPA)
The apparatus for LiCl-CPA training was identical to that in F-CPA. The training procedure was also similar to that of F-CPA. In brief, the training procedure lasted for 5 days. On day 1, mice were placed into the central compartment and allowed to freely explore the entire apparatus for 20 min. Time spent in each compartment and the travel distance in three compartments was recorded. Animals showing baseline preference were discarded from the experiment. On day 2–4, mice were conditioned for 3 consecutive days with 2 pairing sessions each day. In the first session, the animals received saline (10 ml/kg, i.p.) and restricted to one of the conditioning compartments for 35 min in the morning. In the second session in the afternoon, the animals received LiCl (150 mg/kg, i.p.) and restricted to the opposite conditioning compartment for 35 min. On day 5, the animals were tested as day 1. On both day 1 and day 5, the animals' activities were recorded. The travel distance and time in each compartment were analyzed.
In situ hybridization
Mice were anesthetized with sodium pentobarbital (50 mg/kg i.p.) and euthanized by transcardiac perfusion (saline wash, followed by 4% paraformaldehyde in 0.01 M phosphate buffer saline pH 7.4). The mouse brain was removed and post-fixed for 4 hr, then stored in 0.01 M PBS containing 30% sucrose for at least 24 hr for cryoprotection. Thin sections were made using an RNase-free technique. In situ hybridization was performed as described [25].
Statistical analysis
Statistical comparisons were performed with two-way analysis of variance (ANOVA) or Student's t-test. Data are shown as mean ± S.E.M. (standard Error of Mean) and error bars represent S.E.M. In all cases, P < 0.05 was considered statistically significant.
Results
Identification of the ACC-enriched genes
To study the molecular mechanism of pain affection, we have searched the Allen Brain Atlas expression database [24] for genes whose expression is higher in the ACC (Table 1). Three genes, Marcksl1, P311, Etv1, showed the restricted expression pattern in the ACC.
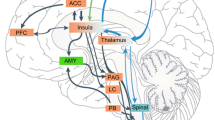
In situ hybridization studies indicated that P311 was abundantly expressed in the ACC as well as in other pain affection related regions of the brain (Fig. 1A, 1C, 1D, data not shown) [2, 7]. P311 was weakly detected in the somatosensory cortex and dorsal spinal cord (Fig. 1B, data not shown). Expression of P311 was also detected in the hippocampus and cerebellum, which is consistent with the previous report [18]. P311 expression was strong in the rostral ventromedial medulla (data not shown), but not detected in the hypothalamus. Despite its extensive expression in the brain, the function of P311 in the nervous system remains largely unknown.
Expression pattern of P311 in mouse brain detected by in situ hybridization. A. P311 is expressed in the anterior cingulate cortex (ACC). B. Weak expression of P311 was detected in the somatosensory cortex (SI). C. P311 is also expressed in the centrolateral thalamic nucleus (CL), the central medial thalamic nucleus (CM) and the paraventricular thalamic nucleus (PV). D. In the amygdala, P311 is expressed in the basolateral amygdala nucleus (BLA) and the lateral amygdala nucleus (LA). Scale bar: A. 100 μm (A-D).
Effects of the P311 mutation on the sensory component of pain perception
Because P311 is expressed in the sensory pathway of pain, we first assessed the role of P311 in pain by comparing thermal, mechanical and inflammatory pain responses of P311-/- mice with their wild-type littermates [25]. We found that the latencies responding to noxious thermal stimuli in Hargreaves test, hotplate, and tail flick test as well as mechanical stimuli produced by graded von Frey filaments were indistinguishable between P311-/- and wild-type mice (Fig. 2A–D). P311-/- mice exhibited normal motor function (Fig. 2E). In formalin test, flinching and licking behaviors in both the first phase (0–10 min) and the second phase (10–60 min) were also comparable between P311-/- and wild-type mice (Fig. 2F), indicating that a loss of P311 does not impact normal inflammatory pain response. Together, these data suggest that P311 is not essential for the sensory component of pain perception.
Normal pain behaviors and locomotor activity in P311-/- mice. A. Acute pain measured by mechanical threshold was comparable between wild-type (n = 8; white bars) and P311-/- mice (n = 9; black bars). B-D. Responses to noxious thermal stimuli were measured by the paw withdrawal latency to Hargreaves test (B), hotplate (C) and the water immersion tail-flick latency (D). Thermal pain in all tests did not differ between wild-type (n = 8; white bars) and P311-/- mice (n = 9; black bars). Student's t-test, P > 0.05. E. Motor function assessed by the rotarod test was not affected in P311-/- mice (n = 9; filled circles) compared with wild-type mice (n = 8; open circles). Repeated measures analysis of variance; P > 0.05. F. Spontaneous pain responses induced by formalin were comparable between wild-type (n = 9; open circles) and P311-/- mice (n = 10; filled circles). Repeated measures analysis of variance; P > 0.05, Phase I (0–10 min); P > 0.05, Phase II (10–60 min). G. Travel distance was monitored during the pre-test (day 1) and post-test day (day 5) of experiment by the ANYMAZE software. There was no significant difference between wild-type and P311-/- mice on both the pre-test and post-test day. And the travel distance was not affected by formalin injection in either wild-type or P311-/- mice. n = 16 for each group. Student's t-test, P > 0.05.
Effects of the P311 mutation on the affective component of pain
We next tested the requirement of P311 in the affective component of pain by F-CPA, which is a well-established model for testing pain affection in rats [6, 7]. When hindpaw formalin injections were paired with a particular compartment in the place-conditioning apparatus, wild-type mice spent significantly less time in this compartment on the post-conditioning test day as compared with the preconditioning test day (Fig. 3A). In the saline-treated group, animals showed no significant avoidance to the conditioned environment (Fig. 3A). The time spent in the treatment-paired compartment of formalin-treated mice was significantly less than that of saline-treated mice on post-test day (Fig. 3A). These results validated the utility of the F-CPA model in mice. When the same training procedure was used in P311-/- mice, CPA was not induced (Fig. 3A). The finding that P311-/- mice, unlike their littermates, displayed no aversive behaviors to formalin injections suggests that P311 is important for the acquisition or expression of F-CPA. In addition, the travel distance on both pre-test and post-test day was comparable between wild-type and P311-/- mice (Fig. 2G), suggesting that lack of F-CPA in P311-/- mice was not caused by a change in locomotor activity.
Defect of pain affection in P311-/- mice. Time spent in each compartment was monitored during the pre-test (day 1) and post-test day (day 5) of the experiments. A. In wild-type mice, formalin (n = 16) but not saline (n = 12) induced conditioned place avoidance. Conditioned place avoidance was induced by formalin in wild-type mice (n = 16), but not in P311-/- mice (n = 16). Student's t-test, *P < 0.05, ***P < 0.001. B. LiCl (150 mg/kg, i.p.) induced conditioned place aversion in both wild-type (n = 10) and P311-/- mice (n = 10) in a comparable manner. Student's t-test, ***P < 0.001.
LiCl-CPA was not affected by the absence of P311
To distinguish whether lack of aversive behavior in P311-/- mice was confounded by a deficit in associative learning and memory, we next examined aversive learning task of P311-/- and wild-type mice by using the LiCl-CPA paradigm [28]. LiCl induced CPA in both wild-type and P311-/- mice (Fig. 3B), suggesting that the P311 mutation did not reduce the animal's ability to acquire a CPA when the stimulus was unpainfully aversive. This result further suggests that the absence of P311 did not impact the animal's ability to associate the aversive stimulus with the distinct environmental context. Therefore, we conclude that the absence of P311 does not alter the learning ability in the place-conditioning paradigm, but rather results in a deficit concerning the acquisition or expression of pain-related aversion.
Discussion
In this study, we have identified several genes including P311 which are highly enriched in the ACC. We set out to examine whether P311 is important for pain perception. Despite P311 is also expressed in neural pathways required for mediating sensory component of pain, we found that mice lacking p311 showed normal acute and persistent pain behaviors in several pain paradigms. Remarkably, P311-/- mice showed deficits in pain affection assessed by the F-CPA paradigm.
Our finding that the P311 mutation abolished CPA induced by formalin suggests that P311 is important for the aversion behavior induced by painful stimuli. Since it is well accepted that the avoidance behavior associated with noxious stimuli can serve as a reflex of affective component of pain, it is reasonable to believe that P311 is necessary for the acquisition or expression of avoidance behavior induced by the noxious stimulus, and thus pain affection. Importantly, this compromised affective pain in the mutant mice is not due to a deficit in associate learning and memory. This is evidenced by the result of the LiCl-CPA paradigm, in which CPA was induced at a similar manner in both wild-type and P311-/- mice. This result further indicates that P311 is not involved in the aversion behavior associated with non-painful stimuli. Although P311 is also expressed in the rostral ventromedial medulla and the dorsal spinal cord, the findings that the pain behaviors of P311-/- mice were normal in several thermal, mechanical and chemical pain paradigms suggest that P311 is dispensable for the sensory processing of pain.
Involvement of P311 in pain affection might not be restricted to the ACC since P311 is also expressed in the regions important for pain affection such as the amygdala, and the thalamus (mainly in the medial/intralaminar thalamic nuclei) [2]. In addition to its role in fear conditioning [29, 30], the amygdala has also been implicated in processing pain affect. Bilateral lesion of the amygdala significantly reduced conditioned response in the laser-pain conditioning model [31] and F-CPA model [7, 32] without affecting normal behavior or baseline nociceptive responses, suggestive of an involvement of the amygdala in pain affection. The medial/intralaminar thalamic nuclei which relay the information from the dorsal spinal cord to the ACC and the insular cortex has been proposed to process pain-related unpleasantness [2, 33, 34]. Although there is no direct evidence from animal experiments showing the involvement of the insular cortex in pain affect, the widespread connections among the insular cortex, thalamus, amygdala, hippocampus and cortical regions related to sensory modalities and autonomic functions suggest the involvement of the insular cortex in autonomic reactions to noxious stimuli and in pain-related learning and memory [2, 35]. Given the presence of P311 in these areas, it is unclear if P311 is required for processing the affective dimension of pain in all of the regions or only in one particular area. Future experiments such as the site-specific deletion of P311 or overexpression of P311 are necessary to determine the site of P311 deficiency that may account for the deficit in pain affection.
The mechanism by which P311 exerts its function in pain affection might involve the modulation of strength of the neural circuits processing or storing affective pain information. There are at least two possibilities. First, P311 may modulate the neuroplasticity by remodeling the spine of the neurons. For example, activity-dependent structural remodeling of dendritic spines in the cortex has been shown to be important for LTP and Long-term depression [36, 37], and has been postulated as a cellular basis of learning and memory [38]. In this regard, recent work indicating that P311 can promote neurite outgrowth of postnatal neurons [20], possibly by the reorganization of cytoskeleton [39] is of interest. Second, P311 may modulate the neural activity in the ACC. Recent studies have shown that NMDA and AMPA receptors are important for pain processing in the ACC [10, 15], so it is likely that P311 may regulate the neuronal activity by directly or indirectly interacting with the excitatory receptors. Nevertheless, the actual mechanism by which P311 regulates the affective pain remains to be explored.
Conclusion
Regardless of mechanisms involved, our findings suggest for the first time that sensory and affective pain may be dissociated from each other at the molecular level. To our best knowledge, our report represents the first to suggest that a unique set of genes may be required for the function of neural circuits underlying the pain affection, thereby providing the molecular logic for explaining the partially dissociable brain networks which are responsible for two distinct components of pain perception [2, 3]. Identification of genes involved in the affective but not in the sensory component of pain may have therapeutic value in the management of pain affection. Since P311 is highly conserved between the rodents and human and formalin injection represents a noxious stimuli to human [18, 40], it is conceivable that P311 may be a potential target for alleviating the affective component of pain.
References
Melzack RaC KL: Sensory, motivational, and central control determinants of pain: a new conceptual model. Kenshalo DR (ed), The Skin Senses. Springfield, IL, CC Thomas; 1968:423–439.
Price DD: Psychological and neural mechanisms of the affective dimension of pain. Science 2000, 288: 1769–1772. 10.1126/science.288.5472.1769
Treede RD, Kenshalo DR, Gracely RH, Jones AK: The cortical representation of pain. Pain 1999, 79: 105–111. 10.1016/S0304-3959(98)00184-5
Rainville P, Carrier B, Hofbauer RK, Bushnell MC, Duncan GH: Dissociation of sensory and affective dimensions of pain using hypnotic modulation. Pain 1999, 82: 159–171. 10.1016/S0304-3959(99)00048-2
Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC: Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science 1997, 277: 968–971. 10.1126/science.277.5328.968
Johansen JP, Fields HL, Manning BH: The affective component of pain in rodents: direct evidence for a contribution of the anterior cingulate cortex. Proc Natl Acad Sci USA 2001, 98: 8077–8082. 10.1073/pnas.141218998
Gao YJ, Ren WH, Zhang YQ, Zhao ZQ: Contributions of the anterior cingulate cortex and amygdala to pain- and fear-conditioned place avoidance in rats. Pain 2004, 110: 343–353. 10.1016/j.pain.2004.04.030
Lagraize SC, Fuchs PN: GABA(A) but not GABA(B) receptors in the rostral anterior cingulate cortex selectively modulate pain-induced escape/avoidance behavior. Exp Neurol 2007, 204: 182–194. 10.1016/j.expneurol.2006.10.007
LaBuda CJ, Fuchs PN: A behavioral test paradigm to measure the aversive quality of inflammatory and neuropathic pain in rats. Exp Neurol 2000, 163: 490–494. 10.1006/exnr.2000.7395
Zhuo M: A synaptic model for pain: long-term potentiation in the anterior cingulate cortex. Mol Cells 2007, 23: 259–271.
Zhuo M: Molecular mechanisms of pain in the anterior cingulate cortex. J Neurosci Res 2006, 84: 927–933. 10.1002/jnr.21003
Sikes RW, Vogt BA: Nociceptive neurons in area 24 of rabbit cingulate cortex. J Neurophysiol 1992, 68: 1720–1732.
Wei F, Zhuo M: Potentiation of sensory responses in the anterior cingulate cortex following digit amputation in the anaesthetised rat. J Physiol 2001, 532: 823–833. 10.1111/j.1469-7793.2001.0823e.x
Ji RR, Kohno T, Moore KA, Woolf CJ: Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci 2003, 26: 696–705. 10.1016/j.tins.2003.09.017
Wu LJ, Xu H, Ren M, Cao X, Zhuo M: Pharmacological isolation of postsynaptic currents mediated by NR2A- and NR2B-containing NMDA receptors in the anterior cingulate cortex. Mol Pain 2007, 3: 11. 10.1186/1744-8069-3-11
Toyoda H, Zhao MG, Xu H, Wu LJ, Ren M, Zhuo M: Requirement of extracellular signal-regulated kinase/mitogen-activated protein kinase for long-term potentiation in adult mouse anterior cingulate cortex. Mol Pain 2007, 3: 36. 10.1186/1744-8069-3-36
Julius D, Basbaum AI: Molecular mechanisms of nociception. Nature 2001, 413: 203–210. 10.1038/35093019
Studler JM, Glowinski J, Levi-Strauss M: An abundant mRNA of the embryonic brain persists at a high level in cerebellum, hippocampus and olfactory bulb during adulthood. Eur J Neurosci 1993, 5: 614–623. 10.1111/j.1460-9568.1993.tb00527.x
Taylor GA, Hudson E, Resau JH, Woude GF: Regulation of P311 expression by Met-hepatocyte growth factor/scatter factor and the ubiquitin/proteasome system. J Biol Chem 2000, 275: 4215–4219. 10.1074/jbc.275.6.4215
Fujitani M, Yamagishi S, Che YH, Hata K, Kubo T, Ino H, Tohyama M, Yamashita T: P311 accelerates nerve regeneration of the axotomized facial nerve. J Neurochem 2004, 91: 737–744. 10.1111/j.1471-4159.2004.02738.x
Shi J, Badri KR, Choudhury R, Schuger L: P311-induced myofibroblasts exhibit ameboid-like migration through RalA activation. Exp Cell Res 2006, 312: 3432–3442. 10.1016/j.yexcr.2006.07.016
Mariani L, McDonough WS, Hoelzinger DB, Beaudry C, Kaczmarek E, Coons SW, Giese A, Moghaddam M, Seiler RW, Berens ME: Identification and validation of P311 as a glioblastoma invasion gene using laser capture microdissection. Cancer Res 2001, 61: 4190–4196.
Roschier M, Kuusisto E, Kyrylenko S, Salminen A: Expression of seizure-related PTZ-17 is induced by potassium deprivation in cerebellar granule cells. Biochem Biophys Res Commun 1998, 252: 10–13. 10.1006/bbrc.1998.9589
Lein ES, et al.: Genome-wide atlas of gene expression in the adult mouse brain. Nature 2007, 445: 168–176. 10.1038/nature05453
Chen ZF, Rebelo S, White F, Malmberg AB, Baba H, Lima D, Woolf CJ, Basbaum AI, Anderson DJ: The paired homeodomain protein DRG11 is required for the projection of cutaneous sensory afferent fibers to the dorsal spinal cord. Neuron 2001, 31: 59–73. 10.1016/S0896-6273(01)00341-5
Zhao ZQ, Chiechio S, Sun YG, Zhang KH, Zhao CS, Scott M, Johnson RL, Deneris ES, Renner KJ, Gereau RWt, Chen ZF: Mice lacking central serotonergic neurons show enhanced inflammatory pain and an impaired analgesic response to antidepressant drugs. J Neurosci 2007, 27: 6045–6053. 10.1523/JNEUROSCI.1623-07.2007
Malmberg AB, Gilbert H, McCabe RT, Basbaum AI: Powerful antinociceptive effects of the cone snail venom-derived subtype-selective NMDA receptor antagonists conantokins G and T. Pain 2003, 101: 109–116. 10.1016/S0304-3959(02)00303-2
Skoubis PD, Lam HA, Shoblock J, Narayanan S, Maidment NT: Endogenous enkephalins, not endorphins, modulate basal hedonic state in mice. Eur J Neurosci 2005, 21: 1379–1384. 10.1111/j.1460-9568.2005.03956.x
Garcia R, Vouimba RM, Baudry M, Thompson RF: The amygdala modulates prefrontal cortex activity relative to conditioned fear. Nature 1999, 402: 294–296. 10.1038/46286
Killcross S, Robbins TW, Everitt BJ: Different types of fear-conditioned behaviour mediated by separate nuclei within amygdala. Nature 1997, 388: 377–380. 10.1038/41097
Kung JC, Su NM, Fan RJ, Chai SC, Shyu BC: Contribution of the anterior cingulate cortex to laser-pain conditioning in rats. Brain Res 2003, 970: 58–72. 10.1016/S0006-8993(02)04276-2
Tanimoto S, Nakagawa T, Yamauchi Y, Minami M, Satoh M: Differential contributions of the basolateral and central nuclei of the amygdala in the negative affective component of chemical somatic and visceral pains in rats. Eur J Neurosci 2003, 18: 2343–2350. 10.1046/j.1460-9568.2003.02952.x
Vogt BA, Sikes RW: The medial pain system, cingulate cortex, and parallel processing of nociceptive information. Prog Brain Res 2000, 122: 223–235.
Sewards TV, Sewards MA: The medial pain system: neural representations of the motivational aspect of pain. Brain Res Bull 2002, 59: 163–180. 10.1016/S0361-9230(02)00864-X
Schnitzler A, Ploner M: Neurophysiology and functional neuroanatomy of pain perception. J Clin Neurophysiol 2000, 17: 592–603. 10.1097/00004691-200011000-00005
Matsuzaki M: Factors critical for the plasticity of dendritic spines and memory storage. Neurosci Res 2007, 57: 1–9. 10.1016/j.neures.2006.09.017
Tanaka J, Horiike Y, Matsuzaki M, Miyazaki T, Ellis-Davies GC, Kasai H: Protein synthesis and neurotrophin-dependent structural plasticity of single dendritic spines. Science 2008, 319: 1683–1687. 10.1126/science.1152864
Yuste R, Bonhoeffer T: Morphological changes in dendritic spines associated with long-term synaptic plasticity. Annu Rev Neurosci 2001, 24: 1071–1089. 10.1146/annurev.neuro.24.1.1071
McDonough WS, Tran NL, Berens ME: Regulation of glioma cell migration by serine-phosphorylated P311. Neoplasia 2005, 7: 862–872. 10.1593/neo.05190
Dubuisson D, Dennis SG: The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain 1977, 4: 161–174. 10.1016/0304-3959(77)90130-0
Acknowledgements
The work was supported by an NIH RO1 (NS046036-01A1) to Z.F.C and NNSFC 30500153, 05KJB180100 to Y.J.G.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
YGS performed pain behavioral experiments and in situ hybridization studies, YJG performed F-CPA and LiCl-CPA experiments, ZQZ and BH contributed to CPA experiment, YJ performed genotyping of mice, GAT provided P311 mutant mice, ZFC, YJG and YGS prepared the manuscript. All authors read and approved the manuscript.
Yan-Gang Sun, Yong-Jing Gao contributed equally to this work.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Sun, YG., Gao, YJ., Zhao, ZQ. et al. Involvement of P311 in the affective, but not in the sensory component of pain. Mol Pain 4, 23 (2008). https://doi.org/10.1186/1744-8069-4-23
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1744-8069-4-23