Abstract
Animals detect environmental changes through sensory neural mechanisms that enable them to differentiate the quality, intensity and temporal characteristics of stimuli. The 'doctrine of specific nervous energies' postulates that the different sensory modalities experienced by humans result of the activation of specific nervous pathways. Identification of functional classes of sensory receptors provided scientific support to the concept that somatosensory modalities (touch, pain, temperature, kinesthesis) are subserved by separate populations of sensory receptor neurons specialized in detecting innocuous and injurious stimuli of different quality (mechanical forces, temperature, chemical compounds). The identification of receptor proteins activated by different physicochemical stimuli, in particular ion channels of the Transient Receptor Potential (TRP) superfamily, has put forward the concept that specificity of peripheral sensory receptor neurons is determined by their expression of a particular "molecular sensor" that confers to each functional type its selectivity to respond with a discharge of nerve impulses to stimuli of a given quality. Nonetheless, recent experimental data suggest that the various molecular sensors proposed as specific transducer molecules for stimuli of different quality are not as neatly associated with the distinct functional types of sensory receptors as originally proposed. First, many ion channel molecules initially associated to the transduction of only one particular form of energy are also activated by stimuli of different quality, implying a limited degree of specificity in their transducing capacities. Second, molecular sensors associated with a stimulus quality and hence to a sensory receptor type and ultimately to a sensory modality may be concomitantly expressed in sensory receptor neurons functionally defined as specific for another stimulus quality. Finally, activation of voltage gated channels involved primarily in nerve impulse generation can also influence the gating of transducing channels, dramatically modifying their activation profile. Thus, we propose that the capacity exhibited by the different functional types of somatosensory receptor neurons to preferentially detect and encode specific stimuli into a discharge of nerve impulses, appears to result of a characteristic combinatorial expression of different ion channels in each neuronal type that finally determines their transduction and impulse firing properties. Transduction channels don't operate in isolation and their cellular context should also be taken into consideration to fully understand their function. Moreover, the inhomogeneous distribution of transduction and voltage-gated channels at soma, axonal branches and peripheral endings of primary sensory neurons influences the characteristics of the propagated impulse discharge that encodes the properties of the stimulus. Alteration of this concerted operation of ion channels in pathological conditions may underlie the changes in excitability accompanying peripheral sensory neuron injuries.
Similar content being viewed by others
Review
The continuous interplay between living organisms and the environment has played a critical role in the evolution and optimization of their sensory capacities. Animals have developed sophisticated neural mechanisms to detect and discriminate environmental changes that enable them to differentiate the quality, location, intensity and temporal characteristics of the various classes of stimuli acting on the body surface and internal organs [1]. Stimuli are detected and encoded by peripheral sensory receptors into trains of nerve impulses that contain the information about those parameters of the stimulus that are particularly relevant for evolutionary success. This neural activity is subsequently processed at different levels of the central nervous system and interpreted finally by the brain as sensations of different perceptual characteristics (i.e. sensory modalities), such as temperature, touch or pain.
In the past few years we have seen a remarkable explosion of studies that are starting to provide a detailed view of the molecules involved in the transduction of the different somatosensory stimuli [2, 3]. The quality of the studies and their impact on the field of pain research and sensory neuroscience is unquestionable. However, the physiological interpretation of some of these findings is often, in our opinion, misguided. The main purpose of this review is to alert about some common misconceptions which are rapidly propagating through the literature.
First, we warn about automatically extending the classical concept of specificity of sensory receptor neurons to single transducing molecules. In fact, the picture arising from the study of many putative transduction molecules is complex and rather perplexing considering the extreme polymodality that they exhibit in many cases.
Second, we consider fallacious to assign the functional specificity of a class of sensory afferents to a particular stimulus quality to the presence in their somas of an individual transducing molecule, even in the case when this molecule complies with specificity. The physiological unit of transduction is the sensory ending which, in many cases, coexpress different transduction channels with different activation profiles.
Finally, we caution about the uncritical translation of functional properties of ion channels observed in recombinant systems to the physiological properties of the terminals harbouring those channels. In order to elucidate the coding rules for specific somatosensory stimuli, still much needs to be learned about the transduction process and the properties of native channels and their functional interactions with other molecular elements on nerve terminals.
Sensations of different modality are conveyed by separate neural pathways
In 1837, Johannes Müller proposed the doctrine of "the specific nervous energies". He postulated that the different modalities of sensation experienced by humans result of the stimulation of specific nervous pathways, now referred to as "labelled lines". These were best activated by a particular type of physical or chemical stimulus, but the perceptual quality of the final sensation remain the same even when that pathway was activated by a different form of energy. In other words, sensation was a function of the active neural pathway not the stimulus. Sensations evoked by stimulation of well-defined sensory organs (vision, audition, smell and taste) were clear examples of this tenet. Those elicited by stimulation of the skin and other structures of the body also appeared to follow the same principle. Thus, on the skin surface, it was possible to identify distinct spots whose natural or electrical stimulation evoked separate tactile, warm or cold sensations, suggesting the existence of specific receptors. These findings were published independently and almost simultaneously in the early 1880s by Magnus Blix, Alfred Goldscheider and Henry Donalson [reviewed by [4]]. Pain was somewhat an exception to this rule and was initially interpreted as the result of "excessive" activation of the other sensory pathways [5]. However, 20 years later, Max von Frey discovered discrete pain points ("Schmerzpunkte") in human skin, extending the concept of "Peripheral Specificity", also termed "Specific Irritability", to all somatosensory modalities [6].
Soon after these proposals were made, researchers tried to establish the anatomical and functional basis of sensory specificity in the somatic system. In the skin, morphologically distinct nerve terminals were identified but the efforts to correlate the anatomy of the various cutaneous receptor types with particular elementary sensory experiences were unsuccessful [7]. With the advent of nerve recording techniques, it was feasible to proof experimentally that peripheral sensory receptors encoded the parameters of the stimulating energy into a discharge of nerve impulses, whose firing frequency reflected with a variable degree of fidelity the characteristics of the stimulus [8]. Furthermore, cutaneous sensory nerve fibers exhibited a marked degree of functional specialization, and different fiber types responding preferentially to touch or to cold and warm temperatures were distinguished [9]. These observations provided a direct proof of the existence, in somatic tissues, of functionally specific peripheral sensory receptors, each of them transducing a particular form of stimulating energy, lending support to the specificity theory of somesthesia [10]. Some of the cutaneous fiber endings were classified as low-threshold mechanoreceptors because they responded preferentially to weak mechanical forces while cold and warm thermoreceptors were primarily activated by moderate cooling or warming of their receptive field. Subsequently, two main populations of nociceptor nerve fibers were distinguished: mechano-nociceptors, excited only by injurious mechanical forces and polymodal nociceptors that also responded to noxious heat, exogenous chemicals and inflammatory mediators [for review see [11]]. More recently, 'silent' (mechanically-insensitive) nociceptors activated only after inflammation develops have been identified [12].
The existence of different subclasses of receptors for low intensity mechanical energy explained the different submodalities of tactile sensations. Cold and warm thermoreceptors would be the origin of sensations of cutaneous cooling and warming while the identification of nociceptors as a functionally distinct group of sensory terminals that responded to a variety of high-intensity injurious stimuli, supported the interpretation that pain, like other somatosensory modalities was subserved by a separate population of receptors specialized in detecting potentially injurious stimuli of different nature [11].
Transduction molecules are activated by different forms of energy
The search for individual molecular entities associated with the detection of the different stimulating energies was the logical further step in the reasoning that specificity of somatosensory pathways arises at the receptor level. Specificity would be ultimately determined by the presence of a particular "molecular sensor" that confers to each functional class of sensory receptor its selectivity for a particular form of energy. This approach proved to be essentially correct for the visual, olfactory and gustatory sensory pathways, where highly specific transduction molecules, belonging to the family of G protein-coupled receptors, and located respectively in photoreceptor, olfactory and taste receptor cells, were associated in each case with the detection of photons or of particular chemical groups that act as odorants or taste molecules [13]. Activation of each receptor led to the synthesis of cyclic nucleotide second messengers and modulation of the activity of cation channels.
The extrapolation of the same principle to the receptors of somatic and visceral sensory pathways of mammals was experimentally more challenging due to the small size of peripheral sensory nerve terminals. Nonetheless, recordings of single polymodal nociceptor fibers evidenced that sensitivity to heat and acid in an individual fiber could be selectively inactivated without affecting responsiveness to mechanical forces (Figure 1), thus providing an indirect proof that transduction of heat, chemical and mechanical stimuli in individual nociceptive terminals depended on separate molecular entities [14]. Some years later, Cesare and McNaughton discovered a heat-activated cationic current in a subpopulation of nociceptors [15].
Separate transduction mechanisms for mechanical and chemical/thermal stimuli in a single polymodal nociceptor terminal. Example of single fiber recordings from polymodal nociceptive endings innervating the cornea of the cat. Responses to acid, heat and mechanical stimuli, before and after topical application of capsaicin at an inactivating concentration (330 μ M). Note the abolition of the response to heat and the marked reduction in the response to local acidic solution (10 mM acetic acid) without affecting the response to mechanical stimulation.
The isolation and subsequent characterization of a functional cDNA encoding a protein involved in the detection of irritant chemicals and heat led to the identification of the first transducing molecule in the mammalian somatosensory system, the 'capsaicin or vanilloid receptor', now named TRPV1 [16]. This membrane receptor is a Ca2+-permeable, non-selective cationic channel that belongs to the Transient Receptor Potential (TRP) ion channel superfamily, which were first described in the fruit fly Drosophila melanogaster [17]. The functional receptor is probably a tetramer and each subunit is predicted to have 6 transmembrane (TM) spanning domains with a short pore-forming hydrophobic loop between TM domains 5 and 6 (Figure 2). This landmark study showed that the channel was gated by capsaicin [16, 18], the pungent component of red peppers long since known as a selective activator of polymodal nociceptor endings [reviewed by [19, 20]]. The channel was also opened by temperatures over 43°C and activated by protons (Figure 2) [reviewed by [21]]. It was primarily expressed in small size, unmyelinated neurons identified as putative nociceptors. Therefore TRPV1 was proposed as the molecular substrate for polymodality in these neurons, providing them with the ability to respond to heat and to potentially injurious chemical stimuli causing pain [16, 22]. Sustained activation, posttranslational modifications and membrane translocation of TRPV1 channels by a variety of endogenous agents released after injury or inflammation, including bradykinin and endogenous lipids, mediates the sensitizing/excitatory action of polymodal nociceptor neurons during these pathological states [23–28].
The capsaicin receptor, a true polymodal sensory receptor molecule. A. Schematic representation of the topology of a TRPV1 protein subunit. The functional channel is a tetramer formed by the ensemble of four such subunits. Marked in orange are residues influencing capsaicin binding. Marked in blue are two extracellular residues critical for activation by protons. The C-terminal region (shaded green) has been implicated in activation by heat but the critical residues are still unknown. B. Whole-cell I-V relationships of a HEK293 cell transfected with rat TRPV1 showing the activation of currents by low pH (6.0), heat (42°C) and capsaicin (100 nM) (A. Mälkiä, unpublished).
Soon afterwards, other channels of the TRP family were cloned and characterized in heterologous expression systems. Several of them (TRPV2, TRPV3, TRPV4, TRPM8 and TRPA1) were also gated by temperature, with thresholds ranging from 52°C down to 18°C [29–36]. All of them were expressed in primary sensory neurons, or skin keratinocytes, and because they exhibited different temperature thresholds, varying from noxious cold to noxious heat, it was proposed that each of these channel molecules may confer to individual cold and warm thermal receptors and to nociceptors, the ability to respond selectively to innocuous or noxious temperatures across a wide thermal range (Figure 3). These ideas have been summarized in several recent reviews [37–44].
Hypothetical correspondence between activation of TRP channels, body surface temperature and evoked sensations. Upper part: Schematic representation of the thermal activation profile of various TRP channels when expressed in recombinant systems. All of them have been located in sensory neurons and/or skin cells. (Adapted from Patapoutian et al. 2003). Middle part: Schematic representation of impulse activity in various cutaneous sensory receptors during application to their receptive fields of temperatures indicated in the thermal scale. Lower part: Quality of sensations evoked in humans by application to the skin of different temperature values. Adapted from Von Frey.
The discovery and characterization of TRPM8, as a transduction channel apparently restricted to a small subpopulation of sensory receptor neurons activated by moderate/non-noxious cold (threshold ~25°C) and by a number of compounds such as menthol, eucalyptol or icilin, known by their ability to induce cold sensations in humans, lend further support to the hypothesis that restricted expression of individual classes of TRP channels in the terminals of subpopulations of primary sensory neurons is the molecular substrate of the specific sensations evoked by each somatosensory stimulus [31, 33]. TRPM8 is a non-selective cation channel of the TRPM (melastatine) family with a modest permeability to calcium. The channel is specifically expressed in small diameter trigeminal and dorsal root ganglion neurons in which cooling and menthol evoke inward depolarizing currents and intracellular calcium rises [45–47]. Thus, TRPM8 has been proposed as the cold sensor molecule that confers to peripheral thermoreceptor neurons their specific ability to respond to small temperature reductions, ultimately causing well-defined innocuous cold temperature sensations [41, 39]. The characterization of transgenic mice lacking TRPM8 provided direct evidence for the critical role of this channel in cold temperature sensing [48–50].
In contrast to the swift progress in the identification of thermoreceptor proteins, the molecular identity of ion channels involved in the transduction of mechanical stimuli by mammalian somatosensory endings is still uncertain [51]. The fact that mechanosensation requires the concerted function of several proteins acting in an ensemble (transduction apparatus) make these studies particularly challenging. The list of candidate transducer molecules for mechanotransduction also includes several mammalian TRP channels (TRPA1, TRPC1, TRPV4) [reviewed by [52]] and members of the acid-sensing ion channel (ASIC) family [53] that are mammalian homologs of DEG/ENaC channels with a known mechanosensory function in invertebrates. In addition, modulatory roles in mechanotransduction have been assigned to other channels, like two-pore-domain K+ channels [54, 55] and P2X purinergic receptors [56].
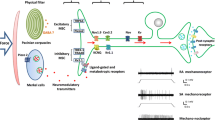
Thus, the concept of specificity to a particular form of stimulating energy, based on the electrophysiological evidence that sensory afferent fibers preferentially detect and encode a particular type of physical or chemical stimulus, was now extended to a molecular level, meaning that the differential sensitivity of receptor endings resulted of the expression of a particular protein whose presence would be necessary and sufficient to determine the capacity to transduce the specific stimulus into a propagated sensory message and ultimately the modality of the evoked sensation. Figure 4 summarizes schematically these ideas, representing the various subtypes of sensory neurons that respond to specific stimuli, evoking defined sensations and the hypothetical channel molecules in their endings associated to such transduction characteristics. With the exception of TRPV1, recognized from the start as a polymodal receptor, initial descriptions of TRP channel activation have emphasized the selectivity of their activation mechanism, matching this specificity with the sensory modality of a defined subpopulation of sensory fibers [31–33, 57, 58]. This categorization of transduction channels leads to what could be named in colloquial terms, a "clear picture" of the molecular basis of sensory specificity (Figure 4).
Gating promiscuity of transduction channels
Nonetheless, a wealth of recent experimental data suggest that specificity of molecular sensors is not as neatly associated with the sensitivity to stimuli of different quality in primary sensory neurons as was originally proposed. First, there is evidence that channel subunits initially associated to the transduction of a single form of energy in a functional class of sensory neuron are also activated by other types of physical and chemical stimuli, suggesting a limited degree of specificity in their transducing capacities. As already indicated, this was quite obvious for TRPV1, recognized since its discovery as a multimodal transduction molecule for chemical and thermal stimuli [16, 22], and more recently to mechanical distension of the bladder [59] and to osmotic stimuli in the hypothalamus [60]. In this case, the expression of an N-truncated product of the Trpv1 gene gives rise to a functional channel with distinct transducing properties that differ from the full-length TRPV1 protein [60]. This result indicates that alternative splicing of the same gene can add diversity to the coding potential of ion channels derived from a single gene locus.
However, multimodality appears to be also present to a variable degree in many of the members of the TRP channel superfamily implicated in cellular sensing (see Table 1). Unlike for TRPV1, in many of the other TRP channels multimodal gating still lacks a clear physiological interpretation. We will briefly summarize the evidence for several candidate thermotransducers. TRPV2, a relatively close TRPV1 homolog, in addition to heat (threshold >52°C) [29] is activated by cell swelling and membrane stretch [61], and also by growth factors [62] and Δ9-tetrahydrocannabinol [63]. TRPV3, a channel transcribed from a gene adjacent to TRPV1 but lacking sensitivity to capsaicin was identified first as a thermosensitive channel (threshold 34–39°C) [34, 35, 64]. Subsequent studies identified camphor, a natural medical compound found in the bark of the camphor laurel tree (Cinnamomum camphora), and pungent components of oregano and clove as potent agonists of TRPV3 channels [65, 66] implying a chemosensory function. Recently, the channel was shown to be directly gated by arachidonic acid and other unsaturated fatty acids [67]. TRPV4 is a channel expressed in a variety of epithelial cells, peripheral sensory ganglia and hypothalamic neurons. It was originally classified as a mechanosensor, activated by hypotonic cell swelling [57, 58] and fluid shear stress [68]. However, later studies also established its activation by warm temperatures (threshold ranging from 25–34°C), acidic pH and a variety of chemical compounds [69, 30, 36, 70]. As is the case for other TRPs, mechanical and chemical sensitivity of TRPV4 is strongly modulated by temperature [71]. The gating of TRPV4 by the different agonists (hyposomolality, heat, and many endogenous metabolites) is somewhat special in that separate second messenger pathways are involved in the activation mechanism for a particular stimulus [72].
The channel subunit TRPA1 was initially proposed as a specific transduction molecule for noxious cold (threshold ~18°C) in peripheral nociceptive neurons [32]. However, recent work has shown that the channel is also gated by various natural pungent compounds such as cinnamaldehyde, allylisothiocyanate and allycin, active ingredients of cinnamon oil, mustard oil and garlic respectively [73–76]. Environmental irritants are also potent activators of TRPA1 [77, 78]. While early work on cochlear hair cells also supported a role for TRPA1 in mechanotransduction [79, 80], its function as a mechanosensor in the somatosensory system is controversial and unsupported by the behavioural characterization of knockout animals [81, 82].
In the case of TRPM8, originally identified as a sensor for mild cold temperatures [31, 33], and modulated by a number of chemical compounds, recent work also indicates that it is gated by lysophospholipds and other chemical agents that induce mechanical deformations in the membrane bilayer, suggesting a possible mechanosensitive role [83, 84].
The multimodality of TRP channels is a well established concept now and can be traced back to ancient channels (e.g. OCR-2 and OSM-9) in invertebrates [85]. Genetic and molecular studies in Caenorhabditis elegans have provided strong evidence that selective activation of TRPV channels to specific stimuli are segregated on different domains of the protein [86]. Similar multimodal gating mechanisms are also frequent among two-pore domain K+ channels, another extended family of ion channels with prominent sensing roles in the somatosensory system (Table 1) [87]. For instance, TREK channels are activated by heat, polyunsaturated free fatty acids including arachidonic acid, phospholipids, volatile anaesthetics, mechanical stretch and intracellular acidification [88–90].
Therefore, many molecular sensors originally ascribed to the response to a given form of energy and primarily associated to the transduction of that stimulus type in the corresponding functional class of sensory neuron, are often also sensitive to physical or chemical stimuli, physiologically linked to a different sensory modality. This gating promiscuity seems to be a common feature of many "transduction" channels and represents in principle an important challenge for an extension of the specificity theory to the molecular level. However, it should be pointed out that for most channels multimodal activation has been observed in heterologous systems. It is unclear whether native channels will show similar properties and in what degree they are activated by different stimuli.
More significant data speaking against extending the specificity hypothesis to transduction molecules were obtained in studies of genetically modified animals. Thus, gene ablation of individual TRPs frequently manifest in a complex sensory phenotype. In TRPV1(-/-) the first deficit identified was a suppression of inflammatory thermal hyperalgesia with no alteration of inflammatory mechanical hyperalgesia [91, 92]. However, a more extensive characterization has shown that TRPV1(-/-) mice have clear alterations in thermoregulation [93, 94], in the transduction of osmotic responses [60] and mechanical stimuli in the gut [95, 96] and bladder [59]. TRPV3(-/-) mice show impaired responses to both, innocuous and noxious heat, suggesting expression in more than one subset of sensory neurons [65]. In the case of TRPV4(-/-) animals, sensory deficits include reduced sensitivity to noxious mechano-osmotic stimuli [97], altered osmotic sensitivity, disturbance of thermal preferences and hearing loss [reviewed by [98, 99]]. A complex phenotype is also observed in TRPA1-deficient mice, with alterations in chemical, thermal and mechanical sensitivity [81, 82]. The phenotype of TRPM8(-/-) mice, although clearly linked to cold temperature sensing is also heterogeneous. Depending on the behavioural context, the animals can show deficits in responses to unpleasant cold stimuli and deficits in cold-mediated analgesia of inflammatory and neuropathic pain [48–50]. Interpretation of these complex phenotypes in mutant mice may be complicated further by development of compensatory mechanisms which are a common occurrence in conventional global gene inactivation approaches used do far. For example, specific pharmacological inhibition strongly suggests a role of TRPA1 in the sensitization of nociceptors to mechanical stimuli, but compensatory mechanisms completely mask this role in TRPA1 deficient mice [100]
'Modality-specific' peripheral sensory neurons also express transduction molecules for different stimuli
Not only are many transducing molecules multimodal for different energy forms. Several of the molecular sensors associated to a stimulus and hence to a sensory modality, are concomitantly expressed in sensory neurons that are functionally defined as specific for another stimulus quality, thus resembling pain-signaling, polymodal nociceptor terminals. These terminals, in addition to TRPV1 possess stretch-activated channels of still unknown molecular nature, which mediate their sensibility to noxious mechanical forces, and ASIC channels that contribute to their response to acidic pH [101, 102]. This is also the case of cold-sensitive thermoreceptor neurons expressing TRPM8; about 50% of them are also activated by capsaicin, a specific activator of TRPV1 channels [39, 103–107]. This dual expression possibly explains the responsiveness of a part of cold sensitive terminals to heat and capsaicin [108]. Indeed, careful experimental scrutiny shows that many thermosensitive afferents can be activated by low and high temperatures, a finding that could explain several interesting perceptual phenomena like paradoxical cold or synthetic heat [reviewed by [109]]. It is also well known that a fraction of low threshold cutaneous mechanoreceptors are activated by cooling [110–114] thus suggesting that they possess some of the transducing mechanisms for temperature present in specific thermoreceptor neurons.
'Modality-specific' peripheral sensory neurons express multiple transduction molecules for the same type of stimulus
Additional evidence illustrating the biological complexity in the organization of transducing processes in the somatosensory system stems from the observation that separate molecular sensors detecting the same type of physical or chemical stimulus may co-exist in an individual sensory receptor type. This overlap was soon realised upon phenotypic characterization of null mutants for single transduction molecules. For instance, the persistence of sensitivity to heat in polymodal sensory endings of TRPV1(-/-) mice has been explained by co-expression in polymodal neurons of other heat-sensitive TRP channels [91, 92, 115]. Specifically, TRPV3 [35] and TRPV2 [116, 117] channels have been shown to co-express, and even form heteromultimers, with TRPV1 subunits. Also, the recent development of potent TRPV1 antagonists has illustrated their lack of effect on acute thermosensory responses [118], strongly suggesting the overlap between peripheral thermosensitive mechanisms operating in vivo. Expression of transducers is not the only factor determining the physiological response of afferents to peripheral stimuli. Of note, the variable sensitivity to noxious heat among many different strains of mice is mediated by the differential expression of the CGRP gene, further illustrating the complex interactions between thermal transducers and other signal modulators in vivo [119].
Similarly, the excitatory effects of protons on nociceptors are mediated by multiple channel subunits including TRPV1, ASICs, and TASK potassium channels [101, 102, 120–123]. Targeted disruption of individual subunits produces variable deficits in the response to low pH in different classes of nociceptors [91, 124] with no obvious deficit in some of them [124], suggesting a significant overlap in the pattern of expression of these transduction channels [125].
In neurons sensitive to non-noxious cold, temperature reductions open TRPM8 channels [31, 46, 47, 126] but also close thermosensitive background K+ channels [104, 127, 128] and, as commented below, the final depolarization induced by cold is the result of the summation in the same neuron of separate transduction mechanisms activated by the specific stimulus. Consistent with this view, cold-evoked responses are not abrogated in TRPM8(-/-) mice; the remaining cold-sensitivity varied in the three studies [48–50]. A large fraction of neurons known to express TRPM8 remain cold sensitive even after genetic ablation of TRPM8 [129]. Additional evidence for this concept of redundancy in transduction molecules for a stimulus modality has been obtained with pharmacological approaches. In a recent study, performed in cold-sensitive nerve terminals of the cornea, selective pharmacological blockade of TRPM8 channel function abolished the activation by menthol of the terminals but left largely unaffected their ability to be excited by cold stimuli [47]. The presence in the same neuron of multiple thermal sensors is also evident in developing sensory neurons, that can respond massively to cooling even before detectable expression of known thermoTRP channels [130].
It is tempting to speculate teleologically that this functional overlap provides sensory neurons with a larger operating range than that offered by a single transduction channel and additionally offers the possibility of different responses to external and internal modulators, adding flexibility to the system.
Limited 'molecular' specificity of sensory receptor terminals
Taken together, the available experimental evidence speaks against the generalization that in the somatosensory system there is a specific molecule for every stimulus type and a specific class of neuron for each transducing molecule. Rather, the capacity exhibited by the different types of primary sensory neurons to preferentially detect and encode the specific stimuli into a discharge of nerve impulses appears to depend on a characteristic combination in each neuronal class of different transduction mechanisms provided by a combinatorial expression of molecular sensors. We see a more "blurry picture", schematized in Figure 5.
Molecular basis of somatosensory specificity: The "Blurry Picture". Schematic representation of various subpopulations of modality specific primary sensory neurons, and of some of the putative transduction molecules that could be involved in their detection capacities for different stimuli. Data for the different channels potentially involved in nociception have been lumped together into an oversimplified model of polymodal neuron. Also, the stimuli refer to the preferred stimulus for each class of neuron but does not exclude the activation by other types.
The limited specificity of transduction molecules and of single primary sensory neurons, contributes to explain at the peripheral level some perplexing aspects of the sensations experienced when two stimuli of different quality act simultaneously on a particular sensory pathway. Cold sensitivity of low threshold mechanoreceptors is apparently the origin of the 'Weber paradox' or Silver Thaler Illusion (i.e. the feeling that cold objects feel heavier than when they are warm [131]). The presence of TRPV1 in a fraction of innocuous cold receptor neurons [107] may be the explanation for the sensation of cold experienced when heat is applied to cutaneous cold spots (von Frey, 1895) and for the response of a fraction of cold-sensitive fibers to heating over 42–45°C [132]. These remarkable psychophysical phenomena may also originate centrally, by the convergence of sensory information of different modality, as exemplified by the thermal grill illusion [133].
Response of nerve terminals is shaped by the interaction of transduction channels with other molecular elements
Additionally, we would like to emphasize that the processes of transduction and encoding of the stimulus take place in the same physical structure, the peripheral nerve ending, where both processes interact. Thus, currents generated by subthreshold and supratherhold activation of voltage gated channels involved in nerve impulse generation can influence, directly or indirectly, the gating of transducing channels. In this respect, it is important to note that many TRP channels are also voltage sensitive [134, 135], and the interaction of chemical and thermal stimuli with the voltage-sensing mechanism plays a key role in their gating mechanism [136–140].
A clear example of the functional interaction between voltage-gated and transducer channels is exemplified in Figure 6. In a subpopulation of primary sensory neurons that do not respond to a cooling stimulus, application of low concentrations of 4-aminopyridine (4-AP), a K+ channel blocker, results in the appearance of a novel excitatory response to cooling [104, 141]. The cellular mechanism that gives rise to this emerging phenotype is the blockade of a slow transient outward K+ current that is acting as an excitability break [104]. In other words, in these neurons the presence of such current prevents the depolarizing effect exerted by an excitatory cold-sensitive conductance restricting their activation profile [104].
Phenotypic transformation of sensory neuron by K+ channel blockade. A. Blocking effect of 100 μ M 4-AP on a slowly-inactivating K+ current that is responsible for preventing the response to cooling in cold insensitive trigeminal ganglion neurons. B. Simultaneous recording of [Ca2+]i and bath temperature in two trigeminal sensory neurons. The application of 100 μ M 4-AP unmasked a [Ca2+]i response in n2 during the second cooling step, transforming a cold-insensitive neuron into a cold-sensitive one. Adapted from Viana et al., 2002.
An additional example of subtractive interactions between two thermosensitive channels has been found recently in C-type nociceptors. Many of these neurons co-express the background K+ channel TREK-1 and TRPV1. The activity of both channels is augmented by heat [89, 16] but, while activation of TREK-1 acts as a brake, TRPV1 drives the excitation. Accordingly, heat-sensitive fibers in TREK1(-/-) animals show a much enhanced response to warm stimuli compared to wildtype animals [87].
Another dramatic example on functional interactions between channels in the peripheral nervous system comes from studies on mutations in the sodium channel Nav1.7 which lead to primary erythromelalgia [142]. Remarkably, a single misense mutation (F1449V) can produce opposing phenotypes in two classes of peripheral neurons where Nav1.7 is expressed, hyperexcitability in DRG neurons and hypoexcitability in sympathetic neurons [143]. The differences depend on the co-expression of Nav1.8 (in DRG) or lack of it (in sympathetic neurons) with Nav1.7.
Direct evidence for functional interactions between transduction and encoding ion channels residing on the same nerve terminal is limited, primarily due to the extreme difficulties in recording from somatosensory endings. In corneal cold receptor terminals, the degree of inactivation of sodium channels by the variable membrane potential determines their excitability [144]. In cutaneous sensory terminals, receptor potentials generated by cold interact with TTX-insensitive NaV1.8 channels and are a critical element of their selective excitability during cooling [145]. This means that expression of a particular transducer (e.g. a cold sensor) could be general but transmission of a propagated cold sensory message would be restricted to specific subpopulations of nerve fibers by its combinatorial expression with voltage-gated channels (i.e. K+ and Na+ channels [104, 145].
The cellular environment can also play an important role in shaping the transducing capacities of ion channels. In the case of TRPM8, it is clear that native channels have temperature activation thresholds that are significantly warmer than heterologously expressed channels [146, 47, 147]. Thus, mean static discharge of many trigeminal cold receptors peaks at 28–30°C, temperatures higher than the threshold of recombinant TRPM8 channels [148]. The intrinsic signals modulating the shift in thermal sensitivity of endogenous TRPM8 channels are currently unknown. TRPM8 activity depends on the presence of PIP2 [149–151]. It is also worth noting that TRPM8 responses to cold are abolished by phospholipase A2 (PLA2) inhibitors and sensitized by PLA2 products such as lysophospholipids (LPLs) (lysophosphatidylcholine, lysophosphatidylinositol, and lysophosphatidylserine) [84]. Another example of context-dependent function is observed in TRPV4. In artificial expression systems, TRPV channels are activated by hypotonic cell swelling [57, 58, 72]. In contrast, expression of TRPV4 in OSM-9 mutant worms can rescue the hypertonicity avoidance behaviour [152].
Concluding remarks
The evidence outlined above is contrary to the temptation in many contemporary neuroscientists to assign particular sensations to single molecular entities and illustrates the convenience of avoiding an oversimplified scheme of the mechanisms used by the various sensory receptors to detect a particular form of energy to finally evoke a specific perceptual experience. Sensory transduction channels don't operate in isolation but rather show important direct and indirect interactions with other ion channels and signalling molecules. Thus, we caution against the tendency to disregard cellular context in the investigations on their function.
Moreover, in any given peripheral sensory neuron type, the presence of a specific molecular structure responding to the same physical or chemical perturbations that act as the natural stimuli for its receptor terminals, does not prove unequivocally that this is the sole or even the principal transduction mechanism used by that particular receptor neuron. As the activation profiles of single molecular sensors do not appear to recapitulate the transduction capacities of single nerve terminals, it seems more likely that discrimination capacity for the adequate stimulus is dictated by a more complex interaction between specific transduction molecules and other ion channel proteins. Their presence and inhomogeneous distribution at soma, axon and peripheral endings will ultimately determine the characteristics of the propagated impulse discharge that encodes the properties of the stimulus, conferring functional specificity to the various types of sensory receptor neurons.
Thus, although the experimental evidence that primary sensory neurons represent "labelled lines" responding preferentially to an energy form remains strong today, an extrapolation of the classical specificity theory to the molecular level in the somatosensory system lacks, at present, solid experimental support. Moreover, a rigid extension of the specificity theory to every mechanism involved in stimulus detection conflicts with the complexity and multimodal characteristics of many peripheral receptor responses to thermal, mechanical and chemical stimuli. Very likely, this is also applicable to higher levels of the nervous system [153], where the presence of 'cross talk' between sensory pathways adds variability and richness to the different conscious sensations finally elicited by a complex multifaceted environment.
It can be expected that the combined efforts of many laboratories approaching the problem at different conceptual levels and applying a range of powerful techniques (gene manipulation, cell imaging, electrophysiology, pharmacology, behavioural analysis) will lead to rapid progress in deciphering the intimacies governing the transduction mechanisms of ambient stimuli at mammalian somatosensory endings.
References
Fain GL: Sensory Transduction. Volume 1. Sunderland, MA, USA, Sinauer Associates Inc; 2003:1–340.
Lumpkin EA, Caterina MJ: Mechanisms of sensory transduction in the skin. Nature 2007, 445(7130):858–865. 10.1038/nature05662
Julius D, Basbaum AI: Molecular mechanisms of nociception. Nature 2001, 413(6852):203–210. 10.1038/35093019
Norrsell U, Finger S, Lajonchere C: Cutaneous sensory spots and the "law of specific nerve energies": history and development of ideas. Brain Res Bull 1999, 48(5):457–465. 10.1016/S0361-9230(98)00067-7
Goldscheider A: Physiologie der Hautsinnesnerven. Leipzig, Johann Ambrosius Barth; 1898:1–432.
Von Frey M: Untersuchungen über die Sinnesfunktionen der menschlichen Haut: Druckempfindung un Schmerz. Hirzel, S. Sensory Transduction. Leipzig 1896.
Weddell G, Pallie W, Palmer E: Studies on the innervation of skin. I. The origin, course and number of sensory nerves supplying the rabbit ear. J Anat 1955, 89(2):162–174.
Adrian ED: The basis of sensation. In Sensory Transduction. London, Christophers; 1928:1–122.
Hunt CC, McIntre AK: Properties of cutaneous touch receptors in cat. J Physiol 1960, 153: 88–98.
Mountcastle VB: Physiology of sensory receptors: introduction to sensory processes. In Mountcastle, V. B. Medical Physiology. St. Louis, Mosby; 1968:1345–1371.
Perl ER: Cutaneous polymodal receptors: characteristics and plasticity. Prog Brain Res 1996, 113: 21–37.
Schaible HG, Schmidt RF: Responses of fine medial articular nerve afferents to passive movements of knee joints. Journal of Neurophysiology 1983, 49(5):1118–1126.
Shepherd GM: Sensory transduction: entering the mainstream of membrane signaling. Cell 1991, 67(5):845–851. 10.1016/0092-8674(91)90358-6
Belmonte C, Gallar J, Pozo MA, Rebollo I: Excitation by irritant chemical substances of sensory afferent units in the cat's cornea. J Physiol 1991, 437: 709–725.
Cesare P, McNaughton P: A novel heat-activated current in nociceptive neurons and its sensitization by bradykinin. Proc Natl Acad Sci USA 1996, 93(26):15435–15439. 10.1073/pnas.93.26.15435
Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D: The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 1997, 389(6653):816–824. 10.1038/39807
Montell C: The TRP superfamily of cation channels. Sci STKE 2005, 2005(272):re3. 10.1126/stke.2722005re3
Jordt SE, Julius D: Molecular basis for species-specific sensitivity to "hot" chili peppers. Cell 2002, 108(3):421–430. 10.1016/S0092-8674(02)00637-2
Szolcsanyi J: Forty years in capsaicin research for sensory pharmacology and physiology. Neuropeptides 2004, 38(6):377–384. 10.1016/j.npep.2004.07.005
Szolcsanyi J: A pharmacological approach to elucidation of the role of different nerve fibres and receptor endings in mediation of pain. J Physiol (Paris) 1977, 73(3):251–259.
Tominaga M, Tominaga T: Structure and function of TRPV1. Pflugers Arch 2005.
Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D: The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 1998, 21(3):531–543. 10.1016/S0896-6273(00)80564-4
Planells-Cases R, Garcia-Sanz N, Morenilla-Palao C, Ferrer-Montiel A: Functional aspects and mechanisms of TRPV1 involvement in neurogenic inflammation that leads to thermal hyperalgesia. Pflugers Arch 2005, 451(1):151–159. 10.1007/s00424-005-1423-5
Di M V, Blumberg PM, Szallasi A: Endovanilloid signaling in pain. Curr Opin Neurobiol 2002, 12(4):372–379. 10.1016/S0959-4388(02)00340-9
Zhang X, Huang J, McNaughton PA: NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J 2005, 24(24):4211–4223. 10.1038/sj.emboj.7600893
Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di M V, Julius D, Hogestatt ED: Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 1999, 400(6743):452–457. 10.1038/22761
Bhave G, Gereau RW: Posttranslational mechanisms of peripheral sensitization. J Neurobiol 2004, 61(1):88–106. 10.1002/neu.20083
Szallasi A, Cortright DN, Blum CA, Eid SR: The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat Rev Drug Discov 2007, 6(5):357–372. 10.1038/nrd2280
Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D: A capsaicin-receptor homologue with a high threshold for noxious heat. Nature 1999, 398(6726):436–441. 10.1038/18906
Guler AD, Lee H, Iida T, Shimizu I, Tominaga M, Caterina M: Heat-evoked activation of the ion channel, TRPV4. J Neurosci 2002, 22(15):6408–6414.
McKemy DD, Neuhausser WM, Julius D: Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 2002, 416(6876):52–58. 10.1038/nature719
Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A: ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 2003, 112(6):819–829. 10.1016/S0092-8674(03)00158-2
Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A: A TRP channel that senses cold stimuli and menthol. Cell 2002, 108(5):705–715. 10.1016/S0092-8674(02)00652-9
Xu H, Ramsey IS, Kotecha SA, Moran MM, Chong JA, Lawson D, Ge P, Lilly J, Silos-Santiago I, Xie Y, DiStefano PS, Curtis R, Clapham DE: TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature 2002, 418(6894):181–186. 10.1038/nature00882
Smith GD, Gunthorpe MJ, Kelsell RE, Hayes PD, Reilly P, Facer P, Wright JE, Jerman JC, Walhin JP, Ooi L, Egerton J, Charles KJ, Smart D, Randall AD, Anand P, Davis JB: TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature 2002, 418(6894):186–190. 10.1038/nature00894
Watanabe H, Vriens J, Suh SH, Benham CD, Droogmans G, Nilius B: Heat-evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. J Biol Chem 2002, 277(49):47044–47051. 10.1074/jbc.M208277200
Benham CD, Gunthorpe MJ, Davis JB: TRPV channels as temperature sensors. Cell Calcium 2003, 33(5–6):479–487. 10.1016/S0143-4160(03)00063-0
Jordt SE, McKemy DD, Julius D: Lessons from peppers and peppermint: the molecular logic of thermosensation. Curr Opin Neurobiol 2003, 13(4):487–492. 10.1016/S0959-4388(03)00101-6
McKemy DD: How cold is it? TRPM8 and TRPA1 in the molecular logic of cold sensation. Mol Pain 2005, 1(1):16. 10.1186/1744-8069-1-16
Patapoutian A, Peier AM, Story GM, Viswanath V: ThermoTRP channels and beyond: mechanisms of temperature sensation. Nat Rev Neurosci 2003, 4(7):529–539. 10.1038/nrn1141
Reid G: ThermoTRP channels and cold sensing: what are they really up to? Pflugers Arch 2005, 451(1):250–263. 10.1007/s00424-005-1437-z
Voets T, Nilius B: TRPs make sense. J Membr Biol 2003, 192(1):1–8. 10.1007/s00232-002-1059-8
Caterina MJ: Transient receptor potential ion channels as participants in thermosensation and thermoregulation. Am J Physiol Regul Integr Comp Physiol 2007, 292(1):R64-R76.
Dhaka A, Viswanath V, Patapoutian A: Trp ion channels and temperature sensation. Annu Rev Neurosci 2006, 29: 135–161. 10.1146/annurev.neuro.29.051605.112958
Reid G, Babes A, Pluteanu F: A cold- and menthol-activated current in rat dorsal root ganglion neurones: properties and role in cold transduction. J Physiol 2002, 545(Pt 2):595–614. 10.1113/jphysiol.2002.024331
Okazawa M, Takao K, Hori A, Shiraki T, Matsumura K, Kobayashi S: Ionic basis of cold receptors acting as thermostats. J Neurosci 2002, 22(10):3994–4001.
Madrid R, Donovan-Rodriguez T, Meseguer V, Acosta MC, Belmonte C, Viana F: Contribution of TRPM8 channels to cold transduction in primary sensory neurons and peripheral nerve terminals. J Neurosci 2006, 26(48):12512–12525. 10.1523/JNEUROSCI.3752-06.2006
Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A: TRPM8 Is Required for Cold Sensation in Mice. Neuron 2007, 54(3):371–378. 10.1016/j.neuron.2007.02.024
Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D: The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 2007, 448(7150):204–208. 10.1038/nature05910
Colburn RW, Lubin ML, Stone DJ Jr, Wang Y, Lawrence D, D'Andrea MR, Brandt MR, Liu Y, Flores CM, Qin N: Attenuated Cold Sensitivity in TRPM8 Null Mice. Neuron 2007, 54(3):379–386. 10.1016/j.neuron.2007.04.017
Lewin GR, Moshourab R: Mechanosensation and pain. J Neurobiol 2004, 61(1):30–44. 10.1002/neu.20078
Lin SY, Corey DP: TRP channels in mechanosensation. Curr Opin Neurobiol 2005, 15(3):350–357. 10.1016/j.conb.2005.05.012
Kellenberger S, Schild L: Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev 2002, 82(3):735–767.
Kim D: Physiology and pharmacology of two-pore domain potassium channels. Curr Pharm Des 2005, 11(21):2717–2736. 10.2174/1381612054546824
Lesage F, Lazdunski M: Molecular and functional properties of two-pore-domain potassium channels. Am J Physiol Renal Physiol 2000, 279(5):F793-F801.
Cockayne DA, Hamilton SG, Zhu QM, Dunn PM, Zhong Y, Novakovic S, Malmberg AB, Cain G, Berson A, Kassotakis L, Hedley L, Lachnit WG, Burnstock G, McMahon SB, Ford AP: Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature 2000, 407(6807):1011–1015. 10.1038/35039519
Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD: OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Biol 2000, 2(10):695–702. 10.1038/35036318
Liedtke W, Choe Y, Marti-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S: Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 2000, 103(3):525–535. 10.1016/S0092-8674(00)00143-4
Birder LA, Nakamura Y, Kiss S, Nealen ML, Barrick S, Kanai AJ, Wang E, Ruiz G, De Groat WC, Apodaca G, Watkins S, Caterina MJ: Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat Neurosci 2002, 5(9):856–860. 10.1038/nn902
Sharif NR, Witty MF, Seguela P, Bourque CW: An N-terminal variant of Trpv1 channel is required for osmosensory transduction. Nat Neurosci 2006, 9(1):93–98. 10.1038/nn1614
Muraki K, Iwata Y, Katanosaka Y, Ito T, Ohya S, Shigekawa M, Imaizumi Y: TRPV2 is a component of osmotically sensitive cation channels in murine aortic myocytes. Circ Res 2003, 93(9):829–838. 10.1161/01.RES.0000097263.10220.0C
Kanzaki M, Zhang YQ, Mashima H, Li L, Shibata H, Kojima I: Translocation of a calcium-permeable cation channel induced by insulin-like growth factor-I. Nat Cell Biol 1999, 1(3):165–170. 10.1038/11086
Neeper MP, Liu Y, Hutchinson TL, Wang Y, Flores CM, Qin N: Activation Properties of Heterologously Expressed Mammalian TRPV2: EVIDENCE FOR SPECIES DEPENDENCE. J Biol Chem 2007, 282(21):15894–15902. 10.1074/jbc.M608287200
Peier AM, Reeve AJ, Andersson DA, Moqrich A, Earley TJ, Hergarden AC, Story GM, Colley S, Hogenesch JB, McIntyre P, Bevan S, Patapoutian A: A heat-sensitive TRP channel expressed in keratinocytes. Science 2002, 296(5575):2046–2049. 10.1126/science.1073140
Moqrich A, Hwang SW, Earley TJ, Petrus MJ, Murray AN, Spencer KS, Andahazy M, Story GM, Patapoutian A: Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science 2005, 307(5714):1468–1472. 10.1126/science.1108609
Xu H, Delling M, Jun JC, Clapham DE: Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat Neurosci 2006, 9(5):628–635. 10.1038/nn1692
Hu HZ, Xiao R, Wang C, Gao N, Colton CK, Wood JD, Zhu MX: Potentiation of TRPV3 channel function by unsaturated fatty acids. J Cell Physiol 2006, 208(1):201–212. 10.1002/jcp.20648
O'Neil RG, Heller S: The mechanosensitive nature of TRPV channels. Pflugers Arch 2005, 451(1):193–203. 10.1007/s00424-005-1424-4
Chung MK, Lee H, Caterina MJ: Warm temperatures activate TRPV4 in mouse 308 keratinocytes. J Biol Chem 2003, 278(34):32037–32046. 10.1074/jbc.M303251200
Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B: Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature 2003, 424(6947):434–438. 10.1038/nature01807
Gao X, Wu L, O'Neil RG: Temperature-modulated diversity of TRPV4 channel gating: activation by physical stresses and phorbol ester derivatives through protein kinase C-dependent and -independent pathways. J Biol Chem 2003, 278(29):27129–27137. 10.1074/jbc.M302517200
Vriens J, Watanabe H, Janssens A, Droogmans G, Voets T, Nilius B: Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc Natl Acad Sci USA 2004, 101(1):396–401. 10.1073/pnas.0303329101
Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A: Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 2004, 41(6):849–857. 10.1016/S0896-6273(04)00150-3
Macpherson LJ, Geierstanger BH, Viswanath V, Bandell M, Eid SR, Hwang S, Patapoutian A: The pungency of garlic: activation of TRPA1 and TRPV1 in response to allicin. Curr Biol 2005, 15(10):929–934. 10.1016/j.cub.2005.04.018
Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Julius D: Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 2004, 427(6971):260–265. 10.1038/nature02282
Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, Hogestatt ED, Julius D, Jordt SE, Zygmunt PM: Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci USA 2005, 102(34):12248–12252. 10.1073/pnas.0505356102
Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, Patapoutian A: Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature 2007, 445(7127):541–545. 10.1038/nature05544
Hinman A, Chuang HH, Bautista DM, Julius D: TRP channel activation by reversible covalent modification. Proc Natl Acad Sci USA 2006, 103(51):19564–19568. 10.1073/pnas.0609598103
Corey DP, Garcia-Anoveros J, Holt JR, Kwan KY, Lin SY, Vollrath MA, Amalfitano A, Cheung EL, Derfler BH, Duggan A, Geleoc GS, Gray PA, Hoffman MP, Rehm HL, Tamasauskas D, Zhang DS: TRPA1 is a candidate for the mechanosensitive transduction channel of vertebrate hair cells. Nature 2004, 432(7018):723–730. 10.1038/nature03066
Nagata K, Duggan A, Kumar G, Garcia-Anoveros J: Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J Neurosci 2005, 25(16):4052–4061. 10.1523/JNEUROSCI.0013-05.2005
Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D: TRPA1 Mediates the Inflammatory Actions of Environmental Irritants and Proalgesic Agents. Cell 2006, 124(6):1269–1282. 10.1016/j.cell.2006.02.023
Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, Corey DP: TRPA1 Contributes to Cold, Mechanical, and Chemical Nociception but Is Not Essential for Hair-Cell Transduction. Neuron 2006, 50(2):277–289. 10.1016/j.neuron.2006.03.042
Abeele FV, Zholos A, Bidaux G, Shuba Y, Thebault S, Beck B, Flourakis M, Panchin Y, Skryma R, Prevarskaya N: Ca(2+)-independent phospholipase A(2)-dependent gating of TRPM8 by lysophospholipids. J Biol Chem 2006, 281(52):40174–40182. 10.1074/jbc.M605779200
Andersson DA, Nash M, Bevan S: Modulation of the cold-activated channel TRPM8 by lysophospholipids and polyunsaturated fatty acids. J Neurosci 2007, 27(12):3347–3355. 10.1523/JNEUROSCI.4846-06.2007
Kahn-Kirby AH, Bargmann CI: TRP channels in C. elegans. Annu Rev Physiol 2006, 68: 719–736. 10.1146/annurev.physiol.68.040204.100715
Sokolchik I, Tanabe T, Baldi PF, Sze JY: Polymodal sensory function of the Caenorhabditis elegans OCR-2 channel arises from distinct intrinsic determinants within the protein and is selectively conserved in mammalian TRPV proteins. J Neurosci 2005, 25(4):1015–1023. 10.1523/JNEUROSCI.3107-04.2005
Alloui A, Zimmermann K, Mamet J, Duprat F, Noel J, Chemin J, Guy N, Blondeau N, Voilley N, Rubat-Coudert C, Borsotto M, Romey G, Heurteaux C, Reeh P, Eschalier A, Lazdunski M: TREK-1, a K(+) channel involved in polymodal pain perception. EMBO J 2006.
Kim D: Fatty acid-sensitive two-pore domain K+ channels. Trends Pharmacol Sci 2003, 24(12):648–654. 10.1016/j.tips.2003.10.008
Maingret F, Lauritzen I, Patel AJ, Heurteaux C, Reyes R, Lesage F, Lazdunski M, Honore E: TREK-1 is a heat-activated background K(+) channel. EMBO J 2000, 19(11):2483–2491. 10.1093/emboj/19.11.2483
Talley EM, Sirois JE, Lei Q, Bayliss DA: Two-pore-Domain (KCNK) potassium channels: dynamic roles in neuronal function. Neuroscientist 2003, 9(1):46–56. 10.1177/1073858402239590
Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D: Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 2000, 288(5464):306–313. 10.1126/science.288.5464.306
Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K, Hughes SA, Rance K, Grau E, Harper AJ, Pugh PL, Rogers DC, Bingham S, Randall A, Sheardown SA: Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature 2000, 405(6783):183–187. 10.1038/35012076
Steiner AA, Turek VF, Almeida MC, Burmeister JJ, Oliveira DL, Roberts JL, Bannon AW, Norman MH, Louis JC, Treanor JJ, Gavva NR, Romanovsky AA: Nonthermal activation of transient receptor potential vanilloid-1 channels in abdominal viscera tonically inhibits autonomic cold-defense effectors. J Neurosci 2007, 27(28):7459–7468. 10.1523/JNEUROSCI.1483-07.2007
Iida T, Shimizu I, Nealen ML, Campbell A, Caterina M: Attenuated fever response in mice lacking TRPV1. Neurosci Lett 2005, 378(1):28–33. 10.1016/j.neulet.2004.12.007
Jones RC III, Xu L, Gebhart GF: The mechanosensitivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require transient receptor potential vanilloid 1 and acid-sensing ion channel 3. J Neurosci 2005, 25(47):10981–10989. 10.1523/JNEUROSCI.0703-05.2005
Rong W, Hillsley K, Davis JB, Hicks G, Winchester WJ, Grundy D: Jejunal afferent nerve sensitivity in wild-type and TRPV1 knockout mice. J Physiol 2004, 560(Pt 3):867–881. 10.1113/jphysiol.2004.071746
Alessandri-Haber N, Dina OA, Yeh JJ, Parada CA, Reichling DB, Levine JD: Transient receptor potential vanilloid 4 is essential in chemotherapy-induced neuropathic pain in the rat. J Neurosci 2004, 24(18):4444–4452. 10.1523/JNEUROSCI.0242-04.2004
Ramsey IS, Delling M, Clapham DE: An introduction to trp channels. Annu Rev Physiol 2006, 68: 619–647. 10.1146/annurev.physiol.68.040204.100431
Liedtke W: Transient receptor potential vanilloid channels functioning in transduction of osmotic stimuli. J Endocrinol 2006, 191(3):515–523. 10.1677/joe.1.07000
Petrus MJ, Peier AM, Bandell M, Hwang SW, Huynh T, Olney N, Jegla T, Patapoutian A: A role of TRPA1 in mechanical hyperalgesia is revealed by pharmacological inhibition. Molecular Pain 2007., 3(40):
Sutherland SP, Benson CJ, Adelman JP, McCleskey EW: Acid-sensing ion channel 3 matches the acid-gated current in cardiac ischemia-sensing neurons. Proc Natl Acad Sci USA 2001, 98(2):711–716. 10.1073/pnas.011404498
Ugawa S, Ueda T, Ishida Y, Nishigaki M, Shibata Y, Shimada S: Amiloride-blockable acid-sensing ion channels are leading acid sensors expressed in human nociceptors. J Clin Invest 2002, 110(8):1185–1190.
Babes A, Zorzon D, Reid G: Two populations of cold-sensitive neurons in rat dorsal root ganglia and their modulation by nerve growth factor. Eur J Neurosci 2004, 20(9):2276–2282. 10.1111/j.1460-9568.2004.03695.x
Viana F, de la Pena E, Belmonte C: Specificity of cold thermotransduction is determined by differential ionic channel expression. Nat Neurosci 2002, 5(3):254–260. 10.1038/nn809
Xing H, Ling J, Chen M, Gu JG: Chemical and Cold Sensitivity of Two Distinct Populations of TRPM8-Expressing Somatosensory Neurons. Journal of Neurophysiology 2006, 95(2):1221–1230. 10.1152/jn.01035.2005
Xing H, Chen M, Ling J, Tan W, Gu JG: TRPM8 mechanism of cold allodynia after chronic nerve injury. J Neurosci 2007, 27(50):13680–13690. 10.1523/JNEUROSCI.2203-07.2007
Okazawa M, Inoue W, Hori A, Hosokawa H, Matsumura K, Kobayashi S: Noxious heat receptors present in cold-sensory cells in rats. Neurosci Lett 2004, 359(1–2):33–36. 10.1016/j.neulet.2004.01.074
Acosta MC, Madrid R, Luna C, Valero M, Belmonte C, Viana F: Activation by heat and capsaicin of peripheral nerve endings and soma of cold primary sensory neurons. Program No.862.8.2005 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience, 2005.Online 2005.
Green BG: Temperature perception and nociception. J Neurobiol 2004, 61(1):13–29. 10.1002/neu.20081
Hensel H, Zotterman Y: The response of mechanoreceptors to thermal stimulation. J Physiol 1951, 115(1):16–24.
Booth CS, Hahn JF: Thermal and mechanical stimulation of type II receptors and field receptors in cat. Exp Neurol 1974, 44(1):49–59. 10.1016/0014-4886(74)90045-4
Duclaux R, Kenshalo DR: The temperature sensitivity of the type I slowly adapting mechanoreceptors in cats and monkeys. J Physiol 1972, 224(3):647–664.
Iggo A: Cutaneous thermoreceptors in primates and sub-primates. J Physiol (Lond) 1969, 200(2):403–430.
Cahusac PM, Noyce R: A pharmacological study of slowly adapting mechanoreceptors responsive to cold thermal stimulation. Neuroscience 2007, 148(2):489–500. 10.1016/j.neuroscience.2007.06.018
Woodbury CJ, Zwick M, Wang S, Lawson JJ, Caterina MJ, Koltzenburg M, Albers KM, Koerber HR, Davis BM: Nociceptors lacking TRPV1 and TRPV2 have normal heat responses. J Neurosci 2004, 24(28):6410–6415. 10.1523/JNEUROSCI.1421-04.2004
Liapi A, Wood JN: Extensive co-localization and heteromultimer formation of the vanilloid receptor-like protein TRPV2 and the capsaicin receptor TRPV1 in the adult rat cerebral cortex. Eur J Neurosci 2005, 22(4):825–834. 10.1111/j.1460-9568.2005.04270.x
Rutter AR, Ma QP, Leveridge M, Bonnert TP: Heteromerization and colocalization of TrpV1 and TrpV2 in mammalian cell lines and rat dorsal root ganglia. Neuroreport 2005, 16(16):1735–1739. 10.1097/01.wnr.0000185958.03841.0f
Almasi R, Petho G, Bolcskei K, Szolcsanyi J: Effect of resiniferatoxin on the noxious heat threshold temperature in the rat: a novel heat allodynia model sensitive to analgesics. Br J Pharmacol 2003, 139(1):49–58. 10.1038/sj.bjp.0705234
Mogil JS, Miermeister F, Seifert F, Strasburg K, Zimmermann K, Reinold H, Austin JS, Bernardini N, Chesler EJ, Hofmann HA, Hordo C, Messlinger K, Nemmani KV, Rankin AL, Ritchie J, Siegling A, Smith SB, Sotocinal S, Vater A, Lehto SG, Klussmann S, Quirion R, Michaelis M, Devor M, Reeh PW: Variable sensitivity to noxious heat is mediated by differential expression of the CGRP gene. Proc Natl Acad Sci USA 2005, 102(36):12938–12943. 10.1073/pnas.0503264102
Reeh PW, Kress M: Molecular physiology of proton transduction in nociceptors. Curr Opin Pharmacol 2001, 1(1):45–51. 10.1016/S1471-4892(01)00014-5
Krishtal O: The ASICs: signaling molecules? Modulators? Trends Neurosci 2003, 26(9):477–483. 10.1016/S0166-2236(03)00210-8
Cooper BY, Johnson RD, Rau KK: Characterization and function of TWIK-related acid sensing K+ channels in a rat nociceptive cell. Neuroscience 2004, 129(1):209–224. 10.1016/j.neuroscience.2004.06.066
Jiang N, Rau KK, Johnson RD, Cooper BY: Proton sensitivity Ca2+ permeability and molecular basis of Acid-sensing ion channels expressed in glabrous and hairy skin afferents. Journal of Neurophysiology 2006, 95(4):2466–2478. 10.1152/jn.00861.2005
Leffler A, Monter B, Koltzenburg M: The role of the capsaicin receptor TRPV1 and acid-sensing ion channels (ASICS) in proton sensitivity of subpopulations of primary nociceptive neurons in rats and mice. Neuroscience 2006, 139(2):699–709. 10.1016/j.neuroscience.2005.12.020
Ugawa S, Ueda T, Yamamura H, Shimada S: In situ hybridization evidence for the coexistence of ASIC and TRPV1 within rat single sensory neurons. Brain Res Mol Brain Res 2005, 136(1–2):125–133. 10.1016/j.molbrainres.2005.01.010
Reid G, Flonta ML: Physiology. Cold current in thermoreceptive neurons. Nature 2001, 413(6855):480. 10.1038/35097164
Reid G, Flonta M: Cold transduction by inhibition of a background potassium conductance in rat primary sensory neurones. Neurosci Lett 2001, 297(3):171–174. 10.1016/S0304-3940(00)01694-3
Kang D, Choe C, Kim D: Thermosensitivity of the two-pore domain K+ channels TREK-2 and TRAAK. J Physiol 2005, 564(Pt 1):103–116. 10.1113/jphysiol.2004.081059
Dhaka A, Earley TJ, Watson J, Patapoutian A: Visualizing cold spots: TRPM8-expressing sensory neurons and their projections. J Neurosci 2008, 28(3):566–575. 10.1523/JNEUROSCI.3976-07.2008
Hjerling-Leffler J, Alqatari M, Ernfors P, Koltzenburg M: Emergence of functional sensory subtypes as defined by transient receptor potential channel expression. J Neurosci 2007, 27(10):2435–2443. 10.1523/JNEUROSCI.5614-06.2007
Weber EH: E.H. Weber on the Tactile Senses. Edited by: Ross HE, Murray AH. Psychology Press; 1896:1–260.
Dodt E, Zotterman Y: The discharge of specific cold fibres at high temperatures; the paradoxical cold. Acta Physiol Scand 1952, 26(4):358–365.
Craig AD, Reiman EM, Evans A, Bushnell MC: Functional imaging of an illusion of pain. Nature 1996, 384(6606):258–260. 10.1038/384258a0
Weil A, Moore SE, Waite NJ, Randall A, Gunthorpe MJ: Conservation of functional and pharmacological properties in the distantly related temperature sensors TRPV1 and TRPM8. Mol Pharmacol 2005.
Gunthorpe MJ, Harries MH, Prinjha RK, Davis JB, Randall A: Voltage- and time-dependent properties of the recombinant rat vanilloid receptor (rVR1). J Physiol 2000, 525(Pt 3):747–759. 10.1111/j.1469-7793.2000.t01-1-00747.x
Nilius B, Talavera K, Owsianik G, Prenen J, Droogmans G, Voets T: Gating of TRP channels: a voltage connection? J Physiol 2005, 567(Pt 1):35–44. 10.1113/jphysiol.2005.088377
Voets T, Owsianik G, Janssens A, Talavera K, Nilius B: TRPM8 voltage sensor mutants reveal a mechanism for integrating thermal and chemical stimuli. Nat Chem Biol 2007, 3(3):174–182. 10.1038/nchembio862
Brauchi S, Orio P, Latorre R: Clues to understanding cold sensation: thermodynamics and electrophysiological analysis of the cold receptor TRPM8. Proc Natl Acad Sci USA 2004, 101(43):15494–15499. 10.1073/pnas.0406773101
Matta JA, Ahern GP: Voltage is a partial activator of rat thermosensitive TRP channels. J Physiol 2007, 585(Pt 2):469–482. 10.1113/jphysiol.2007.144287
Latorre R, Brauchi S, Orta G, Zaelzer C, Vargas G: ThermoTRP channels as modular proteins with allosteric gating. Cell Calcium 2007, 42(4–5):427–438. 10.1016/j.ceca.2007.04.004
Roza C, Belmonte C, Viana F: Cold sensitivity in axotomized fibers of experimental neuromas in mice. Pain 2006, 120(1–2):24–35. 10.1016/j.pain.2005.10.006
Waxman SG, Dib-Hajj S: Erythermalgia: molecular basis for an inherited pain syndrome. Trends Mol Med 2005, 11(12):555–562. 10.1016/j.molmed.2005.10.004
Rush AM, Dib-Hajj SD, Liu S, Cummins TR, Black JA, Waxman SG: A single sodium channel mutation produces hyper- or hypoexcitability in different types of neurons. Proc Natl Acad Sci USA 2006, 103(21):8245–8250. 10.1073/pnas.0602813103
Carr RW, Pianova S, Brock JA: The effects of polarizing current on nerve terminal impulses recorded from polymodal and cold receptors in the guinea-pig cornea. J Gen Physiol 2002, 120(3):395–405. 10.1085/jgp.20028628
Zimmermann K, Leffler A, Babes A, Cendan CM, Carr RW, Kobayashi J, Nau C, Wood JN, Reeh PW: Sensory neuron sodium channel Nav1.8 is essential for pain at low temperatures. Nature 2007, 447(7146):856–859. 10.1038/nature05880
de la Pena E, Malkia A, Cabedo H, Belmonte C, Viana F: The contribution of TRPM8 channels to cold sensing in mammalian neurones. J Physiol 2005, 567(Pt 2):415–426. 10.1113/jphysiol.2005.086546
Malkia A, Madrid R, Meseguer V, de la PE, Valero M, Belmonte C, Viana F: Bidirectional shifts of TRPM8 channel gating by temperature and chemical agents modulate the cold sensitivity of mammalian thermoreceptors. J Physiol 2007, 581(Pt 1):155–174. 10.1113/jphysiol.2006.123059
Hensel H: Neural processes in thermoregulation. Physiol Rev 1973, 53(4):948–1017.
Brauchi S, Orta G, Salazar M, Rosenmann E, Latorre R: A hot-sensing cold receptor: C-terminal domain determines thermosensation in transient receptor potential channels. J Neurosci 2006, 26(18):4835–4840. 10.1523/JNEUROSCI.5080-05.2006
Rohacs T, Lopes CM, Michailidis I, Logothetis DE: PI(4,5)P2 regulates the activation and desensitization of TRPM8 channels through the TRP domain. Nat Neurosci 2005, 8(5):626–634. 10.1038/nn1451
Liu BY, Qin F: Functional control of cold- and menthol-sensitive TRPM8 ion channels by phosphatidylinositol 4,5-bisphosphate. Journal of Neuroscience 2005, 25(7):1674–1681. 10.1523/JNEUROSCI.3632-04.2005
Liedtke W, Tobin DM, Bargmann CI, Friedman JM: Mammalian TRPV4 (VR-OAC) directs behavioral responses to osmotic and mechanical stimuli in Caenorhabditis elegans. Proc Natl Acad Sci USA 2003, 100(Suppl 2):14531–14536. 10.1073/pnas.2235619100
Craig AD: Pain mechanisms: labeled lines versus convergence in central processing. Annu Rev Neurosci 2003, 26: 1–30. 10.1146/annurev.neuro.26.041002.131022
Acknowledgements
This work was supported by grants from the Spanish Ministry of Education and Science: project BFU2007-61855 to F.V and projects CONSOLIDER-INGENIO 2010 CSD2007-00023 and BFI2002-03788 to C.B. We thank Stuart Ingham for help with the illustrations.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Belmonte, C., Viana, F. Molecular and cellular limits to somatosensory specificity. Mol Pain 4, 14 (2008). https://doi.org/10.1186/1744-8069-4-14
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1744-8069-4-14