Abstract
Gene therapy with adeno-associated virus (AAV) has advanced in the last few years from promising results in animal models to >100 clinical trials (reported or under way). While vector availability was a substantial hurdle a decade ago, innovative new production methods now routinely match the scale of AAV doses required for clinical testing. These advances may become relevant to translational research in the chronic pain field. AAV for pain targeting the peripheral nervous system was proven to be efficacious in rodent models several years ago, but has not yet been tested in humans. The present review addresses the steps needed for translation of AAV for pain from the bench to the bedside focusing on pre-clinical toxicology. We break the potential toxicities into three conceptual categories of risk: First, risks related to the delivery procedure used to administer the vector. Second, risks related to AAV biology, i.e., effects of the vector itself that may occur independently of the transgene. Third, risks related to the effects of the therapeutic transgene. To identify potential toxicities, we consulted the existing evidence from AAV gene therapy for other nervous system disorders (animal toxicology and human studies) and from the clinical pharmacology of conventional analgesic drugs. Thereby, we identified required preclinical studies and charted a hypothetical path towards a future phase I/II clinical trial in the oncology-palliative care setting.
Similar content being viewed by others
Introduction
Unrelieved chronic pain is a critical health problem in the US and worldwide. A report by the Institute of Medicine estimated that 116 million Americans suffer from pain that persists for weeks to years, with resulting annual costs exceeding $560 million [1]. Pain is an especially common problem in patients with advanced cancer, with some studies reporting that the majority of patients dying from metastatic solid tumors experience severe unrelieved pain despite treatment with available analgesics [2, 3]. Antineoplastic treatment options have often been exhausted for these patients, placing palliation of symptoms at the center of treatment goals for the limited remaining life span, typically weeks to a few months. The combination of the great need for pain relief and of the presence of an underlying incurable disease creates scenarios for early clinical testing of the most novel (and potentially risky) new pain treatment strategies, first in selected patients with cancer pain as a phase I clinical trial. Therefore, the subsequent review on clinical translation and preclinical toxicology studies is predicated on a future plan to translate AAV gene therapy first in the palliative oncology setting.
Two vector systems have been most extensively studied in gene therapy for pain: Herpes simplex virus (HSV), which has already been tested in cancer pain patients [4]; and adeno-associated virus (AAV). AAV, the subject of this review, has not been tested clinically for pain. However, AAV is arguably the most advanced and widely studied vector in terms of clinical trials seeking long-term gene expression in non-malignant tissues [5]. The first AAV therapeutic product, alipogene tiparvovec, is now marketed in Europe [6]. Therefore, substantial pre-clinical toxicology data and clinical phase I/II toxicology research exists for AAV that appears applicable to the translational development of this vector in the field of pain.
Defining the scope of possible toxicities
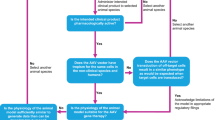
The potential toxicities of AAV for pain can be conceptually broken down into mutually exclusive categories of risk. This review divides toxicities as follows: the procedure employed for vector delivery to the target tissue, i.e., a surgical/interventional risks (section 3); the AAV vector itself (sin transgene), i.e., viral and immune-biology (section 4); and the specific therapeutic gene, i.e., pharmacology (section 5). An overview is provided as a semantic “mind map” in Figure 1.
Potential toxicities of adeno-associated virus (AAV) gene therapy for chronic pain. Risks were sorted into three mutually exclusive categories (blue). For each type of risk existing evidence is highlighted (red) and future studies towards pre-clinical toxicology are proposed (green). MTD, maximum tolerated dose; CNS, central nervous system; IG, intraganglionic; IT, intrathecal; DRG, dorsal root ganglia; ppßE, prepro-ß-endorphin; IL-10, interleukin-10.
Potential toxicities related to the procedure employed for vector delivery
Two routes of delivery have been used in rodent models to demonstrate analgesic efficacy of AAV through gene transfer to the nociceptive neurons in the DRG: intrathecal (IT) or intraganglionic (IG) administration.
The IT route delivers the vector to the cerebrospinal fluid (CSF) and has been achieved in rats by inserting an IT catheter through the cisterna magna and advancing it caudally to the lumbar level [7–10]. The same approach has been used in dogs [11, 12]. In pigs, we recently reported an alternative: direct injection into the lumbar CSF by a lateral lumbar puncture under interventional image guidance with a computed tomography (CT) scanner [13]. In humans, IT delivery can be easily performed by lumbar puncture (LP), a routine bedside procedure with excellent safety profile [14].
Therefore, no additional toxicity studies would be needed in regards to the LP procedure risk, if a phase I trial of AAV for pain were to test vector administration by the IT route.
The IG route delivers AAV directly into the DRG parenchyma. IG studies in rats accessed the DRG by an open neurosurgical procedure [15–19], which would not be attractive in humans because it would require a complicated and invasive procedure. We therefore developed a minimally invasive, CT imaging-guided technique to safely target the DRG in the pig model. Using a customized needle assemble for convection enhanced delivery (CED), we then demonstrated efficacy of CED to deliver AAV into the DRG parenchyma [20]. Comparative anatomy analysis of the human and the porcine spine suggested that the technique will be easily translatable to humans. As a matter of fact, the imaging equipment used in our study was identical with the state-of-the art equipment currently used for neuroradiological spine interventions in humans.
In the field of interventional neuroradiology, it is common practice to target small structures such as the DRG for drug injections or biopsy. Ultrasound ablation of the spinal sensory nerve root has also been reported [21]. Therefore, while IG drug delivery is a new clinical procedure, it will resemble clinical practice of similar interventions. Accordingly, we conclude that only a limited scope of additional preclinical toxicity assessment of the IG injection procedure might be needed, e.g., to determine absence of DRG tissue damage, and that such studies can be performed in the pig model of the procedure that we have already in place.
Potential toxicities associated with the AAV vector: Viral- and immune biology
Clinical translation of AAV for pain faces the issue of general vector toxicity: the risk of AAV itself (without the transgene, which will be discussed in the Section 4 below). This section is predicated on the conservative assumption that vector may spread systemically to all organ sites, even though the IT and IG route of administration attempts to limit such spread.
Previous experience with AAV gene therapy identified several mechanisms by which the vectors trigger an adverse reaction; each is addressed in a separate subsection below.
Vector related general toxicities: insertional mutagenesis, germ line transmission, and replication escape
AAV vectors persist in cells in two states, as episomal concatamers and, for a small minority of vector copies, chromosomal integration [22]. In a specific experimental setting of newborn mice, AAV vectors were found to integrate into the genomes of hepatocytes at a rate of 0.1 – 1% of transduction events [23, 24] resulting in the development of hepatocellular carcinoma [25]. While this report generated substantial concern in the field, subsequent investigation by numerous laboratories have suggested that the observation is likely not applicable to AAV gene therapy in adult humans. No evidence of insertional mutagenesis was reported after systemic delivery of AAV in humans or large animals models or after selective delivery to the CNS in any species.
Transmission to the germ line has not been found in any AAV clinical trial. While AAV particles were detected in the semen of subjects receiving AAV2 into the hepatic artery [26], this was temporary.
The potential of replication escape of the recombinant AAV was shown in vitro in the setting of co-infection with the wild type AAV2 and a helper virus [27]. However, it has not been reported in any of the AAV preclinical toxicology studies or subsequent clinical trials.
The most serious adverse event in the setting of a AAV human trial occurred in 2007 when a patient died 22 days after receiving intra-articular AAV encoding etanercept, a tumor necrosis factor receptor blocker [28, 29], from a large retroperitoneal hematoma and systemic histoplasmosis. The FDA stopped the clinical trial but, after an investigation, allowed the trial to resume. Similar adverse events were not seen in any of the other patients.
In regards to AAV for pain, the above evidence suggests that issues of insertional mutagenesis, germ line transmission, replication escape, and dissemination of the vector to the public domain, have been so thoroughly addressed in the field. The adverse event in the AAV-etanercept trial suggests that in the case of IL-10, a transgene that is similar to etanercept in regards to its immune-suppressive effect, measurement of systemic levels should be an important goal of preclinical toxicity assessments. Furthermore, a pain trial in patient with incurable cancer could answer some of the above questions in humans, at least if (some of the) patients may decide that after their (at trial entry anticipated) death from cancer (i.e., at the end of the trial) autopsy tissue may be sampled for research purposes.
Immune response to the AAV vector (sin transgene)
AAV for pain by the IT or IG route will trigger a serological immune response to the vector capsid. In addition, cellular immunity to the vector capsid has to be carefully considered, although (or because) fewer predictions may be possible in regards to the cellular immunity one may anticipate in humans.
Serological immunity
AAV delivered IT or by direct infusion into the brain parenchyma led to >100-fold increase in the titer of circulating NAb in large animal models as shown for AAV2 [30, 31], AAV1 [32], AAVrh10 [33], and AAV9 [34, 35]. The measured titers of circulating NAb seemed to be proportionate to the total dose of AAV vector administered. The clinical trials employing intraparenchymal infusion of AAV to the brain also demonstrated a rise of circulating NAb in some but not all patients [36–42]. NAb titers in the CSF were examined only in some of these studies. When measured, the CSF NAb levels were consistently lower compared to the systemic NAb, suggesting the systemic origin of the humoral immune response. Importantly, while NAb occur, they have no pathological consequence: No clinical symptoms or adverse effects were associated with the presence of NAb in any animal or human study.
The presence of pre-existing circulating NAb inhibits AAV transduction when the vector is delivered systemically [43]. In regards to IT AAV, studies in large animal models suggest a threshold effect for the capacity of systemic NAb to prevent AAV transduction. In non-human NHP, transduction by IT AAV9 was inhibited by NAb titer of ≥ 1:200 but not affected by titers of ≤ 1:128 [35, 44]. Studies in dogs performed by Haurigot et al. [34] demonstrated only moderately reduced CNS- and PNS transduction rates for NAb titers as high as 1:1000, suggesting that an immune privilege-like status may apply to the PNS. Pre-existing systemic NAb did not affect intraparenchymal gene transfer to the brain by any serotype in either NHP [31] or humans [41].
For the development of AAV for pain, we conclude that the vector will induce NAb without any pathological consequences. Pre-existing NAb may diminish IT transduction rates, but the extent or threshold is a clinical research question (of minor importance) that may be answered in a clinical trial. Pre-existing NAb will not affect IG delivery of AAV. Taken together, we propose that a phase I trial of AAV for pain should collect specimen to measure NAb before and after vector administration, while no further preclinical studies are required towards that plan. Preexisting NAb should not be an exclusion criterion for individual patients considered for IT or IG AAV for cancer pain.
Cellular immunity
The clinical significance of CD8 mediated cytotoxicity directed against the AAV capsid and leading to a destruction of the transduced cells arose as an issue in a clinical trial of AAV2 for hemophilia B [26]. Importantly, the precedent preclinical studies had failed to predict the cellular immune response in animal models [45].
Subsequent animal and human studies demonstrated that the extent of cellular immune response against AAV might depend on the host species, the AAV serotype, and the target tissue. A comparison of rodents, large animal species, and humans showed that humans are prone to mount a cellular immune response to systemic AAV more readily than other species [46, 47]. The choice of AAV serotype may affect the cellular immunity through direct, antigen presenting cell-independent activation of T-lymphocytes by an AAV capsid [48, 49]. When compared to systemic administration, delivery of AAV directly to the nervous system did not result in cell-mediated immunity to the AAV capsid for either the intraparenchymal route, tested in both large animals and humans [32–34, 36, 41, 42, 50–56] or the IT route, tested to date in large animals only [34, 57, 58].
Based on this evidence, the occurrence of a cellular immune response against the AAV capsid in the PNS cannot be definitely excluded on the basis of large animal studies. It will therefore be a critical focus of a phase I clinical trial of AAV for pain, which will need to include collection of peripheral mononuclear blood cells and extensive post-mortem histopathological analysis for possible immune-toxicity. This approach appears particularly justified by the absence of cellular immunity-associated adverse effects in the context of previous AAV studies in the CNS. Furthermore, a cellular immune response could be effectively managed by immunosuppression [36, 49], suggesting that this option, i.e., use of systemic corticosteroids, should be allowed in a clinical trial of AAV for pain if immune toxicity, e.g., a Guillain-Barre like syndrome is seen in a patient. In addition, an AAV for pain human trial may provide unique opportunities for insights into the cellular immunity against AAV in the nervous system, if collection of post-mortem tissue can be accomplished.
AAV serotypes and off-target biodistribution
In order to avoid toxicities and facilitate clinical translation of AAV for pain, selection of AAV serotypes will play a critical role. The ideal serotype for AAV for pain would effectively transduce PNS neurons after IT or IG administration; show low levels of escape to peripheral organ sites; and have precedent safety data available from a clinical trial (for another disease).
AAV8 effectively transduced the DRG neurons, while showing limited brain penetration upon IT administration in rodents [59–61], which might limit toxicities associated with CNS transduction. However, AAV8 manifests strong hepatotropism after systemic administration (and has thus been a leading contender in the preclinical and clinical studies of hemophilia B) [49]. While biodistribution of IT AAV8 has not been examined in a large animal model, any systemic spread of the vector would likely lead to transduction of the liver, raising the issue of systemic transgene effects.
IT AAV9 transduces both the PNS and CNS [34, 35, 44, 62, 63] and is in the final stages of preclinical development for mucopolysaccharidosis IIIA and giant axonal neuropathy [34, 58], with studies suggesting an excellent safety profile for IT doses of up to 2 × 1013 genome copies (GC) in NHP (with projected dose of 3.5 × 1013 GC in humans). Widespread CNS transduction may be a characteristic of AAV9 and may appear less attractive for gene therapy for pain, at least for the current approaches that specifically target the PNS and spinal cord. AAV9 has also been reported to transduce the CNS after intravascular delivery, suggesting the ability of this serotype to cross the blood brain barrier (BBB) [43, 44].
AAV2 and AAVrh10 are the only serotypes that have been clinically tested in humans in the CNS. The safety of intraparenchymal infusion of up to 1 × 1012 GC of AAV2 to the brain has been demonstrated in the setting of Canavan disease [64], late infantile neuronal ceroid lipofuscinosis, LINCL [40], Parkinson disease [37, 41, 65–67], and Alzheimer disease [54]. AAVrh10 showed comparable safety for doses up to 7.2 × 1011 GC, while achieving higher brain penetration after intraparenchymal infusion compared to AAV2 [33] and was clinically tested for mucopolysaccharidosis IIIA [36] and LINCL [68].
Recent advances in AAV biology showed promise in developing AAV particles with improved transduction efficiency or tissue tropism. The improved transduction efficiency has been demonstrated by using self-complementary (sc-) AAV vectors [69] and while no sc-AAV has so far been used in the CNS in humans, systemic delivery of sc-AAV8 showed safety in the clinical trial for hemophilia B [49]. The improved tissue tropism of AAV has been achieved by rational capsid engineering [70]. While most of these studies are in the early phases of development, one of the novel serotypes termed AAV2.5, selected for muscle tropism, has been brought to a phase I clinical trial for Duchenne muscle dystrophy [71]. IT administration of AAV2.5 in NHP resulted in a transduction pattern similar to that of AAV9 [35], making AAV2.5 an appealing alternative to AAV9 for AAV for pain.
In regards to AAV for pain, we would arrive at different conclusions for the two delivery routes under consideration: IG administration of AAV2 or AAVrh10 as well as IT administration of AAV9 might not need any further investigations of serotype-specific toxicities and might only need to be further assessed in regards to the toxicities of a delivered therapeutic gene (as detailed in Section 5 below). IT AAV8 requires further biodistribution studies to confirm whether its feasible characteristics observed rodents also apply to large animals, which will then determine whether AAV9 or AAV8 would the vector of choice for the IT route in the clinics.
Potential toxicities associated with the therapeutic genes: Pharmacology
In this section, the review will focus on two transgenes that appear particularly promising for translating AAV gene therapy for pain: prepro-ß-endorphin (ppßE) and interleukin-10 (IL-10). The therapeutic efficacies of these two genes have been investigated and validated in multiple vector systems including AAV by several independent groups including ours [61, 72–74]. The ppßE gene was originally developed by us for the use in pain gene therapy [75]. A number of other analgesic genes have been reported in a variety of vector systems and might be alternative candidates for the clinical translation of AAV for pain, whereby the pharmacological assessment will follow similar principles as laid out here for ppßE and IL-10.
Dose–response relationship and therapeutic index: Transgene as a drug
Effects of the transgene products can be assessed by applying the principles established in pain pharmacology for conventional (non-gene therapy) drugs. Most analgesic drugs, including the peptide ß-endorphin (ßE, considered as a gene-encoded version here), show dose dependence in regards to both their analgesic- and toxic effect. Low doses of analgesic drugs thereby exert low (if any) pharmacological effect and dose escalation results in an analgesic effect and eventually, if doses exceed a certain threshold, the gradual emergence of toxicity. The drug level needed to produce the toxic effect relative to a therapeutic effect is the therapeutic index.
The emphasis on the dose–response relationship and the therapeutic index in pain pharmacology provides important guidance in regards to the development of AAV for pain. A certain degree of toxicity may be expected at high-therapeutic doses and may be not only acceptable but even the goal of dose-escalation in order to optimize the therapeutic benefit. The rationale for delivering the ppßE gene by AAV is the expectation of a favorable therapeutic index. The basis for its safe development will be the appropriate choice of the initial vector dose in a phase I/II trial.
Tissue-specific transgene expression: improving the therapeutic index
Conventional analgesic drugs, such as opioids, administered orally or intravenously expose all sites of the body to similar drug concentrations. In contrast, AAV gene therapy for pain by the IT or IG route aims at targeting anatomical sites known to mediate analgesic efficacy of opioids but not toxicity: the spinal compartment of the nervous system including the DRGs and the posterior horn of the spinal cord.
IT delivery improves the therapeutic index of conventional opioids. This principle is firmly established through animal studies [11, 12] and the use of IT pump-mediated delivery of opioids in patients with the most severe pain states [76]. Vector systems that were initially used in gene therapy for pain, such as adenovirus [77, 78] or plasmids [74, 79] transduced only meningeal lining fibroblasts, which secreted the transgene product into the spinal CSF emulating an IT pump.
An important observation in the field of AAV for pain, however, has been the marked tropism of AAV for DRG neurons if administered IT or IG. This was a very favorable, while unexpected, finding and has subsequently been confirmed by several groups in different animal models [16, 18, 59–61, 80–82]. It has an important promising implication for the pharmacology of AAV for pain: the potential to not only match the therapeutic index of IT opioids but to improve further upon it. AAV-mediated transgene pharmacokinetics can be formalized as follows. If the transgene encodes a secreted protein such as ppßE and IL-10, the protein can reach the target cells by two routes: (i) through release into the CSF, i.e., emulating an IT pump, and (ii) in an autocrine/paracrine fashion within the immediate surrounding of the transduced cells. While the ratio of biological effects (ii)/(i) cannot be directly measured in animal models, it may be an important consideration when assessing the viability of AAV gene therapy for pain explicating why the therapeutic index of AAV will likely be improved not only over systemic opioids but also when compared with IT pump delivery of opioids.
Of note, a high degree of DRG-neuron selectivity has been the fundamental rationale for the HSV-based approach to pain gene therapy by the subcutaneous route of vector administration [4, 83]. IT AAV may be less selective for DRG neurons than subcutaneous HSV, but will likely improve the therapeutic index over IT delivery of conventional opioids (as discussed above). Furthermore, the IG route will very likely yield the most DRG neuron-selective transgene activity achievable with AAV potentially matching the DRG neuron-selectivity of subcutaneous HSV.
Specific consideration for ppßE
ßE, the secreted peptide product of ppßE, is a μ-opioid receptor agonist and thereby shares its pharmacological activity with the most commonly used conventional opioids such as morphine. The toxicological assessment of ppßE can therefore be guided by the robust clinical experience with exogenous opioids. In addition, ßE was tested in clinical trials.
The principal sites where opioid toxicity occurs at high doses are the CNS and the periphery. The most common adverse effects of opioids in the CNS are nausea and vomiting, mental status changes, pruritus, urinary retention, and respiratory depression [84–87], while the peripheral adverse effects include constipation, cardiovascular adverse events, derangement of hypothalamo-pituitary axis, and immune-modulation [87–91]. Both the CNS and peripheral adverse effects are dependent on the drug concentration at a given site and may be reversed by naloxone, a μ-, κ-, and δ-opioid receptor antagonist.
When IT administration of ß-endorphin was tested in humans, it resulted in profound analgesia similar to that attainable with exogenous opioids [92–97]. Dose escalation led to a toxicity profile that would be expected with an IT opioid drug, whereby doses of IT ßE of up to 7.5 mg resulted in miosis, drowsiness, and elevation plasma prolactin levels in some patients. No systemic adverse effects related to the treatment were found in those studies, reflecting the inability of ß-endorphin to cross the BBB, keeping it from escaping from the IT space to the systemic circulation.
In conclusion, the therapeutic and toxic effects of ppßE are expected to be vector dose-related and the specific toxicities can be predicted from the previous clinical experience with IT opioids and ßE. What remains to be defined in the preclinical large animals studies is the maximum tolerated dose (MTD) of the vector encoding ppßE for both the IT and the IG route. A phase I/II clinical would then test safety for vector doses starting with the lowest potentially efficacious dose and sequentially being escalated towards the MTD established in animals.
Specific consideration for IL-10
IL-10 is of interest for pain therapy because it may be the first/only available agent to target a mechanism of chronic pain first described about a decade ago, glial activation [98]. Recombinant IL-10 administered IT has been found to be efficacious in animal models of chronic pain [99, 100]. The use of recombinant protein, however, has not been tested in humans due to its short half-life of IL-10 in the CSF, its inability to cross the BBB, and the impracticality of continuous IT delivery over a prolonged period of time [78]. A pharmaceutical formulation of IL-10 needed for delivery by an IT pump, i.e. the stability at ambient temperature and the high concentration, has proved to be difficult. Therefore, gene therapy may provide the best (or only) strategy for testing targeted spinal delivery of IL-10 for pain in the clinical setting.
While toxicity of IL-10 delivery targeted to the PNS by either the IT or the IG route has not been addressed in large animal models, rodent data suggest good tolerability of IL-10 in the CSF [61, 74, 101, 102]. Furthermore, systemic toxicities of IL-10 have been thoroughly characterized in trials of recombinant IL-10 for Crohn disease, rheumatoid arthritis, Wegener granulomatosis, and psoriatic arthritis [103–107]. Safety studies showed that a single intravenous injection of up 100 μg/kg IL-10 in healthy humans led to transient, dose-related flu-like symptoms accompanied by neutrophilia, monocytosis, lymphopenia, and down-regulation of pro-inflammatory cytokines IL-1 and TNF-α [108]. Long-term intravenous or subcutaneous administration of IL-10 in rats and NHP confirmed hematological effects of IL-10 and also showed its inhibitory effect on T-lymphocytes [109].
Establishing the MTD for AAV encoding IL-10 will be an important goal for both the IT and the IG route. Clinical testing would then start at a lower vector dose with subsequent dose escalation to establish the MTD in humans.
Transgene-specific immune response
Syngeneic transgenes will not induce serological or cellular immunity. Therefore, no immunity is to be expected in a clinical trial using human IL-10 as therapeutic gene and no special studies appear to be necessary towards assessing it.
The case of ppβE may require additional consideration, because the gene includes an artificial fusion site. Specifically, the pre-pro sequence of ppβE (the amino-terminal “pp” part of the protein) is taken from nerve growth factor (NGF); thus, this portion of the gene by itself is not of concern because it can be made syngeneic (by taking the pre-pro sequence from human NGF). The carboxy terminal portion of ppβE gene encodes the pharmacologically active βE peptide. By itself this portion of the gene is not of concern either, because it can also be made syngeneic (by using the βE sequence as it occurs in the precursor protein human pro-opiomelanocortin (POMC)). In the ppβE gene, these two syngeneic peptides are fused creating a potentially non-syngeneic epitope.
The rationale for using ppβE rather than POMC is to circumvent the requirement of POMC to be processed in acidified secretory vesicles found only in certain cell types and its reliance on the regulated secretory pathway. ppβE can be processed into βE by all cell types and secretion of βE occurs via the constitutive secretory pathway yielding continuous release of the pharmacologically active βE peptide, which is the desired property of this therapeutic gene. Because the processing of the ppβE peptide into its syngeneic components, the pre-pro-sequence and βE, occurs immediately following its ribosomal synthesis and co-translational transport into the ER, the fusion epitope may not even have a chance to become immunologically apparent. Therefore, to evaluate the possibility of cellular immune toxicity, pre-clinical toxicity studies of ppβE will have to assess the integrity of transduced tissues such as neuropathological evaluation of DRG.
A challenge for pre-clinical toxicology assessments is whether to perform animal testing with syngeneic transgenes, i.e., specifically adapted to the animal species employed, or to perform testing of the human-type vector slated to be used in a clinical trial, i.e. xenogeneic to the animal model. Xenogeneic transgenes are well-tolerated in rodents, which express even the bacterial EGFP gene long term. However, xenogeneic transgenes induced immunity in large animals [32]. As a consequence, an AAV vector encoding a species-specific, i.e., syngeneic transgene could be used for preclinical toxicology studies of AAV for pain. The vector that is ultimately used in the clinic could also be given to animals, but the latter type of studies should be limited to effects observable within approximately one week because all later effects can be expected to be confounded by a xenogeneic immune response that is an artifact of the testing scenario and not applicable to the anticipated scenario of a clinical trial.
Conclusions
Responsible and effective preclinical-to-clinical translation of AAV for pain represents a substantial scientific and regulatory challenge. Yet, the goal appears to be supported by two considerations: The growing clinical research experience with AAV (for non-pain applications) and the need for innovative clinical research in patients with intractable pain from incurable cancer. The following two sections follow these two notions in an attempt to turn our review findings into a proposed action plan.
Preclinical toxicity assessment
Additional studies required
For the IT route of AAV delivery, preclinical animals studies are needed to
● Establish the MTD of AAV vectors
◦ Assess health of animals clinically
◦ Neuropathological assessment of neural structures such as the DRG
◦ Repeat independent experiments for each specific vector design: transgene, serotype
◦ If MTD cannot be reached, document absence of toxicity at a dose exceeding the highest concentration anticipated in humans, e.g., if dose range contemplated in humans is 1011 to 1013 GC, then test up to 1014 GC in a large animal
● Establish biodistribution
We propose that testing in large animals would be more meaningful than testing in rodents, even if performed in a significantly smaller number of animals, because a species such as the pig better resembles the mechanical-anatomical and immunological characteristics of humans than rats. We propose that testing for the first transgene-capsid combination should be done in two iterations: a long-term experiment (such as 3 months follow up) with the syngeneic transgene (such as made for the pig) and a short-term experiment (such as ten days) with the human transgene (that would be xenogeneic in pigs). For subsequent assessments of other transgene-vector combinations, it may be justified to limit studies to the vector that is to be used in the clinical trials expressing the human transgene version.
For the IG route of AAV delivery, additional preclinical animal studies are needed to
● Confirm the safety of placing a CED needle into the DRG for vector infusion
Existing studies to cross-reference
Existing studies reviewed above have already addressed the following issues:
● General vector related toxicities including insertional mutagenesis, germ line transmission, replication escape, and dissemination of the vector to the public domain—AAV vector administration per se appears non-hazardous
● Serologic response against AAV (sin transgene)
Issues outside the realms of animal models
Modeling human immunity to vectors in animal models has remained challenging and uncertain. While numerous experimental approaches and assays have been used or proposed, predictions from rodents appear to be misleading if applied to humans and predictions from various large animal models uncertain at best. The conservative assumption therefore has to be that immune toxicity is an anticipated untoward effect requiring close monitoring in the clinical setting to protect patients and learn more about AAV vector biology in the clinical setting.
Hypothetical design of a phase I/II clinical trial
Most gene therapy trials for non-malignant disorders have been conducted in patients with a near-normal life expectancy, such as in the case of hemophilia B. The clinical trial of AAV for pain that we envision would substantially differ in terms of target population: Patients with an unacceptably high symptom burden from intractable pain at the end of life in the setting of a progressing cancer that is incurable or even untreatable (by antineoplastic therapies). Enrollment requirements could include a minimum- and a maximum life expectancy. Both could be shorter for early patient cohorts to limit the impact that unexpected adverse effects could have, such as a range of 4 to 8 weeks. As safety is established throughout the course of the trial, the minimum life expectancy could become longer, e.g., 3 months, to allow collection of data on longer-term effects. While the same high scientific standards should apply to a palliative-care clinical trial in cancer patients as to trials in patients with non-malignant diseases, the proposed clinical trial setting may be the most appropriate for a pre-clinical to clinical research transition in a case such as AAV gene therapy, where toxicities cannot be modeled perfectly in animals.
A phase I/II clinical trial would likely focus first on the transgene with the best-known pharmacology, i.e., an opioid. ppßE may therefore be the most suitable candidate for early clinical translation thanks to the extensive clinical experience with exogenous IT opioids. In addition, the spread of the vector suspension in the IT space for a given volume can be guided by the experience with IT drugs such as methotrexate, which distribute within the CSF-filled space and are routine given as in a volume of 5 cc and may be safe for injection volumes of up to 10 ml in adults.
An exciting medium- to long-term impact of studying AAV for pain in the cancer center setting may be the opportunity to gain insights into AAV biology in humans. Given that some patients may consent to postmortem tissue collection (an autopsy) a trial in this setting may offer a unique chance for analyzing tissue samples from human subjects receiving AAV at a markedly more rapid turnaround than in previous AAV clinical trials.
Another long-term potential from the perspective of analgesic treatment development is that AAV gene therapy may serve as a platform for investigating novel analgesic targets. For instance, the efficacy of IL-10 would test the analgesic effects of counter-acting glial activation in the setting of the human pain state, which could then guide development of conventional (small molecule) drugs harnessing the same mechanism of action. While IL-10 has been proposed as an analgesic strategy more than a decade ago, clinical testing could not be performed to date presumably because prolonged IT delivery of recombinant IL-10 is impractical. AAV could be the means to bring novel analgesic strategies such as IL-10 from the laboratory into clinical trials.
References
Institute of Medicine: Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press; 2011.
Van den Beuken-van Everdingen MHJ, de Rijke JM, Kessels AG, Schouten HC, van Kleef M, Patijn J: High prevalence of pain in patients with cancer in a large population-based study in The Netherlands. Pain 2007, 132: 312–320.
Green CR, Hart-Johnson T, Loeffler DR: Cancer-related chronic pain: examining quality of life in diverse cancer survivors. Cancer 2011, 117: 1994–2003.
Fink DJ, Wechuck J, Mata M, Glorioso JC, Goss J, Krisky D, Wolfe D: Gene therapy for pain: results of a phase I clinical trial. Ann Neurol 2011, 70: 207–212.
Ginn SL, Alexander IE, Edelstein ML, Abedi MR, Wixon J: Gene therapy clinical trials worldwide to 2012 - an update. J Gene Med 2013, 15: 65–77.
Gaudet D, Méthot J, Déry S, Brisson D, Essiembre C, Tremblay G, Tremblay K, de Wal J, Twisk J, van den Bulk N, Sier-Ferreira V, van Deventer S: Efficacy and long-term safety of alipogene tiparvovec (AAV1-LPLS447X) gene therapy for lipoprotein lipase deficiency: an open-label trial. Gene Ther 2013, 20: 361–369.
De la Calle JL, Paino CL: A procedure for direct lumbar puncture in rats. Brain Res Bull 2002, 59: 245–250.
Storkson RV, Kjorsvik A, Tjolsen A, Hole K: Lumbar catheterization of the spinal subarachnoid space in the rat. J Neurosci Methods 1996, 65: 167–172.
Malkmus SA, Yaksh TL: Intrathecal catheterization and drug delivery in the rat. Methods Mol Med 2004, 99: 109–121.
Mestre C, Pelissier T, Fialip J, Wilcox G, Eschalier A: A method to perform direct transcutaneous intrathecal injection in rats. J Pharmacol Toxicol Methods 1994, 32: 197–200.
Rijsdijk M, van Wijck AJ, Kalkman CJ, Meulenhoff PC, Grafe MR, Steinauer J, Yaksh TL: Safety assessment and pharmacokinetics of intrathecal methylprednisolone acetate in dogs. Anesthesiology 2012, 116: 170–181.
Yaksh TL, Rathbun ML, Dragani JC, Malkmus S, Bourdeau AR, Richter P, Powell H, Myers RR, Lebel CP: Kinetic and safety studies on intrathecally infused recombinant-methionyl human brain-derived neurotrophic factor in dogs. Fundam Appl Toxicol 1997, 38: 89–100.
Pleticha J, Maus TP, Jeng-Singh C, Marsh MP, Al-Saiegh F, Christner JA, Lee KH, Beutler AS: Pig lumbar spine anatomy and imaging-guided lateral lumbar puncture: A new large animal model for intrathecal drug delivery. J Neurosci Methods 2013, 216: 10–15.
Evans RW: Complications of lumbar puncture. Neurol Clin 1998, 16: 83–105.
Sapunar D, Kostic S, Banozic A, Puljak L: Dorsal root ganglion - a potential new therapeutic target for neuropathic pain. J Pain Res 2012, 5: 31–38.
Yu H, Fischer G, Ferhatovic L, Fan F, Light AR, Weihrauch D, Sapunar D, Nakai H, Park F, Hogan QH: Intraganglionic AAV6 Results in Efficient and Long-Term Gene Transfer to Peripheral Sensory Nervous System in Adult Rats. PLoS One 2013, 8: e61266.
Puljak L, Kojundzic SL, Hogan QH, Sapunar D: Targeted delivery of pharmacological agents into rat dorsal root ganglion. J Neurosci Methods 2009, 177: 397–402.
Towne C, Pertin M, Beggah AT, Aebischer P, Decosterd I: Recombinant adeno-associated virus serotype 6 (rAAV2/6)-mediated gene transfer to nociceptive neurons through different routes of delivery. Mol Pain 2009, 5: 52.
Xu Y, Gu Y, Wu P, Li G-W, Huang L-YM: Efficiencies of transgene expression in nociceptive neurons through different routes of delivery of adeno-associated viral vectors. Hum Gene Ther 2003, 14: 897–906.
Pleticha J, Maus TP, Christner JA, Marsh MP, Lee KH, Hooten WM, Beutler AS: Minimally invasive convection enhanced delivery of biologics into dorsal root ganglia: validation in the pig model and prospective modeling in humans: technical note. J Neurosurg 2014, 4: 1–8.
Gofeld M, Brown MN, Bollag L, Hanlon JG, Theodore BR: Magnetic positioning system and ultrasound guidance for lumbar zygapophysial radiofrequency neurotomy: a cadaver study. Reg Anesth Pain Med 2014, 39: 61–66.
Gonçalves MA: Adeno-associated virus: from defective virus to effective vector. Virol J 2005, 2: 43.
Paulk NK, Wursthorn K, Wang Z, Finegold MJ, Kay MA, Grompe M: Adeno-associated virus gene repair corrects a mouse model of hereditary tyrosinemia in vivo. Hepatology 2010, 51: 1200–1208.
Miller DG, Wang P-R, Petek LM, Hirata RK, Sands MS, Russell DW: Gene targeting in vivo by adeno-associated virus vectors. Nat Biotechnol 2006, 24: 1022–1026.
Donsante A, Miller DG, Li Y, Vogler C, Brunt EM, Russell DW, Sands MS: AAV vector integration sites in mouse hepatocellular carcinoma. Science 2007, 317: 477.
Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, Rasko J, Ozelo MC, Hoots K, Blatt P, Konkle B, Dake M, Kaye R, Razavi M, Zajko A, Zehnder J, Rustagi PK, Nakai H, Chew A, Leonard D, Wright JF, Lessard RR, Sommer JM, Tigges M, Sabatino D, Luk A, Jiang H, Mingozzi F, Couto L, Ertl HC, et al.: Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med 2006, 12: 342–347.
Hewitt FC, Li C, Gray SJ, Cockrell S, Washburn M, Samulski RJ: Reducing the risk of adeno-associated virus (AAV) vector mobilization with AAV type 5 vectors. J Virol 2009, 83: 3919–3929.
Frank KM, Hogarth DK, Miller JL, Mandal S, Mease PJ, Samulski RJ, Weisgerber GA, Hart J: Investigation of the cause of death in a gene-therapy trial. N Engl J Med 2009, 361: 161–169.
Evans CH, Ghivizzani SC, Robbins PD: Arthritis gene therapy’s first death. Arthritis Res Ther 2008, 10: 110.
Cunningham J, Pivirotto P, Bringas J, Suzuki B, Vijay S, Sanftner L, Kitamura M, Chan C, Bankiewicz KS: Biodistribution of adeno-associated virus type-2 in nonhuman primates after convection-enhanced delivery to brain. Mol Ther 2008, 16: 1267–1275.
Herzog CD, Brown L, Gammon D, Kruegel B, Lin R, Wilson A, Bolton A, Printz M, Gasmi M, Bishop KM, Kordower JH, Bartus RT: Expression, bioactivity, and safety 1 year after adeno-associated viral vector type 2-mediated delivery of neurturin to the monkey nigrostriatal system support cere-120 for Parkinson’s disease. Neurosurgery 2009, 64: 602–612. discussion 612–3
Hadaczek P, Forsayeth J, Mirek H, Munson K, Bringas J, Pivirotto P, McBride JL, Davidson BL, Bankiewicz KS: Transduction of nonhuman primate brain with adeno-associated virus serotype 1: vector trafficking and immune response. Hum Gene Ther 2009, 20: 225–237.
Sondhi D, Johnson L, Purpura K, Monette S, Souweidane MM, Kaplitt MG, Kosofsky B, Yohay K, Ballon D, Dyke J, Kaminksy SM, Hackett NR, Crystal RG: Long-term expression and safety of administration of AAVrh.10hCLN2 to the brain of rats and nonhuman primates for the treatment of late infantile neuronal ceroid lipofuscinosis. Hum Gene Ther Meth 2012, 23: 324–335.
Haurigot V, Marcó S, Ribera A, Garcia M, Ruzo A, Villacampa P, Ayuso E, Añor S, Andaluz A, Pineda M, García-Fructuoso G, Molas M, Maggioni L, Muñoz S, Motas S, Ruberte J, Mingozzi F, Pumarola M, Bosch F: Whole body correction of mucopolysaccharidosis IIIA by intracerebrospinal fluid gene therapy. J Clin Invest 2013, 123: 3254–3271.
Gray SJ, Nagabhushan Kalburgi S, McCown TJ, Jude Samulski R: Global CNS gene delivery and evasion of anti-AAV-neutralizing antibodies by intrathecal AAV administration in non-human primates. Gene Ther 2012, 2013: 1–10.
Tardieu M, Zerah M, Husson B, de Bournonville S, Deiva K, Adamsbaum C, Vincent F, Hocquemiller M, Broissand C, Furlan V, Ballabio A, Fraldi A, Crystal R, Baugnon T, Roujeau T, Heard J-M, Danos O: Intracerebral administration of AAV rh.10 carrying human SGSH and SUMF1 cDNAs in children with MPSIIIA disease: results of a phase I/II trial. Hum Gene Ther 2014, 25: 506–516.
Hwu W-L, Muramatsu S, Tseng S-H, Tzen K-Y, Lee N-C, Chien Y-H, Snyder RO, Byrne BJ, Tai C-H, Wu R-M: Gene therapy for aromatic L-amino acid decarboxylase deficiency. Sci Transl Med 2012, 4: 134ra61.
Marks WJ, Bartus RT, Siffert J, Davis CS, Lozano A, Boulis N, Vitek J, Stacy M, Turner D, Verhagen L, Bakay R, Watts R, Guthrie B, Jankovic J, Simpson R, Tagliati M, Alterman R, Stern M, Baltuch G, Starr PA, Larson PS, Ostrem JL, Nutt J, Kieburtz K, Kordower JH, Olanow CW: Gene delivery of AAV2-neurturin for Parkinson’s disease: a double-blind, randomised, controlled trial. Lancet Neurol 2010, 9: 1164–1172.
Christine CW, Starr PA, Larson PS, Eberling JL, Jagust WJ, Hawkins RA, VanBrocklin HF, Wright JF, Bankiewicz KS, Aminoff MJ: Safety and tolerability of putaminal AADC gene therapy for Parkinson disease. Neurology 2009, 73: 1662–1669.
Worgall S, Sondhi D, Hackett NR, Kosofsky B, Kekatpure MV, Neyzi N, Dyke JP, Ballon D, Heier L, Greenwald BM, Christos P, Mazumdar M, Souweidane MM, Kaplitt MG, Crystal RG: Treatment of late infantile neuronal ceroid lipofuscinosis by CNS administration of a serotype 2 adeno-associated virus expressing CLN2 cDNA. Hum Gene Ther 2008, 19: 463–474.
Kaplitt MG, Feigin A, Tang C, Fitzsimons HL, Mattis P, Lawlor PA, Bland RJ, Young D, Strybing K, Eidelberg D, During MJ: Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson’s disease: an open label, phase I trial. Lancet 2007, 369: 2097–2105.
McPhee SWJ, Janson CG, Li C, Samulski RJ, Camp AS, Francis J, Shera D, Lioutermann L, Feely M, Freese A, Leone P: Immune responses to AAV in a phase I study for Canavan disease. J Gene Med 2006, 8: 577–588.
Gray SJ, Matagne V, Bachaboina L, Yadav S, Ojeda SR, Samulski RJ: Preclinical differences of intravascular AAV9 delivery to neurons and glia: a comparative study of adult mice and nonhuman primates. Mol Ther 2011, 19: 1058–1069.
Samaranch L, Salegio EA, San Sebastian W, Kells AP, Foust KD, Bringas JR, Lamarre C, Forsayeth J, Kaspar BK, Bankiewicz KS: Adeno-Associated Virus Serotype 9 Transduction in the Central Nervous System of Nonhuman Primates. Hum Gene Ther 2012,389(April):382–389.
Mingozzi F, High KA: Immune responses to AAV vectors: overcoming barriers to successful gene therapy. Blood 2013, 122: 23–36.
Nguyen DH, Hurtado-Ziola N, Gagneux P, Varki A: Loss of Siglec expression on T lymphocytes during human evolution. Proc Natl Acad Sci U S A 2006, 103: 7765–7770.
Li H, Lasaro MO, Jia B, Lin SW, Haut LH, High KA, Ertl HCJ: Capsid-specific T-cell Responses to Natural Infections With Adeno-associated Viruses in Humans Differ From Those of Nonhuman Primates. Mol Ther 2011, 19: 2021–2030.
Vandenberghe LH, Wang L, Somanathan S, Zhi Y, Figueredo J, Calcedo R, Sanmiguel J, Desai RA, Chen CS, Johnston J, Grant RL, Gao G, Wilson JM: Heparin binding directs activation of T cells against adeno-associated virus serotype 2 capsid. Nat Med 2006, 12: 967–971.
Nathwani AC, Tuddenham EGD, Rangarajan S, Rosales C, McIntosh J, Linch DC, Chowdary P, Riddell A, Pie AJ, Harrington C, O’Beirne J, Smith K, Pasi J, Glader B, Rustagi P, Ng CYC, Kay MA, Zhou J, Spence Y, Morton CL, Allay J, Coleman J, Sleep S, Cunningham JM, Srivastava D, Basner-Tschakarjan E, Mingozzi F, High KA, Gray JT, Reiss UM, et al.: Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med 2011, 365: 2357–2365.
Ellinwood NM, Ausseil J, Desmaris N, Bigou S, Liu S, Jens JK, Snella EM, Mohammed EE, Thomson CB, Raoul S, Joussemet B, Roux F, Chérel Y, Lajat Y, Piraud M, Benchaouir R, Hermening S, Petry H, Froissart R, Tardieu M, Ciron C, Moullier P, Parkes J, Kline KL, Maire I, Vanier M-T, Heard J-M, Colle M-A: Safe, efficient, and reproducible gene therapy of the brain in the dog models of Sanfilippo and Hurler syndromes. Mol Ther 2011, 19: 251–259.
Herzog CD, Dass B, Gasmi M, Bakay R, Stansell JE, Tuszynski M, Bankiewicz K, Chen E-Y, Chu Y, Bishop K, Kordower JH, Bartus RT: Transgene expression, bioactivity, and safety of CERE-120 (AAV2-neurturin) following delivery to the monkey striatum. Mol Ther 2008, 16: 1737–1744.
Hackett NR, Redmond DE, Sondhi D, Giannaris EL, Vassallo E, Stratton J, Qiu J, Kaminsky SM, Lesser ML, Fisch GS, Rouselle SD, Crystal RG: Safety of direct administration of AAV2(CU)hCLN2, a candidate treatment for the central nervous system manifestations of late infantile neuronal ceroid lipofuscinosis, to the brain of rats and nonhuman primates. Hum Gene Ther 2005, 16: 1484–1503.
Bartus RT, Baumann TL, Siffert J, Herzog CD, Alterman R, Boulis N, Turner DA, Stacy M, Lang AE, Lozano AM, Olanow CW: Safety/feasibility of targeting the substantia nigra with AAV2-neurturin in Parkinson patients. Neurology 2013, 80: 1698–1701.
Rafii MS, Baumann TL, Bakay RA, Ostrove JM, Siffert J, Fleisher AS, Herzog CD, Barba D, Pay M, Salmon DP, Chu Y, Kordower JH, Bishop K, Keator D, Potkin S, Bartus RT: A phase1 study of stereotactic gene delivery of AAV2-NGF for Alzheimer’s disease. Alzheimers Dement 2014,10(5):571–581.
Leone P, Shera D, McPhee SWJ, Francis JS, Kolodny EH, Bilaniuk LT, Wang D-J, Assadi M, Goldfarb O, Goldman HW, Freese A, Young D, During MJ, Samulski RJ, Janson CG: Long-term follow-up after gene therapy for canavan disease. Sci Transl Med 2012, 4: 165ra163.
Mittermeyer G, Christine CW, Rosenbluth KH, Baker SL, Starr P, Larson P, Kaplan PL, Forsayeth J, Aminoff MJ, Bankiewicz KS: Long-term evaluation of a phase 1 study of AADC gene therapy for Parkinson’s disease. Hum Gene Ther 2012, 23: 377–381.
Samaranch L, Sebastian WS, Kells AP, Salegio EA, Heller G, Bringas JR, Pivirotto P, Dearmond S, Forsayeth J, Bankiewicz KS: AAV9-mediated Expression of a Non-self Protein in Nonhuman Primate Central Nervous System Triggers Widespread Neuroinflammation Driven by Antigen-presenting Cell Transduction. Mol Ther 2013, 22: 329–337.
Sames L, Bonnemann C, Gray SJ, Samulski RJ, Dastgir J: Giant Axonal Neuropathy Gene Therapy. National Institutes of Health: Recombinant Advisory Committee; 2013.
Jacques SJ, Ahmed Z, Forbes A, Douglas MR, Vigenswara V, Berry M, Logan A: AAV8(gfp) preferentially targets large diameter dorsal root ganglion neurones after both intra-dorsal root ganglion and intrathecal injection. Mol Cell Neurosci 2012, 49: 464–474.
Vulchanova L, Schuster DJ, Belur LR, Riedl MS, Podetz-Pedersen KM, Kitto KF, Wilcox GL, McIvor RS, Fairbanks CA: Differential adeno-associated virus mediated gene transfer to sensory neurons following intrathecal delivery by direct lumbar puncture. Mol Pain 2010, 6: 31.
Storek B, Reinhardt M, Wang C, Janssen WGM, Harder NM, Banck MS, Morrison JH, Beutler AS: Sensory neuron targeting by self-complementary AAV8 via lumbar puncture for chronic pain. Proc Natl Acad Sci U S A 2008, 105: 1055–1060.
Bucher T, Colle M-A, Wakeling E, Dubreil L, Fyfe J, Briot-Nivard D, Maquigneau M, Raoul S, Cherel Y, Astord S, Duque S, Marais T, Voit T, Moullier P, Barkats M, Joussemet B: scAAV9 intracisternal delivery results in efficient gene transfer to the central nervous system of a feline model of motor neuron disease. Hum Gene Ther 2013, 24: 670–682.
Federici T, Taub JS, Baum GR, Gray SJ, Grieger JC, Matthews KA, Handy CR, Passini MA, Samulski RJ, Boulis NM: Robust spinal motor neuron transduction following intrathecal delivery of AAV9 in pigs. Gene Ther 2011, 19: 852–589.
Janson C, McPhee S, Bilaniuk L, Haselgrove J, Testaiuti M, Freese A, Wang D-J, Shera D, Hurh P, Rupin J, Saslow E, Goldfarb O, Goldberg M, Larijani G, Sharrar W, Liouterman L, Camp A, Kolodny E, Samulski J, Leone P: Clinical protocol. Gene therapy of Canavan disease: AAV-2 vector for neurosurgical delivery of aspartoacylase gene (ASPA) to the human brain. Hum Gene Ther 2002, 13: 1391–1412.
Marks WJ, Ostrem JL, Verhagen L, Starr PA, Larson PS, Bakay RA, Taylor R, Cahn-Weiner DA, Stoessl AJ, Olanow CW, Bartus RT: Safety and tolerability of intraputaminal delivery of CERE-120 (adeno-associated virus serotype 2-neurturin) to patients with idiopathic Parkinson’s disease: an open-label, phase I trial. Lancet Neurol 2008, 7: 400–408.
Eberling JL, Jagust WJ, Christine CW, Starr P, Larson P, Bankiewicz KS, Aminoff MJ: Results from a phase I safety trial of hAADC gene therapy for Parkinson disease. Neurology 2008, 70: 1980–1983.
Muramatsu S, Fujimoto K, Kato S, Mizukami H, Asari S, Ikeguchi K, Kawakami T, Urabe M, Kume A, Sato T, Watanabe E, Ozawa K, Nakano I: A phase I study of aromatic L-amino acid decarboxylase gene therapy for Parkinson’s disease. Mol Ther 2010, 18: 1731–1735.
Crystal RG: Direct CNS Administration of a Replication Deficient Adeno-associated Virus Gene Transfer Serotype rh.10 Expressing the Human CLN2 cDNA to Children with Late Infantile Neuronal Ceroid Lipofuscinosis. Bethesda, MD: Recombinant DNA Advisory Committee, National Institutes of Health; 2009.
McCarty DM: Self-complementary AAV vectors; advances and applications. Mol Ther 2008, 16: 1648–1656.
Asokan A, Schaffer DV, Samulski RJ: The AAV vector toolkit: poised at the clinical crossroads. Mol Ther 2012, 20: 699–708.
Bowles DE, McPhee SWJ, Li C, Gray SJ, Samulski JJ, Camp AS, Li J, Wang B, Monahan PE, Rabinowitz JE, Grieger JC, Govindasamy L, Agbandje-McKenna M, Xiao X, Samulski RJ: Phase 1 gene therapy for Duchenne muscular dystrophy using a translational optimized AAV vector. Mol Ther 2012, 20: 443–455.
Milligan ED, Sloane EM, Langer SJ, Cruz PE, Chacur M, Spataro L, Wieseler-Frank J, Hammack SE, Maier SF, Flotte TR, Forsayeth JR, Leinwand LA, Chavez R, Watkins LR: Controlling neuropathic pain by adeno-associated virus driven production of the anti-inflammatory cytokine, interleukin-10. Mol Pain 2005, 1: 9.
Lau D, Harte SE, Morrow TJ, Wang S, Mata M, Fink DJ: Herpes simplex virus vector-mediated expression of interleukin-10 reduces below-level central neuropathic pain after spinal cord injury. Neurorehabil Neural Repair 2012, 26: 889–897.
Milligan ED, Sloane EM, Langer SJ, Hughes TS, Jekich BM, Frank MG, Mahoney JH, Levkoff LH, Maier SF, Cruz PE, Flotte TR, Johnson KW, Mahoney MM, Chavez RA, Leinwand LA, Watkins LR: Repeated intrathecal injections of plasmid DNA encoding interleukin-10 produce prolonged reversal of neuropathic pain. Pain 2006, 126: 294–308.
Beutler AS, Banck MS, Bach FW, Gage FH, Porreca F, Bilsky EJ, Yaksh TL: Retrovirus-mediated expression of an artificial beta-endorphin precursor in primary fibroblasts. J Neurochem 1995, 64: 475–481.
Koulousakis A, Kuchta J, Bayarassou A, Sturm V: Intrathecal opioids for intractable pain syndromes. Acta Neurochir Suppl 2007,97(Pt 1):43–48.
Finegold AA, Mannes AJ, Iadarola MJ: A paracrine paradigm for in vivo gene therapy in the central nervous system: treatment of chronic pain. Hum Gene Ther 1999, 10: 1251–1257.
Milligan ED, Langer SJ, Sloane EM, He L, Wieseler-Frank J, O’Connor K, Martin D, Forsayeth JR, Maier SF, Johnson K, Chavez RA, Leinwand LA, Watkins LR: Controlling pathological pain by adenovirally driven spinal production of the anti-inflammatory cytokine, interleukin-10. Eur J Neurosci 2005, 21: 2136–2148.
Soderquist RG, Milligan ED, Harrison JA, Chavez RA, Johnson KW, Watkins LR, Mahoney MJ: PEGylation of interleukin-10 for the mitigation of enhanced pain states. J Biomed Mater Res A 2010, 93: 1169–1179.
Mason MRJ, Ehlert EME, Eggers R, Pool CW, Hermening S, Huseinovic A, Timmermans E, Blits B, Verhaagen J: Comparison of AAV serotypes for gene delivery to dorsal root ganglion neurons. Mol Ther 2010, 18: 715–724.
Fischer G, Kostic S, Nakai H, Park F, Sapunar D, Yu H, Hogan Q: Direct injection into the dorsal root ganglion: technical, behavioral, and histological observations. J Neurosci Methods 2011, 199: 43–55.
Storek B, Harder NM, Banck MS, Wang C, McCarty DM, Janssen WG, Morrison JH, Walsh CE, Beutler AS: Intrathecal long-term gene expression by self-complementary adeno-associated virus type 1 suitable for chronic pain studies in rats. Mol Pain 2006, 2: 4.
Goss JR, Mata M, Goins WF, Wu HH, Glorioso JC, Fink DJ: Antinociceptive effect of a genomic herpes simplex virus-based vector expressing human proenkephalin in rat dorsal root ganglion. Gene Ther 2001, 8: 551–556.
Chaney MA: Side effects of intrathecal and epidural opioids. Can J Anaesth 1995, 42: 891–903.
Noble M, Treadwell JR, Tregear SJ, Coates VH, Wiffen PJ, Akafomo C, Schoelles KM: Long-term opioid management for chronic noncancer pain. Cochrane Database Syst Rev 2010, ᅟ: CD006605.
King S, Forbes K, Hanks GW, Ferro CJ, Chambers EJ: A systematic review of the use of opioid medication for those with moderate to severe cancer pain and renal impairment: a European Palliative Care Research Collaborative opioid guidelines project. Palliat Med 2011, 25: 525–552.
Baldini A, Von Korff M, Lin EHB: A review of potential adverse effects of long-term opioid therapy: a practitioner’s guide. Prim Care Companion CNS Disord 2012, 14: 1–12.
Bell TJ, Panchal SJ, Miaskowski C, Bolge SC, Milanova T, Williamson R: The prevalence, severity, and impact of opioid-induced bowel dysfunction: results of a US and European Patient Survey (PROBE 1). Pain Med 2009, 10: 35–42.
Vuong C, Van Uum SHM, O’Dell LE, Lutfy K, Friedman TC: The effects of opioids and opioid analogs on animal and human endocrine systems. Endocr Rev 2010, 31: 98–132.
Carman WJ, Su S, Cook SF, Wurzelmann JI, McAfee A: Coronary heart disease outcomes among chronic opioid and cyclooxygenase-2 users compared with a general population cohort. Pharmacoepidemiol Drug Saf 2011, 20: 754–762.
Al-Hashimi M, Scott SWM, Thompson JP, Lambert DG: Opioids and immune modulation: more questions than answers. Br J Anaesth 2013, 111: 80–88.
Foley KM, Kourides IA, Inturrisi CE, Kaiko RF, Zaroulis CG, Posner JB, Houde RW, Li CH: beta-Endorphin: analgesic and hormonal effects in humans. Proc Natl Acad Sci U S A 1979, 76: 5377–5381.
Matsuki A, Kudo T, Ishihara H, Jin T, Taneichi T, Oyama T, Guillemin R, Ling N: Plasma beta-endorphin levels during obstetrical analgesia with synthetic beta-endorphin. Agressologie 1986, 27: 133–137.
Oyama T, Fukushi S, Jin T: Epidural beta-endorphin in treatment of pain. Can Anaesth Soc J 1982, 29: 24–26.
Oyama T, Jin T, Yamaya R, Ling N, Guillemin R: Profound analgesic effects of beta-endorphin in man. Lancet 1980, 1: 122–124.
Oyama T, Matsuki A, Taneichi T, Ling N, Guillemin R: beta-Endorphin in obstetric analgesia. Am J Obstet Gynecol 1980, 137: 613–616.
Wen HL, Mehal ZD, Ong BH, Ho WK, Wen DY: Intrathecal administration of beta-endorphin and dynorphin-(1–13) for the treatment of intractable pain. Life Sci 1985, 37: 1213–1220.
Milligan ED, Penzkover KR, Soderquist RG, Mahoney MJ: Spinal interleukin-10 therapy to treat peripheral neuropathic pain. Neuromodulation 2012, 15: 520–526.
Chacur M, Milligan ED, Sloan EM, Wieseler-Frank J, Barrientos RM, Martin D, Poole S, Lomonte B, Gutiérrez JM, Maier SF, Cury Y, Watkins LR: Snake venom phospholipase A2s (Asp49 and Lys49) induce mechanical allodynia upon peri-sciatic administration: involvement of spinal cord glia, proinflammatory cytokines and nitric oxide. Pain 2004, 108: 180–191.
Laughlin TM, Bethea JR, Yezierski RP, Wilcox GL: Cytokine involvement in dynorphin-induced allodynia. Pain 2000, 84: 159–167.
Plunkett JA, Yu CG, Easton JM, Bethea JR, Yezierski RP: Effects of interleukin-10 (IL-10) on pain behavior and gene expression following excitotoxic spinal cord injury in the rat. Exp Neurol 2001, 168: 144–154.
Yu C-G, Fairbanks CA, Wilcox GL, Yezierski RP: Effects of agmatine, interleukin-10, and cyclosporin on spontaneous pain behavior after excitotoxic spinal cord injury in rats. J Pain 2003, 4: 129–140.
Mani R, Paulus H, Breedveld F: RHuIL-10 in subjects with active rheumatoid arthritis: a phase I and cytokine response study [abstract]. Arthritis Rheum 1997, 40: 224.
Schreiber S, Fedorak RN, Nielsen OH, Wild G, Williams CN, Nikolaus S, Jacyna M, Lashner BA, Gangl A, Rutgeerts P, Isaacs K, van Deventer SJ, Koningsberger JC, Cohard M, LeBeaut A, Hanauer SB: Safety and efficacy of recombinant human interleukin 10 in chronic active Crohn’s disease. Crohn's Disease IL-10 Cooperative Study Group. Gastroenterology 2000, 119: 1461–1472.
Kimball AB, Kawamura T, Tejura K, Boss C, Hancox AR, Vogel JC, Steinberg SM, Turner ML, Blauvelt A: Clinical and immunologic assessment of patients with psoriasis in a randomized, double-blind, placebo-controlled trial using recombinant human interleukin 10. Arch Dermatol 2002, 138: 1341–1346.
Friedrich M, Döcke W-D, Klein A, Philipp S, Volk H-D, Sterry W, Asadullah K: Immunomodulation by interleukin-10 therapy decreases the incidence of relapse and prolongs the relapse-free interval in Psoriasis. J Invest Dermatol 2002, 118: 672–677.
National Institute of Allergy and Infectious Diseases: Phase I Trial of Recombinant Human Interleukin-10 (SCH 52000) in Patients With Wegener’s Granulomatosis. In Clinical Trials.gov [Internet]. Bethesda (MD): National Library of Medicine (US); 2008. [cited 2014 Apr 11]. Available from: http://clinicaltrials.gov/ct2/show/NCT00001761?term=NCT00001761&rank=1
Huhn RD, Radwanski E, O’Connell SM, Sturgill MG, Clarke L, Cody RP, Affrime MB, Cutler DL: Pharmacokinetics and immunomodulatory properties of intravenously administered recombinant human interleukin-10 in healthy volunteers. Blood 1996, 87: 699–705.
Rosenblum IY, Johnson RC, Schmahai TJ: Preclinical safety evaluation of recombinant human interleukin-10. Regul Toxicol Pharmacol 2002, 35: 56–71.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests. RJS is a founder of Asklepios Biopharmaceutical, Inc.
Authors’ contributions
JP and ASB wrote the manuscript. LFH prepared the figure. CHE, AA, and RJS contributed sections on adeno-associated viral vectors. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Pleticha, J., Heilmann, L.F., Evans, C.H. et al. Preclinical toxicity evaluation of AAV for pain: evidence from human AAV studies and from the pharmacology of analgesic drugs. Mol Pain 10, 54 (2014). https://doi.org/10.1186/1744-8069-10-54
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1744-8069-10-54