Abstract
A lytic phage for Pseudomonas aeruginosa belongs to the Myoviridea family was isolated from urine for use in therapeutics. Pair of hepatitis C virus (HCV) primers highlighted segments on the genome of this phage. The sequence of these PCR products as well as the possible serological cross reactivity/relationship between HCV and the phage were investigated. One hundred HCV positive human sera were analyzed by ELISA. Ninety six well plates were coated with multiple epitopes of HCV proteins (Kit), phage and Pseudomonas cells. Initially the positive and negative control sera supplied in the test kit were used to evaluate the cross reactivity between the phage and anti-HCV antibodies. The results suggested a value over than 0.105 for a HCV positive reaction. Of the 100 HCV positive sera tested, sixty five and thirty percent showed cross reaction with phage lysate and Pseudomonas aeruginosa, respectively. High HCV antibody titer correlated to high cut off value for phage cross reaction, whereas no such correlation existed between HCV antibody titer and Pseudomonas cross reaction. The PCR products were sequenced and aligned with the HCV genome of H77. Sequence homology was detected in the 5', 3' UTRs and NS3 regions. Further these products showed similarity with HIV-1 Env, Pol & 3'LTR regions as well.
Similar content being viewed by others
Introduction
Hepatitis C virus (HCV) currently infects an estimated 3% of people worldwide encodes several proteins. HCV displays numerous interactions with the immune systems. Consequently a number of auto-antibodies are observed during the course of hepatitis C [1]. Many studies have detected the presence of antibodies reactive to a cloned host derived auto-antigen GOR and are highly correlated with the presence of antibodies to HCV. In chronic hepatitis C presence of these cross reactive antibodies is not merely due to sequence homology but also due to cross reactivity at the molecular level [2–5]. Antibody antigen reactions usually occur when an antigen combines with a corresponding antibody to produce an immune complex [6]. Specificity of this reaction refers to the ability of an individual antibody combining site to react with only one antigenic determinant or the ability of a population of antibody molecules to react with only one antigen. In general, there is a high degree of specificity in the antigen-antibody reactions [7]. However, cross reactivity refers to the ability of an individual antibody combining site to react with more than one antigenic determinant or the ability of a population of antibody molecules to react with more than one antigen [8]. Antigen antibody reaction is highly specific in some cases whereas cross reactivity is exhibited due to sharing of antigenic determinants by two unrelated microbes. For example, cross reactive anti HCV antibodies triggered by an epitope on HCV core protein which exhibits homology with auto antigen GOR 47-1 epitope. Similarly anti GOR antibodies, distinct from anti HCV core antibodies were revealed to have dual specificities. They target both the core gene product and host liver cell components [9]. Another cross reactive epitope shared by HCV NS3 protein and Influenza A (IV) virus. NS3- 1073 and influenza neuraminidase peptides displayed a high degree of sequence homology. These determinants are recognized by cytotoxic T lymphocytes with similar affinity. These heterologous antigens induce cross reactive CD8+ T cells [10].
The reasons for the cross-reactivity between Pseudomonas phage lysate and human HCV positive sera are not known. We are the first to observe cross reactivity between HCV positive sera and newly isolated Pseudomonas phage antigens. The present study was undertaken to determine the reason for the cross reactivity or the non-specific reaction between HCV positive sera and the phage antigen. The findings are presented in this report.
Materials and methods
i. Determination of phage activity in clinical sample on bacterial lawn
Urine sample (50.ml) of a patient was centrifuged at 6000 rpm to remove solid matter and was then filter sterilized through a 0.45 μm membrane. 50 μl of the urine filtrate and 100 μl of 4 hrs young culture of Pseudomona aeruginosa were added to 3 ml of melted L.B soft agar and plaque assayed. All phages were purified by successive single plaque isolation until homogeneous plaques were obtained.
ii. Isolation of phage from clinical specimen
One ml of the 4 hrs young culture of the respective hosts (Pseudomonas aeruginosa (P5& P6 strains), E. coli, E. coli4MD, E. coli-N all local strains) was mixed with 100 μl of urine, incubated for 2 hrs. at 37°C and centrifuged at 4000 rpm for 5 min. The pellet was washed twice with 500 μl of LB broth and suspended in 500 μl broth. E.coli lysogen cells exposed to UV for 1 min and incubated at 37°C for 2 hrs. The lysate was filtered, sterilized and plaque assay was performed.
ii. Transmission Electron Microscopy
Particle morphology was studied by precipitating the lysate with PEG 6000 (Promega Co.) and NaCl to final concentration of 8% and 4%, respectively and incubated at 4°C overnight. The pellet resuspended in 100 μl of double de-ionized distill water. Four hundred (400) mesh carbon coated grids were negatively stained with 2% uranyl acetate for 30 seconds and examined in a GOEL-JEM-1200 EX II transmission electron microscope.
iii. Desalting of lysate by micro dialysis
. Desalting of the phage suspension was carried out in an Eppendrof tube with the cap replaced with 2 cm of dialysis membrane that was held by a rubber band and floated in cold sterile distilled water in upside-down position. Water was changed after one hour and dialysis continued overnight at 4°C.
iv. Coating of ELISA 96 wells plate by lysa
Phage PS5Φ concentrated by 0.95% PEG 8000 and 8% NaCl precipitation and suspended in 300 μl of sterile distilled water. The Phage suspension was then dialyzed, diluted in coating buffer (1:5) and coated on 96 wells plate as prescribed [11]. HCV positive serum (100 μl) was added to the coated wells and incubated for 1 hr. at 37°C. Assay was done as suggested by AutoBio Diagnostic Co.
v. Coating of ELISA 96 wells plate by Pseudomonas cells:
The bacteria were scooped off from the lawn on BHI agar, washed several times with PBS buffer and a100 μl of cell suspension (1:10 in coating buffer) was added into each well of the ELISA plate and assay was performed.
vi. Phage DNA purification
DNA was extracted by using Promega kit.
vii. Polymerase Chain Reaction (PCR) amplification
Phage DNA was isolated from lysates PS5 Φ, and used as PCR templates. PCR products were generated using Taq DNA polymerase (Promega Co.) by following the manufacture's protocols. A range of primer sets were used (Table 1) The PCR conditions used were denaturation at 94°C for 4 min, followed by 35 cycles of denaturation at 94°C for 30 seconds, annealing temperature as 58°C (for C1& C3) and 60°C (for Ac2 & Sc2) for 30 seconds and finally extension at 72°C for 2 min. Amplified products were separated by 2% agarose gel electrophoresis and photographed.
viii. DNA sequencing
Amplified PCR products separated by 2% agarose gel electrophoresis and fragments were selected and purified for sequencing. The selected PCR mixtures were prepared in 50 μl for each reaction and amplified products sequenced using the original amplification primers.
Results
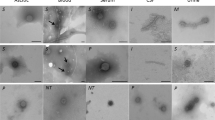
In this study, phages were isolated from the urine of a 24 years old female athlete with E. coli urinary tract infection (UTI). (Figure 1). Electron microscopy indicated the presence of Siphoviridiae and Myoviridiae- like morphology in the initial lysates prepared by using Pseudomonas aeruginosa strain (PS5) as host bacteria. Phage was further purified by single plaque assay (Figure 2). The presence of E. coli and lytic phages of Pseudomonas in the urine sample indicated the lysogenic status of E. coli which seems to be infected with a temperate phage which was lytic for Pseudomonas specie.
C1, C3 primers present in our collection were used contingently to highlight the PCR product from the amplified phage genome. Presence of discrete bands of 640-1169 bps (Figure four, lane 6 & 7; Table 2) warranted us to evaluate possible genetic or serological relation between phage and HCV. In order to confirm the possible serological cross-reactivity/relation, between phage and HCV +ve anti-sera, ELISA and PCR were performed.
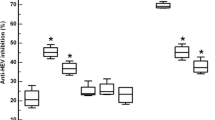
Interestingly HCV positive control provided in the kit showed a strong reaction on phage coated plate (Figure 3). It was found that 83% of HCV positive sera tested reacted to the phage coated plate. This value is comparable to validity of HCV antigen coated plate. It has been shown that presence of antibodies in HCV positive sera is highly correlated with respective antigens Core/NS as indicated with ELISA (Figure 3)
Similarly the ranges for the Pseudomonas aeruginosa cross reactions with HCV positive sera (Figure 3) were found to be in the order of 30% sera. A plausible reason for the high Pseudomonas cutoff value for a HCV +ve serum (OD = 0.13) could be attributed to invasive Pseudomonas aeruginosa secondary infection in this patient. However a correlation between the HCV antibody titer and high cutoff value due to phage lysate was observed (Figure 3). This was further strengthened by the antigenic relation between phage and HCV in addition to the secondary infections of Pseudomonas in the HCV patients.
Results of ELISA suggest a serological relation between cascade (NS3, NS5, and Core) and Ps. phage (PS5 Φ). The enhanced ELISA cutoff value of phage lysate compared with Pseudomonas aeruginosa cell suggested significant cross-reactivity of HCV antibodies and phage antigens. High cut off value for cross reaction between phage antigen and 83% of the HCV sero-positive samples reflects the possible sequence similarities between antigen determinant region in the HCV genome and the phage genome.
The HCV 5'-UTR specific primers and universal primers (Ac2, Sc2, A5 and S7) were used to highlight the genome segment of Pseudomonas phage (PS5Φ) (Figure 4). These PCR products were sequenced and compared by Quick Alignment data exhibited the similarity among different HCV genotypes database (1a, 2a, 1b, 2b); (Figure 4). Gene locator software has highlighted reverse complement of nucleotide position (Similarity 90%) on 3'-UTR, 5'-UTR and NS3 region of H77 (shown as black bar in Figure 5A, C, 6A, B).
QuickAlign: QuickAlign Analysis of PCR Products of Φ PS5 Genome. Homologous with HCV Sequences in LosaAlamos-NCBI. Database. A: QuickAlign: QuickAlign analysis of PCR product of Φ PS5 genome raised by Universa (Ac2/Sc2) primers. B: QuickAlign: QuickAlign analysis of PCR product of ΦPS5 genome raised by Universal (Ac2/Sc2) primers. C: QuickAlign: QuickAlign analysis of PCR product of Φ PS5 genome raised designed (Forward) primer.(1st band)
Preliminary data suggests that HCV 3'-UTR binds cellular proteins such as the 52 kDa La auto-antigen, the 57 kDa polypyrimidine tract-binding protein, and other proteins [12]. These results predict that isolated PS5 Φ may have regulatory machinery similar to HCV type translation-regulation. The similarity of phage translation-regulation regions and regulatory 5'and 3'-UTRs of HCV was substantiated by Quick Align and Gene locator analysis using non-HCV primers Reverse and Forward, (Table 2; Figure 5B) designed in our laboratory. This analysis showed variable matched size of PS5 Φ and 5'-UTR and 3'-UTR regulatory region of HCV genome.
It is worth noting that, 5'-UTR and the extreme end of the 3'-UTR of HCV have the lowest sequence diversity among various genotypes and subtypes. The relatively conserved nature of these regions is significant in their functional importance in the life cycle of the virus [13].
Quick Align and Gene locator analysis of PCR products of Φ genome raised by HCV 5'-UTR specific primers have highlighted the region of phage that has similarity with domain I and II of 5'-UTR of HCV genome (Figure 5B & 6). This un-translated, conserved region is involved in viral RNA translation and plays an important role in replication as well [14]. Analyses of the results have shown the similarity on region of 3'-UTR (Figure 5B, 6A, B), all these PCR products except (Figure 6A raised by Sc2) raised by C1 or C3 5'UTR specific primers. (Figure 5C & 6B) showed homologous region extended into NS5B 3' end. These products were raised by C1+C3 pair. (Figure 5A & 7A) indicated homologous region on NS3 of HCV and Env of HIV. These viral regions are highlighted by a PCR product of PS5Φ genome raised by Ac2 primer. (Figure 7) Exhibited relation between phage PCR product with HIV Pol gene. (Figure 7B) indicated the similarity between U5 regions of 3'LTR of HIV with PCR product raised by C3 primer of HCV. The comparative analysis confirmed that PS5 Φ has got the flexible regions on the genome, which is similar to the 5'-UTR IRES of HCV RNA, and may involved in the cap-independent translation.
QuickAlign: QuickAlign Analysis of PCR products of Φ PS5 genome homologous to HIV sequences in LosAlamos database. QuickAlign analysis of PCR product of Φ PS5 genome raised by single primer (Sc2) A: QuickAlign: QuickAlign analysis of PCR product of ΦPS5 genome raised by pair primers (Ac2/Sc2) B: QuickAlign: QuickAlign: analysis of PCR product of ΦPS5 genome raised by single primer(C3)
Discussion
Most of the PCR products raised by 5'UTR specific primers (C1, C3; Table 1, 2), highlighted from phage genome showed homology with 3' UTR region of HCV (Figure 5C, 6B). The entire 3'UTR is matched with these PCR products. However (Figure 5C & 6B) exhibited homologous region extended into NS5B's 3' end. A stretch of 398 nts (reverse complement) of PS5Φ genome is similar to not only 3' UTR but 3' end of HCV genomic RNA. This homology has reflected significant value, since the conserved sequence and stem and loop structures of HCV function as cis acting signals that interact with viral and cellular proteins to initiate the synthesis of minus strand RNA during viral replication [15]. Recently Cristina [16], (2009) demonstrated the role of stem loop structure at 3' end of RNA, the 5BSL 3.2 motif which is embedded in cruciform structure at 3' end of NS5B coding sequence contribute to the 3 D folding of the entire 3' end of the HCV genome. It is essential in the initiation of replication. Presence of such functional/structural sequence in DNA genome of phage remains elusive and provides the incentive for investigation.
PCR product of PS5Φ genome raised by Ac2 HCV primer (Table 1 & 2) exhibited sequence to NS3 at the HCV genome's location 4508←4636 (reverse complement) Figure 5B. Interestingly same PCR product is homologous to HIV genome at the location 7469→7600, which specify envelop gene (Figure 7). These 131 nts are highlighting the gp 120 outer-domain (OD) variable region comprised of several important immune epitopes. However V3 immuno dominance is the property of OD immunogens [17, 18]. This stretch of Φ genome provides the compelling genetic evidence of sharing of some functional determinant between NS3 central domain and HIV gp120 region V3.
Large body of data indicated the implications of NS3/4A on adaptive immune response which cause inhibition of cytokines/chemokines [19]. Similarly gp120 interaction with cell chemokine receptor CCRS5 and CXCR4 are the key events for HIV-1 infection [20, 21]. These results presented herein providing insight into possible mechanism by which HCV can acquires tissue tropism and can infect lymphoid cells i.e. monocytes/macrophages. Furthermore this shared region of HCV and HIV-1 is immunogenic can induce neutralizing antibodies [22, 23]. These antibodies could be cross reactive too. Phillip [24] demonstrated the highly processive helicase activity on DNA by NS3. Presumably this may have the capacity to effect HIV DNA phase.
According to our results one of the PCR product raised by single primer Sc2 of HCV highlighting the PS5Φ genome segment homologous to HIV Pol gene at a location of HXB2 genome 3046←3273 (Figure 7). This segment of 227 nucleotides is a reverse complement and is complementary to the entire palm sub domain of HIV reverse transcriptase (RT). It is interesting to note that this sub domain of RT carries active site for replication [25–28]. Previous analysis of retroviral RT has indicated that palm domain in addition to finger and thumb sub domains facilitate the flow of genetic information moving in the reverse direction. It is worth to note that DNA genome of PS5Φ carries homologous sequence to palm sub domain of RT provide the insight into phylogenetic niches between this triad, HIV, HCV and DNA genome.
Interestingly PCR product raised by Ac2 primer highlighted two segments from PS5 Φ genome, as exhibited 2 bands on DNA gel (Figure 4, lane 2, 4); Analysis of the data from the first band showed similarity with HCV genome. A stretch of 128 nts exhibited similarity with NS3 region of HCV genome. The non-structural protein NS3, which is an essential component of HCV replication complex, is a protease that mediates NS2/3 cleavage. Serological cross-reaction between phage and HCV positive sera was found to be related to the presence of NS3 and this was further substantiated with HCV-Quick Align analysis of the PCR products (Figure 2). Clustal W alignment of the PCR products raised by Ac2 and retrieved NS3 sequence has confirmed that this homology is present close to N-terminal of NS3 region. Therefore, we predict that the genome of phage PS5 may code a protein having similar domain to NS3.
Epidemiological data shows that many HCV-infected patients are co-infected by HIV. Despite their remarkable differences, HIV and HCV share common characteristics (genetic, replicative, and pathological) that make real their interaction and facilitate the exacerbation of their related diseases [29]. It has been reported that hyper variable region of the NS1 protein of HCV may share similarities with the hyper-variable region of the envelope protein of human immunodeficiency virus HIV [30]. These results are in accordance to our finding as V3 region of envelop of HIV share determinant with NS3 of HCV. Serological tests and sequencing have shown that Pseudomonas phage (PS5 Φ) has similar antigenic epitopes to HCV and HIV variable immunogenic regions which contribute in complexity and diversity of these viruses. Previous reports have been demonstrated that a high proportion of blood donors exhibited false positive anti HCV antibodies [31]. Recently Jung-ah Kwon [32] used microarray assay for HCV detection and found same false results in few individuals. Therefore it can be concluded that PS5Φ presence in an individual may attribute to false positive results despite higher diagnostic accuracy of HCV/HIV infection. A healthy individual having this phage may unnecessarily receive interferon or antiviral drug. Therefore, diagnosis should be based strictly on HCV antigen detection and sequence analysis of the PCR products.
Based on computational analysis indicating 3'-UTR, 5'-UTR, NS3 of HCV and V3 of OD, palm sub domain of RT and U5 of 3' LTR of HIV are homologous with genome segments of PS5Φ. Which predicts that the genome of the Pseudomonas aeruginosa phage share similar regulatory stem and loop structure or common functional domains with the HIV/HCV genome. This can facilitate protein-protein interaction between prokaryotes and eukaryotes.
Horizontal transfer of genetic material between distantly related prokaryotes has been shown to play a major role in the evolution of bacterial genomes, but exchange of genes between prokaryotes and eukaryotes is not as well understood [33] and it cannot be ignored.
In this context, studies of retroviruses could be the best example illustrating the first demonstrated synthesis of DNA from RNA templates, a fundamental mode for transferring genetic material that occurs in both eukaryotes and prokaryotes. It has been noted that retrovirus infection which, introduces additional viral oncogenes into the cells, and transformed proteins leads to the conversion of normal cells to tumor cells [34, 35].
Through this genetic mixing retrovirus and animal cells have been swapping genes for millions of years [36]. There is best circumstantial evidence suggesting retroviruses has been involved in other major evolutionary innovation too. McIntoch [37] (2008) proposed that the genes of DNA viruses were recruited in the evolution of eukaryotic machinery. Current work is consistent with this finding as PS5Φ has DNA genome carry a stretch of sequence homologous to entire palm sub domain of HIV RT, in fact exhibiting the evolutionary niches of most unrelated organism. Recent advances in understanding the molecular relation of host-pathogen and their interactions which highlighted the role in microbial evolution could play a significant role in the emergence of bacterial pathogens.
The exchange of genes between phage and virus should be visualized in this scenario. HIV/HCV patients are prone to any infections. Pseudomonas is a very common etiologic agent in infections particularly of the urinary and respiratory tracts. Therefore the body of HIV/HCV patient could be the re-union site for these prokaryotes i.e. phage and virus for the exchange of genetic material. Their flexible nucleic acids defiantly have their own modification to evade the nucleases of the host. HCV, HIV and PS5Φ triad proposed that fluxes of genetic material between prokaryotes and eukaryotes cannot be ruled out.
Finally, recent research by the virology and cell biology communities have developed the understanding of the co-infection (HCV/HIV) implication on complexity and diversity of HCV by ignoring other bacterial pathogens and their respective phages. Present study predicts that co-infection (HCV/HIV) with Pseudomonas phage PS5Φ may result in a higher rate of viral persistence. This may enhance the predisposing factors for complexity and diversity for HCV.
References
Manns MP, Straub PO: Viral induction of autoimmunity: mechanisms and examples in hepatology. J Viral Hep 2008, 4: 42-47. 10.1111/j.1365-2893.1997.tb00179.x
Quiroga AJ, Castillo I, Bartolomé J, Carreño V: Serum immunoglobulin G antibodies to the GOR auto-epitope are present in patients with occult hepatitis C virus (HCV) infection despite lack of HCV-specific antibodies. Clin Vaccine Immunol 2007, 14: 1302-1306. 10.1128/CVI.00128-07
Koike R, Iizuka T, Watanabe T, Miyasaka N: The GOR gene product cannot cross react with hepatitis C virus in human. Clin Exp Immunol 2001, 124: 429-434. 10.1046/j.1365-2249.2001.01508.x
Markus R, Norah T, David N, Masashi M, Linda F, John LR, John RP, Nancy AL, Tresa WL, Johnson LYN: Antibody to the host cellular gene-derived epitope GOR-1 in liver transplant recipient with hepatitis C virus. Infect Transpl 1997,63(4):609-6112.
Zang ZX: Molecular basis for antibody cross reactivity between the hepatitis core protin and the host derived GOR protein. Clin Exp Immunol 1994, 96: 403-409. 10.1111/j.1365-2249.1994.tb06042.x
C M, Summerfield A: Basic concept of immune response and defense development. ILAR J 2005,46(3):230-240.
Mayer G: Immunoglobulins antigen-antibody reactions and selected tests. Microbiology and Immunology University of South Carolina School of Medicine; 2006. Immunology chapter 7 [http://pathmicro.med.sc.edu/mayer/IgStruct2000.htm]
Male D, Roth D, Roitt I: Immunology. 7th edition. Elsevier Inc press; 2009:67-74.
Mishiro S, Takeda K, Hoshi Y, Yoshikawa A, Itoh : An antibody cross reactive to hepatitis C virus core and a host nuclear antigen. Autoimmun 1991, 10: 269-273. 10.3109/08916939109001900
Wedmeyer H, Mizokoshi E, Dovis AR, Bennink JR, Rehermann B: Cross reactivity between hepatitis C virus and influenza A virus determinant-specific cytotoxic T cell. J Virol 2001, 75: 11392-11400. 10.1128/JVI.75.23.11392-11400.2001
Wu R, Hu S, Xiao Y, Li Z, Shi D, Bi D: Development of indirect enzyme-linked immunosorbent assay with nucleoprotein as antigen for detection and quantification of antibodies against avian influenza virus. Vet Res Comm 2007, 31: 631-641. 10.1007/s11259-007-3510-x
Karakasiliotis I: Analysis of RNA-Protein interactions involved in calicivirus translation and replication. PhD Thesis 2008.
Easton LE, Locker N, Lukavsky PJ: Conserved functional domains and a novel tertiary interaction near the pseudo knot derive translational activity of hepatitis C virus and hepatitis-like virus internal ribosome entry sites. Nucleic Acid Res 2009,37(16):5537-5549. 10.1093/nar/gkp588
Tan Luo, Xin S, Cai Z: Role of the 5'-proximal stem-loop structure of the 5' untranslated region in replication and translation of hepatitis C virus RNA. J Virol 2003, 77: 3312-3318. 10.1128/JVI.77.23.12562-12571.2003
Cheng JC, Chang FM, Chang SC: Specific interaction between the hepatitis C virus NSB5 S polymerase and the 3'end of the viral RNA. J Virol 1999, 73: 7044-7049.
Lopez RC, Herranz AB: A long range RNA-RNA interaction between the 5' and 3' ends of the hepatitis genome. RNA 2009, 15: 1740-175. 10.1261/rna.1680809
Wyatt R, Sodroski : The HIV-1 envelop glycoprotein: fusogen, antigens and immunogens. Science 1998, 280: 1884-1888. 10.1126/science.280.5371.1884
Panzer-Zolla S, Cohen SS, Krachmorov C, Wang S, Lus PA: Focus on immune response on the V3 loop a neutralizing epitope of HIV-1 gp120 envelop. Virol 2008,15(372):233-246.
Kaukinen P, Sillanpää M, Kotenko S, Lin R, Hiscott J, Melĕn K, Julkunen I: Hepatitis C virus NS2and NS3/4A proteins are potent inhibitors of host cell cytokine/chemokine gene expression. Virol J 2006.,3(66):
Galanakis PA, Spyroulias GA, Rizos A, Samolis P, Krambovitis E: Conformational properties of HIV-1 gp 120/V3 immunogenic domain. Curr Med Chem 2005,12(13):1551-1568. 10.2174/0929867054038982
Fenouillet E, Barbauch R, Jones IM: Cell entry by enveloped viruses: redox consideration for HIV and SARS-Corona virus. Antioxidant Redox Signalling 2007, 9: 1009-1034. 10.1089/ars.2007.1639
Bell CH, Pantophlet R, Schiefner A, Cavacini LA, Stanfield RL, Burton DR, Wilson IA: Structure of antibody F425-B4e8 in complex with a V3 peptide reveals a new binding mode for HIV-1 neutralization. J Mol Biol 2008,375(4):969-978. 10.1016/j.jmb.2007.11.013
Panzer-Zolla S, Cohen S, Pinter A, Krachmaroü C, Wrin T, Wang S, Lu S: Cross clad neutralizing antibodies against HIV-1 induced in rabbits by focusing the immune response on a neutralizing epitope. Viro J 2009,15 392(1):82-93. 10.1016/j.virol.2009.05.039
Pang SP, Jankowsky E, Planet PJ, Pyle AM: The hepatitis C viral NS3 protein is a proccessive DNA helicase with cofactor enhanced RNA unwinding. EMBO J 2002,21(5):1168-1176. 10.1093/emboj/21.5.1168
Hughes SH: Molecular match making: NNRTIs can enhance the dimerization of HIV-1 reverse transcriptase. PNAS 2001,98(13):6991-6992. 10.1073/pnas.141222698
Ntemgwa M, Wainberg MA, Divera M, Moisi D, Lalonde R, Micheli V, Brenner BC: Variation in reverse transcriptase and RNAase H domain mutation in human immunodeficiency virus type 1 clinical isolates are associated with divergent phenotypic resistance to zidovudine. Antimicrobial Agent Chemotherpy 2007,51(11):3861-3869. 10.1128/AAC.00646-07
Abbondanzieri EA, Bokinsky G, Rausch JW, Zhang JX, Stuart FJ, Grice L, Zhuang X: Dynamic binding orientation direct activity of HIV reverse transcriptase. Nature 2008, 453: 184-189. 10.1038/nature06941
Basu VP, Song M, Gao L, Rigby ST, Hanson MN, Bambra RA: Strand transfer event during HIV-1 reverse transcriptase. Virus Res 2008,134(1-2):19-38. 10.1016/j.virusres.2007.12.017
Ogata N, Harvey J, Miller ARH, Purcell RH: Nucleotide sequence and mutation rate of the H strain of hepatitis C virus. Proc Natl Acad Sci USA 1991, 88: 3392-3396. 10.1073/pnas.88.8.3392
Neumann MG, Monteiro M, Rehm J: Drug interactions between psychoactive substances and antiretroviral therapy in individuals infected with human immunodeficiency and hepatitis viruses. Informa Healthcare 2006,41(10-12):1395-1463. [http://www.informaworld.com/smpp/content~db=all~content=a769423083~tab=citations]
Shany BS, Green MS, Shinar E: False positive test for anti hepatitis C antibodies and problem of notifying blood donors. Int J Epidemiol 1996,25(3):674-678. 10.1093/ije/25.3.674
Kown JA, Lee H, Lee KN, Chae K, Lee S, Lee DK, Kim Sec : High diagnostic accuracy of antigen microarray for sensitive detection of hepatitis C virus inf tion. Clin Chem 2008, 54: 424-4. 10.1373/clinchem.2007.090464
Rogers BM, Patron NJ, Keeling PJ: Horizontal transfer of a eukaryotic plastid-targeted gene to cyanobacteria. BMC Biol 2007.,5(26):
Kurt R: Oncogenes in retroviruses and cells. Natur wissenschaften 1983,70(9):439-450. 10.1007/BF01079610
Gifford RJ: Evolution at the host retrovirus interface. Bioassays 2006,28(12):1153-1156. 10.1002/bies.20504
Bambra RA: Gene hijacked by HIV ancestor suggest new way to block viral reproduction.News Room: University of Rochester Medical Centre; 2009. [http://www.urmc.rochester.edu/news/story/index.cfm?id=2702]
McIntosh M: Retroviruses & Evolution. Gene expression.com 2008. [http://www.gnxp.com/blog/2008/02/retroviruses-evolution.php]
Acknowledgments
We are thankful to Prof. Dr. Adibul Hassan Rizvi director SIUT for allowing us to use the Transmission Electron Microscope. We further thank Prof. Dr. Qasim Mehdi director (ex) KIBGE for the use of ELISA Reader. And finally, thankful to Essa lab for providing HCV positive sera for this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
ZG carried out complete bench work, participate in sequence analysis by applying bioinformatics tools and involved in partial drafting of manuscript. NJ conceived of the study, carried out design, inference of computational analysis and drafted the manuscript. Both authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Golkar, Z., Jamil, N. Hepatitis C virus, human immunodeficiency virus and pseudomonas phage PS5 triad share epitopes of immunogenic determinants. Virol J 7, 346 (2010). https://doi.org/10.1186/1743-422X-7-346
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1743-422X-7-346