Abstract
Chikungunya has resurged in the form of unprecedented explosive epidemic in 2006 after a long gap in India affecting 1.39 million of persons. The disease continued for the next two consecutive years affecting 59,535 and 64,548 persons during 2007 and 2008 respectively. The 2008 outbreak being the second largest among these three years the information regarding the etiology and the mutations involved are useful for further control measures. Among the 2008 outbreaks the Coastal Karnataka accounts for the 46,510 persons. An in-depth investigation of Chikungunya epidemic of Coastal Karnataka, India, 2008 by serology, virus isolation, RT-PCR and genome sequencing revealed the presence and continued circulation of A226V mutant Chikungunya virus. The appearance of this mutant virus was found to be associated with higher prevalence of vector Aedes albopictus and the geographical proximity of coastal Karnataka with the adjoining Kerala state. This is the first report regarding the appearance of this mutation in Karnataka state of India. The present study identified the presence and association of A226V mutant virus with Chikungunya outbreak in India during 2008.
Similar content being viewed by others
Findings
Chikungunya fever is an acute arthropod borne viral illness reported from many parts of Africa and south east Asia. The causative agent is Chikungunya virus (CHIKV), a member of the genus Alphavirus of the family Togaviridae and is primarily transmitted by the Aedes aegypti mosquito [1–3]. CHIKV illness in humans is often characterized by sudden onset of fever, headache, fatigue, nausea, vomiting, rash, myalgia and severe arthralgia. The arthralgia may persist in a small proportion of cases even for months. These clinical symptoms mimic with that of dengue fever and therefore, many cases of Chikungunya are misdiagnosed as dengue infections [4–6]. At present, there is no vaccine or antiviral therapy available against Chikungunya infection.
An outbreak of Chikungunya virus infection occurred during 2006 in 15 states or union territories in India affecting more than 1.39 million of persons [7–10]. The epidemic started from December 2005 and since then continued for the next three consecutive years. Among the various outbreaks in different states, the 2007 outbreak of Kerala was unique in the sense that it affected more than 25,000 persons with higher epidemic potential and reported mortalities [11]. The investigation of full genome sequence of 2007 Kerala isolates and its comparison with 2006 Indian isolates revealed the presence of A226V mutation in E1 gene of the virus and was found to be associated with evolutionary success due to adaptation in the Aedes albopictus mosquito vector with progression of epidemic from 2006 to 2007 [11, 12].
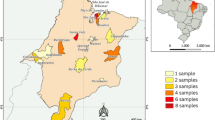
In 2008, an outbreak of fever with severe arthralgia occurred in costal Karnataka, in India adjoining the state of Kerala, affecting 46,510 persons [10]. The affected areas include Puttur, Mangalore, Sulya and other parts of the Dakshina Kannada district. The outbreak seems similar to Kerala 2007 outbreak with respect to higher epidemic potential and reported mortalities. The geographical proximity of coastal Karnataka to Kerala coupled with the higher prevalence of Aedes albopictus created an apprehension regarding involvement of similar etiology. To rule out any confusion, an in depth virological, serological and molecular investigation was carried out to identify the etiology of this unprecedented outbreak, which is considered to be the second highest in terms of number of persons affected since its resurgence in 2006.
A total of 100 blood samples from patients suspected of having Chikungunya fever were brought from District Surveillance Unit, Mangalore which were collected from Mangalore, Puttur, Sulya and other parts of the Dakshina Kannada district for this study. Two sets of blood samples were collected with and without anticoagulant for virus isolation and serology respectively. All these samples were investigated for the presence of Chikungunya specific RNA by RT-PCR and for the presence of CHIKV specific IgM antibodies by using recombinant E1 and E2 protein based IgM ELISA.
The presence of CHIKV specific RNA in clinical samples was detected using the Access quick one-step RT-PCR kit (Promega, USA) employing a primers pair targeting the E1 gene (CHIK13: TTACATCACGTGCGAATAC genome position 10128-10146 and CHIK14: CTTTGCTCTCAGGCGTGCGACTTT genome position 10604-10627); designed from the nucleotide sequence of the reference S27 strain, GenBank Acc No. AF490259[8, 11]. The 8 representative RT-PCR positive samples were again amplified through RT-PCR using a different set of primer targeting E1 gene (E1-10145F:ACAAAACCGTCATCCCGTCTC genome position 10145-10165, E1-11158R: TGACTATGTGGTCCTTCGGAGG genome position 11137-11158, E1-11011F:CGGGAAGCTGAGATAGAAGTTGAA Genome position 11011-11034, 3'NTR-11669R: TTGATTTTTATTAGTTTTATGTTT genome position 11645-11669) for sequencing and were subjected to double stranded sequencing employing Big dye terminator cycle sequencing ready reaction kit with ABI 310 sequencer (Applied Biosystems, USA). The nucleotide sequences were aligned edited and analysed using Seqscape V.3 software (Applied Biosystem, USA). CLUSTALW version 1.83 [13] was used to perform multiple nucleotide and amino acid sequence allignment of E1 gene (1044 nt). A The phylogenetic analysis was performed based on partial E1 gene (837 nt) sequences of CHIK viruses using MEGA version 3.1 [14].
For the construction of phylogenetic trees, the neighbour-joining algorithm and the Kimura two-parameter distance modelwere utilized. The reliability of the analysiswas evaluated by a bootstrap test with 10,000 replications
The isolation of virus was also attempted in C6/36 cells from selected RT-PCR positive samples following the virus adsorption technique [15]. The serological analysis was carried out using an in-house developed recombinant E1 & E2 protein based indirect format IgM ELISA.
The serological analysis of the samples indicated overall 28% seropositivity for IgM antibodies. A total of 40 (40%) serum samples were found positive for the presence of CHIKV specific RNA, through demonstration of CHIKV specific 500 bp amplicon by RT-PCR. A representative of 20 RT-PCR positive samples were subjected to virus isolation in C6/36 cells, which yielded 8 CHIKV isolates. The isolation of the virus was further confirmed by RT-PCR and nucleotide sequencing.
Nucleotide sequencing of the partial E1 gene of 8 representative CHIKV strains was determined and were compared with 27 other globally diverse CHIKV isolates including Chikungunya isolates from 2006, 2007 outbreaks of India and Reunion islands (Table 1). The BLAST search revealed > 99% identity with CHIKV isolates from 2006-07, French Reunion isolates. All the Eight representative isolates from this outbreak revealed A226V shift in the E1 gene as observed in the 2007 Kerala isolates (Fig. 1). The presence of this A226V shift in 2008, coastal Karnataka isolates as well as the geographical proximity with adjoining karala state where this mutation was reported and the results of phylogenetic analysis suggest that the current outbreak might have spread from the Kerala. As E1 gene sequences were available for additional isolates and also because of its importance in phylogenetic analysis, a Neighbour-joining phylogenetic was constructed (Fig. 2). It revealed that all the DRDE-08 isolates from this epidemic grouped along with the DRDE-07, and other 2007 Kerala isolates within the Indian Cluster of ECSA genotype, whereas all the Reunion isolates form a RU cluster with in the ECSA genotype as reported earlier [11].
Since early 2005, a major epidemic of CHIK started in many Indian Ocean island nations; and towards end of 2005, it reemerged in several parts of India [7–9, 16]. The resurgence of CHIK epidemic after a gap of 32 years and the subsequent hiatus of explosive epidemic for three consecutive years is a point of major concern. In addition, the implication of A226V mutation with increased severity, non classical symptoms, reported mortality and large epidemic in Kerala during 2007 has warranted the continues monitoring and surveillance of the activity of the mutant virus in this particular region [11]. The investigation of 2008 coastal Karnataka outbreak clearly showed the involvement of A226V mutant in large scale morbidity.
The present study revealed less seropositivity in comparison to RT-PCR which may be attributed to the collection of samples at very early or acute stage of the illness. The demonstration of CHIKV RNA in 40% samples by RT-PCR and detection of IgM antibodies in 28% of sample confirmed the causative agent of this epidemic to be CHIKV. The isolation of CHIKV from clinical samples further confirmed this etiology. The sequence of CHIKV were directly determined from clinical samples without risk of altering the genome by in vitro passaging. During this outbreak, patients also reported non classical symptoms including hemorrhage, lymphadenitis, ictures and liver involvement, etc. similar to the cases in Reunion 2005-06 and Kerala India-2007 [11, 16]. These types of unusual cases, geographical similarity, proximity and higher prevalence of Aedes albopictus leads to the speculation for the involvement of Kerala 2007 strain for the current outbreak. The sequencing of CHIKV strains from the current 2008 outbreak led to identification of A226V shift in these isolates. The E1:A226V mutation was earlier correlated with vector specificity as well as epidemic potential [17]. This shift in the present outbreak may be attributed to the higher epidemic potential and higher prevalence of Aedes albopictus vector in Coastal Karnataka in 2008. However, the complete genome sequencing is required to see other mutations other than A226V which are unique for the current 2008 outbreak
The phylogenetic analysis revealed that all isolates from the current outbreak were very closely related to analogous strains from Kerala 2007 outbreak. All these isolates harbor valine at E1-226 position compared to alanine in the 2006 Indian isolates
In summary, to the best of our knowledge, this is the first report regarding the appearance of this mutation in Karnataka state of India. The involvement of A226V mutant virus was attributed to the continued circulation of the 2007 Kerala strain in the current outbreak due its geographical proximity coupled with higher prevalence of Aedes albopictus vector, supporting the higher epidemic potential of A226V mutant virus. However, a continuous surveillance is warranted to monitor its spread and track possible evolution of the virus during the epidemic.
References
Peters C, Dalrymple J: Alphaviruses. In Fields virology. 2nd edition. Edited by: Fields BN, Knipe DM. New York: Raven Press; 1990:713-761.
Strauss EG, Strauss JH: Structure and replication of the alphavirus genome. In The Togaviridae and Flaviviridae. Edited by: Schlesinger S, Schlesinger MJ. New York: Plenum Press; 1986:35-90.
Porterfield JH: Antigenic characteristics and classification of the Togaviridae. In The Togaviruses. Edited by: Schlesinger R. New York: Academic Press; 1980:13-46.
Johnston RE, Peters CJ: Alphaviruses associated primarily with fever and polyarthritis. In Fields virology. Edited by: Fields BN, Knipe DM, Howley PM. Philadelphia: Lippincott-Raven Publishers; 1996:843-898.
Jupp PG, McIntosh BM: Chikungunya disease. In The Arboviruses: Epidemiology and ecology. Edited by: Monath TP. Boca Raton (Florida): CRC Press; 1988:137-157.
Carey DE: Chikungunya and dengue: a case of mistaken identity? Journal of the History of Medicine and Allied Sciences 1971, 26: 243-62. 10.1093/jhmas/XXVI.3.243
Yergolkar PN, Tandale BV, Arankalle VA, Sathe PS, Sudeep A, Gandhe SS: Chikungunya outbreaks caused by African genotype, India. Emerg Infect Dis 2006, 12: 1580-1583.
Dash PK, Parida MM, Santhosh SR, Verma SK, Tripathi NK, Ambuj NK: East Central South African genotype as the causative agent in reemergence of Chikungunya outbreak in India. Vector Borne Zoonotic Dis 2007, 7: 519-527. 10.1089/vbz.2006.0648
Arankalle VA, Shrivastava S, Cherian S, Rashmi S, Gunjikar AM, Walimbe SM: Genetic divergence of Chikungunya viruses in India (1963-2006) with special reference to the 2005-2006 explosive epidemic. J Gen Virol 2007, 88: 1967-1976. 10.1099/vir.0.82714-0
NVBDCP: State wise status of Chikungunya fever in India. (prov.) New Delhi: National Vector Borne Disease Control Programme; 2008. [http://www.nvbdcp.gov.in/Chikun-cases.html]
Santhosh SR, Dash PK, Parida MM, Khan M, Tiwari M, Lakshmana Rao PV: Comparative full genome analysis revealed E1: A226V shift in 2007 Indian Chikungunya virus isolates. Virus Research 2008, 135: 36-41. 10.1016/j.virusres.2008.02.004
Pradeep Kumar N, Rajan J, Kamataj T, Jambulingam P: A226V mutation in the virus during the 2007 chikungunya outbreak in Kerala, India. J Gen Virol 2008, 89: 1945-1948. 10.1099/vir.0.83628-0
Thompson JD, Higgins DG, Gibson TJ, Clustal W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalities and weight matrix choice. Nucl Acid Res 1994, 22: 4673-4680. 10.1093/nar/22.22.4673
Kumar S, Tamura K, Nei M: MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinform 2004, 5: 150-163. 10.1093/bib/5.2.150
Yamada K, Takasaki T, Nawa M, Kurane I: Virus isolation as one of the diagnostic methods for dengue virus infection. J Clin Virol 2002, 24: 203-209. 10.1016/S1386-6532(01)00250-5
Schuffenecker I, Iteman I, Michault A, Murri S, Frangeul L, Vaney M: Genome microevolution of chikungunya viruses causing the Indian Ocean outbreak. PLoS Med 2006, 3: e263. 10.1371/journal.pmed.0030263
Konstantin A, Tsetsarkin DL, Vanlandingham CE, McGee SH: A single mutation in chikungunya virus affects vector specificity and epidemic potential. PLoS Pathogens 2007,3(12):e201. 10.1371/journal.ppat.0030201
Acknowledgements
The authors are thankful to Defence Research and Development Organization (DRDO), Ministry of Defence, Govt. of India for providing necessary facilities and financial grant for this study. The authors are also thankful to the District Health officer and District Surveillance officer, Mangalore, Dakshina kannada District, Kranataka, India for providing clinical samples.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
SRS defined the study and carried out the laboratory experiments, interpreted the results and wrote the manuscript. PKD designed the primers, analyzed the data. MMP co-interpreted the results and co-wrote the manuscript. PVLR and MK contributed their ideas to the design of the study and the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Santhosh, S., Dash, P.K., Parida, M. et al. Appearance of EI: A226V mutant Chikungunya virus in Coastal Karnataka, India during 2008 outbreak. Virol J 6, 172 (2009). https://doi.org/10.1186/1743-422X-6-172
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1743-422X-6-172