Abstract
An outbreak of orf virus infection in dairy goats in Korea was investigated. Suspected samples of the skin and lip of affected goats were sent to the laboratory for more exact diagnosis. Orf virus was detected by electron microscopy and viral DNA was identified by PCR. To reveal the genetic characteristics of the Korean strain (ORF/09/Korea), the sequences of the major envelope protein (B2L) and orf virus interferon resistance (VIR) genes were determined and then compared with published reference sequences. Phylogenetic analysis revealed that the ORF/09/Korea strain was closest to the isolates (Taiping) from Taiwan. This is believed to be the first report on the molecular characterization of orf virus in Korea.
Similar content being viewed by others
Background
Contagious ecthyma (contagious pustular dermatitis; orf) is a common epitheliotrophic viral disease of sheep, goats, and wild ruminants and is characterized by the formation of papules, nodules, or vesicles that progress into thick crusts or heavy scabs on the lips, gingiva, and tongue. Orf virus is an oval, enveloped virus containing dsDNA genome within the genus Parapoxvirus, family Poxviridae[1]. The genus also includes pseudocowpox virus (PCPV) and bovine papular stomatitis virus (BPSV) in cattle and parapoxvirus of red deer in New Zealand. Zoonotic infection with orf virus is characterized by nodular and papillomatous lesions mainly on the hands, face, and mouth [2, 3].
To reveal the genetic variation and characterization of parapoxvirus, the major virus envelope protein B2L and virus interferon resistance (VIR) genes have been used recently [4–8]. Orf virus infection has been diagnosed occasionally in Korea since the outbreak of orf was reported clinically in the 1990s. Although a few studies have been conducted, molecular epidemiology based on gene sequences of orf virus has not been performed because the population of sheep and goats is low in Korea. In the present study, orf virus infection in dairy goats was identified by clinical diagnosis and PCR. The complete B2L and VIR genes were sequenced, and their phylogenetic trees were constructed.
Case presentation
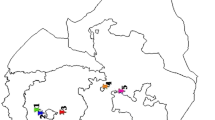
In April 2009, an exanthematic outbreak occurred in a farm with 400 dairy goats in the Chungbuk province. Sixty dairy goats presented with wart-like lesions on the lips, tongue, and around the anus (Fig. 1A and 1B). The clinical diagnosis was contagious ecthyma. Morbidity was 15% (60/400), and goats of all the ages ranging from 2 weeks to 1 month were affected. Dried scabs collected from affected goats were stored in a -70°C freezer until the samples were used for further study.
Typical clinical cases of orf virus infection in diary goats (A, B), histopathological findings (C) showing intracytoplasmic eosinophilic inclusion bodies (arrows) in the keratinocytes indicated virus-induced lesions (HE, bar = 35 μm) and electron micrograph of the orf virus particle (D) showing typical oval-shaped morphology (bar = 100 nm).
The tissue samples were either fixed in 10% buffered formalin for histological examination, or were homogenized mechanically in PBS in a tube using a pellet pestle device. The homogenates were centrifuged at 3000 g for 5 min, and the supernatant was collected and negatively stained with 2% phosphotungstic acid for electron-microscopic examination of orf virus.
A PCR with OVS and OVA primers was performed as described previously [9]. In brief, the sequences of OVS and OVA primers are 5'-AGGCGGTGGAATGGAAAGA-3' and 5'-CCAGCAGGTATGCCAGGATG-3', respectively. The total viral DNA was extracted from scab samples using QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) following the manufacturer's instructions. The amplication were performed in a GeneAmp PCR System 2700 (Applied Biosystems) using the following conditions: a preliminary heating for 5 min at 94°C; 10 cycles of 30 s at 94°C, 45 s at 55°C, and 1 min at 72°C; 20cycles of 30 s at 94°C, 45 s at 57°C, and 1 min at 72°C; and the final elongation of 5 min at 72°C. For phylogenetic analysis, a PCR was also performed using previously described primers [4, 5]. The amplified gene was purified using an agarose gel DNA extraction kit (INtRON, Seongnam, Korea) and further cloned into pGEM-T vector (Promega, Madison, WI, USA) according to the manufacturer's instructions. Automated nucleotide sequencing of the VP60 gene insert was performed using an ABI 3130XL genetic analyzer with the BigDye® Terminator cycle sequencing kit (Applied Biosystems, Foster City, CA, USA). The nucleotides at all positions were confirmed by three or more independent sequencing reactions in both directions. The published sequences of parapoxviruses were retrieved from GenBank for phylogenetic analyses (Table 1).
The B2L and VIR gene sequences of the orf virus, Korean strain were aligned with those of parapoxvirus sequences obtained from GenBank using Bioedit software (Ibis Biosciences, Carlsbad, CA, USA). A phylogenetic analysis was conducted using the Bioedit software and Molecular Evolutionary Genetics Analysis (MEGA) 3.1 software, with bootstrap values calculated from 1000 replicates [10]. The phylogenetic algorithm used for the building of the tree was the neighbor-joining method.
All clinical samples collected from the orf outbreak were positive using the previously described PCR method. The amplified PCR products were separated by electrophoresis and visualized by staining with ethidium bromide (EtBr). The size of amplified PCR product was 708 bp (data not shown). Keratinocytes in the stratum spinosum showed vacuolation and ballooning degeneration. Intracytoplasmic eosinophilic inclusion bodies were found characteristically in keratinocytes (Fig 1C). Negatively stained orf virus particles were examined by electron microscopy, which revealed the oval-shaped morphology of Parapoxvirus. The size of the observed virus particles was approximately 150-200 nm (Fig. 1D).
Additional PCR reactions were performed to obtain the full-length B2L and partial VIR genes. The amplified PCR products were 1206 bp for the full-length B2L gene and 552 bp for the partial VIR gene. The purified DNA was sequenced for comparative analysis. The sequence comparison of the ORF/09/Korea strain demonstrated a high degree of identity among our Korean strain and other orf virus strains. In phylogenetic analysis based on the complete B2L gene, the ORF/09/Korea strain was closer to the Taiping isolate (EU327506) from Taiwan (Fig. 2A). The ORF/09/Korea strain and Taiping isolate showed 99.82% and 99.48% similarity at the nucleotide and amino acid levels, respectively. The deduced amino acid sequences of the complete B2L gene of the ORF/09/Korea strain and the other strains were aligned using Bioedit software. Unique amino acid substitutions were found at two positions (A/R250→ D, I275→ M).
Phylogenetic analysis of different parapoxviruses based on nucleotide sequences of B2L gene (A) and VIR gene (B). The nucleotide sequences of diverse orf viruses were aligned using the Bioedit program and Mega 3 software. One thousand bootstrap replicates were subjected to nucleotide sequence distance and neighbor-joining methods, and the consensus phylogenetic tree is shown. All bootstrap values are displayed above the tree branches, and only bootstrap values >70% are shown.
In phylogenetic analysis based on the partial VIR gene, the ORF/09/Korea strain also was closer to the Taiping isolate (EU327508) from Taiwan (Fig. 2B). The ORF/09/Korea strain and Taiping isolate showed 97.8% and 97.6% similarity at the nucleotide and amino acid level, respectively. The deduced amino acid sequences of the partial VIR gene of the ORF/09/Korea strain and the other strains were aligned, and unique amino acid substitutions were found at two positions (N/D250→ E, F275→ S).
Conclusion
Orf infection is endemic in Korea, and no vaccination has been implemented to control this disease. An increase in the dairy goat population in Korea has been observed over the past several years. Clinical diagnosis and electron microscopy have been used routinely to identify orf viruses in Korea. Recently, PCR methods have been used to detect the viruses [11, 12]. The dairy goat is not a native species in Korea and has been imported from abroad. Several causes of orf outbreaks exist, and the phylogenetic analysis may indicate a hypothetical origin of the viral strains involved.
Although only one case from dairy goats that showed clinical signs was used in the present study, the ORF/09/Korea strain was closest to the isolates (Taiping) from Taiwan, according to phylogenetic analysis based on the B2L and VIR genes. However, it is difficult to conclude the precise route by which the Korean orf virus strain was introduced into Korea based on molecular analysis alone. Also, at present, all ruminants from Taiwan have been prohibited from importation into Korea because of the outbreak of foot-and-mouth disease in Taiwan. Therefore, more epidemiological data concerning the distribution of orf viruses in Korea and neighboring countries are needed. Our continuing work to characterize more isolates in Korea and neighboring countries should prove valuable in that regard.
References
Robinson AJ, Balassu TC: Contagious pustular dermatitis (orf). Vet Bull 1981, 51: 771-82.
Haig DM, Thomson J, Mclnnes C, McCaughan C, Imlach W, Mercer A, Fleming S: Orf virus immuno-modulation and the host immune response. Vet Immunol Immunopathol 2002, 87: 395-99. 10.1016/S0165-2427(02)00087-9
Haig DM, Mercer AA: Ovine diseases. Orf. Vet Res 1998, 29: 311-26.
Hosamani M, Bhanuprakash V, Scagliarini A, Singh RK: Comparative sequence analysis of major envelope protein gene (B2L) of Indian orf viruses isolated from sheep and goats. Vet Microbiol 2006, 116: 317-24. 10.1016/j.vetmic.2006.04.028
Guo J, Zhang Z, Edwards JF, Ermel RW, Taylor C Jr, de la Concha-Bermejillo A: Characterization of a North American orf virus isolated from a goat with persistent, proliferative dermatitis. Virus Res 2003, 93: 169-79. 10.1016/S0168-1702(03)00095-9
Chan KW, Lin JW, Lee SH, Liao CJ, Tsai MC, Hsu WL, Wong ML, Shih HC: Identification and phylogenetic analysis of orf virus from goats in Taiwan. Virus Genes 2007, 35: 705-12. 10.1007/s11262-007-0144-6
Kottaridi C, Nomikou K, Teodori L, Savini G, Lelli R, Markoulatos P, Mangana O: Phylogenetic correlation of Greek and Italian orf virus isolates based on VIR gene. Vet Microbiol 2006, 116: 310-16. 10.1016/j.vetmic.2006.04.020
Mondal B, Bera AK, Hosamani M, Tembhurne PA, Bandyopadhyay SK: Detection of Orf virus from an outbreak in goats and its genetic relation with other parapoxviruses. Vet Res Commun 2006, 30: 531-39. 10.1007/s11259-006-3270-z
Zheng M, Liu Q, Jin N, Guo J, Huang X, Li H, Zhu W, Xiong Y: A duplex PCR assay for simultaneous detection and differentiation of capripoxvirus and orf virus. Mol Cell Probes 2007, 21: 276-81. 10.1016/j.mcp.2007.01.005
Kumar S, Tamura K, Nei M: MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinform 2004, 5: 150-63. 10.1093/bib/5.2.150
Inoshima Y, Morooka A, Sentsui H: Detection and diagnosis of parapoxvirus by the polymerase chain reaction. J Virol Methods 2000, 84: 201-208. 10.1016/S0166-0934(99)00144-5
Chan KW, Hsu WL, Wang CY, Yang CH, Lin FY, Chulakasian S, Wong ML: Differential diagnosis of orf viruses by a single-step PCR. J Virol Methods 2009, 160: 85-9. 10.1016/j.jviromet.2009.04.025
Sullivan JT, Mercer AA, Fleming SB, Robinson AJ: Identification and characterization of an orf virus homologue of the vaccinia virus gene encoding the major envelope antigen p37K. Virol 1994, 202: 968-73. 10.1006/viro.1994.1420
Delhon G, Tulman ER, Afonso CL, Lu Z, de la Concha-Bermejillo A, Lehmkuhl HD, Piccone ME, Kutish GF, Rock DL: Genomes of the parapoxviruses ORF virus and bovine papular stomatitis virus. J Virol 2004, 78: 168-77. 10.1128/JVI.78.1.168-177.2004
Mercer AA, Ueda N, Friederichs SM, Hofmann K, Fraser KM, Bateman T, Fleming SB: Comparative analysis of genome sequences of three isolates of Orf virus reveals unexpected sequence variation. Virus Res 2006, 116: 146-58.
McInnes CJ, Wood AR, Mercer AA: Orf virus encodes a homolog of the vaccinia virus interferon-resistance gene E3L. Virus Genes 1998, 17: 107-15. 10.1023/A:1026431704679
Chan KW, Yang CH, Lin JW, Wang HC, Lin FY, Kuo ST, Wong ML, Hsu WL: Phylogenetic analysis of parapoxviruses and the C-terminal heterogeneity of viral ATPase proteins. Gene 2009, 432: 44-53. 10.1016/j.gene.2008.10.029
Acknowledgements
This study was supported by the National Veterinary Research and Quarantine Service, Ministry of Agriculture, Anyang, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
JKO*, YHJ, and OSL participated in the planning of the project. KKL and HRK performed the PCR and phylogenetic analysis. ISR and KHL collected the samples and performed electron microscopy and histopathological examination. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Oem, JK., Roh, IS., Lee, KH. et al. Phylogenetic analysis and characterization of Korean orf virus from dairy goats: case report. Virol J 6, 167 (2009). https://doi.org/10.1186/1743-422X-6-167
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1743-422X-6-167