Abstract
This review summarises theoretical studies attempting to assess the population impact of antiretroviral therapy (ART) use on mortality and HIV incidence. We describe the key parameters that determine the impact of therapy, and argue that mathematical models of disease transmission are the natural framework within which to explore the interaction between antiviral use and the dynamics of an HIV epidemic. Our review focuses on the potential effects of ART in resource-poor settings. We discuss choice of model type and structure, the potential for risk behaviour change following widespread introduction of ART, the importance of the stage of HIV infection at which treatment is initiated, and the potential for spread of drug resistance. These issues are illustrated with results from models of HIV transmission. We demonstrate that HIV transmission models predicting the impact of ART use should incorporate a realistic progression through stages of HIV infection in order to capture the effect of the timing of treatment initiation on disease spread. The realism of existing models falls short of properly reproducing patterns of diagnosis timing, incorporating heterogeneity in sexual behaviour, and describing the evolution and transmission of drug resistance. The uncertainty surrounding certain effects of ART, such as changes in sexual behaviour and transmission of ART-resistant HIV strains, demands exploration of best and worst case scenarios in modelling, but this must be complemented by surveillance and behavioural surveys to quantify such effects in settings where ART is implemented.
Similar content being viewed by others
Introduction
The epidemiological impact of widescale use of (highly active) antiretroviral therapy (HAART, or ART) among HIV patients in industrialised countries has been explored by a number of mathematical modelling studies [1–5]. The consequences of ART use are far from intuitive. Successful ART decreases plasma [6] and seminal viral load [7, 8] and so is thought to reduce HIV infectiousness. However, its main function is to increase the life expectancy of infected individuals [9, 10], and over time this causes the pool of potential transmitters of infection to grow. These two factors – decreased infectivity but increased duration of infectiousness – have opposing effects on transmission. In addition, increases in risk behaviour could result from increased optimism about HIV prognosis due to the availability of ART. This is an area of uncertainty, with contradictory evidence [11–15].
Mathematical models can be used to address questions regarding the potential impact and effectiveness of various strategies. In terms of ART use, they can be used to investigate:
-
1)
optimising the efficient use of ART;
-
2)
the epidemiological consequences of ART and interaction with behavioural changes/interventions;
-
3)
the likely course of drug resistance evolution
-
a.
Within the individual;
-
b.
Between individuals;
-
4)
achievable levels of coverage and effectiveness;
-
5)
the effective and efficient use of second line treatments; and
-
6)
demographic/health care impact.
This review will briefly describe a range of models investigating the impact of ART use in various settings and evaluate the utility of these dynamic models.
The range of ART models
Mathematical models examining the epidemiological impact of ART broadly fall into two categories; those incorporating HIV transmission dynamics, where incidence of new infections is dependent on HIV prevalence [1, 2, 4, 5], and simpler linear models [16–18]. A summary of ART models is provided in Table 1. Aalen et al [17] constructed a model describing men who have sex with men (MSM) in England and Wales and the use of ART. This Markov multi-stage model represented stages of HIV infection based on CD4 count. The authors considered a variety of treatment scenarios, and incorporated asymptomatic and symptomatic individuals and the concept of eligibility for treatment, making the simulation of treatment uptake and its impact more realistic than previous work. Wood et al [16] constructed a health economic model to predict the future impact of low-level ART use in South Africa from 2000 to 2005. The authors modelled total drug cost, cost per life year gained and the proportion of per person healthcare expenditure required to finance ART in each scenario. The study involved a cost effectiveness analysis comparing the epidemiological impact of ART with other interventions such as prevention of mother-to-child transmission (PMTCT). Freedberg et al [18] also used stages of disease determined by CD4 count and predicted the incremental cost per quality-adjusted year of life gained by ART in the US. Wilson and Blower [19] used a spatial mathematical model to explore ART allocation strategies among health care facilities in the province of KwaZulu-Natal, South Africa, with an emphasis on maximising equity in access to treatment.
In an investigation into the impact of an expanded response (incorporating prevention interventions and care and support activities) on the HIV/AIDS pandemic, Stover et al 2002 did not include the effect of ART because, "there is little empirical data available on the magnitude of the preventive effect of treatment (reduced viral load and hence infectiousness) and care" [20]. However in a later publication, the authors investigated the effects of combining treatment with effective prevention efforts, using the same model (the Goals model [21]), calibrated to sub-Saharan Africa [22]. The Goals model is a Microsoft Excel™ spreadsheet model using linear equations, designed to improve resource allocation for national HIV/AIDS programmes. It feeds into the dynamic epidemic projection package (EPP) and Spectrum, used by the UNAIDS/WHO to produce national HIV/AIDS estimates [23, 24], to predict the impact of an intervention. The authors concluded that a prevention-centred strategy provides greater reductions in incidence, but more modest mortality benefits, than treatment-centred scenarios. A combined approach would yield further benefits, but focusing on treatment at the expense of prevention could diminish this effect.
Auvert et al 2004 used a linear model to estimate the proportion of the South African population requiring ART under the then current WHO guidelines (treating all individuals with a CD4 cell count less than 200 cells/mm3 [25]) and to predict the impact of ART on the short term spread of HIV in this setting [26].
Such linear models have generally been used to inform policy makers on issues such as resource allocation, and typically involve only short-term predictions of the effect of ART for health care providers, as estimated by cost-effectiveness analysis [16, 18]. The models are relatively straightforward in that they look at the health states of individuals, associated treatments and events that individuals experience, but fail to take account of the non-linear feedback process underlying infectious disease epidemics. Linear models are limited by the accuracy of estimates of HIV incidence used to parameterise the models, which is all the more important because their predictions and conclusions are usually more quantitative in nature than those provided by dynamic models, which have tended to be used to give more qualitative insight. Models incorporating HIV transmission dynamics typically investigate the impact of ART over a longer time frame and are used to address more general questions surrounding ART use, such as whether the benefits of ART provision outweigh the problems and risks, and which approaches to ART provision are most effective. Both types of model are required, to inform policy makers in resource-poor settings about the costs of ART provision in the short term (Wood et al [16], for example), as well as to predict the likely impact of scaling up ART use.
To date, policies designed to ameliorate the HIV/AIDS epidemic in Africa have been heavily based on policies from industrialised countries [27]. However, the epidemiological and economic contexts are so different that there is an urgent requirement to assess whether existing policy options and targets are optimal for resource-poor settings.
Dynamic model structures
Most dynamic models of HIV transmission investigating the impact of ART are deterministic, with a frequency-dependent (density-independent) transmission term. This means that the rate of (sexual) contact between one individual and others within a population does not depend on the density of the population, as it would, for example, in the case of contacts for air-borne infection transmission. HIV transmission models often incorporate relatively complex patterns of sexual behaviour, with model populations stratified into sexual activity groups by rate of partner change, and assuming different degrees of mixing between groups. However, to date most models specifically designed to examine ART impact have assumed homogeneous risk behaviour (although some of these models have investigated changes in risk behaviour of the general population as a result of ART introduction and/or a change upon diagnosis of HIV [4, 5]). More realistic incorporation of sexual behaviour is likely to improve the ability of models to capture the observed timescale of African HIV epidemics, namely steady state being reached over decades rather than centuries. Figure 1 shows projections from a homogeneous sexual activity model, illustrating how, with a homogeneous population, realistic prevalence levels (representing epidemics in sub-Saharan Africa) can only be reached over unrealistic timescales (a full description of the model is provided in the Endnote). However, such homogeneous models can simulate HIV epidemics over realistic timescales if they are assumed to represent the 'at-risk proportion' of the total population only. This means that the population is crudely divided into two groups; one group practices no risky behaviour at all, whereas the other has a relatively high rate of (unprotected) sexual partner change. This structure produces an epidemic curve over a realistic timeframe (decades rather than centuries), without producing unreasonably high prevalence levels for the entire population (at-risk and not at-risk).
Model predictions of the effect of ART on a mature epidemic, under various assumptions. Model simulations of the potential impact of ART on a mature epidemic, varied by treatment uptake rate, reduction in infectivity due to treatment and impact on risk behaviour at the population level (see Endnote for model description). The model used only incorporates one stage of HIV infection and so individuals initiate treatment at an earlier stage of infection than is realistic, and there is homogeneous sexual mixing. Scenario A – ART uptake = 50% per year, sexual activity post ART = unchanged (2.5 partners per year), reduction in infectivity due to ART = 50-fold. Scenario B – ART uptake = 50% per year, sexual activity post ART halves (1.25 partners per year), reduction in infectivity due to ART = 50-fold. Scenario C – ART uptake = 50% per year, sexual activity post ART = unchanged (2.5 partners per year), reduction in infectivity due to ART = 1000-fold. Scenario D – ART uptake = 90% per year, sexual activity post ART = unchanged (2.5 partners per year), reduction in infectivity due to ART = 1000-fold. Scenario E – ART uptake = 90% per year, sexual activity post ART = reduced by 20% (2 partners per year), reduction in infectivity due to ART = 1000-fold.
More sophisticated models incorporating sexual behaviour include partner models [28, 29] and network models [30, 31]. Gray et al [2] use a stochastic simulation incorporating individuals and their contacts, although some assumptions are not clear in the available publication. The need for complexity will depend on the nature of the research question [32]. For example, where changes in sexual behaviour as a result of ART are to be investigated, a more sophisticated description of sexual behaviour is required [30]. Where the effect of ART on transmission is to be investigated, a more realistic pattern of infectivity is required [2, 4]. However, while increased complexity can make models more realistic, it also makes them more difficult to parameterise and it more difficult to analyse and interpret model output.
Behaviour change
The possibility of widescale use of ART leading to changes in patterns of risk behaviour, particularly a disinhibition effect, has been of considerable concern. There are competing possible effects; at the individual level, treated patients may increase the frequency of sexual activity due to the severity of their symptoms decreasing, but may receive effective prevention counselling upon treatment initiation, which would decrease the frequency of risky activities. At the population level, in areas with substantial treatment coverage and successful treatment outcomes, there may be an increase in complacency among the general population regarding an HIV diagnosis, leading to increases in risk behaviour. Despite considerable debate [11–15], this relationship has not been convincingly demonstrated in industrialised countries where ART is readily available. A recent paper suggests that recent increases in risk-taking behaviour among MSM may be the result of non-volitional changes at the individual level over time [33]. The depletion of the pool of high-risk individuals in the pre-ART era made it more difficult for the remaining high risk-taking individuals to find partners to engage in risky sex with, but ART has facilitated the differential replenishment of this group. Therefore individuals who previously had to reduce their levels of risky sex could resume their initial high-risk behaviours.
The threat of behavioural disinhibition it is unlikely to be an immediate concern as ART is rolled out in high prevalence, resource-poor settings, where initial coverage is likely to be low and the effectiveness of ART programmes remains to be seen. Furthermore, the behavioural effects resulting from ART use in resource-poor settings are unlikely to follow patterns of industrialised countries. A person's decision to have sex, protected or unprotected, is influenced by a different set of considerations in resource-poor settings than those common in industrialised countries. Key is an individual's ability to negotiate her or his own sexual activity – as defined by fear of stigma, financial need, or the status of women within society. An individual is also less likely to be aware of his or her serostatus, due to lack of testing facilities and/or fears regarding a positive result. The provision of treatment may increase interest in voluntary counselling and testing (VCT) services, which may in turn lead to a decrease in frequency of risk behaviour by those infected. In Cote d'Ivoire for example, individuals reported low sexual activity following an HIV diagnosis, and this was not increased by the offer of ART [34]. Despite the inaccuracies of sexual behaviour data, these results are encouraging.
Given that it is difficult to predict how individuals might change their sexual behaviour as a result of ART introduction in different regions, models are faced with either estimating behavioural parameters from epidemiological data, or exploring pessimistic and optimistic scenarios using parameter values assumed to be at the ends of the spectrum of possible outcomes. Law et al 2001 modelled the effect of ART on the HIV epidemic in Australia in 1996 among the homosexual population [4] and predicted the outcome of the competing effects of increased life expectancy, decreased infectiousness and increases in unsafe sex of uninfected MSM on HIV incidence. Their assumption of a range of no change to a doubling of risky sex was essentially arbitrary, but demonstrated that increases in sexual behaviour (and life expectancy) could negate the beneficial impact of decreased infectiousness on incidence. Blower et al 2000 produced similar results for the homosexual population in San Francisco [5], again using the range of no change to a doubling in sexual risk-taking.
Velasco-Hernandez et al have investigated the conditions under which ART in HIV infected individuals may drive an epidemic to extinction [1]. As can be shown by the model output in Figure 1, for ART to eliminate HIV, an extensive reduction in risk activity at the population level, accompanying ART use (such as a 50% reduction in the partner acquisition rate) is required, together with high levels of treatment uptake and large decreases in infectiousness induced by ART. As behaviour change is notoriously difficult to generate and initial coverage rates for ART in resource-poor settings are likely to be low, this optimistic scenario is highly unlikely.
The early impact of widescale ART use in resource-poor settings where HIV prevalence is currently high will probably not involve substantial population-level increases in risky behaviour. The effectiveness of local ART programmes will likely have to be demonstrated across a broad swath of the population before the perceived threat of AIDS as a disease declines. In lower prevalence regions where high coverage rates are feasible, such changes may occur. Careful monitoring of potential changes in risk behaviour would be very useful, if feasible. Any model designed to explore the impact of sexual behaviour change in resource-poor settings, be it an increase or a decrease, should explicitly model HIV diagnosis separately from treatment initiation, as shown by Law et al [4], because 1) it is knowledge of HIV status and the associated counselling that may change behaviour, 2) the advent of therapy in the sick may change their desire and/or ability for sexual functioning and 3) the attitude of those who know they are infected with HIV may change between not being treated, where they perceive a risk of transmitting to partners, to being treated, where the magnitude of risk may be perceived as smaller. This is one area where the introduction of ART could be used for prevention as well as treatment, through facilitating VCT.
Stage of HIV infection
Some researchers believe that ART could be used as a direct prevention tool due to its effect on viral load leading to a decrease in infectivity and therefore incidence [26, 35, 36]. However, the competing effects of increasing prevalence due to the effect of ART on life expectancy and potential behavioural disinhibition would make this a risky strategy. Furthermore, models that predict dramatic reductions in incidence due to ART have used unrealistic treatment uptake rates. As described, some have argued that even high prevalence (30%) epidemics can be driven to extinction by ART, when assuming a treatment coverage rate of 50% to 90% [1, 5]. The 50% level was estimated from data collected in a telephone sample interview of 462 MSM from four US cities conducted between November 1996 and February 1998 [37]. This was when HAART was in its infancy and treatment was initiated in a large proportion of HIV positive individuals, regardless of infection stage or CD4 count, because a "hit hard, hit early" consensus existed for patient management. Furthermore, the study only included self-identified, HIV-positive MSM, and so individuals unaware of or reluctant to admit their serostatus would have been missed. It is now more common to initiate treatment at a later stage of infection, due to side-effects and the risk of evolution of drug resistance. The proportion of HIV-infected people currently being treated, even in industrialised countries, is likely to be substantially below that required for any prospect of disease elimination.
More realistic patterns of ART use are incorporated in the models of Law et al [4, 38] and Gray et al [2], where the proportion of individuals treated increases with severity of HIV disease as determined by CD4 count [4] or plasma viral load [2]. By explicitly modelling changes in infectiousness and sexual activity over time, it has been shown that ART alone cannot be relied upon as a sole prevention tool.
Gray et al [2] and Nagelkerke et al [39] explicitly modelled the impact of ART in resource-poor settings (Uganda, and Botswana and India, respectively). Nagelkerke et al 2002 assumed that those receiving ART and infected with drug-sensitive virus had zero infectivity, which does not reflect the true situation, despite viral load being substantially reduced [39]. Assumed rates of resistance evolution seem optimistically low for ART use in resource-poor settings, only being varied between 5% and 25% of those on ART failing treatment per year, whereas rates as high as 60% have been predicted by others [40–42]. Despite this the model predicted that after transient success, ART would be rendered ineffective within 30 years due to wide-scale emergence of drug resistance, based on resistant virus being as transmissible as sensitive virus.
Gray et al's conclusions were relatively pessimistic [2], contrasting with Blower et al [5]. Gray et al concluded that ART alone cannot control mature HIV epidemics such as that in Rakai, Uganda. This conclusion concurs with Garnett et al 2002 [43], who believe that ART cannot make an impact on a mature epidemic unless treatment is initiated with high coverage and earlier in infection (i.e. with higher CD4 cell counts) than is currently recommended in treatment guidelines. Such early treatment is unfeasible financially and unwarranted clinically, since it would lead to earlier evolution of resistance and treatment failure, leaving individuals running out of treatment options, perhaps even before the onset of AIDS.
The dependence of the epidemiological impact of ART use on the timing of treatment initiation is worth considering in more detail. The progress of HIV infection to AIDS can broadly be divided into four stages: primary infection, incubation, the period preceding AIDS ("pre-AIDS") and AIDS. While there is much between- and within-patient variation, on average, infectiousness is highest during primary infection, pre-AIDS and AIDS. Some experts believe that primary infection carries the highest risk of transmission, because it is associated with high plasma HIV RNA levels and continued sexual activity [44]. However, while some studies are aiming to evaluate the effect of treating individuals in primary infection [3], the vast majority of HIV infections are not diagnosed until well into the incubation period. If primary infection is defined as the period before detectable antibodies against the virus emerge, then testing can only identify those who have completed the primary stage. However, if primary infection is used to describe the high initial viraemia then infection could be diagnosed before this has ended. Treatment could not start earlier than the incubation stage which follows primary infection except in rare circumstances where exposure is known to have occurred. In resource-poor settings, diagnosis is frequently at a very late stage of infection [45, 46], partly because of the non-specific nature of symptoms and the difficulty in accessing healthcare. Therefore, initiating treatment at diagnosis or when CD4 counts descend to a certain benchmark, such as 350 or 200 cells/mm3 (as recommended by current guidelines [25, 47]), will mean that the highly infectious period of primary infection and the long period of incubation escape the controlling effects of treatment.
In models examining the impact of ART on HIV incidence, inclusion of the variation in infectiousness as a function of infection stage is crucial for producing realistic predictions. As ART can only be initiated upon HIV diagnosis, it will have no effect on transmission from most individuals undergoing primary infection, when risk of transmission is high. By the time an individual has developed AIDS, their sexual activity will have decreased, and so this group of infected individuals will not contribute as much to HIV transmission as the duration of this phase would suggest. Figure 2 shows runs from a four-stage HIV infection model, with various treatment coverage scenarios, determined by stage of infection. Treatment is introduced into a population with a mature HIV epidemic and a high basic reproductive number for the at-risk fraction of the population (R0~5), so it is not surprising that even aggressive implementation of ART to individuals, regardless of stage of infection, cannot lead to elimination. Figure 2 illustrates that ART under more realistic assumptions regarding treatment delivery, in terms of treatment initiation, will have far less impact on incidence. In this model, there is a single treatment regimen and high, but plausible, rates of drug resistance evolution (30% per year), meaning that the effects on transmission are short-lived, coinciding with the effectiveness of the regimen. This illustrates the urgent need for cheap and reliable second-line treatment options to be available for ART roll-out.
Predictions of the impact of ART by stage of infection at which treatment is initiated. Predictions of the impact of the introduction of ART in terms of HIV incidence, by stage of infection at which treatment is initiated (for a brief description of the four stage infection model used, see Endnote). Scenario A – No treatment. Scenario B – ART uptake: AIDS patients only (after a mean of 1 month). Scenario C – ART uptake: AIDS patients (after mean 1 month) and pre-AIDS (after mean 6 months). Scenario D – ART uptake: AIDS patients (after mean 1 month) and pre-AIDS (after mean 6 months) and incubation stage (after mean 4 years). Scenario E – ART uptake: all four stages, after mean 1 month.
Despite our view that Blower et al are over-optimistic [5], Gray et al's assumptions of the effects of ART may similarly be over-pessimistic [2]. The authors assume that ART leads to an average proportional reduction in HIV log viral load of between 26.8% and 43.6%, based on data from the Women's Interagency HIV Study (WIHS) [48] and the John Hopkins Clinic [49] respectively. However, other studies distinguish between patients who respond to a regimen (who typically experience reduction in viral load to undetectable levels (<50 copies/ml)), those who do not respond and those who subsequently experience treatment failure (viral rebound). With these distinctions, an individual responding successfully to ART will have a far greater reduction in viral load than Gray et al assume. Furthermore, the proportion reduction was the value recorded one month and three months after treatment initiation for the WIHS and John Hopkins Clinic patients, respectively. It can take much longer than this for complete reduction of viral load, often to undetectable limits [50] (models generally do not explicitly account for a delay between treatment initiation and effect, but it can be assumed that this is implicitly accounted for in the treatment uptake rate). Gray et al also included the possibility of behavioural disinhibition; the average number of partners for those on treatment was increased by 50% or 100%. Again, the values appear pessimistic and were essentially chosen arbitrarily, probably in order to complement other models [4, 5].
Emergence of ART drug resistance
Many models of ART have concentrated on predicting the emergence and spread of ART drug resistance, which has been of concern [51–54]. Once again it is very difficult to make such predictions, as the spread of drug-resistant virus is highly dependent on the replicative fitness of the resistant strains that evolve and their ability to superinfect individuals infected with wild-type strains (i.e. to co-infect someone already infected with wild-type virus, and successfully replicate). Superinfection is perhaps only likely in the successfully treated individual, where suppression of viral load allows the target cell population to recover, hence increasing the chance of successful replication and establishment of a new strain. In the untreated individual, it is unlikely that low frequency resistant virus, typically less fecund than wild type in this environment, would be able to compete against the established viral population sufficiently successfully to allow long-term persistence of the invading strain.
In a context where ART use is common in core groups, the possibility of superinfection of those on ART means that the likely maximum rate of spread of resistance epidemics may be similar to the speed of the initial HIV epidemic. HIV co-infection with different wild-type viruses [55, 56], and by wild-type strains re-infecting patients harbouring drug-resistant viruses after a short period of treatment interruption [57, 58], have both been documented. Chakraborty et al postulate that it is possible for patients infected with wild type HIV-1 isolates and under successful ART to become exposed to drug-resistant strains that would have significant selective advantage, leading them to outcompete the original wild-type strain and instigate treatment failure [59]. They concede that the probability of an individual undergoing successful treatment of a wild-type strain being exposed to a drug-resistant strain is low, but the large-scale roll-out of ART in high prevalence, resource-poor settings may increase this probability substantially.
The rate at which drug resistance evolves within the individual is likely to become higher in resource-poor settings than industrialised countries; even though there are reports of patient adherence being no lower than in the West [60], potential interruptions in supply due to transport problems and a lack of sophisticated laboratory monitoring systems will limit the success of any ART regimen. However, even with high levels of drug resistance evolving within the individual ("secondary resistance"), transmission of such strains ("primary resistance"), while increasing in many industrialised settings [54, 61], is reported to be substantially less frequent than for wild-type HIV [61, 62], because there is usually a fitness cost for mutations. Mathematical models may have the ability to predict best- and worst-case scenarios for resistance spread [39, 63], but it must be conceded that the degree to which drug resistance and risky behaviour increase as ART use rolls out in Africa and other resource-poor areas cannot yet be quantified.
Blower et al 2001 predicted that acquired resistance will continue to rise, but transmitted resistance is likely to increase only gradually, with a doubling time of around four years and a predicted median of 15.6% of new HIV infections likely to be resistant to antiretroviral drugs by 2005 [63]. This conclusion was due to an assumption that of all possible ART-resistant HIV strains that could possibly evolve, none could be as transmissible as wild-type. The study also assumed that individuals infected with ART-sensitive virus undergoing treatment cannot be co-infected or superinfected by an ART-resistant strain.
Despite the conclusion that transmitted ART resistance will stabilise at low levels, the predicted range around the 15.6% value is very wide (0.05% to 73.21%) [63]. The authors argue that the higher values in the range generated from their sensitivity analysis have a very low probability. However, the choice of parameter distributions in the Monte Carlo sampling of parameter space undertaken in their study was arbitrary (in the sense of not being motivated by prior data) and entirely determines the probability of pessimistic scenarios.
The authors themselves acknowledge that they are "predicting the unpredictable" [63], but argue that their theoretical predictions [5] are in close agreement with empirical data [64]. Both display an increase in primary resistance between 1997 and 2001, but this is a short time period and the increase may reflect the expansion in use of ART over this time. Furthermore, the uncertainty interval around predictions made by the authors is large enough for a wide range of empirical data to fit the model. Blower et al acknowledge that transmission of ART resistance may vary widely by location and that frequent comparison to empirical data is necessary. However, Blower et al in 2005 recommend that large-scale surveillance for detecting transmitted resistance in Africa will be unnecessary for the next decade because transmitted drug resistance will not reach more than 5% during that time [65]. This is due to the assumption that ART use will remain at low levels, although the authors suggest that in urban locations rates of treatment may be higher. They recommend close monitoring of treated patients, but in areas where resources are constrained, this is unlikely to be practicable (WHO guidelines do not consider resistance testing, or even viral load testing, to be a priority in these regions [25, 66]). We would argue that surveillance of the prevalence of drug resistance among patients is required in all locations where ART is used, and that the predictive utility of models with high degrees of uncertainty in their input parameters, and hence also their results, is limited.
In this context, it should be noted that increases in levels of acquired resistance are not inevitable – in Switzerland, where more than 80% of prescribed ART is dispensed by one of the highly experienced Swiss HIV Cohort centres, prevalence of drug-resistant HIV in newly infected individuals has been decreasing since 1996 [67]. However, we would argue that such an effect is less likely in resource-poor settings with restricted access to high-quality care and laboratory facilities and potential problems of drug sharing, black market resale of drugs and inappropriate prescribing of mono and dual therapy outside of official ART programmes [53]. The differences between ART programmes in industrialised and developing countries will be so marked that predicting programme impact and patterns of drug resistance from those in former setting is not necessarily informative.
If one relaxes the assumption that no resistant strain can exceed the transmission fitness of wild-type even in the presence of ART use, even greater variation in predicted levels of transmitted drug resistance after 10 years of ART provision is possible (Figure 3). The scenarios illustrated assume a conservative rate of 10% per year for the evolution of resistance in the treated patient and do not allow for the possible enhancement of that rate in individuals suffering viral rebound (an increase in viral load following a previous decrease due to ART) without initial resistance, but who are then maintained on the same regimen (due to a lack of virological testing). Nevertheless, the results show that if a relatively fit variant emerged, the effectiveness of current ART regimens could be compromised after a very short period. There appears to be little change in model results when superinfection alone is allowed to occur, but when heterogeneous sexual activity is incorporated, resistance transmission is predicted to emerge more rapidly. This is due to superinfection allowing resistance to be transmitted through core groups receiving ART.
Model predictions of transmission of ART drug resistance by relative fitness of strains. Predictions of the spread of transmitted (primary) ART resistance under various scenarios, using a simplified ART model (see Endnote). Model output is 10 years after ART introduction. ART is introduced once the epidemic has reached equilibrium. Superinfection refers to the infection with ART-resistant HIV of individuals previously infected with ART-sensitive HIV and successfully undergoing treatment. These are the only individuals without viral outgrowth, and thus will have a pool of target cells rendering them susceptible to infection. Evolution of drug resistance within an individual is at a rate of 10% per year.
These results do not suggest a likelihood of drug resistance transmission but merely demonstrate the potential effects of various scenarios. It is more for biological studies (in particular resistance testing) and within-host models of HIV infection to examine the possibility that such strains could emerge [68–70]. Even in instances where laboratory tests reveal infections with virus deemed "resistant" to more than one drug class (either phenotypically or genotypically), these infections often still respond to treatment. A multidrug-resistant HIV strain would require a large number of compensatory mutations to be of a comparable fitness to wild type strains. However, ongoing treatment pressure in the presence of viral rebound could lead to sequential mutations increasing the fitness of a resistant strain; therefore, there is an argument for close monitoring of patients and implementation of drug resistance surveillance systems as ART is rolled out in resource-poor settings.
Parameterisation
To aid the design of successful ART programmes in resource-poor settings, more information on the impact of ART provision is crucial – data on morbidity and mortality, tolerability, treatment failure and the possible emergence of drug resistant strains are very important. This information also increases the reliability of model predictions by providing more accurate ranges of parameter estimates. Population-level monitoring for changes in risk behaviour and patterns of ARV drug resistance are also required. Information on the performance of ART programmes in these settings is starting to be generated. Pilot programmes such as the Médecins Sans Frontières (MSF) initiatives (established in seven low- and middle-income countries: Malawi, Kenya, South Africa, Cameroon, Cambodia, Thailand and Guatemala) are starting to report back preliminary findings. The six-month outcomes from the MSF projects were positive [71]: the probability of survival at six months was estimated as 89.5% (95% CI 86.8–92.1), with high patient attendance and adherence rates comparable to those in industrialised countries. However, pilot programmes may not be representative of future large-scale ART roll-out, where health-care infrastructure and expertise are likely to be poorer. Other programmes, such as those of the Drug Access Initiative (DAI), formed in 1998 by UNAIDS in collaboration with the Ministries of Health of Chile, Côte d'Ivoire, Uganda and Vietnam, have been running for longer. Reports from the first two years of the initiatives in Côte d'Ivoire have been positive [72], with immunologic and virologic outcomes similar to those reported from industrialised countries. The only resource-poor country to implement ART provision on a large scale is Brazil, which has made ART available free of charge to all eligible patients since 1996, and has produced positive outcomes [73]. The experience of Brazil can give information on the impact of long-term, large-scale ART provision, but is a very different setting to sub-Saharan Africa. As with data from industrialised countries, care must be taken in interpreting and assessing the applicability of results. Modelling provides the tools to predict the consequences of possible activity; by constraining ourselves to examining only those scenarios that are supported by current, gathered data, we can be ignoring other, distinct possibilities, such as the case in which an ART-resistant strain as fit as wild-type could evolve.
Conclusion
We have argued that HIV transmission models predicting the impact of ART use should incorporate a realistic progression through stages of HIV infection in order to realistically capture the timing of treatment initiation. Further elaboration of models is required (depending on the research question being posed), in areas such as time of diagnosis, sexual behaviour and assumptions regarding drug resistance evolution and transmission. All modelling studies are eventually dependent on the availability of setting-specific surveillance and behavioural data, and collection of such data is important for all regions where large scale ART use is introduced.
More investigation is required in order to determine the effect of introducing ART on a substantial scale in resource-poor settings with different stages and magnitudes of HIV epidemic. Models addressing questions of ART implementation in such settings, utilising data from fledgling ART projects where possible, will be of great use in designing cost effective programmes.
Endnote
Model assuming one stage of HIV infection
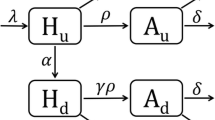
The model assuming one-stage of HIV infection, used to produce Figure 1, is illustrated in Figure 4, with state variables, parameter symbols and model equations given below. Superinfection with an ART-resistant strain is possible for individuals undergoing successful treatment only ( , S = ART-sensitive, T = treated), as these are the only individuals without viral outgrowth, and thus will have a pool of target cells rendering them susceptible to infection. Treatment failure can occur with (κ) or without (f) the evolution of drug resistance. Individuals who have developed treatment failure not accompanied by resistance (
, S = ART-sensitive, T = treated), as these are the only individuals without viral outgrowth, and thus will have a pool of target cells rendering them susceptible to infection. Treatment failure can occur with (κ) or without (f) the evolution of drug resistance. Individuals who have developed treatment failure not accompanied by resistance ( , F = treatment failure) are at increased risk of developing drug resistance (κ
F
) because of viral replication in the presence of continued drug pressure. Figure 3 uses a version of this one-stage model, modified to incorporate heterogeneous sexual mixing, with four different sexual activity groups.
, F = treatment failure) are at increased risk of developing drug resistance (κ
F
) because of viral replication in the presence of continued drug pressure. Figure 3 uses a version of this one-stage model, modified to incorporate heterogeneous sexual mixing, with four different sexual activity groups.
Model of HIV transmission and treatment, with one stage of infection only. Schematic illustration of the structure of the one stage HIV transmission model. 1° Res designates those with primary (transmitted) resistance, while 2° Res designates those with secondary (acquired) resistance. ART-Sens denotes people infected with ART-sensitive virus. For clarity, death rates are not shown.
The advantage of one-stage models of infection is that they are relatively simple and analytically tractable; that is, the relationship between each parameter and the outcome of the models, for example in terms of R0[1], can be exactly specified without recourse to simulation and sensitivity analysis. However, while a model should not incorporate complexity for its own sake, stages of HIV infection play a crucial role in the impact of ART, because treatment is only initiated at late stages of infection, and infectivity and sexual activity vary with the course of infection. Similarly, incorporating heterogeneous sexual activity within a model is more important for some research questions than for others. If we want to investigate the potential impact of a transmissible ARV-resistant HIV strain through a population, its spread would appear very different in a model of homogeneous sexual activity, compared to one with heterogeneity and various assumptions regarding mixing between activity classes, where infection would travel through core groups first before spreading into the general population.
State Variables
S = susceptible individuals
 = ART-sensitive HIV infected individuals, untreated
= ART-sensitive HIV infected individuals, untreated
 = ART-sensitive HIV infected individuals, treated
= ART-sensitive HIV infected individuals, treated
 = ART-sensitive HIV infected individuals, treated but viral rebound
= ART-sensitive HIV infected individuals, treated but viral rebound
 = ART-sensitive HIV infected individuals, suffered viral rebound, withdrawn from treatment
= ART-sensitive HIV infected individuals, suffered viral rebound, withdrawn from treatment
 = ART-resistant HIV infected individuals, treated
= ART-resistant HIV infected individuals, treated
 = ART-resistant HIV infected individuals, untreated
= ART-resistant HIV infected individuals, untreated
 = ART-resistant HIV infected individuals (transmitted resistance), untreated
= ART-resistant HIV infected individuals (transmitted resistance), untreated
 = ART-resistant HIV infected individuals (transmitted resistance), treated
= ART-resistant HIV infected individuals (transmitted resistance), treated
 = ART-resistant HIV infected individuals (transmitted resistance), withdrawn from treatment
= ART-resistant HIV infected individuals (transmitted resistance), withdrawn from treatment
λ S = force of infection for ART-sensitive virus phenotype
λ R = force of infection for ART-resistant virus phenotype (and force of (super)infection for ART-resistant virus phenotype, infecting an HIV wild type infected individual under treatment pressure)
Parameters
For individuals of infection status  :
:
i refers to viral phenotype (S (ART-sensitive) or R (ART-resistant));
j refers to treatment status (U (untreated) or T (treated)).
σN0 entry into model population (rate of recruitment into sexually active class). N0 is the size of the population at time t = 0.
μ death rate due to causes other than HIV infection
 excess death rate due to HIV infection for an infected individual in class
excess death rate due to HIV infection for an infected individual in class 
γ S treatment uptake rate for individuals infected with ART-sensitive HIV
γ R treatment uptake rate for individuals infected with ART-resistant HIV
f rate of treatment failure (viral rebound) without ART resistance evolution
κ rate of resistance evolution without previous viral rebound
κ F rate of resistance evolution with previous viral rebound
α S rate of treatment withdrawal for individuals initially infected with ART-sensitive HIV
α R rate of treatment withdrawal for individuals initially infected with ART-resistant HIV
 HIV transmission probability per partnership for an infected individual in class
HIV transmission probability per partnership for an infected individual in class 
c Number of sexual partnerships per year
 Probability of
Probability of  transmitting ART-resistant HIV
transmitting ART-resistant HIV
 Probability of
Probability of  transmitting ART-resistant HIV
transmitting ART-resistant HIV
 Probability of
Probability of  transmitting ART-resistant HIV
transmitting ART-resistant HIV
 Probability of
Probability of  transmitting ART-resistant HIV
transmitting ART-resistant HIV
ι Factor reduction in transmissibility for an infected individual in class 
Transmission equations

The forces of infection for ART-sensitive (λ S ) and ART-resistant (λ R ) HIV are given below. They are determined by the infectiousness of individuals in each class (β) and the probability that ART-resistant rather than ART-sensitive virus is transmitted in the case of mixed infections (ω).

Model assuming four stages of HIV infection
The model used to produce Figure 2 is essentially the same as for the one stage infection model, but with infection divided into four stages (primary infection, incubation, pre-AIDS and AIDS) as shown in Figure 5. The forces of infection are determined by the infectiousness of individuals in each class, the probability that ART-resistant rather than ART-sensitive virus is transmitted in the case of mixed infections and the rates of sexual partner change, which decrease as individuals reach the final stage (AIDS). The rest of the model structure is as for the one-stage model. Full details of this model, equations and parameter estimates are not shown here but are available on request.
Model of HIV transmission and treatment, with four stages of infection. Schematic illustration of the structure of the stages of HIV infection for the four stage model. Individuals progress from one stage to the next exponentially, with an average duration in each stage as shown in the figure. Individuals have no HIV-related mortality during primary infection or incubation, a very slightly elevated baseline death rate during pre-AIDS and an average one year life expectancy once AIDS has developed.
Abbreviations
- AIDS:
-
Acquired Immunodeficiency Syndrome
- ART:
-
Antiretroviral therapy
- HAART:
-
Highly Active Antiretroviral Therapy
- HIV:
-
Human Immunodeficiency Virus
- MSM:
-
Men who have Sex with Men
- PMTCT:
-
Prevention of Mother to Child Transmission
- VCT:
-
Voluntary Counselling and Testing
References
Velasco-Hernandez JX, Gershengorn HB, Blower SM: Could widespread use of combination antiretroviral therapy eradicate HIV epidemics?. Lancet Infect Dis. 2002, 2: 487-493. 10.1016/S1473-3099(02)00346-8
Gray RH, Li X, Wawer MJ, Gange SJ, Serwadda D, Sewankambo NK, Moore R, Wabwire-Mangen F, Lutalo T, Quinn TC: Stochastic simulation of the impact of antiretroviral therapy and HIV vaccines on HIV transmission; Rakai, Uganda. AIDS. 2003, 17 (13): 1941-1951. 10.1097/00002030-200309050-00013
Xiridou M, Geskus R, De Wit J, Coutinho R, Kretzschmar M: Primary HIV infection as a source of HIV transmission within steady and casual partnerships among homosexual men. AIDS. 2004, 18: 1311-1320. 10.1097/00002030-200406180-00010
Law MG, Prestage G, Grulich A, Van de Ven P, Kippax S: Modelling the effect of combination antiretroviral treatments on HIV incidence. AIDS. 2001, 15 (10): 1287-1294. 10.1097/00002030-200107060-00011
Blower SM, Gershengorn HB, Grant RM: A tale of two futures: HIV and antiretroviral therapy in San Francisco. Science. 2000, 287: 650-654. 10.1126/science.287.5453.650
Zhang L, Ramratnam B, Tenner-Racz K, He Y, Vesanen M, Lewin S, Talal A, Racz P, Perelson AS, Korber BT, et al: Quantifying residual HIV-1 replication in patients receiving combination antiretroviral therapy. N Engl J Med. 1999, 340 (21): 1605-1613. 10.1056/NEJM199905273402101
Vernazza PL, Gilliam BL, Dyer J, Fiscus SA, Eron JJ, Frank AC, Cohen MS: Quantification of HIV in semen: correlation with antiviral treatment and immune status. AIDS. 1997, 11 (8): 987-993. 10.1097/00002030-199708000-00006
Gupta P, Mellors J, Kingsley L, Riddler S, Singh MK, Schreiber S, Cronin M, Rinaldo CR: High viral load in semen of human immunodeficiency virus type 1-infected men at all stages of disease and its reduction by therapy with protease and nonnucleoside reverse transcriptase inhibitors. J Virol. 1997, 71 (8): 6271-6275.
Mocroft A, Vella S, Benfield TL, Chiesi A, Miller V, Gargalianos P, d'Arminio Monforte A, Yust I, Bruun JN, Phillips AN, et al: Changing patterns of mortality across Europe in patients infected with HIV-1. EuroSIDA Study Group. The Lancet. 1998, 352 (9142): 1725-1730. 10.1016/S0140-6736(98)03201-2. 10.1016/S0140-6736(98)03201-2
Weidle PJ, Holmberg SD, DeCock KM: Changes in HIV and AIDS epidemiology from new generation antiretroviral therapy. Aids. 1999, 13 (Suppl A): S61-68.
HIV incidence among young men who have sex with men – seven U.S. cities, 1994–2000. MMWR. 2001, 50 (21): 440-444.
Dodds J, Nardone A, Mercey D, Johnson A: Increase in high risk sexual behaviour among homosexual men, London 1996–8: cross sectional, questionnaire study. BMJ. 2000, 320: 1510-1511. 10.1136/bmj.320.7248.1510
Bouhnik AD, Moatti JP, Vlahov D, Gallais H, Dellamonica P, Obadia Y: Highly active antiretroviral treatment does not increase sexual risk behaviour among French HIV infected injecting drug users. J Epidemiol Community Health. 2002, 56 (5): 349-353. 10.1136/jech.56.5.349
Murphy G, Charlett A, Jordan LF, Osner N, Gill ON, Parry JV: HIV incidence appears constant in men who have sex with men despite widespread use of effective antiretroviral therapy. AIDS. 2004, 18 (2): 265-272. 10.1097/00002030-200401230-00016
Ostrow DE, Fox KJ, Chmiel JS, Silvestre A, Visscher BR, Vanable PA, Jacobson LP, Strathdee SA: Attitudes towards highly active antiretroviral therapy are associated with sexual risk taking among HIV-infected and uninfected homosexual men. AIDS. 2002, 16 (5): 775-780. 10.1097/00002030-200203290-00013
Wood E, Braitstein P, Montaner JS, Schechter MT, Tyndall MW, O'Shaughnessy MV, Hogg RS: Extent to which low-level use of antiretroviral treatment could curb the AIDS epidemic in sub-Saharan Africa. The Lancet. 2000, 355 (9221): 2095-2100. 10.1016/S0140-6736(00)02375-8. 10.1016/S0140-6736(00)02375-8
Aalen OO, Farewell VT, De Angelis D, Day NE, Gill ON: New therapy explains the fall in AIDS incidence with a substantial rise in number of persons on treatment expected. AIDS. 1999, 13 (1): 103-108. 10.1097/00002030-199901140-00014
Freedberg KA, Losina E, Weinstein MC, Paltiel AD, Cohen CJ, Seage GR, Craven DE, Zhang H, Kimmel AD, Goldie SJ: The cost effectiveness of combination antiretroviral therapy for HIV disease. N Engl J Med. 2001, 344 (11): 824-831. 10.1056/NEJM200103153441108
Wilson DP, Blower SM: Designing Equitable Antiretroviral Allocation Strategies in Resource-Constrained Countries. PLoS Med. 2005, 2 (2): 132-141. 10.1371/journal.pmed.0020050. 10.1371/journal.pmed.0020050
Stover J, Walker N, Garnett GP, Salomon JA, Stanecki KA, Ghys PD, Grassly NC, Anderson RM, Schwartlander B: Can we reverse the HIV/AIDS pandemic with an expanded response?. The Lancet. 2002, 360 (9326): 73-77. 10.1016/S0140-6736(02)09339-X. 10.1016/S0140-6736(02)09339-X
Stover J, Bollinger L, Cooper-Arnold K: Goals model: for estimating the effects of resource allocation decisions on the achievement of goals of the HIV/AIDS strategic plan. 2001
Salomon JA, Hogan DR, Stover J, Stanecki KA, Walker N, Ghys PD, Schwartlander B: Integrating HIV Prevention and Treatment: From Slogans to Impact. PLoS Med. 2005, 2 (1): e16. 10.1371/journal.pmed.0020016
Improved methods and assumptions for estimation of the HIV/AIDS epidemic and its impact: Recommendations of the UNAIDS Reference Group on Estimates, Modelling and Projections. Aids. 2002, 16 (9): W1-14.
Ghys PD, Brown T, Grassly NC, Garnett G, Stanecki KA, Stover J, Walker N: The UNAIDS Estimation and Projection Package: a software package to estimate and project national HIV epidemics. Sex Transm Infect. 2004, 80 (Suppl 1): i5-9. 10.1136/sti.2004.010199
WHO: Scaling up antiretroviral therapy in resource limited settings: Guidelines for a public health approach. WHO. 2002, 1-115.
Auvert B, Males S, Puren A, Taljaard D, Carael M, Williams B: Can Highly Active Antiretroviral Therapy Reduce the Spread of HIV?: A Study in a Township of South Africa. J Acquir Immune Defic Syndr. 2004, 36 (1): 613-621.
De Cock K, Mbori-Ngacha D, Marum E: Shadow on the continent: public health and HIV/AIDS in Africa in the 21st century. The Lancet. 2002, 360 (9326): 67-72. 10.1016/S0140-6736(02)09337-6. 10.1016/S0140-6736(02)09337-6
Kretzschmar M, Morris M: Measures of concurrency in networks and the spread of infectious disease. Math Biosci. 1996, 133 (2): 165-195. 10.1016/0025-5564(95)00093-3
Bauch CT: A versatile ODE approximation to a network model for the spread of sexually transmitted diseases. J Math Biol. 2002, 45 (5): 375-395. 10.1007/s002850200153
Boily MC, Bastos FI, Desai K, Masse B: Changes in the transmission dynamics of the HIV epidemic after the wide-scale use of antiretroviral therapy could explain increases in sexually transmitted infections: results from mathematical models. Sex Transm Dis. 2004, 31 (2): 100-113.
Eames KT, Keeling MJ: Modeling dynamic and network heterogeneities in the spread of sexually transmitted diseases. Proc Natl Acad Sci U S A. 2002, 99 (20): 13330-13335. 10.1073/pnas.202244299
Anderson RM, Garnett GP: Mathematical models of the transmission and control of sexually transmitted diseases. Sex Transm Dis. 2000, 27 (10): 636-643.
Boily MC, Godin G, Hogben M, Sherr L, Bastos FI: The impact of the transmission dynamics of the HIV/AIDS epidemic on sexual behaviour: a new hypothesis to explain recent increases in risk taking-behaviour among men who have sex with men. Med Hypotheses. 2005, 65 (2): 215-226. 10.1016/j.mehy.2005.03.017
Moatti JP, Prudhomme J, Traore DC, Juillet-Amari A, Akribi HA, Msellati P: Access to antiretroviral treatment and sexual behaviours of HIV-infected patients aware of their serostatus in Cote d'Ivoire. AIDS. 2003, 17 (Suppl 3): S69-77.
Cameron DW: Can we reduce HIV transmission by providing healthcare and HIV therapy to commercial sex workers?. J Int Assoc Physicians AIDS Care. 1998, 4 (11): 24-26.
Hosseinipour M, Cohen MS, Vernazza PL, Kashuba AD: Can antiretroviral therapy be used to prevent sexual transmission of human immunodeficiency virus type 1?. Clin Infect Dis. 2002, 34 (10): 1391-1395. 10.1086/340403
Stall R, Pollack L, Mills TC, Martin JN, Osmond D, Paul J, Binson D, Coates TJ, Catania JA: Use of antiretroviral therapies among HIV-infected men who have sex with men: a household-based sample of 4 major American cities. Am J Public Health. 2001, 91 (5): 767-773.
Clements MS, Prestage G, Grulich A, Van De Ven P, Kippax S, Law MG: Modeling Trends in HIV Incidence Among Homosexual Men in Australia 1995–2006. J Acquir Immune Defic Syndr. 2004, 35 (4): 401-406.
Nagelkerke N, Jha P, de Vlas S, Korenromp E, Moses S, Blanchard J, Plummer F: Modelling HIV/AIDS epidemics in Botswana and India: impact of interventions to prevent transmission. Bull World Health Org. 2002, 80: 89-96.
Garnett G, Bartley L, Cameron D, Anderson R: Both a 'magic bullet' and good aim are required to link public health interests and health care need in HIV infection. Nat Med. 2000, 6 (3): 261-262. 10.1038/73104
Paredes R, Mocroft A, Kirk O, Lazzarin A, Barton S, van Lunzen J, Katzenstein T, Antunes F, Lundgren J, Clotet B: Predictors of virological success and ensuing failure in HIV-positive patients starting highly active antiretroviral therapy in Europe: results from the EuroSIDA study. Arch Intern Med. 2000, 160: 1123-1132. 10.1001/archinte.160.8.1123
Descamps D, Flandre P, V C, Peytavin G, Meiffredy V, Collin G, Delaugerre C, Robert-Delmas S, Bazin B, Aboulker J, et al: Mechanisms of virologic failure in previously untreated HIV-infected patients from a trial of induction-maintenance therapy. JAMA. 2000, 283: 205-211. 10.1001/jama.283.2.205
Garnett GP, Bartley L, Grassly NC, Anderson RM: Antiretroviral therapy to treat and prevent HIV/AIDS in resource-poor settings. Nat Med. 2002, 8 (7): 651-654. 10.1038/nm0702-651
Koopman JS, Jacquez JA, Welch GW, Simon CP, Foxman B, Pollock SM, Barth-Jones D, Adams AL, Lange K: The role of early HIV infection in the spread of HIV through populations. J Acquir Immune Defic Syndr Hum Retrovirol. 1997, 14 (3): 249-258.
Sungkanuparph S, Vibhagool A, Mootsikapun P, Chetchotisakd P, Tansuphaswaswadikul S, Bowonwatanuwong C: Opportunistic infections after the initiation of highly active antiretroviral therapy in advanced AIDS patients in an area with a high prevalence of tuberculosis. AIDS. 2003, 17 (14): 2129-2131. 10.1097/00002030-200309260-00018
Laurent C, Diakhate N, Gueye NF, Toure MA, Sow PS, Faye MA, Gueye M, Laniece I, Toure Kane C, Liegeois F, et al: The Senegalese government's highly active antiretroviral therapy initiative: an 18-month follow-up study. AIDS. 2002, 16 (10): 1363-1370. 10.1097/00002030-200207050-00008
USDHHS: Guidelines for the use of ARV agents in HIV-infected adults and adolescents. US Department of Health and Human Services. Washington, DC; 2002.
Barkan SE, Melnick SL, Preston-Martin S, Weber K, Kalish LA, Miotti P, Young M, Greenblatt R, Sacks H, Feldman J: The Women's Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology. 1998, 9 (2): 117-125. 10.1097/00001648-199803000-00001
Moore RD: Understanding the clinical and economic outcomes of HIV therapy: the Johns Hopkins HIV clinical practice cohort. J Acquir Immune Defic Syndr Hum Retrovirol. 1998, 17 (Suppl 1): S38-41.
Ledergerber B, Egger M, Opravil M, Telenti A, Hirschel B, Battegay M, Vernazza P, Sudre P, Flepp M, Furrer H, et al: Clinical progression and virological failure on highly active antiretroviral therapy in HIV-1 patients: a prospective cohort study. Swiss HIV Cohort Study. The Lancet. 1999, 353 (9156): 863-868. 10.1016/S0140-6736(99)01122-8. 10.1016/S0140-6736(99)01122-8
Brenner B, Turner D, Wainberg M: HIV-1 drug resistance: can we overcome?. Expert Opin Biol Ther. 2002, 2 (7): 751-761. 10.1517/14712598.2.7.751
Cohen OJ, Fauci AS: Transmission of drug-resistant strains of HIV-1: unfortunate, but inevitable. The Lancet. 1999, 354 (9180): 697-698. 10.1016/S0140-6736(99)90106-X. 10.1016/S0140-6736(99)90106-X
Harries AD, Nyangulu DS, Hargreaves NJ, Kaluwa O, Salaniponi FM: Preventing antiretroviral anarchy in sub-Saharan Africa. The Lancet. 2001, 358 (9279): 410-414. 10.1016/S0140-6736(01)05551-9. 10.1016/S0140-6736(01)05551-9
Little S, Holte S, Routy J, Daar E, Markowitz M, Collier A, Koup R, Mellors J, Connick E, Conway B, et al: Antiretroviral-drug resistance among patients recently infected with HIV. N Engl J Med. 2002, 347 (6): 385-394. 10.1056/NEJMoa013552
Ramos A, Hu DJ, Nguyen L, Phan KO, Vanichseni S, Promadej N, Choopanya K, Callahan M, Young NL, McNicholl J, et al: Intersubtype human immunodeficiency virus type 1 superinfection following seroconversion to primary infection in two injection drug users. J Virol. 2002, 76 (15): 7444-7452. 10.1128/JVI.76.15.7444-7452.2002
Jost S, Bernard MC, Kaiser L, Yerly S, Hirschel B, Samri A, Autran B, Goh LE, Perrin L: A patient with HIV-1 superinfection. N Engl J Med. 2002, 347 (10): 731-736. 10.1056/NEJMoa020263
Altfeld M, Allen TM, Yu XG, Johnston MN, Agrawal D, Korber BT, Montefiori DC, O'Connor DH, Davis BT, Lee PK, et al: HIV-1 superinfection despite broad CD8+ T-cell responses containing replication of the primary virus. Nature. 2002, 420 (6914): 434-439. 10.1038/nature01200
Koelsch KK, Smith DM, Little SJ, Ignacio CC, Macaranas TR, Brown AJ, Petropoulos CJ, Richman DD, Wong JK: Clade B HIV-1 superinfection with wild-type virus after primary infection with drug-resistant clade B virus. AIDS. 2003, 17 (7): F11-16. 10.1097/00002030-200305020-00001
Chakraborty B, Kiser P, Rangel HR, Weber J, Mirza M, Marotta ML, Asaad R, Rodriguez B, Valdez H, Lederman MM, et al: Can HIV-1 superinfection compromise antiretroviral therapy?. AIDS. 2004, 18 (1): 132-134.
Laniece I, Ciss M, Desclaux A, Diop K, Mbodj F, Ndiaye B, Sylla O, Delaporte E, Ndoye I: Adherence to HAART and its principal determinants in a cohort of Senegalese adults. AIDS. 2003, 17 (Suppl 3): S103-108.
Leigh Brown AJ, Frost SD, Mathews WC, Dawson K, Hellmann NS, Daar ES, Richman DD, Little SJ: Transmission fitness of drug-resistant human immunodeficiency virus and the prevalence of resistance in the antiretroviral-treated population. J Infect Dis. 2003, 187 (4): 683-686. 10.1086/367989
Yerly S, Jost S, Telenti A, Flepp M, Kaiser L, Chave JP, Vernazza P, Battegay M, Furrer H, Chanzy B, et al: Infrequent transmission of HIV-1 drug-resistant variants. Antivir Ther. 2004, 9 (3): 375-384.
Blower S, Aschenbach A, Gershengorn H, Kahn J: Predicting the unpredictable: transmission of drug-resistant HIV. Nat Med. 2001, 7: 1016-1020. 10.1038/nm0901-1016
Grant R, Hecht F, Warmerdam M, Liu L, Liegler T, Petropoulos C, Hellmann N, Chesney M, Busch M, Kahn J: Time trends in primary HIV-1 drug resistance among recently infected persons. JAMA. 2002, 288 (2): 181-188. 10.1001/jama.288.2.181
Blower S, Bodine E, Kahn J, McFarland W: The antiretroviral rollout and drug-resistant HIV in Africa: insights from empirical data and theoretical models. AIDS. 2005, 19: 1-14. 10.1097/00002030-200501030-00001
WHO: The use of antiretroviral therapy: a simplified approach for resource-constrained countries. 2002, [http://w3.whosea.org/LinkFiles/Publications_aids-133.pdf]
Yerly S, Kaiser L, Race E, Bru JP, Clavel F, Perrin L: Transmission of antiretroviral-drug-resistant HIV-1 variants. The Lancet. 1999, 354 (9180): 729-733. 10.1016/S0140-6736(98)12262-6. 10.1016/S0140-6736(98)12262-6
Ghani AC, Ferguson NM, Fraser C, Donnelly CA, Danner S, Reiss P, Lange J, Goudsmit J, Anderson RM, De Wolf F: Viral replication under combination antiretroviral therapy: a comparison of four different regimens. J Acquir Immune Defic Syndr. 2002, 30 (2): 167-176.
Vergu E, Mallet A, Golmard JL: The role of resistance characteristics of viral strains in the prediction of the response to antiretroviral therapy in HIV infection. J Acquir Immune Defic Syndr. 2002, 30 (3): 263-270.
Ferguson NM, deWolf F, Ghani AC, Fraser C, Donnelly CA, Reiss P, Lange JM, Danner SA, Garnett GP, Goudsmit J, et al: Antigen-driven CD4+ T cell and HIV-1 dynamics: residual viral replication under highly active antiretroviral therapy. Proc Natl Acad Sci U S A. 1999, 96 (26): 15167-15172. 10.1073/pnas.96.26.15167
Tassie JM, Szumilin E, Calmy A, Goemaere E: Highly active antiretroviral therapy in resource-poor settings: the experience of Medecins Sans Frontieres. AIDS. 2003, 17 (13): 1995-1997. 10.1097/00002030-200309050-00023
Diomande FV, Bissagnene E, Nkengasong JN, Maurice C, Monga B, Laga M, Nolan ML: The most efficient use of resources to identify those in need of antiretroviral treatment in Africa: empirical data from Cote d'Ivoire's Drug Access Initiative. AIDS. 2003, 17 (Suppl 3): S87-93.
Bastos FI, Kerrigan D, Malta M, Carneiro-da-Cunha C, Strathdee SA: Treatment for HIV/AIDS in Brazil: strengths, challenges, and opportunities for operations research. AIDScience (Science knowledge environment). 2001, I (15):
Zaric GS, Brandeau ML, Bayoumi AM, Owens DK: The effects of protease inhibitors on the spread of HIV and the development of drug-resistance HIV strains: a simulation study. Simulation. 1998, 71 (4): 262-275.
Wood E, Schechter MT, Tyndall MW, Montaner JS, O'Shaughnessy MV, Hogg RS: Antiretroviral medication use among injection drug users: two potential futures. AIDS. 2000, 14 (9): 1229-1235. 10.1097/00002030-200006160-00021
Tchetgen E, Kaplan EH, Friedland GH: Public health consequences of screening patients for adherence to highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2001, 26 (2): 118-129. 10.1097/00042560-200102010-00003
Dangerfield BC, Fang Y, Roberts CA: Model-based scenarios for the epidemiology of HIV/AIDS: the consequences of highly active antiretroviral therapy. System Dynamics Review. 2001, 17 (2): 119-150. 10.1002/sdr.211. 10.1002/sdr.211
Law MG, Prestage G, Grulich A, Van de Ven P, Kippax S: Modelling HIV incidence in gay men: increased treatment, unsafe sex and sexually transmissible infections. AIDS. 2002, 16 (3): 499-501. 10.1097/00002030-200202150-00029
Johnson L, Dorrington R: The demographic and epidemiological impact of HIV/AIDS treatment and prevention programmes: an evaluation based on the ASSA2000 model. Paper presented at the Demographic Association of Southern Africa Conference, Cape Town, 26–27 September 2002. 2002.
Nagelkerke NJ, Jha P, de Vlas SJ, Korenromp EL, Moses S, Blanchard JF, Plummer FA: Modelling HIV/AIDS epidemics in Botswana and India: impact of interventions to prevent transmission. Bull World Health Organ. 2002, 80 (2): 89-96.
Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, Meehan MO, Lutalo T, Gray RH: Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000, 342 (13): 921-929. 10.1056/NEJM200003303421303
Xiridou M, Geskus R, De Wit J, Coutinho R, Kretzschmar M: The contribution of steady and casual partnerships to the incidence of HIV infection among homosexual men in Amsterdam. AIDS. 2003, 17 (7): 1029-1038. 10.1097/00002030-200305020-00012
Acknowledgements
Supported by an unrestricted educational grant from GlaxoSmithKline.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
RB was supported by an unrestricted education grant from GlaxoSmithKline.
Authors' contributions
RB drafted the manuscript and constructed the mathematical models. All authors reviewed articles and read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Baggaley, R.F., Ferguson, N.M. & Garnett, G.P. The epidemiological impact of antiretroviral use predicted by mathematical models: a review. Emerg Themes Epidemiol 2, 9 (2005). https://doi.org/10.1186/1742-7622-2-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1742-7622-2-9