Abstract
Background
Several host-encoded antiviral factors suppress HIV-1 replication in a cell-autonomous fashion in vitro. The relevance of these defenses to the control of HIV-1 in vivo remains to be elucidated. We hypothesized that cellular restriction of HIV-1 replication plays a significant role in the observed suppression of HIV-1 in "elite controllers", individuals who maintain undetectable levels of viremia in the absence of antiretroviral therapy (ART). We comprehensively compared the expression levels of 34 host restriction factors and cellular activation levels in CD4+ T cells and sorted T cell subsets between elite controllers, HIV-1-infected (untreated) non-controllers, ART-suppressed, and uninfected individuals.
Results
Expression of schlafen 11, a codon usage-based inhibitor of HIV-1 protein synthesis, was significantly elevated in CD4+ T cells from elite controllers as compared to both non-controllers (p = 0.048) and ART-suppressed individuals (p = 0.024), with this effect most apparent in central memory CD4+ T cells. Schlafen 11 expression levels were comparable between controllers and uninfected individuals. Cumulative restriction factor expression was positively correlated with CD4+ T cell activation (r2 = 0.597, p < 0.0001), viral load (r2 = 0.34, p = 0.015), and expression of ISG15 (r2 = 0.73, p < 0.0001), a marker of interferon exposure. APOBEC3C, APOBEC3D, CTR9, TRIM26, and TRIM32 were elevated in elite controllers with respect to ART-suppressed individuals, while levels were comparable to uninfected individuals and non-controllers.
Conclusions
Host restriction factor expression typically scales with cellular activation levels. However, the elevated mRNA and protein expression of schlafen 11, despite low activation and viral load, violates the global pattern and may be a signature characteristic of HIV-1 elite control.
Similar content being viewed by others
Background
To date, there is no effective cure or prophylactic vaccine for HIV-1 infection. Antiretroviral therapy (ART) has dramatically decreased the morbidity and mortality associated with HIV-1 infection. However, there is a demand for alternative clinical management strategies due to the necessity of lifelong therapy, evolution of antiretroviral resistance, toxicity issues, and substantial costs of current regimens [1]. HIV-1 "elite controllers" naturally suppress HIV-1 to undetectable levels in the absence of ART, and therefore represent a promising model for a functional cure. The immunological determinants of control in these individuals may serve as foundations for novel antiretroviral strategies.
HIV-1 elite controllers appear to be a heterogeneous group, and the observed suppression of HIV-1 in these individuals has been attributed to a number of virologic, immunologic and genetic characteristics [2, 3]. Although there are indications that viruses in elite controllers may be less virulent than strains in non-controllers [4], HIV-1 isolates from controllers often exhibit typical replication kinetics in vitro suggesting that reduced viral fitness cannot fully explain this phenomenon [5]. Multiple studies suggest that HIV-specific CD4+ [6] and CD8+ T cells [7] play a key role, and exhibit high functionality and proliferative capacity in elite controllers. Elite controllers have higher CD8+ T cell activation levels than ART-suppressed individuals, despite maintaining clinically undetectable levels of viral replication and lower CD4+ T cell activation levels [8]. Certain human leukocyte antigen (HLA) class I alleles such as HLA-B*57 and HLA-B*27 are overrepresented in elite controllers, and the protective effects of these alleles are thought to be CD8+ cell-mediated. However, not all elite controllers have protective HLA alleles [9], and some individuals with established protective alleles progress to disease rapidly [10]. CD8+ T cell responses alone cannot explain the elite controller phenotype, and other immunologic and molecular mechanisms likely play a role [9].
In addition to adaptive immune responses against HIV-1, cell-intrinsic mechanisms may play an important role in mediating resistance to HIV-1 infection in elite controllers. Genome wide mRNA expression studies suggest that a transcriptional profile signature of CD4+ T cells is associated with HIV-1 elite control and viral set point in viremic individuals [11, 12]. In support of target cell-associated signature characteristics, CD4+ T cells from elite controllers may exhibit decreased susceptibility to HIV-1 infection ex vivo as compared to cells from viremic individuals, and cellular susceptibility to HIV-1 in controllers is predictive of reservoir size (total cell-associated HIV-1 DNA levels) [13, 14]. However, this observation is controversial, and other studies report conflicting results [15–17]. Cell-intrinsic factors that contribute to HIV-1 control may include a number of recently identified proteins that restrict HIV-1 replication in target cells, and provide the host with a pre-mobilized defense against retroviral infection. The most widely recognized restriction factors are TRIM5α [18], APOBEC3G [19], and BST2/tetherin [20, 21], and a number of additional factors with anti-HIV-1 activity have been identified and characterized in recent years. Our group recently published data suggesting that the BST-2/tetherin restriction factor plays a critical role in the interferon-mediated suppression of HIV-1 viremia in chronically infected individuals [22]. Although a few reports have examined the relevance of single factors (e.g. APOBEC3G) to HIV-1 plasma viral load and elite control [23], the overall contribution of host restriction mechanisms to HIV-1 elite control remains to be elucidated [2].
To address the hypothesis that cellular restriction of HIV-1 replication plays a significant role in the observed suppression of HIV-1 in elite controllers, we comprehensively compared restriction factor expression patterns and cellular activation levels in CD4+ T cells and T cell subsets between elite controllers, HIV-1-infected (untreated) non-controllers, ART-suppressed, and uninfected individuals enrolled in the UCSF SCOPE cohort. Restriction mechanisms suppress HIV-1 replication, while target cell activation promotes HIV-1 transactivation, replication, and production [24, 25]; therefore, consideration of these two parameters in a synchronous fashion will allow us to gauge overall cell-intrinsic susceptibility to HIV-1 infection. We designed and implemented a custom TaqMan Low Density Array (TLDA) to measure the expression of 34 anti-HIV-1 restriction genes. The precise prerequisites for achieving the designation of host restriction factor are somewhat controversial. Moreover, this repertoire is by definition a moving target as new restriction mechanisms come to light. We relied on the following two minimal criteria for inclusion in our "Cumulative Restriction" or "CuRe" TLDA: 1) Peer-reviewed, published evidence of direct inhibition of HIV-1 replication in vitro, and 2) Detectable expression in human peripheral blood mononuclear cells. In addition to bona fide, extensively characterized restriction factors such as APOBEC3G and BST-2/tetherin, the CuRe array measures a number of recently identified candidate anti-HIV-1 restriction factors to maximize the breadth, impact and generalizability of our translational study. Some of these factors may play functional roles outside of antiretroviral defense, and as of yet, co-evolutionary studies revealing canonical signatures indicative of historical host-pathogen conflict have not been performed across all sampled gene targets. However, all factors in the CuRe array meet the essential, minimal definition of a host restriction factor, and function in a cell-autonomous manner to suppress HIV-1 replication.
In addition to considering the individual expression of 34 anti-HIV-1 restriction genes, we created an intuitive mathematical construct to represent the overall, cumulative anti-HIV-1 restriction capacity associated with each sample. This metric, or "CuRe score", captures the cumulative fold-difference in antiviral gene expression with respect to a control individual.
Results
Schlafen 11 expression is elevated in elite controllers
We implemented the "CuRe" (Cumulative Restriction) TLDA to measure the mRNA expression of 34 anti-HIV-1 genes in CD4+ T cells from 48 subjects equally representing four HIV-1 disease states (12 elite controllers, 12 non-controllers, 12 ART-suppressed, and 12 HIV-1-uninfected individuals). The complete list of surveyed genes along with their respective reported anti-HIV-1 roles is presented in Table 1, precise disease state definitions are described in Table 2, and individual subject characteristics are documented in Additional file 1: Table S1. To represent overall HIV-1 inhibitory potential, we defined the CuRe score as the cumulative fold-difference in restriction factor expression with respect to a control individual (described in greater detail in the Methods section).
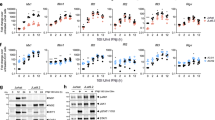
CD4+ T cells are the primary HIV-1 target cells within peripheral tissues, and therefore gene expression in this cellular subset is likely to be most relevant to viral production and propagation. CD4+ T cells were negatively-selected from freshly-collected blood with near 100% purity as described previously [22]. Expression of anti-HIV-1 genes in CD4+ T cells, as summarized by the CuRe score, was significantly higher in non-controllers as compared to elite controllers (p = 0.007), ART-suppressed (p = 0.002), or uninfected controls (p = 0.003) (Figure 1). A complete list of relative copy numbers for each restriction factor, and p-values for all inter-disease state gene expression comparisons are presented in Table 3 and Additional file 1: Table S2, respectively. These data suggest that overall, global restriction factor expression is not enhanced in elite controllers with respect to other HIV-1 disease groups, in contrast to the near-global induction of antiviral restriction mechanisms observed in individuals undergoing interferon-α therapy (unpublished observations, M. Abdel-Mohsen et al.).
Relationship between restriction factor expression and HIV-1 disease state. (A) CuRe (Cumulative Restriction) scores across HIV-1 disease states. Reported p-values were obtained using unpaired t tests. (B) Heat map representing expression of individual restriction genes across HIV-1 disease states. Fold-differences of each gene’s expression level in relation to the median values for each gene in the HIV-negative control group are reported. Yellow coloring indicates value of 1 (expression values equivalent to the median of HIV-negative controls). Red coloring indicates elevated relative expression, and green coloring indicates suppressed relative expression. Each column represents a single individual.
Although CuRe scores and the majority of individual gene-specific mRNA measurements were highest in viremic non-controllers with respect to other groups represented in our study (Figure 1), we sought to determine if particular anti-HIV-1 restriction factors violated this average behavior and exhibited high mRNA expression levels in HIV-1 elite controllers. We performed iterative univariate analyses to identify genes that were maximally expressed in elite controllers with respect to other HIV-1-infected individuals. Our analyses revealed that a single factor, schlafen 11, was expressed at a significantly higher level in CD4+ T cells from elite controllers with respect to viremic, untreated non-controllers (p = 0.048) and ART-suppressed (p = 0.024) individuals (Figure 2A). Schlafen 11 is a recently identified anti-HIV-1 restriction factor that potently suppresses HIV-1 replication by codon usage-based inhibition of HIV-1 protein synthesis [48].
Elevated expression of schlafen 11 in HIV-1 elite controllers. (A) schlafen 11 (SLFN11) expression in unfractionated CD4+ T cells across disease states. Data points involved in subsequent protein characterization are highlighted with black bordering. (B) SLFN11 expression in central memory CD4+ T cells. (C) SLFN11 expression in effector memory CD4+ T cells. Reported p-values in panels A-C were obtained using unpaired t tests. (D) SLFN11 protein expression in elite controllers and non-controllers, as determined by western blot. Immunoblotting bands were quantified with ImageJ64 software. The quantified SLFN11 protein expression levels were normalized to corresponding GAPDH protein levels to ensure equal loading. (E) Correlation between SLFN11 normalized protein level and mRNA relative copy number. A Spearman’s rank test was used to evaluate the significance of the correlation.
We next performed an exploratory analysis of the mRNA expression of schlafen 11 in central memory and effector memory CD4+ T cell subsets. Multiple reports suggest that elite controllers and long-term non-progressors harbor higher relative numbers of central memory CD4+ T cells, and these cells exhibit distinct phenotypic properties from viremic non-controllers [49, 50]. Flow-based sorting was used to isolate central and effector memory populations in a subset of 20 individuals (5 of each disease state), and the CuRe array was implemented to measure restriction gene expression. Our data from these cellular subsets reveal that central memory CD4+ T cells from elite controllers (Figure 2B), but not effector memory cells (Figure 2C), express significantly higher levels of schlafen 11 (p = 0.031) than corresponding cellular subsets from viremic non-controllers (although a trend was apparent in effector memory cells).
To confirm that our schlafen 11 mRNA expression data were recapitulated at the protein level, we measured schlafen 11 protein expression in CD4+ T cells from a subset of four elite controllers and four non-controllers, chosen based on range of mRNA expression and availability of remaining specimens (data points in Figure 2A corresponding to samples chosen for subsequent protein characterization are highlighted with black bordering). Our western blots demonstrate that schlafen 11 protein expression (normalized to GAPDH protein level to control for loading) is elevated in elite controllers with respect to non-controllers, validating our mRNA-based observations (Figure 2D). Moreover, schlafen 11 normalized protein levels and mRNA relative copy numbers exhibit a near-perfect correlation, suggesting that mRNA quantitation is a reliable strategy for measuring schlafen 11 expression in primary cells (Figure 2E).
Restriction factor expression in vivois correlated with CD4+ T cell activation
Based on our observation that restriction factor expression was highest in non-controllers, we hypothesized that restriction gene expression fluctuates in tandem with CD4+ T cell activation levels. We used flow cytometry to determine the relative levels of CD4+ T cell activation in all study subjects. T cell activation levels were reported as a percentage of CD4+ T cells co-expressing HLA-DR and CD38, as well as the mean fluorescence intensity (MFI) of HLA-DR. In our study population, non-controllers had a higher frequency of activated CD4+ T cells compared to elite controllers (p < 0.0001), ART-suppressed (p = 0.003), or uninfected individuals (p < 0.0001) (Figure 3, Figure 4A). We additionally observed a higher frequency of activated CD4+ T cells in the ART-suppressed group compared to elite controllers (p = 0.033) and uninfected individuals (p < 0.015) (Figure 4A). The CD4+ activation profiles reported here recapitulate previously reported data [8]. Focusing on all HIV-1 infected, untreated individuals, we identified statistically significant correlations between the CuRe score and frequency of activated CD4+ T cells (r2 = 0.597, p < 0.0001) (Figure 4B), and HIV-1 plasma viral load (r2 = 0.336, p = 0.015) (Figure 4C). We also examined the relationship between the CuRe score and the expression of interferon-stimulated gene 15 (ISG15), a marker of interferon exposure, and observed a strong correlation (r2 = 0.73, p < 0.0001) (Figure 4D).
Frequency of activated CD4+ T cells across HIV-1 disease states. CD38 and HLA-DR expression on the surface of CD4+ T cells was measured by flow cytometry. Plots for three representative, median individuals are included for each HIV-1 disease state. Percentages of activated cells (co-expressing CD38 and HLA-DR) are reported in the upper-right quadrant of each plot.
Immunologic and virologic correlates of host restriction factor expression. (A) Frequency of activated (CD38+ HLA-DR+) CD4+ T cells across disease states. Reported p-values were obtained using unpaired t tests. Correlations between CuRe score and CD4+ T cell activation, HIV-1 viral load, and ISG15 expression in HIV-1-infected, untreated individuals (elite controllers and non-controllers) are reported in (B), (C), and (D), respectively. Correlations were evaluated using Pearson’s r tests.
Elite controllers exhibit a distinct restriction factor expression profile from ART-suppressed individuals
As HIV-1 replication apparently drives expression of many restriction factors, we compared gene expression in the two "aviremic" infected groups (controllers and ART-suppressed). Our rationale was that differences in gene expression between these groups would be minimally confounded by differences in HIV-1 antigen levels, and may provide insights into factors which are mechanistically associated with virus control. In this two-way comparison, five anti-HIV-1 restriction genes were significantly elevated in elite controllers when compared to ART-suppressed subjects: APOBEC3C (p = 0.022), APOBEC3D (p = 0.029), CTR9 (p = 0.028), TRIM26 (p = 0.038), and TRIM32 (p = 0.044) (Figure 5A-E). No genes were elevated in ART-suppressed individuals with respect to controllers. The enhanced expression of these five genes in elite controllers violates the typical positive correlation with CD4+ T cell activation, as does schlafen 11. We examined the correlations between the expression of restriction genes and CD4+ T cell activation within the two aviremic, HIV-1-infected groups. CDKN1A/p21 was the only gene that exhibited a significant correlation in elite controllers; its expression was positively correlated with activation in controllers (r2 = 0.42, p < 0.023) (Figure 5F), while there were no significant correlations between gene expression and activation in ART-suppressed individuals.
Elevated expression of restriction factor genes in HIV-1 elite controllers with respect to ART-suppressed individuals. Five restriction factors were significantly elevated in elite controllers with respect to ART-suppressed subjects: (A) APOBEC3C, (B) APOBEC3D, (C) CTR9, (D) TRIM26, and (E) TRIM32. Gene expression between groups was compared using unpaired t tests. (F) The expression of CDKN1A/p21 was positively correlated with CD4+ T cell activation in elite controllers. There were no correlations between gene expression and activation in ART-suppressed individuals. Correlations were evaluated using Pearson’s r tests.
Discussion
Our overall objective was to determine the relevance of host-encoded anti-HIV-1 restriction factors to HIV-1 elite controller status. We measured the relative expression levels of 34 different antiviral factors in CD4+ T cells from elite controllers to ascertain if one or more restriction genes are associated with the control of HIV-1 in vivo. Overall restriction factor expression (as measured by the CuRe score) exhibited significant, pronounced relationships with T cell activation and ISG15 expression (an indicator of interferon levels), and a less pronounced positive correlation with viral load. This pattern likely reflects a scenario in which restriction factor expression is primarily driven by cellular activation and interferon exposure in vivo, mirroring recent data from our group derived from in vitro experiments [51] and studies of HLA-B*57-positive healthy donors [52]. The moderate correlation with viral load likely reflects an indirect association (since activation and interferon expression are driven by viral antigen). This pattern parallels data describing positive correlations between the breadth and magnitude of anti-HIV-1 CD8+ responses and viral load observed in structured treatment interruption studies [53]. Natural variation in restriction factor mRNA expression on a global level, considered independently of other cellular and immunologic parameters, does not appear to be a prognostic indicator of effective viral control in vivo. However, overall restriction factor expression may serve as a prognostic indicator of HIV-1 suppression within the context of exogenous interferon-α treatment, when expression and activity of several factors is induced to supraphysiologic levels (unpublished observations, M. Abdel-Mohsen et al.).
Schlafen 11 [48] was the only gene in our array that exhibited significantly higher expression in elite controllers as compared to both viremic non-controllers and ART-suppressed groups. This suggests that schlafen 11 may play a role in the suppression of HIV-1 in vivo, by selectively inhibiting the synthesis of HIV-1 proteins via tRNA limitation and codon-based discrimination (HIV-1 favors A/T-rich codons in reflection of its skewed base composition). Moreover, expression data from our exploratory analyses of sorted T cell subsets indicate that schlafen 11 may specifically contribute to the distinct phenotypic signature and resilience associated with CD4+ central memory cells from HIV-1 elite controllers [49, 50]. However, despite the fact that we were able to identify statistically significant differences in expression levels between patient groups in these subsets that recapitulated the hierarchy observed in unsorted CD4+ cells, these observations warrant validation using larger sample sizes.
The hierarchy of schlafen 11 gene expression violates the typical linkage with cellular activation levels and interferon exposure. In addition, the lack of a significant difference between uninfected individuals and elite controllers in juxtaposition with significantly reduced expression levels in viremic non-controllers and ART-suppressed HIV-1-infected individuals suggests that controllers may be protected from viral downregulation of the schlafen 11 factor. This pattern may be driven by host and/or viral determinants; elite controllers may harbor a genetic variant of the schlafen 11 protein that is less susceptible to HIV-1 evasion and modulation. Alternatively, elite controllers may be infected by genetic variants of HIV-1 that exhibit attenuated capacity to downregulate schlafen 11, mirroring reported observations that HIV-1 strains in elite controllers express weak or defective versions of the Nef and Vif proteins [4]. However, as of yet, this is speculative, since a schlafen 11 antagonistic factor in HIV-1 remains to be identified. Another possibility stems from the characteristics of uninfected controls included in our study; this population, reflecting the composition of uninfected individuals enrolled in the SCOPE cohort, is enriched for highly HIV-exposed seronegative individuals, and factors such as schlafen 11 that contribute to viral control may also protect against HIV-1 acquisition. This is supported by the observation that amongst uninfected controls in our study, highly-exposed individuals express schlafen 11 at significantly higher levels than individuals with minimal exposure (data not shown).
It is important to note that despite statistically significant differences between patient groups, not all elite controllers in our study expressed schlafen 11 at elevated levels. Some controllers exhibit high schlafen 11 mRNA and protein expression, while other controllers are indistinguishable from non-controllers. This pattern mirrors other immunologic measurements of elite controllers; these individuals exhibit highly heterogeneous neutralizing antibody responses and T cell phenotypes [2, 3, 54]. This variability may reflect a scenario whereby some elite controllers are immunologically adapted to retroviral infection, while others simply maintain undetectable viremia due to poor replicative fitness of the infecting viral strain.
Five restriction factors (APOBEC3C, APOBEC3D, TRIM26, TRIM32, and CTR9) were elevated in elites when compared specifically to ART-suppressed individuals, who have similar levels of residual viremia. The elevated expression of these five genes, including APOBEC3 cytidine deaminases and TRIM-family members, constitutes an elite controller-specific signature. Moreover, the expression of CDKN1A/p21 scales with CD4+ T cell activation in elites (while no such correlations were observed in treated patients). CDKN1A/p21, a potent inhibitor of cyclin-dependent kinases, has recently been proposed to exert in vivo anti-HIV-1 activity, and is believed to be overexpressed in CD4+ T cells from elite controllers [13]. However, other groups report that CDKN1A/p21 is not directly involved in HIV-1 restriction [14]. We found no statistically significant difference in the expression levels of CDKN1A/p21 in elite controllers compared to any of the other three disease states. This observation remained consistent when the mRNA expression of these factors was examined specifically in central memory and effector memory CD4+ T cell subsets. The observation that expression of p21 was significantly correlated with CD4+ T cell activation in controllers, but not in any of the other groups included in our study, is provocative. This suggests that the inducibility and responsiveness of this defense factor to viral antigen, rather than constitutive overexpression, may contribute to the elite controller phenotype.
There are certain caveats associated with these data and their interpretation. Unlike studies involving in vitro or animal models, it is difficult to unequivocally demonstrate a causal relationship between restriction factor expression and the control of HIV-1 using a patient-based study design. Consideration of our findings within the context of the robust literature describing a causal relationship between these restriction factors and HIV-1 suppression in vitro (Table 1) lends credibility to these in vivo observations. Another caveat involves our characterization of mRNA expression levels. Although concordance between mRNA and protein expression has been demonstrated for several of our studied factors, mRNA may not always accurately predict protein concentrations. Although we performed confirmatory protein quantitation to validate elevated schlafen 11 expression in elite controllers, cellular protein concentrations may not always accurately reflect antiviral activity in vivo. Functional assays using clinical specimens from elite controllers will complement and extend these findings.
Conclusions
Our data suggest that overall restriction factor gene expression, captured in our CuRe score, is highest amongst non-controllers, likely a reflection of increased viral antigen and interferon exposure. However, the codon-usage-based inhibitor of HIV protein synthesis schlafen 11 is a notable exception to this pattern. Elevated schlafen 11 expression is a signature characteristic of HIV-1 elite control and may play a role in the suppression of HIV-1 in vivo. These findings complement earlier reports suggesting that CD4+ T cells from controllers are refractory to HIV-1 infection ex vivo. Continued investigation into the regulation, tissue-specific expression, and antiretroviral capacity of host restriction factors will likely benefit the development of therapeutic and curative strategies for HIV-1 infection.
Methods
Subjects and specimen processing
60 cc of blood was collected prospectively from 48 individuals enrolled in the UCSF SCOPE Cohort. The research was approved by the relevant institutional review boards, and all human participants gave written informed consent. Disease state definitions are described in Table 2, and individual subject characteristics are documented in detail in Additional file1: Table S1. Plasma RNA viral load and CD4+ T cell counts were measured at all patient visits. Total blood from all subjects was processed with Ficoll-Plaque PLUS and 106 PBMCs were immediately subject to CD4+ cell enrichment. CD4+ T cells were enriched from fresh PBMCs using the EasySep Human CD4+ T Cell enrichment magnetic kit (StemCell Technologies), according to the manufacturer’s instructions. Total RNA extraction was performed directly after enrichment.
Cell sorting
A subset of 20 individuals (5 of each disease status) were chosen for sorting; four CD4+ T cell subpopulations (naïve, central memory, transitional memory, and effector memory cells) were isolated using a FACS-based sorting procedure. PBMCs were isolated by Ficoll density gradient, stained with aqua amine reactive dye, and then stained with fluorescently conjugated monoclonal antibodies against CD3, CD4, CD45RO, CD27, CCR7, CD57 and CD14. After gaining on lymphocytes and singlets, and excluding non-viable cells (using the amine reactive dye) and CD14+ monocytes, CD3+CD4+ cells were sorted as follows: [1] Naïve (CD45RO-CCR7+CD27+CD57-); [2] Central Memory (CD45RO+CCR7+CD27+); [3] Transitional Memory (CD45RO+CCR7-CD27+); and [4] Effector Memory (CD45RO+CCR7-CD27-) (Additional file 1: Figure S1).
Gene expression profiling
Total RNA was extracted from enriched CD4+ T cells using 700 μl of Qiazol reagent (Qiagen), followed by 15 min centrifugation at 12,000 g at 4°C. RNA was extracted from the aqueous layer using the miRNeasy Mini kit (Qiagen) with on-column DNAase treatment (Qiagen RNase-Free DNase Set) and eluted in 30 μl of RNase-free water. DNase-treated, clean RNA was transcribed into cDNA using random primers and the SuperScript® VILO™ cDNA Synthesis Kit (Invitrogen), according to manufacturer’s instructions. Quantitative real-time PCR utilized custom-made TaqMan® Low Density Arrays (TLDA) from Applied Biosystems following the manufacturer’s instructions. TaqMan Low Density Array (TLDA) cards (Applied Biosystems, Foster City, CA) are 384-well microfluidic cards with eight ports, each containing 48 connected wells. The primers and probes for each assay were preloaded and dried onto the designated duplicate wells. All probes used on our card are conjugated to 6-carboxyfluorescein (FAM). Black hole quencher 1 (BHQ1) was used as a quencher for all probes. The Cumulative Restriction (CuRe) TLDA card is designed to run 4 samples in duplicate on one card. Each port tests against 34 different antiviral genes and a panel of housekeeping genes. All assays and their respective target genes are listed in Table 1. Thermal cycling was performed using an ABI ViiA™ 7 Real-Time PCR System. Up to 450 ng cDNA in 200 μl of Applied Biosystems TaqMan Universal PCR Master Mix with UNG was loaded onto the designated ports of the TLDA plates. Data was analyzed using ABI ViiA™ 7 software. A panel of 6 housekeeping genes was included in the TLDA plates (GAPDH, 18S, ACTB, PPIA, RPLP0, and UBC). RPLP0 was identified as the most stably expressed gene from those 6 housekeeping genes among all samples using the GeNorm algorithm [55]. Therefore, raw cycle threshold numbers of amplified gene products were normalized to the housekeeping gene, RPLP0, (60S acidic ribosomal protein P0) to control for cDNA input amounts. Fold induction was determined using the comparative Ct method [55].
CuRe score calculation
The expression value for the ith gene is notated as ei, i = 1,2,…,n, assuming there are n genes. Missing values were imputed using the minimum expression value across samples for each gene, respectively. The median gene expression profile is calculated for the HIV-1-uninfected control group. A reference sample with the maximum number of genes that are closest to the median gene expression profile is the chosen from the uninfected control group. The reference expression value for the ith gene is notated as ri, i = 1,2,…,n. The gene expression profile for each sample was standardized by calculating the ratio against the reference sample. The CuRe score for a sample is the cumulative fold-difference in antiviral gene expression with respect to a control individual, expressed by the following formula:
Western blotting
Eight cryopreserved PBMC samples (four elite controller, four non-controller) were chosen for protein quantitation. CD4+ T cells were negatively selected from PBMC as described previously. 15 μl of cellular lysates in NuPAGE LDS Sample Buffer with 2-mercaptoethanol (equal to 0.6 million cells) were resolved by NuPAGE 4-12% gel, then transferred to PVDF membrane. The membrane was blocked by 5% non-fat dry milk/TBST and incubated with anti-SLFN11 mAb (1:500 dilution, Santa Cruz Biotech SLFN11 Antibody (E-4): sc-374339) or anti-GAPDH (1:2500, Cell Signal Tech GAPDH (D16H11) XP® Rabbit mAb #5174) in 5% BSA/TBST at 4C overnight. The next day, the membrane was washed and incubated with Cell Signal Tech anti-mouse or anti-rabbit HRP antibodies respectively and developed with PerkinElmer Western Lightning ECL Pro. The immunoblotting bands were quantified with ImageJ64 software. The quantified SLFN11 protein expression levels were normalized to corresponding GAPDH protein levels to ensure equal loading.
Flow cytometry
Cryopreserved PBMCs were quickly thawed, washed in FACS buffer (2 mM EDTA, 0.5% BSA in ice-cold PBS) and surface stained for 30 min at 4°C. Activation panel included the following antibodies: CD3-ECD (1:50, UCHT1, Beckman Coulter), CD4-PacificBlue (1:50, RPA-T4, Beckman Coulter), CD8-QDot605 (3B5, Invitrogen), CD14-APC/Cy7 (1:100, MΦP9, Becton Dickinson), CD19-APC/Cy7 (1:100, SJ25C1, BD Pharmingen), CD38-PE (1:50, HB7, BD Pharmingen), HLA-DR-FITC (1:200, L243 (G46-6), BD Pharmingen), and a live/dead marker (Invitrogen) emitting in the aqua wavelength. After staining, the cells were washed three times, fixed in 2% paraformaldehyde, and events were collected on a Becton Dickinson LSR-II, using FACS DIVA software (BD Biosciences). Data were analyzed using FlowJo software (version 9.3.2, TreeStar). FMOs were carried out for compensation purposes. T cell activation levels are reported as the percentage of CD4+ T cells co-expressing HLA-DR and CD38 and the mean fluorescence intensity (MFI) of HLA-DR.
Statistical analysis
A battery of statistical tests including unpaired t tests and Pearson’s r correlation coefficient were applied to data using GraphPad Prism v5.0c.
Abbreviations
- APOBEC:
-
Apolipoprotein B mRNA editing enzyme
- BST-2:
-
Bone marrow stromal cell antigen 2
- SAMHD1:
-
SAM domain and HD domain-containing protein 1
- TRIM:
-
Tripartite motif
- ISG:
-
Interferon-stimulated gene
- CDKN1A:
-
Cyclin-dependent kinase inhibitor 1A
- PAF1:
-
RNA polymerase II associated factor
- EIF2AK2:
-
Eukaryotic translation initiation factor 2-alpha kinase 2
- HERC5:
-
HECT domain and RLD 5
- IFITM:
-
Interferon induced transmembrane
- MOV10:
-
Moloney leukemia virus 10 homolog
- SLFN11:
-
Schlafen family member 11
- TLDA:
-
TaqMan® low density array
- GAPDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- 18S:
-
18S ribosomal RNA
- ACTB:
-
Beta-actin
- PPIA:
-
Peptidylprolyl isomerase A
- RPLP0:
-
60S acidic ribosomal protein P0
- UBC:
-
Ubiquitin C
- HN:
-
HIV negative controls
- EC:
-
Elite controllers
- AS:
-
ART suppressed
- NC:
-
Non-controllers.
References
Volberding PA, Deeks SG: Antiretroviral therapy and management of HIV infection. Lancet. 2010, 376: 49-10.1016/S0140-6736(10)60676-9.
Zaunders J, van Bockel D: Innate and adaptive immunity in long-term non-progression in HIV disease. Front Immunol. 2013, 4: 95-
Saag M, Deeks SG: How do HIV elite controllers do what they do?. Clin Infect Dis. 2010, 51: 239-10.1086/653678.
Cruz NV, Amorim R, Oliveira FE, Speranza FA, Costa LJ: Mutations in the nef and vif genes associated with progression to AIDS in elite controller and slow-progressor patients. J Med Virol. 2013, 85: 563-10.1002/jmv.23512.
Blankson JN, Bailey JR, Thayil S, Yang HC, Lassen K, Lai J, Gandhi SK, Siliciano JD, Williams TM, Siliciano RF: Isolation and characterization of replication-competent human immunodeficiency virus type 1 from a subset of elite suppressors. J Virol. 2007, 81: 2508-10.1128/JVI.02165-06.
Dyer WB, Zaunders JJ, Yuan FF, Wang B, Learmont JC, Geczy AF, Saksena NK, McPhee DA, Gorry PR, Sullivan JS: Mechanisms of HIV non-progression; robust and sustained CD4+ T-cell proliferative responses to p24 antigen correlate with control of viraemia and lack of disease progression after long-term transfusion-acquired HIV-1 infection. Retrovirology. 2008, 5: 112-10.1186/1742-4690-5-112.
Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M, Roederer M, Koup RA: HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006, 107: 4781-10.1182/blood-2005-12-4818.
Hunt PW, Brenchley J, Sinclair E, McCune JM, Roland M, Page-Shafer K, Hsue P, Emu B, Krone M, Lampiris H, Douek D, Martin JN, Deeks SG: Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis. 2008, 197: 126-10.1086/524143.
Emu B, Sinclair E, Hatano H, Ferre A, Shacklett B, Martin JN, McCune JM, Deeks SG: HLA class I-restricted T-cell responses may contribute to the control of human immunodeficiency virus infection, but such responses are not always necessary for long-term virus control. J Virol. 2008, 82: 5398-10.1128/JVI.02176-07.
Migueles SA, Laborico AC, Imamichi H, Shupert WL, Royce C, McLaughlin M, Ehler L, Metcalf J, Liu S, Hallahan CW, Connors M: The differential ability of HLA B*5701+ long-term nonprogressors and progressors to restrict human immunodeficiency virus replication is not caused by loss of recognition of autologous viral gag sequences. J Virol. 2003, 77: 6889-10.1128/JVI.77.12.6889-6898.2003.
Vigneault F, Woods M, Buzon MJ, Li C, Pereyra F, Crosby SD, Rychert J, Church G, Martinez-Picado J, Rosenberg ES, Telenti A, Yu XG, Lichterfeld M: Transcriptional profiling of CD4 T cells identifies distinct subgroups of HIV-1 elite controllers. J Virol. 2011, 85: 3015-10.1128/JVI.01846-10.
Rotger M, Dang KK, Fellay J, Heinzen EL, Feng S, Descombes P, Shianna KV, Ge D, Gunthard HF, Goldstein DB, Telenti A: H. I. V. C. S. Swiss, H. I. V. A. V. I. Center for, Genome-wide mRNA expression correlates of viral control in CD4+ T-cells from HIV-1-infected individuals. PLoS Pathog. 2010, 6: e1000781-10.1371/journal.ppat.1000781.
Chen H, Li C, Huang J, Cung T, Seiss K, Beamon J, Carrington MF, Porter LC, Burke PS, Yang Y, Ryan BJ, Liu R, Weiss RH, Pereyra F, Cress WD, Brass AL, Rosenberg ES, Walker BD, Yu XG, Lichterfeld M: CD4+ T cells from elite controllers resist HIV-1 infection by selective upregulation of p21. J Clin Invest. 2011, 121: 1549-10.1172/JCI44539.
Saez-Cirion A, Hamimi C, Bergamaschi A, David A, Versmisse P, Melard A, Boufassa F, Barre-Sinoussi F, Lambotte O, Rouzioux C, Pancino G: Restriction of HIV-1 replication in macrophages and CD4+ T cells from HIV controllers. Blood. 2011, 118: 955-10.1182/blood-2010-12-327106.
Rabi SA, O’Connell KA, Nikolaeva D, Bailey JR, Jilek BL, Shen L, Page KR, Siliciano RF, Blankson JN: Unstimulated primary CD4+ T cells from HIV-1-positive elite suppressors are fully susceptible to HIV-1 entry and productive infection. J Virol. 2011, 85: 979-10.1128/JVI.01721-10.
O’Connell KA, Rabi SA, Siliciano RF, Blankson JN: CD4+ T cells from elite suppressors are more susceptible to HIV-1 but produce fewer virions than cells from chronic progressors. Proc Natl Acad Sci USA. 2011, 108: E689-10.1073/pnas.1108866108.
Julg B, Pereyra F, Buzon MJ, Piechocka-Trocha A, Clark MJ, Baker BM, Lian J, Miura T, Martinez-Picado J, Addo MM, Walker BD: Infrequent recovery of HIV from but robust exogenous infection of activated CD4(+) T cells in HIV elite controllers. Clin Infect Dis. 2010, 51: 233-10.1086/653677.
Stremlau M, Owens CM, Perron MJ, Kiessling M, Autissier P, Sodroski J: The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature. 2004, 427: 848-10.1038/nature02343.
Sheehy AM, Gaddis NC, Choi JD, Malim MH: Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature. 2002, 418: 646-10.1038/nature00939.
Van Damme N, Goff D, Katsura C, Jorgenson RL, Mitchell R, Johnson MC, Stephens EB, Guatelli J: The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe. 2008, 3: 245-10.1016/j.chom.2008.03.001.
Neil SJ, Zang T, Bieniasz PD: Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 2008, 451: 425-10.1038/nature06553.
Pillai SK, Abdel-Mohsen M, Guatelli J, Skasko M, Monto A, Fujimoto K, Yukl S, Greene WC, Kovari H, Rauch A, Fellay J, Battegay M, Hirschel B, Witteck A, Bernasconi E, Ledergerber B, Gunthard HF, Wong JK: Role of retroviral restriction factors in the interferon-alpha-mediated suppression of HIV-1 in vivo. Proc Natl Acad Sci USA. 2012, 109: 3035-10.1073/pnas.1111573109.
Gandhi SK, Siliciano JD, Bailey JR, Siliciano RF, Blankson JN: Role of APOBEC3G/F-mediated hypermutation in the control of human immunodeficiency virus type 1 in elite suppressors. J Virol. 2008, 82: 3125-10.1128/JVI.01533-07.
Tong-Starksen SE, Luciw PA, Peterlin BM: Human immunodeficiency virus long terminal repeat responds to T-cell activation signals. Proc Natl Acad Sci USA. 1987, 84: 6845-10.1073/pnas.84.19.6845.
Siekevitz M, Josephs SF, Dukovich M, Peffer N, Wong-Staal F, Greene WC: Activation of the HIV-1 LTR by T cell mitogens and the trans-activator protein of HTLV-I. Science. 1987, 238: 1575-10.1126/science.2825351.
Zheng YH, Irwin D, Kurosu T, Tokunaga K, Sata T, Peterlin BM: Human APOBEC3F is another host factor that blocks human immunodeficiency virus type 1 replication. J Virol. 2004, 78: 6073-10.1128/JVI.78.11.6073-6076.2004.
Yu Q, Konig R, Pillai S, Chiles K, Kearney M, Palmer S, Richman D, Coffin JM, Landau NR: Single-strand specificity of APOBEC3G accounts for minus-strand deamination of the HIV genome. Nat Struct Mol Biol. 2004, 11: 435-10.1038/nsmb758.
Bishop KN, Holmes RK, Sheehy AM, Davidson NO, Cho SJ, Malim MH: Cytidine deamination of retroviral DNA by diverse APOBEC proteins. Curr Biol. 2004, 14: 1392-10.1016/j.cub.2004.06.057.
Yu Q, Chen D, Konig R, Mariani R, Unutmaz D, Landau NR: APOBEC3B and APOBEC3C are potent inhibitors of simian immunodeficiency virus replication. J Biol Chem. 2004, 279: 53379-10.1074/jbc.M408802200.
Bourara K, Liegler TJ, Grant RM: Target cell APOBEC3C can induce limited G-to-A mutation in HIV-1. PLoS Pathog. 2007, 3: 1477-
Abudu A, Wang X, Dang Y, Zhou T, Xiang SH, Zheng YH: Identification of molecular determinants from Moloney leukemia virus 10 homolog (MOV10) protein for virion packaging and anti-HIV-1 activity. J Biol Chem. 2012, 287: 1220-10.1074/jbc.M111.309831.
Dang Y, Siew LM, Wang X, Han Y, Lampen R, Zheng YH: Human cytidine deaminase APOBEC3H restricts HIV-1 replication. J Biol Chem. 2008, 283: 11606-10.1074/jbc.M707586200.
Berger G, Durand S, Fargier G, Nguyen XN, Cordeil S, Bouaziz S, Muriaux D, Darlix JL, Cimarelli A: APOBEC3A is a specific inhibitor of the early phases of HIV-1 infection in myeloid cells. PLoS Pathog. 2011, 7: e1002221-10.1371/journal.ppat.1002221.
Carthagena L, Bergamaschi A, Luna JM, David A, Uchil PD, Margottin-Goguet F, Mothes W, Hazan U, Transy C, Pancino G, Nisole S: Human TRIM gene expression in response to interferons. PLoS One. 2009, 4: e4894-10.1371/journal.pone.0004894.
Uchil PD, Quinlan BD, Chan WT, Luna JM, Mothes W: TRIM E3 ligases interfere with early and late stages of the retroviral life cycle. PLoS Pathog. 2008, 4: e16-10.1371/journal.ppat.0040016.
Barr SD, Smiley JR, Bushman FD: The interferon response inhibits HIV particle production by induction of TRIM22. PLoS Pathog. 2008, 4: e1000007-10.1371/journal.ppat.1000007.
Zhang J, Attar E, Cohen K, Crumpacker C, Scadden D: Silencing p21(Waf1/Cip1/Sdi1) expression increases gene transduction efficiency in primitive human hematopoietic cells. Gene Ther. 2005, 12: 1444-10.1038/sj.gt.3302544.
Zhang J, Scadden DT, Crumpacker CS: Primitive hematopoietic cells resist HIV-1 infection via p21. J Clin Invest. 2007, 117: 473-10.1172/JCI28971.
Liu L, Oliveira NM, Cheney KM, Pade C, Dreja H, Bergin AM, Borgdorff V, Beach DH, Bishop CL, Dittmar MT, McKnight A: A whole genome screen for HIV restriction factors. Retrovirology. 2011, 8: 94-10.1186/1742-4690-8-94.
Muto NF, Martinand-Mari C, Adelson ME, Suhadolnik RJ: Inhibition of replication of reactivated human immunodeficiency virus type 1 (HIV-1) in latently infected U1 cells transduced with an HIV-1 long terminal repeat-driven PKR cDNA construct. J Virol. 1999, 73: 9021-
Woods MW, Kelly JN, Hattlmann CJ, Tong JG, Xu LS, Coleman MD, Quest GR, Smiley JR, Barr SD: Human HERC5 restricts an early stage of HIV-1 assembly by a mechanism correlating with the ISGylation of Gag. Retrovirology. 2011, 8: 95-10.1186/1742-4690-8-95.
Lu J, Pan Q, Rong L, He W, Liu SL, Liang C: The IFITM proteins inhibit HIV-1 infection. J Virol. 2011, 85: 2126-10.1128/JVI.01531-10.
Pincetic A, Kuang Z, Seo EJ, Leis J: The interferon-induced gene ISG15 blocks retrovirus release from cells late in the budding process. J Virol. 2010, 84: 4725-10.1128/JVI.02478-09.
Furtak V, Mulky A, Rawlings SA, Kozhaya L, Lee K, Kewalramani VN, Unutmaz D: Perturbation of the P-body component Mov10 inhibits HIV-1 infectivity. PLoS One. 2010, 5: e9081-10.1371/journal.pone.0009081.
Malathi K, Dong B, Gale M, Silverman RH: Small self-RNA generated by RNase L amplifies antiviral innate immunity. Nature. 2007, 448: 816-10.1038/nature06042.
Nasr N, Maddocks S, Turville SG, Harman AN, Woolger N, Helbig KJ, Wilkinson J, Bye CR, Wright TK, Rambukwelle D, Donaghy H, Beard MR, Cunningham AL: HIV-1 infection of human macrophages directly induces viperin which inhibits viral production. Blood. 2012, 120: 778-10.1182/blood-2012-01-407395.
Hrecka K, Hao C, Gierszewska M, Swanson SK, Kesik-Brodacka M, Srivastava S, Florens L, Washburn MP, Skowronski J: Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein. Nature. 2011, 474: 658-10.1038/nature10195.
Li M, Kao E, Gao X, Sandig H, Limmer K, Pavon-Eternod M, Jones TE, Landry S, Pan T, Weitzman MD, David M: Codon-usage-based inhibition of HIV protein synthesis by human schlafen 11. Nature. 2012, 491: 125-10.1038/nature11433.
Potter SJ, Lacabaratz C, Lambotte O, Perez-Patrigeon S, Vingert B, Sinet M, Colle JH, Urrutia A, Scott-Algara D, Boufassa F, Delfraissy JF, Theze J, Venet A, Chakrabarti LA: Preserved central memory and activated effector memory CD4+ T-cell subsets in human immunodeficiency virus controllers: an ANRS EP36 study. J Virol. 2007, 81: 13904-10.1128/JVI.01401-07.
van Grevenynghe J, Procopio FA, He Z, Chomont N, Riou C, Zhang Y, Gimmig S, Boucher G, Wilkinson P, Shi Y, Yassine-Diab B, Said EA, Trautmann L, El Far M, Balderas RS, Boulassel MR, Routy JP, Haddad EK, Sekaly RP: Transcription factor FOXO3a controls the persistence of memory CD4(+) T cells during HIV infection. Nat Med. 2008, 14: 266-10.1038/nm1728.
Raposo RA, Abdel-Mohsen M, Bilska M, Montefiori DC, Nixon DF, Pillai SK: Effects of Cellular Activation on Anti-HIV-1 Restriction Factor Expression Profile in Primary Cells. J Virol. 2013, 87 (21): 11924-11929. 10.1128/JVI.02128-13. doi:10.1128/JVI.02128-13. Epub 2013 Aug 21
Raposo RA, Abdel-Mohsen M, Holditch SJ, Kuebler PJ, Cheng RG, Eriksson EM, Liao W, Pillai SK, Nixon DF: Increased expression of intrinsic antiviral genes in HLA-B*57-positive individuals. J Leukoc Biol. 2013, Epub ahead of print
Oxenius A, Price DA, Gunthard HF, Dawson SJ, Fagard C, Perrin L, Fischer M, Weber R, Plana M, Garcia F, Hirschel B, McLean A, Phillips RE: Stimulation of HIV-specific cellular immunity by structured treatment interruption fails to enhance viral control in chronic HIV infection. Proc Natl Acad Sci USA. 2002, 99: 13747-10.1073/pnas.202372199.
Lambotte O, Ferrari G, Moog C, Yates NL, Liao HX, Parks RJ, Hicks CB, Owzar K, Tomaras GD, Montefiori DC, Haynes BF, Delfraissy JF: Heterogeneous neutralizing antibody and antibody-dependent cell cytotoxicity responses in HIV-1 elite controllers. AIDS. 2009, 23: 897-10.1097/QAD.0b013e328329f97d.
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A: Speleman, Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3: RESEARCH0034
Acknowledgments
We thank all UCSF SCOPE Cohort patients who participated in our study, the physicians and study nurses for excellent patient care, Dr. John Guatelli for helpful discussion and suggestions regarding the panel of restriction factors analyzed, and the UCSF CFAR Core Virology Lab and Core Immunology Lab staff for processing and storage of the samples: Alex Carvidi, Christina Gonzaga, David Chung, Khooshbu Shah, Lorrie Epling, Maudi Killian, Montha Pao, Sophie Stephenson, Timothy Ryner, and Timothy Schmidt. This study was supported by grants from the National Institutes of Health (1K01DA024654, R01 AI087145, K24 AI069994, R21 AI93179), the University of California, San Francisco-Gladstone Institute of Virology & Immunology Center for AIDS Research (grant number P30 AI027763), the DARE: Delaney AIDS Research Enterprise (DARE; U19AI096109), the American Foundation for AIDS Research (grant number 106710-40-RGRL), the UCSF Clinical and Translational Research Institute Clinical Research Center (UL1 RR024131), the Center for AIDS Prevention Studies (P30 MH62246), and the CFAR Network of Integrated Systems (R24 AI067039).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
MAM, RASR, TL, ES and SKP performed gene expression and flow cytometry-based experiments, ML and MD performed western blotting, and XD performed statistical analyses. RH and SGD coordinated patient recruitment. MAM, SKP, JKW, DFN, SGD, MSS and HEAG designed the studies. MAM and SKP wrote the paper. All authors read and approved the final manuscript.
Electronic supplementary material
12977_2013_3615_MOESM1_ESM.docx
Additional file 1: Figure S1: FACS-based sorting of CD4+ T cell subpopulations. PBMCs were isolated by Ficoll density gradient, stained with aqua amine reactive dye, and then stained with fluorescently-conjugated monoclonal antibodies against CD3, CD4, CD45RO, CD27, CCR7, CD57 and CD14. After gaining on lymphocytes and singlets, and excluding non-viable cells (using amine reactive dye) and CD14+ monocytes, CD3+CD4+ cells were sorted as illustrated: (1) Naïve (CD45RO-CCR7+CD27+CD57-); (2) Central Memory (CD45RO+CCR7+CD27+); (3) Transitional Memory (CD45RO+CCR7-CD27+); and (4) Effector Memory (CD45RO+CCR7-CD27-). Table S1. Subject characteristics. Table S2. P values for inter-disease state gene expression comparisons. (DOCX 322 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Abdel-Mohsen, M., Raposo, R.A.S., Deng, X. et al. Expression profile of host restriction factors in HIV-1 elite controllers. Retrovirology 10, 106 (2013). https://doi.org/10.1186/1742-4690-10-106
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1742-4690-10-106