Abstract
Background
Methicillin-resistant Staphylococcus aureus (MRSA) is endemic in many hospital settings, posing substantial threats and economic burdens worldwide.
Methods
We propose mathematical models to investigate the transmission dynamics of MRSA and determine factors that influence the prevalence of MRSA infection when antibiotics are given to patients to treat or prevent infections with either MRSA itself or other bacterial pathogens.
Results
Our results suggest that: (i) MRSA always persists in the hospital when colonized and infected patients are admitted; (ii) the longer the duration of treatment of infected patients and the lower the probability of successful treatment will increase the prevalence of MRSA infection; (iii) the longer the duration of contamination of health care workers (HCWs) and the more their contacts with patients may increase the prevalence of MRSA infection; (iv) possible ways to control the prevalence of MRSA infection include treating patients with antibiotic history as quickly and efficiently as possible, screening and isolating colonized and infected patients at admission, and compliance with strict hand-washing rules by HCWs.
Conclusion
Our modeling studies offer an approach to investigating MRSA infection in hospital settings and the impact of antibiotic history on the incidence of infection. Our findings suggest important influences on the prevalence of MRSA infection which may be useful in designing control policies.
Similar content being viewed by others
Background
Methicillin-resistant Staphylococcus aureus (MRSA) is a major nosocomial pathogen that poses substantial threats and economic burdens to patients and hospitals globally. MRSA infection increases the risk of mortality, the length of hospital stay of patients, and extra costs of treatment and a control program for patients and hospital settings[1].
Although MRSA can be transmitted via contaminated objects, that is not the most common means of transmission[2, 3]. Rather, transmission from patient to patient occurs via the hands of health care workers (HCWs) in hospitals. Patients may be categorized into three disease statuses: uncolonized, colonized, and infected with MRSA[4]. MRSA causes sequelae such as sepsis, abscesses, wound infection, skin and soft tissue infection, and bloodstream infection. However, it can also colonize healthy humans without causing infection. Many parts of the body can be colonized such as the axillae, perineum, groin, rectum, skin, and anterior nares. In fact, a third of humans are asymptomatic nasal carriers of Staphylococcus aureus[5, 6]. The risk of colonization increases after contact with colonized and infected individuals[7]. Furthermore, it is more likely that individuals persistently colonized with MRSA will develop infection than those with short-term colonization[8, 9].
Antibiotics are widely prescribed to inhibit bacterial infections. They help to kill bacteria or prevent them from reproducing. However, because bacteria are capable of multiplying rapidly and transferring plasmids, the use of antibiotics may act as an environmental pressure on bacteria to select for resistance mutations, and the resistance trait can be transmitted to their progeny and other recipients by vertical and horizontal gene transfer[10]. Hence, the use of antibiotics may predispose individuals to acquire resistant elements. Consequently, treating infected individuals may become increasingly difficult, especially patients who have previously received antibiotic treatment. The ability to acquire resistance has been noted particularly in S. aureus. Antibiotic treatment of individuals infected with MRSA has been shown to be associated with skin infection, colonization, and treatment failure[8, 11–14]. In fact, a clear association between antibiotic exposure and MRSA isolation has been observed[12].
Various control methods have been suggested and implemented to overcome the spread of MRSA in hospital settings, including hand-washing compliance, staff cohorting, isolation of infected patients, use of rapid screening tests to identify acquisition of MRSA, antibiotic susceptibility tests, and appropriate antibiotic prescriptions.
Mathematical models have been widely used to study the spread of nosocomial bacteria in hospital settings[15–21]. In those studies patients are generally categorized into two groups: uncolonized and colonized by antibiotic-resistant bacteria. Treatments and histories of infected patients have received little attention in such models, although antibiotic exposure and treatment strategies play crucial roles among these patients. Besides, the rate of MRSA infection after colonization has been identified may be as high as 30%, and patients infected with MRSA are more likely to contaminate the hands of HCWs and the environment than colonized patients[22, 23]. Moreover, the mortality rate among patients infected with MRSA may be as high as 40%[24]. Hence, in this work, we clearly separate infected patients from colonized patients to investigate factors that may influence MRSA infection. Our work focuses on MRSA infection and treatment rather than colonization and other control methods, which have been addressed in several previous modeling studies.
We first develop a baseline model, which does not incorporate the history of antibiotic usage in individuals, and study the transmission dynamics, prevalence, and persistence of MRSA infection. We then modify the model to take the history of antibiotic usage into account. In the extended model, patients are separated into two groups, with and without antibiotic history. Patients with antibiotic history are those who have previously been treated for other types of bacterial infections, or have been prescribed antibiotics outside the hospital, or have been treated for MRSA infection. In addition, we study an optimal control strategy for the use of treatment in an attempt to identify an efficient way to control MRSA infection.
Methods
The baseline model for MRSA transmission
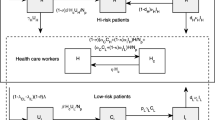
To capture the transmission dynamics of MRSA among patients in hospital settings via the hands of HCWs, we employ the structure of mathematical models for vector-borne diseases[16, 19]. In our case, HCWs are viewed as vectors and patients as definitive hosts. A flowchart and a table of parameter values can be found in Figure1 and Table1. Patients are categorized into three different groups: uncolonized (U), colonized (C), and infected (I) with MRSA[4]. HCWs are separated into two groups: uncontaminated (H) and contaminated (H c ) with MRSA. The rates of change of these two populations are described by the following system of ordinary differential equations:
where N h = H + H c . Patients are admitted to the hospital at a rate of ∧ per day; they are colonized with MRSA with a probability of λ c , infected with MRSA with a probability of λ i , or uncolonized with a probability of (1−λ c −λ i ). We define γ u , γ c , and γ i respectively as the discharge rates of uncolonized and colonized patients, and the death rate of infected patients. Colonized patients become infected at a rate of ϕ per day. Decolonization of MRSA occurs at a rate of ω per day. Complete clearance of MRSA in infected patients does not occur without passing through the colonization state. Antibiotics have been used widely in hospitals. We assume that they are given to all infected patients at a maximum rate of τ to prevent further complications of infection and death due to MRSA. However, it is possible that some patients are successfully treated, while others are resistant to the antibiotics and the treatment fails. Hence, ρ in the model is a scaling parameter reflecting successful treatment. Because antibiotics cannot kill all the bacteria in infected patients, it is assumed that treated patients are still colonized by the bacteria after a successful antibiotic course.
Because patients colonized with MRSA are more likely than uncolonized patients to develop infection, we assume that there is no direct infection of uncolonized patients by the hands of contaminated HCWs. In mathematical models for vector-borne diseases, each mosquito bites at a constant rate (irrespective of the number of humans), whereas the rate at which humans are bitten increases in proportion the number of mosquitoes[30]. We amend these assumptions slightly: we do not assume that each HCW contacts the patients at a constant rate, but that each patient needs a constant number of contacts with HCWs per day. Under this assumption, transmission is frequency-dependent with respect to HCWs, and the rate at which HCWs contact residents increases in proportion to the number (or density) of residents. A particular HCW contacts a particular patient at a rate of a/N h , where a is the number of contacts required by each patient per day and N h is the total number of HCWs. Uncolonized patients become colonized during contact with a contaminated HCW with a probability b p . HCWs become contaminated by contact with colonized patients with a probability b hc , and by contact with infected patients with a probability b hi .
The extended model for MRSA transmission with antibiotic exposure
It has been shown that patients with history of antibiotic exposure are vulnerable to skin infection and are also likely to be colonized by MRSA[12, 31, 32]. Furthermore, the use of antibiotics may exert a selective effect and lead to the emergence of resistant bacteria in patients and failure of treatment as a consequence. As antibiotic resistance can persist for a year, the history of antibiotic exposure of patients may become important. We therefore take this factor into account in the second model. In this extended model, treated patients either become colonized or remain infected with antibiotic history. A flowchart for this model is depicted in Figure2. The system of equations for it is as follows:
Parameters for the extended model
Owing to the consequences of antibiotic exposure, patients may be at risk of having skin rashes, thrush, and gastrointestinal symptoms; and they are more likely to become infected, show a higher death rate, and have a lower probability of successful treatment because of the acquisition of resistance elements and treatment failure, if the duration of decolonization, or the length of stay are greater. Therefore, we assume that and γ cA ≤ γ c [8, 11–14, 31]. The probability of a patient having a history of antibiotic usage on admission (λ uA + λ cA + λ iA ) is estimated to be 0.38[24]. The probability of treatment failure in an infected patient (ρu) (due to resistance for instance) is approximated by the probability that MSSA is replaced by MRSA in that patient[28].
Analysis
Steady states of the baseline model
Because the number of beds in the hospital is fixed, we assume that
Hence, the total number of patients remains constant in the model. Note that the total number of HCWs is also constant.
With the admission of colonized and infected patients, there exists only a disease-present steady state with
where C* satisfies the equation
in which
where N p is the total number of patients. Without admission of colonized and infected patients (λ c = λ i = 0), there are two steady states: disease-free (N p , 0, 0, N h , 0) and disease-present.
The basic reproductive number (R0)
It is important to understand when an outbreak can take place, and hence to calculate the basic reproductive number (R0). In the simplest case, when only uncolonized patients are admitted (λ c = λ i = 0), we follow a method developed by[33, 34] to calculate R0. Let us introduce as a vector of new infections in which is the rate of appearance of new infections in compartment i (i = 1, 2, 3, 4, 5) and as a vector of transfer rates by all other means in which, where represents the transfer of individuals into compartment i and represents the transfer of individuals out of compartment i. To compute R0, we rearrange the order of equations and variables in (1) as C, I, H c U, and H.
Next, we define F and V as the partitioned matrices from the disease-related variables of the Jacobian matrices of and at the disease-free steady state, respectively. Hence, we have
The next generation matrix is
Therefore, the basic reproductive number (R0), which is the positive and maximum eigenvalue of FV -1, is
Note that some authors use the square of this term as the basic reproductive number. By Theorem 2 in[33], we conclude that the disease-free steady state is unstable if and only if R0 < 1. However, when admission of colonized and infected patients is taken into account, there is always an influx of them moving into the C and I compartments. Although R0 < 1, the spread of ARB among patients can still persists.
Optimal control
We use optimal control techniques to study the optimal treatment rate of infected patients. The treatment rate is chosen as a control variable. We consider the following objective functional:
We wish to find the optimal treatment rate that minimizes the number of infected patients (A = 1) or the hospitalization costs (A=$1555 per day[35], for instance) in a limited time interval. The term τ2 is included to prevent the occurrence of a bang-bang control and also to reflect the minimization of the control itself. We follow the steps in[36] to solve the problem. The baseline model for MRSA transmission can be rewritten as follows:
where and. Introducing five adjoint variables, we have the Hamiltonian as follows
The adjoint and transversality conditions are
Because the control is bounded, the optimality condition is
where b is the upper bound of the control variable. The problem is solved by the forward-backward sweep method.
We also employ the optimal control techniques to study the extended model with antibiotic exposure. We follow the similar steps with the baseline model and consider the optimal problem of the following objective functional:
with the Hamiltonian
and the following optimal conditions:
and
Results
The baseline model for MRSA transmission
When the admission of colonized and infected individuals is not included, two situations can occur: MRSA becomes either extinct or endemic in the hospital. The threshold condition is in term of the basic reproductive number (R0)[33, 34]; MRSA dies out if and only if
Hence, some parameters such as ρ, τ, a, and μ may be important in controlling the persistence and spread of MRSA. In Figure3(a), we show that when R0 < 1, MRSA dies out if there is no admission of colonized and infectious individuals, but is endemic if colonized and infected individuals are admitted. Figure3(b) shows that MRSA is endemic when R0 > 1 irrespective of whether colonized and infected individuals are admitted.
Prevalence of MRSA infection. Numerical solutions: (a) the number of infectious patients when R0 = 0.3 that MRSA is endemic when admission of colonized and infectious patients is present (dashed trace) and it dies out when there is no admission of colonized and infectious patients (solid trace): (b) the endemic number of infectious patients when R0 = 1.7 while admission of colonized and infectious patients is present and absent (dashed and solid traces, respectively): (c) the prevalence of MRSA infection in the hospital when duration of treatment and probability of a successful treatment vary: and (d) the prevalence of MRSA infection when duration of contamination in HCWs and the number of required contacts from patients vary.
Furthermore, we demonstrate that the prevalence of MRSA infection increases with longer treatment duration and decreases when the probability of a successful course of treatment increases (see Figure3(c)). It also increases when the number of required contacts with patients is greater and the hands of HCWs are contaminated for longer.
The extended model for MRSA transmission with antibiotic exposure
Because exposure to antibiotics and the history of their usage may lead to the presence of resistant pathogens, some patients may become difficult to treat by the same antibiotics and this may lead to treatment failure. The extended model, which incorporates the history of antibiotic usage, captures differences in pathogenesis and probabilities of treatment failure among patients with and without histories of antibiotic treatment.
Figure4(a) shows that more patients with histories of antibiotic usage become infected over time than patients with no antibiotic history, and the infection becomes endemic in the hospital. In Figure4(b), We vary the duration of treatment of infected patients while fixing other parameters, and investigate the effect on the prevalence of infected patients. We find that the prevalence increases with duration of treatment of infected patients, that prolonged treatment of patients with antibiotic exposure may increase the prevalence of MRSA infection more than patients without antibiotic exposure. We further investigate how the probability of a successful course of treatment influences the prevalence of MRSA infection in the hospital. Figure4(c) shows that the prevalence is reduced when successful treatment is probable, and the success of treatment of patients with antibiotic exposure may reduce the prevalence of MRSA infection more than patients without antibiotic exposure. Hence, our findings suggest that treating infected patients with antibiotic histories as quickly and effectively as possible may help to reduce the incidence of MRSA infection in the hospital. In addition, treatment failure in infected patients without antibiotic exposure may increase the prevalence of MRSA infection (see Figure4(d)). Hence, it may be important to establish the correct treatment at the outset infection so that infected patients will not become a reservoir for MRSA later.
Prevalence of MRSA infection when history of antibiotic usage is taken into account. Numerical solutions: (a) the proportions of infectious patients with and without history of antibiotic exposure (dashed and solid traces, respectively): (b) the prevalence of MRSA infection in the hospital when duration of treatment in patients with and without antibiotics vary: (c) the prevalence of MRSA infection in the hospital when probability of a successful treatment in patients with and without antibiotics vary: and (d) the number of infectious patients according to the probability of treatment failure in the first therapy ().
Optimal control
To seek for an efficient way to control MRSA infection and gain insight into what may affect the control measure over a limited time, we consider the treatment rate of infected patients as a time-dependent control variable and then apply optimal control techniques in order to minimize the number of infected patients (or the cost of treating them). Note that whether we minimize the number of infected patients or their hospital costs, our results suggest similar control strategies and optimal solutions.
Figure5(a) shows that to minimize the number of infected patients or their hospital costs, treatment should be given as quickly as possible at the maximum rate. This maximum effort should be prolonged when colonized and infected patients are admitted. Although the maximum rate of treatment is applied, MRSA is still endemic in the hospital when colonized and infected patients are admitted (see Figure5(b)). However, when they are not, the treatment rate may be reduced and MRSA can be eliminated (see Figure5(a)-(b)).
Optimal control. Numerical studies of optimal control strategies and solutions (A = 1 or $1555, B = 1 or $3000, E = 1/2, F = 1/2): (a) the optimal treatment rates for the baseline model when admission of colonized and infectious patients is present or absent (solid and dashed traces, respectively): (b) the number of infectious patients corresponding to the optimal treatment rates: (c) the optimal treatment rates for patients with and without antibiotic exposure when admission of colonized or infectious patients is present or absent (solid trace=without antibiotic exposure but with admission of colonized or infectious patients, dotted trace=with antibiotic exposure and admission of colonized or infectious patients, dashed trace=without antibiotic exposure and admission of colonized or infectious patients, dashed-dotted trace=with antibiotic exposure but without admission of colonized or infectious patients): (d) the number of infectious patients corresponding to the optimal treatment rates (lines are defined in (c)).
Figure5(c) shows that the maximum treatment rate should be applied to treat infected patients in both groups (with and without antibiotic exposure) to minimize the number of such patients or their costs of treatment. However, MRSA still persists in the hospital with more infected patients with than without antibiotic history (see Figure5(d)). When no colonized and infected patients are admitted, the maximum effort can be reduced after a certain time, but that time needs to be slightly longer for patients with antibiotic exposure (see Figure5(c)-(d)).
Discussion and conclusions
MRSA is a leading cause of nosocomial infections in hospitals. Hence, it is important to understand its dynamics and the factors that influence its spread in order to seek for effective ways of reducing public loss and burdens. In this work, mathematical models were used to investigate the transmission dynamics of MRSA in hospitals. We particularly focused on the prevalence of MRSA infection, the impact of treating patients with antibiotics, and how to control MRSA infection efficiently.
We first studied a baseline model for MRSA transmission that does not include the history of antibiotic usage. This general framework was developed in an attempt to understand the transmission dynamics of MRSA and how the use of antibiotics (expressed via treatment rate and probability of successful treatment) influences the prevalence of MRSA infection in the hospital. In this model, infected patients are distinguished from colonized patients, since colonization is quite widespread among humans and it does not always cause MRSA infection. By excluding the admission of colonized and infected patients, the basic reproductive number (R0) was calculated. This epidemic quantity suggests that: (1) MRSA dies out if and only if R0 < 1, and (2) MRSA colonization and infection are associated (for example) with duration of treatment, probability of successful treatment, duration of contamination in HCWs, and the number of contacts required for each patient, for example. In general, admission of colonized and infected patients can occur. When this is taken into account, our model predictions suggest that MRSA is always endemic in the hospital. Assuming R0 = 0.3, our model suggests that MRSA infection is prevalent in approximately 5% of patients.
Furthermore, we investigated how certain parameters influence the prevalence of MRSA infection. We found that the longer the duration of treatment and the lower the probability of treating infected patients successfully, the greater the resulting prevalence of MRSA infection. Hence, it is important that patients with MRSA infection are treated as quickly and efficiently as possible. Also, the longer the duration of contamination among HCWs and the greater the number of contacts required for patients, the greater the prevalence of MRSA infection. Thus, in a unit in which the number of contacts cannot be reduced (an intensive care unit, for instance), it may be important to reduce the duration of contamination among HCWs instead.
From previously published data, it has been suggested that prior exposure to antibiotics may lead to the emergence of resistant bacteria and treatment failure[12, 14, 32]. We extended the baseline model to incorporate the history of antibiotic usage. In the extended model, patients are separated into two groups, with and without antibiotic exposure. We found that there are likely to be more infected patients with than without antibiotic exposure in the hospital. This result may suggest that these groups of patients may be important determinants of the prevalence of MRSA infection and hospital costs because they are more difficult to treat successfully.
We investigated the relationship between the prevalence of MRSA infection and the duration and effectiveness of treatment. We found that the duration that takes to successfully treat infected patients with antibiotic exposure and the probability of treatment failure in these patients may have a greater impact on the prevalence of MRSA infection than infected patients without antibiotic exposure. Besides, as long as colonized or infected patients are admitted, our predictions suggest that MRSA persists in the hospital and consequently the maximum effort should be made consistently to treat patients as quickly and efficiently as possible. Therefore, on the basis of our findings, possible ways to reduce the prevalence of MRSA infection in the hospital include treating patients with antibiotic exposure as quickly and efficiently as possible, and screening and isolating colonized or infected patients at admission.
Because our results show that failure of treatment and more prolonged treatment may lead to a higher prevalence of MRSA infection, it may be inferred that the fewer the patients with antibiotic exposure, the less prevalent the infection. Hence, it may be important to treat patients correctly from the outset in order to reduce the number of infected patients with antibiotic exposure, and continuously to develop novel drugs to increase the efficacy of treating patients and antimicrobial susceptibility tests that may help to increase the chances of successful treatment. Note that reducing the number of colonized patients may also help to decrease the incidence of MRSA infection. However, recolonization is common, so decolonization regimens (the use of screening tests and mupirocin for instance) may be effective only in the short term during ongoing transmission. Other control measures such as hand-washing, staff cohorting, and environmental decontamination, which have not been discussed here are also very important in controlling MRSA in hospital settings.
References
Wassenberg MWM, de Wit GA, van Hout BA, Bonten MJM: Quantifying cost-effectiveness of controlling nosocomial spread of antibiotic-resistant bacteria: the case of MRSA. PLoS One. 2010, 5: e11562-10.1371/journal.pone.0011562.
Wolinksky E, Lipsitz PJ, Mortimer EA, Rammelkamp CH: Acquisition of staphylococci by newborns: direct versus indirect transmission. Lancet. 1960, 2: 620-622.
Mortimer EA, Lipsitz PJ, Wolinksky E, Gonzaga AJ, Rammelkamp CH: Transmission of Staphylococci between newborns: importance of the hands of personnel. Am J Dis Child. 1962, 104: 289-295.
Cohen AL, Gorwitz R, Jernigan DB: Antimicrobial Resistance and Implications for the 21st Century. 2007, New York: Springer
Kluytmans J, van Belkum A, Verbrugh H: Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risk. Clin Microbiol Rev. 1997, 10: 505-520.
Kuehnert MJ, Kruszon-Moran D, Hill HA, McQuillan G, McAllister SK, Fosheim G, McDougal LK, Chaitram J, Jensen B, Fridkin SK, Killgore G, Tenover FC: Prevalence of Staphylococcus aureus nasal colonization in the United States, 2001-2002. J Infect Dis. 2006, 193: 172-179. 10.1086/499632.
Adcock PM, Pastor P, Medley F, Patterson JE, Murphy TV: Methicillin-resistant Staphylococcus aureus in two child care centers. J Infect Dis. 1998, 178: 577-580. 10.1086/517478.
Ellis MW, Hospenthal DR, Dooley DP, Gray PJ, Murray CK: Natural history of community-acquired methicillin-resistant Staphylococcus aureus colonization and infection in soldiers. Clin Infect Dis. 2004, 39: 971-979. 10.1086/423965.
von Eiff C, Becker K, Machka K, Stammer H, Peters G: Nasal carriage as a source of Staphylococcus aureus bacteremia. N Engl J Med. 2001, 344: 11-16. 10.1056/NEJM200101043440102.
Furuya EY, Lowy FD: Antimicrobial-resistant bacteria in the community setting. Nat Rev Microbiol. 2006, 4: 36-45. 10.1038/nrmicro1325.
Baggett HC, Hennessy TW, Rudolph K, Bruden D, Reasonover A, Parkinson A, Sparks R, Donlan RM, Martinez P, Mongkolrattanothai K, Butler JC: Community-onset methicillin-resistant Staphylococcus aureus associated with antibiotic use and the cytotoxin Panton-Valentine leukocidin during a furunculosis outbreak in rural Alaska. J Infect Dis. 2004, 189: 1565-1573. 10.1086/383247.
Tacconelli E, De Angles G, Cataldo MA, Pozzi E, Cauda R: Does antibiotic exposure increase the risk of methicillin-resistant Staphylococcus aureus (MRSA) isolation? A systematic review and meta-analysis. J Antimicrob Chemother. 2008, 61: 26-38.
Costelloe C, Metcalfe C, Lovering A, Mant D, Hay AD: Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ. 2010, 340: c2096-10.1136/bmj.c2096.
Aston JL, Dortch MJ, Dossett LA, Creech CB, May AK: Risk factors for treatment failure in patients receiveing vancomycin for hospital-acquired methicillin resistant Staphylococcus aureus pneumonia. Surg Infect. 2010, 11: 21-27. 10.1089/sur.2008.100.
Bonhoeffer S, Lipstich M, Levin BR: Evaluating treatment protocols to prevent antibiotic resistance. Proc Natl Acad Sci USA. 1997, 94: 12106-12111. 10.1073/pnas.94.22.12106.
Austin DJ, Bonten MJM, Weinstein RA, S S, Anderson RM: Vancomycin-resistant enterococci in intensive-care hospital settings: Transmission dynamics, persistence, and the impact of infection control programs. Proc Natl Acad Sci USA. 1999, 96: 6908-6913. 10.1073/pnas.96.12.6908.
Lipsitch M, Bergstrom CT, Levin BR: The epidemiology of antibiotic resistance in hospitals: Paradoxes and prescriptions. Proc Natl Acad Sci USA. 2000, 97: 1938-1943. 10.1073/pnas.97.4.1938.
Cooper BS, Medley GF, Stone SP, Kibbler CC, Cookson BD, Roberts JA, Duckworth G, Lai R, Ebrahim S: Methicillin-resistant Staphylococcus aureus in hospitals and the community: Stealth dynamics and control cathastrophes. Proc Natl Acad Sci USA. 2004, 101: 10223-10228. 10.1073/pnas.0401324101.
D’Agata EMC, Webb G, Horn M: A mathematical model quantifying the impact of antibiotic exposure and other interventions on the endemic prevalence of vancomycin-resistant enterococci. J Infect Dis. 2005, 192: 2004-2011. 10.1086/498041.
D’Agata EMC, Webb GF, Horn MA, Moellering RC, Ruan S: Modeling the invasion of Community-Acquired Methicillin-Resistant Staphylococcus aureus into hospitals. Clin Infect Dis. 2009, 48: 274-284. 10.1086/595844.
Chamchod F, Ruan S: Modeling the spread of methicillin-resistant Staphylococcus aureus in nursing homes for elderly. PLoS One. 2012, 7: e29757-10.1371/journal.pone.0029757.
Huang SS, Platt R: Risk of methicillin-resistant Staphylococcus aureus infection after previous infection or colonization. Clin Infect Dis. 2003, 36: 281-285. 10.1086/345955.
Davis KA, Stewart JJ, Crouch HK, Florez CE, Hospenthal DR: Methicillin-resistant Staphylococcus aureus (MRSA) nares colonization at hospital admission and its effect on subsequent MRSA infection. Clin Infect Dis. 2004, 39: 776-782. 10.1086/422997.
Fishbain JT, Lee JC, Nguyen HD, Mikita JA, Mikita CP, Uyehara CFT, Hospenthal DR: Nosocomial transmission of methicillin-resistant staphylococcus aureus: a blind study to establish baseline acquisition rates. Infect Cont Hosp Ep. 2003, 24: 415-421. 10.1086/502224.
Troche’ G, Joly L, Guibert M, Zazzo J: Detection and treatment of antibiotic-resistant bacteria carriage in surgical intensive care unit: a 6-year prospective survey. Infect Cont Hosp Ep. 2005, 26: 161-165. 10.1086/502521.
Libert M, Elkholti M, Massaut J, Karmali R, Mascart G, Cherifi S: Risk factors for methicillin resistance and outcome of Staphylococcus aureus bloodstream infection in a Belgian university hospital. J Hosp Infect. 2008, 68: 17-24. 10.1016/j.jhin.2007.08.021.
Grundmann H, Hori S, Winter B, Tami A, Austin DJ: Risk factors for the transmission of methicillin-resistant staphylococcus aureus in an adult intensive care unit: fitting a model to the data. J Infect Dis. 2002, 185: 481-488. 10.1086/338568.
Schentag JJ, Hyatt JM, Carr JR, Paladino JA, Birmingham MC, Zimmer GS, Cumbo TJ: Genesis of methicillin-resistant Staphylococcus aureus (MRSA), how treatment of MRSA infections has selected for vancomycin-resistant Enterococcus faecium, and the importance of antibiotic management and infection control. Clin Infect Dis. 1998, 26: 1204-1214. 10.1086/520287.
Knaus WA, Wagner DP, Zimmerman JE, Draper EA: Variations in mortality and length of stay in intensive care units. Ann Intern Med. 1993, 118: 753-761.
Keeling MJ, Rohani P: Modeling Infectious Diseases in Humans and Animals. 2007, Princeton: Princeton University Press
Dancer SJ: How antibiotics can make us sick: the less obvious adverse effects of antimicrobial chemotherapy. Lancet Infect Dis. 2004, 4: 611-619. 10.1016/S1473-3099(04)01145-4.
Tacconelli E: Antimicrobial use: risk driver of multidrug resistant microorganisms in healthcare settings. Curr Opin Infect Dis. 2009, 22: 352-358. 10.1097/QCO.0b013e32832d52e0.
van den Driessche P, Watmough J: Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math Biosci. 2002, 285: 29-48.
Diekmann O, Heesterbeek JAP, Roberts MG: The construction of next-generation matrices for compartmental epidemic models. J Royal Soc Interface. 2010, 7: 873-885. 10.1098/rsif.2009.0386.
Cosgrove SE, Qi Y, Kaye KS, Harbarth S, Karchmer AW, Carmeli Y: The impact of methicillin resistance in Staphylococcus aureus bacteremia on patient outcomes: mortality, length of stay, and hospital charges. Infect Cont Hosp Ep. 2005, 26: 166-174. 10.1086/502522.
Lenhart S, Workman JT: Optimal Control Applied to Biological Models. 2007, London: Chapman & Hall/CRC
Acknowledgements
This work was partially supported by NIH grant R01GM083607 and NSF grant DMS-1022728. The authors are very grateful to the reviewers for their helpful comments and suggestions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
Both authors conceived, discussed, and carried out the modeling studies and drafted the manuscript. Both authors read and approved the manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Chamchod, F., Ruan, S. Modeling methicillin-resistant Staphylococcus aureus in hospitals: Transmission dynamics, antibiotic usage and its history. Theor Biol Med Model 9, 25 (2012). https://doi.org/10.1186/1742-4682-9-25
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1742-4682-9-25