Abstract
Many children with Autism Spectrum Diseases (ASD) present with seizure activity, but the pathogenesis is not understood. Recent evidence indicates that neuro-inflammation could contribute to seizures. We hypothesize that brain mast cell activation due to allergic, environmental and/or stress triggers could lead to focal disruption of the blood-brain barrier and neuro-inflammation, thus contributing to the development of seizures. Treating neuro-inflammation may be useful when anti-seizure medications are ineffective.
Similar content being viewed by others
Background
Autism Spectrum Disorders (ASD) are pervasive neurodevelopmental disorders affecting almost 1/100 children and are characterized by difficulties in social skills, concentration, language, learning and stereotypic behaviors [1–3]. About 22-30% of children with ASD also develop seizures with no specific underlying pathology, and no obvious or classic EEG changes [1, 4–7]. These rates of seizures in ASD are about ten times higher than that in the general population [8]. This high rate is not found in other neurologic diseases such as schizophrenia [9]. Alterations in architecture of cortical neurons were recently reported in autism [10] and may contribute to seizures.
Epileptic symptoms in children with ASD were recently considered to be related to immune-mediated pathogenesis [11]. In fact, ASD are associated with some immune dysfunction, such as elevated antibody levels directed against the fetal brain [12–15] suggesting BBB disruption. A recent paper from the Autism Phenome Project reported that 42% of 3 year old children with ASD and controls had plasma antibodies against GABAergic cerebellar neuron proteins, but those control children had high scores on the Child Behavior Check list, suggesting that they may constitute a susceptible subtype for ASD [16]. Moreover, IL-6 expression was elevated in the brains of ASD patients [17], while increased serum IL-6 was linked to the expression of an autistic phenotype in mice [18, 19].
There is new evidence that the environment contributes significant to ASD pathogenesis [20]. Many ASD patients suffer from food allergies [21]. Moreover, 25% of ASD children have "allergic-like" symptomatology [22], but often without positive skin or RAST tests, suggesting mast cell activation by non-allergic triggers [23], including mercury [24]. Many studies delineate the importance of mast cells in both innate and acquired immunity [25], as well as in inflammation [26]. Substances originating in the gut or the brain can trigger mast cells to release mediators that could disrupt the gut-blood barrier and blood-brain barrier (BBB), thus contributing to the pathogenesis of autism [27]. Many mediators, such as IL-6 [28], can be released from mast cells "selectively" [29], making histological evaluation impossible. More importantly, mast cells have been implicated in the pathogenesis of seizures. One study using a mouse model showed that the non-allergic mast cell trigger compound 48/80 significantly increased the rate of seizures in mice induced by electric shock, and this effect was eliminated in mast cell-depleted mice [30]. Moreover, the mast cell trigger neurotensin (NT) [31] can facilitate N-Methyl-D-aspartate (NMDA)-induced excitation of cortical neurons [32] and seizure activity in rodents [33]. NT was increased in young children with autism [34], and was proposed as a possible therapeutic target for autism also due to its ability to induce neurotoxicity [35].
Children with mastocytosis, a spectrum of diseases that present with skin allergies and diarrhea, also complain of learning disabilities, hyperactivity and difficulty focusing ("brain fog"), reminiscent of ASD [36, 37]. In fact, children with mastocytosis have a 10-fold higher prevalence of ASD (1/10 children) than that reported for the general population (1/100 children) [38]. Interestingly, mastocytosis patients also have high serum IL-6 levels [39, 40] and develop seizures [41]. Also, a solitary mastocytoma produced symptoms mimicing seizures [42].
Hypothesis
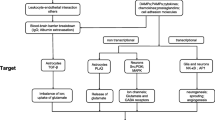
Immune dysfunction and inflammation appear to alter BBB integrity [43, 44]. Recent evidence indicates that the integrity of the BBB, especially leukocyte endothelial adhesion may also be involved in the pathogenesis of epilepsy [45], a phenomenon described as "immunology: barrier to electrical storms" [46]. Mast cells were considered as the "immune gate to the brain" [47]. ASD patients are prone to stress [48], and prenatal stress has been linked to risk of autism [49]. The brain, especially the hypothalamus, contains many mast cells critically located around the BBB, and that stress activates brain mast cells leading to BBB disruption [50]. Moreover, corticotropin-releasing hormone (CRH), secreted under stress, can activate mast cells [51] and is responsible for mast cell-dependent BBB disruption [50, 51]. The possible involvement of mast cells is further supported by the ability of histamine-1 receptors to augment seizures [52]. Brain mast cells also contribute to the pathogenesis of migraine headaches [53] that increase the likelihood of seizures [54]. Local activation of brain mast cells could lead to focal disruption of the BBB, permitting focal neuro-inflammation that could become an epileptogenic site (Figure 1). This process could worsen by activation of Fcgamma receptors (FcγRI) on neurons that could contribute to brain cell death after injection of the epileptogenic kainic acid [55]. Moreover, Fcepsilon receptors (FcεRI), typically thought to be expressed only by mast cells and basophils, were recently identified on neurons [56] implying that allergic triggers may even affect the neurons directly, once the BBB has been disrupted to permit entry of immunoglobulins. A recent publication reported that increased serum level of "high mobility group box 1 protein (HMGB1) in young autistic patients [57]. HMGB1 is released from neurons following neurotoxicity [58] and it was recently shown to constitute a pro-seizure pathway through activation of toll-like receptors (TLR-4) in mice [59]. We recently showed that mast cell activation leads to mitochondrial translocation to the cell surface [60], and secretion of extracellular mtDNA [34]. We also showed that serum of children with autism had increased levels of extracellular mitochondrial DNA [61]. Damaged Associated Molecular Patterns (DAMPs), which include mitochondrial DNA, were reported to be released from damaged cells in trauma patients and activate TLR leading to auto-inflammation [62]. Mitochondrial DNA was also reported to be directly neurotoxic and alter behavior in mice [63].
Mast cells are located perivascularly close to nerve endings and regulate blood-brain barrier permeability. Upon stimulation by allergic and non-immune triggers (e.g, CRH, neurotensin, mercury, mitochondrial (mt) DNA), mast cells release vasodilatory and inflammatory molecules (IL-6, mtDNA, TNF and VEGF), some of which increase the expression of vascular endothelial cell adhesion molecules (VCAMs) and permit exit of circulating lymphocytes in the brain. Focal brain inflammation could then contribute to or exacerbate seizures.
Anticonvulsant medications often are ineffective in both ASD and mastocytosis patients with seizures [64]. It would, therefore, be important to use treatment approaches directed to the core symptoms of ASD and/or brain mast cell activation and inflammation [23]. Use of select, natural, flavonoids, may be useful because of their anti-oxidant and anti-inflammatory ability [65]. Luteolin is a flavone contained in chamomile and chrysanthemum. Increasing evidence indicates that luteolin has potent antioxidant, free radical scavenger, anti-inflammatory and mast cell inhibitory activity [66]. In addition, luteolin inhibits microglia IL-6 release [67, 68], as well as mimics the activity of brain-derived neurotrophic factor (BDNF) [69]. Luteolin also inhibits autistic-like behavior in mice [70]. Luteolin also inhibits mast cell-dependent stimulation of activated T cells [71], as well as activated peripheral blood mononuclear cells from patients with the brain inflammatory disease multiple sclerosis [72]. Moreover, the structural flavone analogue, quercetin, found in citrus pulp and peels, is also a potent mast cell inhibitor [73] and has anti-seizure activity [74], as does its natural glycoside rutin [75]. Luteolin may, therefore, be useful for the treatment of neuroinflammation, including seizures in ASD children, especially if administered in formulations that permit sufficient oral absorption.
Implications
In conclusion, evidence reviewed above indicates a possible association between neuroinflammation, mast cell activation and seizures, through secretion of pro-inflammatory mediators and regulation of the BBB permeability. Mast cell function inhibitors, especially those blocking the effect of NT, such as luteolin, may serve as novel therapeutic agents for the treatment of autism and related seizures.
References
Kogan MD, Blumberg SJ, Schieve LA, Boyle CA, Perrin JM, Ghandour RM, Singh GK, Strickland BB, Trevathan E, van Dyck PC: Prevalence of parent-reported diagnosis of autism spectrum disorder among children in the US, 2007. Pediatrics. 2009, 5: 1395-1403.
Fombonne E: Epidemiology of pervasive developmental disorders. Pediatric Research. 2009, 65: 591-598. 10.1203/PDR.0b013e31819e7203.
Volkmar FR, State M, Klin A: Autism and autism spectrum disorders: diagnostic issues for the coming decade. J Child Psychol Psychiatry. 2009, 50: 108-115. 10.1111/j.1469-7610.2008.02010.x.
Tuchman R, Rapin I: Epilepsy in autism. Lancet Neurol. 2002, 1: 352-358. 10.1016/S1474-4422(02)00160-6.
Trevathan E: Seizures and epilepsy among children with language regression and autistic spectrum disorders. J Child Neurol. 2004, 19 (Suppl 1): S49-S57.
Fombonne E, du Mazaubrun C: Prevalence of infantile autism in four French regions. Soc Psychiatry Psychiatr Epidemiol. 1992, 27: 203-210. 10.1007/BF00789007.
Volkmar FR, Nelson DS: Seizure disorders in autism. J Am Acad Child Adolesc Psychiatry. 1990, 29: 127-129. 10.1097/00004583-199001000-00020.
Clarke DF, Roberts W, Daraksan M, Dupuis A, McCabe J, Wood H, Snead OC, Weiss SK: The prevalence of autistic spectrum disorder in children surveyed in a tertiary care epilepsy clinic. Epilepsia. 2005, 46: 1970-1977. 10.1111/j.1528-1167.2005.00343.x.
Hyde TM, Weinberger DR: Seizures and schizophrenia. Schizophr Bull. 1997, 23: 611-622.
Oblak AL, Rosene DL, Kemper TL, Bauman ML, Blatt GJ: Altered posterior cingulate cortical cyctoarchitecture, but normal density of neurons and interneurons in the posterior cingulate cortex and fusiform gyrus in autism. Autism Res. 2011, 4: 200-211. 10.1002/aur.188.
Specchio N, Fusco L, Claps D, Vigevano F: Epileptic encephalopathy in children possibly related to immune-mediated pathogenesis. Brain Dev. 2010, 32: 51-56. 10.1016/j.braindev.2009.09.017.
Enstrom AM, Van de Water JA, Ashwood P: Autoimmunity in autism. Curr Opin Investig Drugs. 2009, 10: 463-473.
Theoharides TC, Kempuraj D, Redwood L: Autism: an emerging 'neuroimmune disorder' in search of therapy. Exp Opinion on Pharmacotherapy. 2009, 10: 2127-2143. 10.1517/14656560903107789.
Goines P, Van de Water J: The immune system's role in the biology of autism. Curr Opin Neurol. 2010, 23: 111-117. 10.1097/WCO.0b013e3283373514.
Wills S, Cabanlit M, Bennett J, Ashwood P, Amaral DG, Van de Water J: Detection of autoantibodies to neural cells of the cerebellum in the plasma of subjects with autism spectrum disorders. Brain Behav Immun. 2009, 23: 64-74. 10.1016/j.bbi.2008.07.007.
Rossi CC, Van de Water J, Rogers SJ, Amaral DG: Detection of plasma autoantibodies to brain tissue in young children with and without autism spectrum disorders. Brain Behav Immun. 2011, 25: 1123-1135. 10.1016/j.bbi.2011.02.011.
Li X, Chauhan A, Sheikh AM, Patil S, Chauhan V, Li XM, Ji L, Brown T, Malik M: Elevated immune response in the brain of autistic patients. J Neuroimmunol. 2009, 207: 111-116. 10.1016/j.jneuroim.2008.12.002.
Dahlgren J, Samuelsson AM, Jansson T, Holmang A: Interleukin-6 in the maternal circulation reaches the rat fetus in mid-gestation. Pediatr Res. 2006, 60: 147-151. 10.1203/01.pdr.0000230026.74139.18.
Smith SE, Li J, Garbett K, Mirnics K, Patterson PH: Maternal immune activation alters fetal brain development through interleukin-6. Journal of Neuroscience. 2007, 27: 10695-10702. 10.1523/JNEUROSCI.2178-07.2007.
Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, Miller J, Fedele A, Collins J, Smith K, Lotspeich L, Croen LA, Ozonoff S, Lajonchere C, Grether JK, Risch N: Genetic Heritability and Shared Environmental Factors Among Twin Pairs With Autism. Arch Gen Psychiatry. 2011
Jyonouchi H: Autism spectrum disorders and allergy: observation from a pediatric allergy/immunology clinic. Expert Rev Clin Immunol. 2010, 6: 397-411. 10.1586/eci.10.18.
Angelidou A, Alysandratos KD, Asadi S, Zhang B, Francis K, Vasiadi M, Kalogeromitros D, Theoharides TC: Brief Report: "Allergic Symptoms" in Children with Autism Spectrum Disorders. More than Meets the Eye?. J Autism Dev Disord. 2011, 41: 1579-1585. 10.1007/s10803-010-1171-z.
Theoharides TC, Angelidou A, Alysandratos KD, Zhang B, Asadi S, Francis K, Toniato E, Kalogeromitros D: Mast cell activation and autism. Biochim Biophys Acta. 2011
Kempuraj D, Asadi S, Zhang B, Manola A, Hogan J, Peterson E, Theoharides TC: Mercury induces inflammatory mediator release from human mast cells. J Neuroinflammation. 2010, 7: 20-10.1186/1742-2094-7-20.
Galli SJ, Nakae S, Tsai M: Mast cells in the development of adaptive immune responses. Nat Immunol. 2005, 6: 135-142. 10.1038/ni1158.
Theoharides TC, Alysandratos KD, Angelidou A, Delivanis DA, Sismanopoulos N, Zhang B, Asadi S, Vasiadi M, Weng Z, Miniati A, Kalogeromitros D: Mast cells and inflammation. Biochim Biophys Acta. 2010
Theoharides TC, Doyle R, Francis K, Conti P, Kalogeromitros D: Novel therapeutic targets for autism. Trends Pharmacol Sci. 2008, 29: 375-382. 10.1016/j.tips.2008.06.002.
Kandere-Grzybowska K, Letourneau R, Kempuraj D, Donelan J, Poplawski S, Boucher W, Athanassiou A, Theoharides TC: IL-1 induces vesicular secretion of IL-6 without degranulation from human mast cells. J Immunol. 2003, 171: 4830-4836.
Theoharides TC, Kempuraj D, Tagen M, Conti P, Kalogeromitros D: Differential release of mast cell mediators and the pathogenesis of inflammation. Immunol Rev. 2007, 217: 65-78. 10.1111/j.1600-065X.2007.00519.x.
Yillar DO, Kucukhuseyin C: The effects of compound 48/80, morphine, and mast cell depletion on electroshock seizure in mice. J Basic Clin Physiol Pharmacol. 2008, 19: 1-14. 10.1515/JBCPP.2008.19.1.1.
Singh LK, Pang X, Alexacos N, Letourneau R, Theoharides TC: Acute immobilization stress triggers skin mast cell degranulation via corticotropin-releasing hormone, neurotensin and substance P: A link to neurogenic skin disorders. Brain Behav Immunity. 1999, 13: 225-239. 10.1006/brbi.1998.0541.
Antonelli T, Ferraro L, Fuxe K, Finetti S, Fournier J, Tanganelli S, De Mattei M, Tomasini MC: Neurotensin enhances endogenous extracellular glutamate levels in primary cultures of rat cortical neurons: involvement of neurotensin receptor in NMDA induced excitotoxicity. Cereb Cortex. 2004, 14: 466-473. 10.1093/cercor/bhh008.
Shulkes A, Harris QL, Lewis SJ, Vajda JE, Jarrott B: Regional brain concentrations of neurotensin following amygdaloid kindled and cortical suprathreshold stimulation-induced seizures in the rat. Neuropeptides. 1988, 11: 77-81. 10.1016/0143-4179(88)90014-5.
Angelidou A, Francis K, Vasiadi M, Alysandratos K-D, Zhang B, Theoharides A, Lykouras L, Kalogeromitros D, Theoharides TC: Neurotensin is increased in serum of young children with autistic disorder. J Neuroinflammation. 2010, 7: 48-10.1186/1742-2094-7-48.
Ghanizadeh A: Targeting neurotensin as a potential novel approach for the treatment of autism. J Neuroinflammation. 2010, 7: 58-10.1186/1742-2094-7-58.
Valent P, Akin C, Escribano L, Fodinger M, Hartmann K, Brockow K, Castells M, Sperr WR, Kluin-Nelemans HC, Hamdy NA, Lortholary O, Robyn J, van Doormaal J, Sotlar K, Hauswirth AW, Arock M, Hermine O, Hellmann A, Triggiani M, Niedoszytko M, Schwartz LB, Orfao A, Horny HP, Metcalfe DD: Standards and standardization in mastocytosis: consensus statements on diagnostics, treatment recommendations and response criteria. Eur J Clin Invest. 2007, 37: 435-453. 10.1111/j.1365-2362.2007.01807.x.
Metcalfe DD: Mast cells and mastocytosis. Blood. 2008, 112: 946-956. 10.1182/blood-2007-11-078097.
Theoharides TC: Autism spectrum disorders and mastocytosis. Int J Immunopathol Pharmacol. 2009, 22: 859-865.
Brockow K, Akin C, Huber M, Metcalfe DD: IL-6 levels predict disease variant and extent of organ involvement in patients with mastocytosis. Clin Immunol. 2005, 115: 216-223. 10.1016/j.clim.2005.01.011.
Theoharides TC, Boucher W, Spear K: Serum interleukin-6 reflects disease severity and osteoporosis in mastocytosis patients. Int Arch Allergy Immunol. 2002, 128: 344-350. 10.1159/000063858.
Sarrot-Reynauld F, Massot C, Amblard P, Rousset H, Da Costa A, Vital-Durand D, Ninet J, Decousus H: [Systemic mastocytosis: incidence and risks of vasomotor seizures]. Rev Med Interne. 1993, 14: 1034-10.1016/S0248-8663(05)80151-1.
Krowchuk DP, Williford PM, Jorizzo JL, Kandt RS: Solitary mastocytoma producing symptoms mimicking those of a seizure disorder. J Child Neurol. 1994, 9: 451-453. 10.1177/088307389400900428.
Banks WA, Erickson MA: The blood-brain barrier and immune function and dysfunction. Neurobiol Dis. 2010, 37: 26-32. 10.1016/j.nbd.2009.07.031.
Quan N: Immune-to-brain signaling: how important are the blood-brain barrier-independent pathways?. Mol Neurobiol. 2008, 37: 142-152. 10.1007/s12035-008-8026-z.
Fabene PF, Navarro Mora G, Martinello M, Rossi B, Merigo F, Ottoboni L, Bach S, Angiari S, Benati D, Chakir A, Zanetti L, Schio F, Osculati A, Marzola P, Nicolato E, Homeister JW, Xia L, Lowe JB, McEver RP, Osculati F, Sbarbati A, Butcher EC, Constantin G: A role for leukocyte-endothelial adhesion mechanisms in epilepsy. Nat Med. 2008, 14: 1377-1383. 10.1038/nm.1878.
Ransohoff RM: Immunology: Barrier to electrical storms. Nature. 2009, 457: 155-156. 10.1038/457155a.
Theoharides TC: Mast cells: the immune gate to the brain. Life Sci. 1990, 46: 607-617. 10.1016/0024-3205(90)90129-F.
Gillott A, Standen PJ: Levels of anxiety and sources of stress in adults with autism. J Intellect Disabil. 2007, 11: 359-370. 10.1177/1744629507083585.
Kinney DK, Munir KM, Crowley DJ, Miller AM: Prenatal stress and risk for autism. Neurosci Biobehav Rev. 2008, 32: 1519-1532. 10.1016/j.neubiorev.2008.06.004.
Esposito P, Chandler N, Kandere-Grzybowska K, Basu S, Jacobson S, Connolly R, Tutor D, Theoharides TC: Corticotropin-releasing hormone (CRH) and brain mast cells regulate blood-brain-barrier permeability induced by acute stress. J Pharmacol Exp Ther. 2002, 303: 1061-1066. 10.1124/jpet.102.038497.
Theoharides TC, Konstantinidou A: Corticotropin-releasing hormone and the blood-brain-barrier. Front Biosci. 2007, 12: 1615-1628. 10.2741/2174.
Rehni AK, Singh TG, Singh N, Arora S: Tramadol-induced seizurogenic effect: a possible role of opioid-dependent histamine H1 receptor activation-linked mechanism. Naunyn Schmiedebergs Arch Pharmacol. 2010, 381: 11-19. 10.1007/s00210-009-0476-y.
Theoharides TC, Donelan J, Kandere-Grzybowska K, Konstantinidou A: The role of mast cells in migraine pathophysiology. Brain Res Rev. 2005, 49: 65-76. 10.1016/j.brainresrev.2004.11.006.
Ottman R, Lipton RB: Comorbidity of migraine and epilepsy. Neurology. 1994, 44: 2105-2110.
Suemitsu S, Watanabe M, Yokobayashi E, Usui S, Ishikawa T, Matsumoto Y, Yamada N, Okamoto M, Kuroda S: Fcgamma receptors contribute to pyramidal cell death in the mouse hippocampus following local kainic acid injection. Neuroscience. 2010, 166: 819-831. 10.1016/j.neuroscience.2010.01.004.
van der Kleij H, Charles N, Karimi K, Mao YK, Foster J, Janssen L, Chang Yang P, Kunze W, Rivera J, Bienenstock J: Evidence for neuronal expression of functional Fc (epsilon and gamma) receptors. The Journal of Allergy and Clinical Immunology. 2010, 125: 757-760. 10.1016/j.jaci.2009.10.054.
Emanuele E, Boso M, Brondino N, Pietra S, Barale F, Ucelli di Nemi S, Politi P: Increased serum levels of high mobility group box 1 protein in patients with autistic disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2010, 34: 681-683. 10.1016/j.pnpbp.2010.03.020.
Faraco G, Fossati S, Bianchi ME, Patrone M, Pedrazzi M, Sparatore B, Moroni F, Chiarugi A: High mobility group box 1 protein is released by neural cells upon different stresses and worsens ischemic neurodegeneration in vitro and in vivo. J Neurochem. 2007, 103: 590-603. 10.1111/j.1471-4159.2007.04788.x.
Maroso M, Balosso S, Ravizza T, Liu J, Aronica E, Iyer AM, Rossetti C, Molteni M, Casalgrandi M, Manfredi AA, Bianchi ME, Vezzani A: Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat Med. 2010, 16: 413-419. 10.1038/nm.2127.
Zhang B, Alysandratos KD, Angelidou A, Asadi S, Sismanopoulos N, Delivanis DA, Weng Z, Miniati A, Vasiadi M, Katsarou-Katsari A, Miao B, Leeman SE, Kalogeromitros D, Theoharides TC: Human mast cell degranulation and preformed TNF secretion require mitochondrial translocation to exocytosis sites: Relevance to atopic dermatitis. The Journal of Allergy and Clinical Immunology. 2011
Zhang B, Angelidou A, Alysandratos KD, Vasiadi M, Francis K, Asadi S, Theoharides A, Sideri k, Lykouras L, Kalogeromitros D, Theoharides TC: Mitochondrial DNA and anti-mitochondrial antibodies in serum of autistic children. J Neuroinflammation. 2010, 7: 80-10.1186/1742-2094-7-80.
Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ: Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010, 464: 104-107. 10.1038/nature08780.
Lauritzen KH, Moldestad O, Eide L, Carlsen H, Nesse G, Storm JF, Mansuy IM, Bergersen LH, Klungland A: Mitochondrial DNA toxicity in forebrain neurons causes apoptosis, neurodegeneration, and impaired behavior. Mol Cell Biol. 2010, 30: 1357-1367. 10.1128/MCB.01149-09.
Frye RE, Sreenivasula S, Adams JB: Traditional and non-traditional treatments for autism spectrum disorder with seizures: an on-line survey. BMC Pediatr. 2011, 11: 37-10.1186/1471-2431-11-37.
Middleton E, Kandaswami C: The impact of plant flavonoids on mammalian biology: implications for immunity, inflammation and cancer, in the flavonoids. Edited by: Barborne JB. 1993, 619-652.
Kimata M, Shichijo M, Miura T, Serizawa I, Inagaki N, Nagai H: Effects of luteolin, quercetin and baicalein on immunoglobulin E-mediated mediator release from human cultured mast cells. Clin Exp Allergy. 2000, 30: 501-508. 10.1046/j.1365-2222.2000.00768.x.
Jang S, Kelley KW, Johnson RW: Luteolin reduces IL-6 production in microglia by inhibiting JNK phosphorylation and activation of AP-1. Proc Natl Acad Sci USA. 2008, 105: 7534-7539. 10.1073/pnas.0802865105.
Meyer U, Feldon J, Dammann O: Schizophrenia and autism: both shared and disorder-specific pathogenesis via perinatal inflammation?. Pediatr Res. 2011, 69: 26R-33R. 10.1203/PDR.0b013e318212c196.
Jang SW, Liu X, Yepes M, Shepherd KR, Miller GW, Liu Y, Wilson WD, Xiao G, Blanchi B, Sun YE, Ye K: A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc Natl Acad Sci USA. 2010, 107: 2687-2692. 10.1073/pnas.0913572107.
Parker-Athill E, Luo D, Bailey A, Giunta B, Tian J, Shytle RD, Murphy T, Legradi G, Tan J: Flavonoids, a prenatal prophylaxis via targeting JAK2/STAT3 signaling to oppose IL-6/MIA associated autism. J Neuroimmunol. 2009, 217: 20-27. 10.1016/j.jneuroim.2009.08.012.
Kempuraj D, Tagen M, Iliopoulou BP, Clemons A, Vasiadi M, Boucher W, House M, Wolferg A, Theoharides TC: Luteolin inhibits myelin basic protein-induced human mast cell activation and mast cell dependent stimulation of Jurkat T cells. Br J Pharmacol. 2008, 155: 1076-1084.
Sternberg Z, Chadha K, Lieberman A, Drake A, Hojnacki D, Weinstock-Guttman B, Munschauer F: Immunomodulatory responses of peripheral blood mononuclear cells from multiple sclerosis patients upon in vitro incubation with the flavonoid luteolin: additive effects of IFN-beta. J Neuroinflammation. 2009, 6: 28-10.1186/1742-2094-6-28.
Kempuraj D, Madhappan B, Christodoulou S, Boucher W, Cao J, Papadopoulou N, Cetrulo CL, Theoharides TC: Flavonols inhibit proinflammatory mediator release, intracellular calcium ion levels and protein kinase C theta phosphorylation in human mast cells. Br J Pharmacol. 2005, 145: 934-944. 10.1038/sj.bjp.0706246.
Joshi D, Naidu PS, Singh A, Kulkarni SK: Protective effect of quercetin on alcohol abstinence-induced anxiety and convulsions. J Med Food. 2005, 8: 392-396. 10.1089/jmf.2005.8.392.
Nassiri-Asl M, Shariati-Rad S, Zamansoltani F: Anticonvulsive effects of intracerebroventricular administration of rutin in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2008, 32: 989-993. 10.1016/j.pnpbp.2008.01.011.
Acknowledgements
Aspects of our work described above were funded by NIH grants NS38326 and AR47652, as well as the Autism Collaborative, the Autism Research Institute, National Autism Association, Safe Minds and Theta Biomedical Consulting and Development Co., Inc. (Brookline, MA, USA).
This paper is dedicated to the memory of Elia Tembenis, a young boy with autism and seizures.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
TCT is the inventor of US patents No. 6,624,148; 6,689,748; 6,984,667, and EPO 1365777, which cover methods and compositions of mast cell blockers, including flavonoids, US patents 7,906,153 and 12/861,152 (allowed on September, 22, 2011) for treatment of neuroinflammatory conditions, as well as US patent applications No.12/534,571 and No.13/009,282 for the diagnosis and treatment of ASD. TCT is also the inventor of the dietary supplement, NeuroProtek®, which has the US trademark No 3,225,924.
Authors' contributions
TCT and BZ prepared, read, and approved this manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Theoharides, T.C., Zhang, B. Neuro-inflammation, blood-brain barrier, seizures and autism. J Neuroinflammation 8, 168 (2011). https://doi.org/10.1186/1742-2094-8-168
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1742-2094-8-168