Abstract
Background
Recent studies suggest an important role for neurotransmitters as modulators of inflammation. Neuroinflammatory mediators such as cytokines and molecules of the arachidonic acid pathway are generated and released by microglia. The monoamine norepinephrine reduces the production of cytokines by activated microglia in vitro. However, little is known about the effects of norepinephrine on prostanoid synthesis. In the present study, we investigate the role of norepinephrine on cyclooxygenase- (COX-)2 expression/synthesis and prostaglandin (PG)E2 production in rat primary microglia.
Results
Interestingly, norepinephrine increased COX-2 mRNA, but not protein expression. Norepinephrine strongly enhanced COX-2 expression and PGE2 production induced by lipopolysaccharide (LPS). This effect is likely to be mediated by β-adrenoreceptors, since β-, but not α-adrenoreceptor agonists produced similar results. Furthermore, β-adrenoreceptor antagonists blocked the enhancement of COX-2 levels induced by norepinephrine and β-adrenoreceptor agonists.
Conclusions
Considering that PGE2 displays different roles in neuroinflammatory and neurodegenerative disorders, norepinephrine may play an important function in the modulation of these processes in pathophysiological conditions.
Similar content being viewed by others
Background
Microglia, the innate immune cells of the brain, constantly screen their microenvironment and transform into an "activated" state in response to brain lesions, e.g., toxic lesions or debris and degenerating neurons [1, 2] (for review see [3]). Once activated, microglia secrete pro- and anti-inflammatory mediators such as cytokines and prostaglandins (for review see [4]). In vitro stimulation with lipopolysaccharide (LPS), the endotoxin of gram-negative bacteria, results in the secretion of neurotoxic and pro-inflammatory mediators. LPS triggers the activation of microglial cells via the anchored surface myeloid glycoprotein CD14 [5]. CD14 has also been found to bind to amyloid-β (Aβ), the major compound found in amyloid plaques in the brains of patients with Alzheimer's disease (AD) [6].
LPS is known to induce the production of cyclooxygenase-2 (COX-2) enzyme in microglial cells in vitro [7–9]. COX-2 converts arachidonic acid released from membrane phospholipids to prostaglandin (PG) H2. PGH2 is then isomerized to PGE2 by terminal prostaglandin E synthases. COX-2 has emerged as a major player in inflammatory reactions in the brain and increased COX-2 expression has been considered to contribute to neurodegeneration [10, 11]. Elevated COX-2 expression has been described in AD [12–17] and COX-2 protein content in the hippocampus of AD patients may correlate with the severity of dementia [18] On the other hand, COX-2 has been suggested to play a physiological role in the brain for being involved in neuronal plasticity and synaptic transmission [19, 20].
In recent years it has become evident that there may exist a crosstalk between the autonomic nervous system and the immune system during inflammation [21, 22]. The catecholamine norepinephrine (NE) is a classical neurotransmitter with suggested immunomodulatory properties. NE is released by neurons into the synaptic cleft and may exert effects on glial cells that are in close vicinity. NE binds to α- and β-adrenergic receptors, and expression of α1-, α2-, β1- and β2-adrenegic receptors have been identified on microglia [23–29].
An immunosuppressive role has been suggested for NE in vitro since it attenuates LPS-induced microglial production of tumour necrosis factor (TNF)α, interleukin (IL)-1β, IL-6 and nitric oxide in vitro [24, 30, 31]. NE has been shown to reduce microglia-induced neuronal cell death [30, 32].
Although it is known that NE has immunosuppressive properties, information of NE's effect on prostaglandins is scarce. The aim of our study was to further investigate the role of NE on eicosanoid production, namely PGE2, in primary rat microglia.
Methods
Chemicals and reagents
L-(-)-Norepinephrine (+) bitartrate salt monohydrate (A9512), LPS, the α1-adrenergic agonist L-phenylephrine hydrochloride and the β2-adrenergic agonist terbutaline hemisulfate salt were obtained from Sigma-Aldrich (Taufkirchen, Germany). ICI 118,551, a selective β2-antagonist, and CGP 20712A, a selective β1-antagonist, the α2-receptor agonist clonidine, the α1-receptor antagonist nicergoline, and the β1-/β2-receptor agonist dobutamine were obtained from Tocris (distributed by Biotrend, Cologne, Germany).
Cell culture
Primary microglial cell cultures were established from cerebral cortices of one-day-old neonatal Wistar rats as previously described [7, 33]. Briefly, forebrains were minced and gently dissociated by repeated pipetting in Hank's balanced salt solution. Cells were collected by centrifugation, resuspended in Dulbecco's modified Eagle's medium containing 10% fetal calf serum and antibiotics and cultured on 10-cm cell culture dishes (Falcon, 5 × 105 cells/plate) in 5% CO2 at 37°C. Medium was prepared taking extreme care to avoid LPS contamination [34]. Floating microglia were harvested from 10- to 14-day-old mixed (astrocyte-microglia) primary cultures and re-seeded into 35-mm cell culture dishes in fresh complete medium to give pure microglial cultures (2 × 104 cells/dish). Microglial cultures were washed 1 h after seeding to remove non-adherent cells. The purity of the microglial culture was >98% as previously determined by immunofluorescence and cytochemical analysis [34].
RNA extraction and RT-PCR analysis
Total RNA was isolated using the guanidine isothiocyanate method [35]. Two μg of total RNA was reverse transcribed using M-MLV reverse transcriptase and random hexamers (Promega, Mannheim, Germany). One μl of the resulting cDNA was amplified using Taq DNA polymerase (Promega), dNTPs (Invitek, Berlin, Germany) and primers specific for rat COX-1 (forward, 5'-CGG CCT CGA CCA CTA CCA ATG-3'; reverse, 5'-TGC GGG GCG GGA ATG AAC T-3', annealing temperature 60°C, 30 cycles, amplicon size: 426 bp); rat COX-2 (forward: 5' TGC GAT GCT CTT CCG AGC TGT GCT 3', reverse: 5' TCA GGA AGT TCC TTA TTT CCT TTC 3', annealing temperature 55°C, 35 cycles, amplicon size: 479 bp); rat S12 (forward: 5'-ACG TCA ACA CTG CTC TAC A-3', reverse: 5'-CTT TGC CAT AGT CCT TAA C-3', 56°C, 30 cycles, amplicon size: 312 bp), that were designed using Primer Select software (DNA Star Inc., Madison, WI) and synthesized through an in-house facility (Dr. Gabor Igloi, Institute for Biology III, Freiburg, Germany). All PCR amplifications included a final 10-min extension at 72°C. The products were analyzed on a 2% agarose gel. Contamination by genomic DNA was identified by substituting total RNA instead of cDNA in the reaction mixture using S12 primers.
Western blot analysis
After the respective experimental set up, microglial cells were washed with phosphate-buffered saline (PBS) and lysed in 1.3 × SDS- (sodium dodecyl sulfate-) containing sample buffer without DTT or bromophenol blue containing 100 μM orthovanadate [36]. Lysates were homogenized by repeated passage through a 26-gauge needle. Protein contents were measured using the bicinchoninic acid (BCA) method (kit obtained from Pierce, distributed by KFC Chemikalien, München, Germany). Bovine serum albumin (BSA) was used as a protein standard at concentrations ranging from 0.2 μg/μl to 4 μg/μl; the optical density was read at 570 nm using a microplate reader. Before electrophoresis, bromophenol blue and DTT (final concentration, 10 mM) were added to the samples. For COX-1 and COX-2 immunoblotting, 30 to 50 μg of protein from each sample was subjected to SDS-PAGE (polyacrylamide gel electrophoresis) on a 10% gel under reducing conditions. Proteins were then transferred onto a polyvinylidene fluoride (PVDF) membrane (Millipore, Bedford, MA, USA) by semi-dry blotting. The membrane was blocked for 1 or 2 h at room temperature using Rotiblock (Roth, Karlsruhe, Germany) or 5% blocking milk (BioRad, München, Germany), before the overnight incubation at 4°C with the primary antibody. Primary antibodies were goat anti-COX-1 goat (M-20, Santa Cruz, Heidelberg, Germany) and anti-COX-2 diluted 1:500 in Tris-buffered saline (TBS) containing 0.1% Tween 20 (Merck, Darmstadt, Germany) and 1% bovine serum albumin (BSA) and rabbit anti-actin diluted 1:5000 (Sigma, St. Louis, MO, USA). After extensive washing (three times for 15 min each in TBS containing 0.1% Tween 20), proteins were detected with horseradish peroxidase (HRP)-coupled rabbit anti-goat IgG (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA, 1:100,000) or HRP-coupled donkey anti-rabbit (GE Healthcare, Freiburg Germany, 1:25,000) using chemiluminescence (ECL) reagents (GE Healthcare). All western blot experiments were carried out at least three times.
Enzyme immunoassay (EIA)
Supernatants were harvested, centrifuged at 10,000 × g for 10 min, and levels of prostaglandin E2 in the media were measured by enzyme immunoassay (EIA) (Assay design, distributed by Biotrend, Cologne, Germany and Cayman Chemicals, Ann Arbor, MI, USA, respectively) according to the manufacturer's instructions. Standards from 39 to 2500 pg/ml were used; sensitivity of the assay was 36.2 pg/ml.
Statistical analysis
At least three independent experiments were used for data analysis. Original data were converted into %-values of LPS control and mean ± S.E.M. were calculated. Values were compared using t-test (two groups) or one-way ANOVA with post-hoc Student-Newman-Keuls test (multiple comparisons). Differences were considered statistically significant when p < 0.05.
Results
NE enhances LPS-induced production of COX-2 mRNA and protein in primary rat microglial cells
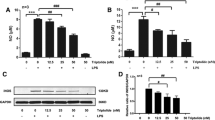
We show here that NE dose-dependently enhanced LPS-induced COX-2 protein levels in primary rat microglia (Fig. 1A). No COX-2 protein levels were detected after 4 h stimulation with LPS (10 ng/ml) alone. Moreover, NE alone showed no effect. Addition of 1-100 nM NE to LPS-stimulated cells did not increase COX-2 protein levels, although 100 nM NE in combination with LPS already showed a tendency to increase COX-2 levels. Significant upregulation of COX-2 immunoreactivity was observed at doses of 1 to 10 μM NE in combination with LPS. Analysis of western blots from three independent experiments showed a 22- and 35-fold increase in COX-2 immunoreactivity in rat primary microglial cells (p < 0.001) using 1 and 10 μM NE, respectively, as compared to 10 ng/ml LPS alone (Fig. 1B). On the other hand, we did not observe any alterations in COX-1 protein levels after adding the same doses of NE along with LPS (Fig. 1A).
Different concentrations of norepinephrine enhance the synthesis of COX-2 protein (A, B) and mRNA (C) in LPS-stimulated primary neonatal microglial cells. Microglial cells were stimulated with different concentrations of norepinephrine (0.001 μM to 10 μM) and LPS (10 ng/ml) for 4 hours. (A) A representative western blot against β-actin (42 kDa) and COX-2 (70 kDa) is shown here. (B) Quantitative densitometric analysis of COX-2 protein expression normalized to β-actin control (n = 3). *P < 0.05, **P < 0.001 with respect to LPS control. (C, D) Semi-quantitative PCR analysis of the effect of different concentrations of norepinephrine alone (C) and of norepinephrine plus LPS on COX-2 and COX-1 mRNA expression (D). [NE = norepinephrine].
Next, we investigated whether our findings on the protein level were due to increased mRNA levels, using qualitative PCR. COX-2 mRNA was marginally detectable in unstimulated microglial cells. In contrast to the finding that NE alone did not influence COX-2 protein levels, a potent induction of COX-2 mRNA expression was observed after 2 h stimulation with NE alone, which resulted in a dose-response upregulation starting with 1 nM that peaked at 10 μM NE (Fig. 1C). Addition of LPS, 10 ng/ml, further enhanced the expression of COX-2 mRNA (Fig. 1D). Thus, in agreement with the effects observed on COX-2 protein levels, we found an increase in COX-2 mRNA when microglial cells were stimulated by both LPS plus NE.
Next we studied time kinetics in COX-2 mRNA and protein synthesis after stimulation with 10 ng/ml LPS or 1 μM NE and the combination of both. At 2 h, a slight increase in COX-2 immunoreactivity was detected when microglia were treated with LPS plus NE, while there was no change in COX-2 protein levels when the cells were treated with LPS or NE alone (Fig. 2A). LPS plus NE further increased COX-2 immunoreactivity significantly at 4 and 8 h compared to LPS treatment alone (p < 0.05). After 24 h, LPS alone reached the same immunoreactivity as the treatment of LPS plus NE (Fig. 2A and 2B).
Time course of treatment with norepinephrine 1 μM alone, LPS 10 ng/ml alone, or a combination of norepinephrine 1 μM plus LPS 10 ng/ml on the expression of COX-2 protein and mRNA (A-C). (A, B) Microglial cells were stimulated for 4, 8, and 24 hours. This was followed by western blot analysis against β-actin (42 kDa) and COX-2 (70 kDa). A representative western blot is shown here (A). Quantitative densitometric analysis of COX-2 normalized to β-actin loading control. *P < 0.05 with respect to LPS control. (C) Semi-quantitative analysis of the effect of the different treatment groups on COX-2 mRNA expression at different time points (15 min to 24 hours). [NE = norepinephrine].
COX-2 mRNA upregulation was already observed at 30 min upon LPS plus NE treatment and reached its maximum by 1 h. In LPS-only treated microglial cells COX-2 mRNA expression levels reached a maximum and the same expression level as LPS plus NE after 6 h. In LPS only and LPS plus NE, COX-2 level did not further increase and stayed at the same level after 24 h. Interestingly, NE alone also led to an increase in COX-2 mRNA expression by 30 min and was still observed after 8 h (Fig. 2C).
NE increases release of PGE2 by LPS-stimulated primary microglial cells
We further investigated whether the augmentation of COX-2 synthesis by NE in the presence of LPS is accompanied by an increased release of PGE2, an important product of the enzymatic activity of COX-2 (Fig. 3). PGE2 was barely detectable in unstimulated primary neonatal microglial cells. Treatment with NE, 1 μM alone, did not lead to an increase in PGE2 over 24 h. LPS, 10 ng/ml alone, significantly increased PGE2 concentration after 24 h (p < 0.05), whereas there was no significant increase after 4 and 8 h stimulation with LPS.
Effect of norepinephrine on PGE 2 concentrations at different time points (0 h, 4 h, 8 h, 24 h) was measured in the supernatants of LPS-stimulated primary microglial cell cultures using EIA (n = 6). Data are depicted as means ± standard error of mean. Statistical analysis was done using ANOVA with post hoc Newman-Keuls for the 4 different time points (*P < 0.001). [NE = norepinephrine].
We observed a significant increase in the secretion of PGE2 after 8 h of stimulation with the combination of LPS (10 ng/ml) and NE (1 μM) compared to unstimulated cells (p < 0.05). At 24 h, LPS plus NE also showed a significant elevation of PGE2 levels compared to treatment with LPS alone (p < 0.001). The enhancement of PGE2 induced by NE in LPS-stimulated microglia, but not by NE alone, confirms the previous results in which NE in combination with LPS further increased COX-2 immunoreactivity whereas NE alone did not increase COX-2 protein levels.
The NE-induced increase in COX-2 is mediated by β-adrenoreceptors
Since microglial cells express α- and β-adrenoreceptors, and NE activates G-protein-coupled receptors, we asked which adrenoreceptor subtype mediates the increase in COX-2 after stimulation with LPS in combination with NE. Thus, we treated microglial cells with LPS, 10 ng/ml, in combination with various adrenoreceptor agonists (Fig. 4A). Co-treatment of LPS with the selective α1-agonists phenylephrine only induced a modest increase in COX-2 protein expression compared to LPS and NE treatment, while clonidine, an α2-adrenergic agonist, did not show any effect at all. In contrast, terbutaline and dobutamine, β2-and unselective β-adrenergic agonists, respectively, mimicked the effect of NE (Fig. 4A). Thus, stimulation of microglial β1- or β2-receptors, or both, seem to be required for the observed effect on COX-2 protein levels, suggesting that treatment with β-adrenergic antagonists should abolish the enhanced COX-2 protein synthesis. Indeed, the selective β1- and β2-receptor antagonists CGP 20712A and ICI 118,551, respectively, reduced COX-2 protein levels induced by the combination of LPS/NE (Fig. 4B). However, they were not able to completely abolish COX-2 immunoreactivity (Fig. 4B). Nicergoline, an α1/α2-adrenoreceptor antagonist, had no effect on COX-2 protein levels induced by NE/LPS, further supporting the absence of role of α1/α2-adrenoreceptors.
Effect of different α- and β-adrenergic agonists and antagonists on COX-2 protein expression. Microglial cells were stimulated with LPS 10 ng/ml and different adrenergic agonists/antagonists for 4 hours, followed by western blot analysis for COX-2 protein expression (70 kDa). (A) LPS-stimulated microglial cells were treated with different α- and β-adrenoreceptor agonists at two different concentrations (1 and 10 μM): α1-agonist phenylephrine; α2-agonist clonidine; β1/β2-agonist dobutamine; β2-agonist terbutaline. (B) LPS-stimulated microglial cells were treated with different α- and β-adrenoreceptor antagonists at two different concentrations (1 and 10 μM): α1/α2-antagonist nicergoline; β1-antagonist CGP 20712A; β2-antagonist ICI118, 551. (C) LPS-stimulated microglial cells were treated both with dobutamine or terbutaline and with the two different β-adrenoreceptor antagonists ICI 118,551 or CGP 20712A. [NE = norepinephrine].
Finally, we treated LPS-stimulated microglial cells with both β-adrenergic receptor agonists and antagonists (Fig. 4C). As shown, ICI 118,551 and CGP 20712A reduced COX-2 expression in LPS- plus dobutamine-stimulated cells. After stimulation with the β2-selective agonist terbutaline plus LPS, addition of the β1-receptor antagonist CGP completely abolished COX-2 expression. Low concentrations of ICI 118,551, however, did not alter COX-2 expression levels, while at higher concentrations a modest decrease was observed.
Discussion
In the present study, basal COX-2 expression in non-stimulated rat primary neonatal microglia was not detectable, suggesting that either COX-2 is generated by de novo synthesis in response to applied stimuli or, alternatively, that basal levels do not reach the threshold of immunoblot detection. NE alone induced COX-2 mRNA expression but did not affect COX-2 protein synthesis. We suppose that the COX-2 mRNA induced by NE is degraded before protein synthesis is initiated. Alternatively, a second stimulus, such as LPS, is required to set off translation of COX-2.
Different kinases are important in the regulation of COX-2 in LPS-stimulated microglia [37], and it has been demonstrated that NE increases the activity of mitogen-activated protein kinases (MAPK) [38] as well as transcription factors [39]. Kan et al. (1999) demonstrated that, in neonatal rat cardiac myocytes, NE alone increases the activity of MAPK and, although IL-1β alone does not induce the same effect, the co-addition of IL-1β and NE results in an enhanced MAPK activity in comparison to the substances alone [38]. Salmeterol and isoproterenol, β2- and non-selective β-adrenergic receptor agonists, respectively, enhance the phosphorylation of p38 MAPK and extracellular signal-regulated kinases (ERK) in peritoneal macrophages and RAW264.7 [40, 41]. Interestingly, pretreatment with isoproterenol decreases the release of TNFα, IL-12 and NO in LPS-stimulated macrophages. On the contrary, isoproterenol reduces the release of these same mediators after PMA stimulation, indicating that the effect of isoproterenol might depend on the stimulus [42].
Recently, Morioka et al. (2009) demonstrated that, in rat spinal microglia, NE reduces phosphorylation of p38 MAPK, induced by ATP, via β1- and β2-receptors [43]. The deactivation of p38 would lead to a decrease in COX-2 protein synthesis, since p38 is involved in COX-2 mRNA stabilization [44]. As such, it is possible that the observed increase in COX-2 mRNA may be due to increased transcription or increased stability of mRNA in rat microglia by activation of certain transcription factors and/or kinases. That could explain the increase in the COX-2 mRNA with incubation of cells with NE alone, or the enhancement of COX-2 protein synthesis when associated with LPS.
On the other hand, many post-transcriptional factors contribute to the translation of mRNA, which might not be affected by NE. This could explain the lack of effect of NE on COX-2 protein synthesis. For example, it is known that LPS induces the activation of the mammalian target of rapamycin (mTOR) [45]. Activation of mTOR induces translation of different mRNA through its downstream targets such as the ribosomal p70S6 kinase and the initiation factor 4E-binding protein 1 [46]. Although we did not test this possibility, it seems feasible that NE per se cannot activate the machinery responsible for the translation of COX-2 in rat microglia, but potentiates protein synthesis by increasing transcription or protein stability.
As shown before, LPS, 10 ng/ml, increases COX-2 mRNA and protein expression at 4 and 8 h, respectively [8]. Co-stimulation with NE already at low concentrations results in earlier and a markedly enhanced induction of COX-2 mRNA and protein levels. Similar to our results, other groups have also shown that NE per se does not increase protein synthesis, but drastically increases the effect induced by LPS. In peripheral blood monocytes and monocyte-derived macrophages, NE and epinephrine alone only show a minor effect on matrix metalloproteinase (MMP)-1 and MMP-9 production [39]. However, a combination of the catecholamine with LPS further enhances the increased production of MMP-1 and MMP-9.
Next, we investigated whether the increased intracellular levels of COX-2 evoked by LPS plus NE causes elevated levels of PGE2. In our experiments, NE plus LPS further increased the levels of PGE2. Interestingly, after 24 h stimulation with NE plus LPS, COX-2 mRNA and protein levels are similar to the increase observed in LPS alone. However, the increase observed in PGE2 is about three-fold higher in LPS plus NE than with LPS alone. Thus, it is possible that the combination of LPS and NE might induce the expression or increase the activity of other enzymes involved in PGE2 synthesis, such as phospholipase A2 and/or PGE synthases.
Dependent on the experimental setting, PGE2 can exert either neuroprotective or neurodetrimental effects. Agents reducing PGE2 synthesis in in vitro and in vivo models of chronic neurodegenerative diseases like Parkinson's disease or AD have been demonstrated to be neuroprotective due to their anti-inflammatory effects [47–52]. On the other hand, exogenous PGE2 protects neurons from LPS-induced cell death by reduction of NO and reactive oxygen species [53]. Direct administration of PGE2 into the brain has also been shown to reduce microglial activation and TNF-α expression in brain parenchyma induced by intraperitoneal LPS injection [54]. In addition, PGE2 protects neurons in culture from different types of noxious stimuli [29, 55, 56].
Localized inflammatory responses in the brain parenchyma have been associated with the pathogenesis and progression of AD. Inhibition of neuroinflammation has been identified as a potential therapeutic target [57–60]. COX-2 expression is elevated in the AD brains [14, 18] and PGE2 is accumulated in the cerebrospinal fluid of AD patients [61]. It was therefore reasonable to hypothesize that inhibition of COX-2 may have a therapeutic potential in AD. Despite convincing evidence in epidemiological studies on the prevention of AD through long-term treatment with non-steroidal anti-inflammatory drugs, most clinical trials have failed to show beneficial effects [62–65], suggesting that either the molecular target or the therapeutic window has been missed.
PGE2 acts on four different receptors, EP1-EP4 [66], of which microglia express three, namely EP1, EP2, and EP3; the latter having been exclusively detected in activated microglia [67]. Microglial EP2 receptors are known to enhance neurotoxic activities [10, 50–52, 68]. This would suggest that enhanced secretion of PGE2 through NE might increase microglial toxicity. However, the role of PGE2 may be far more complex due to the presence of other receptor subtypes on microglia. So far, NE-mediated regulation of EP receptor expression on microglia has not been studied.
In this study, we could show that the observed effect of NE is mediated by β-adrenoreceptor agonists. This corresponds to the findings of Minghetti and Levi, who showed that the non-selective β-adrenergic agonist isoproterenol increases COX-2 protein and PGE2 synthesis in microglial cells [8]. In our experiments, both β1- and β2-receptors seem to mediate the enhanced effect of COX-2 production. Our data also indicate a lack of involvement of α-adrenoreceptors. The use of relatively-selective β-adrenoreceptors allowed us to further confirm the participation of β-adrenoreceptors in the enhancement of LPS-induced COX-2 expression. Based on our data, both β-adrenoreceptors subtypes seem to be involved. The antagonists used in this study, CGP20712A (β1-antagonist) and ICI118,551 (β2-antagonist) are widely used to discern the role of β-adrenoreceptor subtypes [69, 70]. Terbutaline is considered a relatively selective β2-agonist, but it also binds to the β1-adrenoreceptor [71]. As with any pharmacological agent, these compounds are not 100% selective, therefore future studies utilizing knockout animals will be needed to fully clarify the role of distinct β-adrenoreceptor subtypes.
We did not detect that stimulation of α-adrenoreceptors results in any significant increase in COX-2 protein levels. Of note, stimulation of β-adrenoreceptors increases levels of cAMP in microglial cells, mainly through activation of the β2-adrenoreceptor [25]. Raised intracellular cAMP levels are known to suppress activation of microglial cells [54, 72, 73]. Increased PGE2 levels may in addition stimulate the microglial EP2 receptor, which is linked to cAMP formation [74] and thereby contribute to the inactivation of microglia. Other cell culture experiments have shown that exogenous PGE2 results in decreased levels of pro-inflammatory cytokines such as TNF-alpha and IL-12 in LPS-stimulated microglia [75–77]. In concordance with these results, increased cAMP levels via stimulation of β-adrenoreceptors inhibits the production of macrophage inflammatory protein (MIP)-1α, which is known to activate macrophages to secrete pro-inflammatory cytokines [78].
Our data suggest that NE has a strong effect on microglial inflammatory responses, suggesting that NE is an active modulator of microglial activation. This may be important in AD, a disease in which early loss of noradrenergic locus coeruleus (LC) neurons has been observed [79]. Depletion of LC neurons by injection of the neurotoxin DSP4 increases the levels of inflammatory mediators like iNOS, IL-1β and IL-6 [80].
Conclusions
Our study shows that NE increases LPS-induced COX-2 expression and PGE2 secretion by primary rat neonatal microglia in vitro. These results shed new light on the role of NE in CNS inflammation. NE via COX-2 and PGE-2 induction may have a protective physiological function rather than being purely detrimental. However, further investigations in in vivo models, especially in regard to PGE2 and COX-2, may help to clarify the exact role of NE in neuroinflammation.
References
Nimmerjahn A, Kirchhoff F, Helmchen F: Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005, 308: 1314-1318. 10.1126/science.1110647.
Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB: ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005, 8: 752-758. 10.1038/nn1472.
Hanisch UK, Kettenmann H: Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007, 10: 1387-1394. 10.1038/nn1997.
Block ML, Zecca L, Hong JS: Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007, 8: 57-69. 10.1038/nrn2038.
Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC: CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990, 249: 1431-1433. 10.1126/science.1698311.
Fassbender K, Walter S, Kuhl S, Landmann R, Ishii K, Bertsch T, Stalder AK, Muehlhauser F, Liu Y, Ulmer AJ, Rivest S, Lentschat A, Gulbins E, Jucker M, Staufenbiel M, Brechtel K, Walter J, Multhaup G, Penke B, Adachi Y, Hartmann T, Beyreuther K: The LPS receptor (CD14) links innate immunity with Alzheimer's disease. Faseb J. 2004, 18: 203-205.
Akundi RS, Candelario-Jalil E, Hess S, Hull M, Lieb K, Gebicke-Haerter PJ, Fiebich BL: Signal transduction pathways regulating cyclooxygenase-2 in lipopolysaccharide-activated primary rat microglia. Glia. 2005, 51: 199-208. 10.1002/glia.20198.
Minghetti L, Levi G: Induction of prostanoid biosynthesis by bacterial lipopolysaccharide and isoproterenol in rat microglial cultures. J Neurochem. 1995, 65: 2690-2698. 10.1046/j.1471-4159.1995.65062690.x.
Hoozemans JJ, Veerhuis R, Janssen I, van Elk EJ, Rozemuller AJ, Eikelenboom P: The role of cyclo-oxygenase 1 and 2 activity in prostaglandin E(2) secretion by cultured human adult microglia: implications for Alzheimer's disease. Brain Res. 2002, 951: 218-226. 10.1016/S0006-8993(02)03164-5.
Kawano T, Anrather J, Zhou P, Park L, Wang G, Frys KA, Kunz A, Cho S, Orio M, Iadecola C: Prostaglandin E2 EP1 receptors: downstream effectors of COX-2 neurotoxicity. Nat Med. 2006, 12: 225-229. 10.1038/nm1362.
Hewett SJ, Uliasz TF, Vidwans AS, Hewett JA: Cyclooxygenase-2 contributes to N-methyl-D-aspartate-mediated neuronal cell death in primary cortical cell culture. J Pharmacol Exp Ther. 2000, 293: 417-425.
Bazan NG, Colangelo V, Lukiw WJ: Prostaglandins and other lipid mediators in Alzheimer's disease. Prostaglandins Other Lipid Mediat. 2002, 68-69: 197-210. 10.1016/S0090-6980(02)00031-X.
Colangelo V, Schurr J, Ball MJ, Pelaez RP, Bazan NG, Lukiw WJ: Gene expression profiling of 12633 genes in Alzheimer hippocampal CA1: transcription and neurotrophic factor down-regulation and up-regulation of apoptotic and pro-inflammatory signaling. J Neurosci Res. 2002, 70: 462-473. 10.1002/jnr.10351.
Kitamura Y, Shimohama S, Koike H, Kakimura J, Matsuoka Y, Nomura Y, Gebicke-Haerter PJ, Taniguchi T: Increased expression of cyclooxygenases and peroxisome proliferator-activated receptor-gamma in Alzheimer's disease brains. Biochem Biophys Res Commun. 1999, 254: 582-586. 10.1006/bbrc.1998.9981.
Pasinetti GM, Aisen PS: Cyclooxygenase-2 expression is increased in frontal cortex of Alzheimer's disease brain. Neuroscience. 1998, 87: 319-324. 10.1016/S0306-4522(98)00218-8.
Yokota O, Terada S, Ishizu H, Ishihara T, Ujike H, Nakashima H, Nakashima Y, Kugo A, Checler F, Kuroda S: Cyclooxygenase-2 in the hippocampus is up-regulated in Alzheimer's disease but not in variant Alzheimer's disease with cotton wool plaques in humans. Neurosci Lett. 2003, 343: 175-179.
Ho L, Pieroni C, Winger D, Purohit DP, Aisen PS, Pasinetti GM: Regional distribution of cyclooxygenase-2 in the hippocampal formation in Alzheimer's disease. J Neurosci Res. 1999, 57: 295-303. 10.1002/(SICI)1097-4547(19990801)57:3<295::AID-JNR1>3.0.CO;2-0.
Ho L, Purohit D, Haroutunian V, Luterman JD, Willis F, Naslund J, Buxbaum JD, Mohs RC, Aisen PS, Pasinetti GM: Neuronal cyclooxygenase 2 expression in the hippocampal formation as a function of the clinical progression of Alzheimer disease. Arch Neurol. 2001, 58: 487-492. 10.1001/archneur.58.3.487.
Kaufmann WE, Worley PF, Pegg J, Bremer M, Isakson P: COX-2, a synaptically induced enzyme, is expressed by excitatory neurons at postsynaptic sites in rat cerebral cortex. Proc Natl Acad Sci USA. 1996, 93: 2317-2321. 10.1073/pnas.93.6.2317.
Minghetti L: Cyclooxygenase-2 (COX-2) in inflammatory and degenerative brain diseases. J Neuropathol Exp Neurol. 2004, 63: 901-910.
Tracey KJ, Czura CJ, Ivanova S: Mind over immunity. Faseb J. 2001, 15: 1575-1576. 10.1096/fj.01-0148hyp.
Sternberg EM: Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nat Rev Immunol. 2006, 6: 318-328. 10.1038/nri1810.
Pocock JM, Kettenmann H: Neurotransmitter receptors on microglia. Trends Neurosci. 2007, 30: 527-535. 10.1016/j.tins.2007.07.007.
Farber K, Pannasch U, Kettenmann H: Dopamine and noradrenaline control distinct functions in rodent microglial cells. Mol Cell Neurosci. 2005, 29: 128-138. 10.1016/j.mcn.2005.01.003.
Mori K, Ozaki E, Zhang B, Yang L, Yokoyama A, Takeda I, Maeda N, Sakanaka M, Tanaka J: Effects of norepinephrine on rat cultured microglial cells that express alpha1, alpha2, beta1 and beta2 adrenergic receptors. Neuropharmacology. 2002, 43: 1026-1034. 10.1016/S0028-3908(02)00211-3.
Tanaka KF, Kashima H, Suzuki H, Ono K, Sawada M: Existence of functional beta1- and beta2-adrenergic receptors on microglia. J Neurosci Res. 2002, 70: 232-237. 10.1002/jnr.10399.
Prinz M, Hausler KG, Kettenmann H, Hanisch U: beta-adrenergic receptor stimulation selectively inhibits IL-12p40 release in microglia. Brain Res. 2001, 899: 264-270. 10.1016/S0006-8993(01)02174-6.
Chang JY, Liu LZ: Catecholamines inhibit microglial nitric oxide production. Brain Res Bull. 2000, 52: 525-530. 10.1016/S0361-9230(00)00291-4.
Thery C, Dobbertin A, Mallat M: Downregulation of in vitro neurotoxicity of brain macrophages by prostaglandin E2 and a beta-adrenergic agonist. Glia. 1994, 11: 383-386. 10.1002/glia.440110411.
Madrigal JL, Feinstein DL, Dello Russo C: Norepinephrine protects cortical neurons against microglial-induced cell death. J Neurosci Res. 2005, 81: 390-396. 10.1002/jnr.20481.
Dello Russo C, Boullerne AI, Gavrilyuk V, Feinstein DL: Inhibition of microglial inflammatory responses by norepinephrine: effects on nitric oxide and interleukin-1beta production. J Neuroinflammation. 2004, 1: 9-10.1186/1742-2094-1-9.
Madrigal JL, Dello Russo C, Gavrilyuk V, Feinstein DL: Effects of noradrenaline on neuronal NOS2 expression and viability. Antioxid Redox Signal. 2006, 8: 885-892. 10.1089/ars.2006.8.885.
Seregi A, Keller M, Jackisch R, Hertting G: Comparison of the prostanoid synthesizing capacity in homogenates from primary neuronal and astroglial cell cultures. Biochem Pharmacol. 1984, 33: 3315-3318. 10.1016/0006-2952(84)90099-6.
Gebicke-Haerter PJ, Bauer J, Schobert A, Northoff H: Lipopolysaccharide-free conditions in primary astrocyte cultures allow growth and isolation of microglial cells. J Neurosci. 1989, 9: 183-194.
Chomczynski P, Sacchi N: Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987, 162: 156-159. 10.1016/0003-2697(87)90021-2.
Laemmli UK: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970, 227: 680-685. 10.1038/227680a0.
de Oliveira AC, Candelario-Jalil E, Bhatia HS, Lieb K, Hull M, Fiebich BL: Regulation of prostaglandin E2 synthase expression in activated primary rat microglia: evidence for uncoupled regulation of mPGES-1 and COX-2. Glia. 2008, 56: 844-855. 10.1002/glia.20658.
Kan H, Xie Z, Finkel MS: Norepinephrine-stimulated MAP kinase activity enhances cytokine-induced NO production by rat cardiac myocytes. Am J Physiol. 1999, 276: H47-52.
Speidl WS, Toller WG, Kaun C, Weiss TW, Pfaffenberger S, Kastl SP, Furnkranz A, Maurer G, Huber K, Metzler H, Wojta J: Catecholamines potentiate LPS-induced expression of MMP-1 and MMP-9 in human monocytes and in the human monocytic cell line U937: possible implications for peri-operative plaque instability. Faseb J. 2004, 18: 603-605.
Magocsi M, Vizi ES, Selmeczy Z, Brozik A, Szelenyi J: Multiple G-protein-coupling specificity of beta-adrenoceptor in macrophages. Immunology. 2007, 122: 503-513. 10.1111/j.1365-2567.2007.02658.x.
Tan KS, Nackley AG, Satterfield K, Maixner W, Diatchenko L, Flood PM: Beta2 adrenergic receptor activation stimulates pro-inflammatory cytokine production in macrophages via PKA- and NF-kappaB-independent mechanisms. Cell Signal. 2007, 19: 251-260. 10.1016/j.cellsig.2006.06.007.
Szelenyi J, Selmeczy Z, Brozik A, Medgyesi D, Magocsi M: Dual beta-adrenergic modulation in the immune system: stimulus-dependent effect of isoproterenol on MAPK activation and inflammatory mediator production in macrophages. Neurochem Int. 2006, 49: 94-103. 10.1016/j.neuint.2006.01.009.
Morioka N, Tanabe H, Inoue A, Dohi T, Nakata Y: Noradrenaline reduces the ATP-stimulated phosphorylation of p38 MAP kinase via beta-adrenergic receptors-cAMP-protein kinase A-dependent mechanism in cultured rat spinal microglia. Neurochem Int. 2009, 55: 226-234. 10.1016/j.neuint.2009.03.004.
Ridley SH, Dean JL, Sarsfield SJ, Brook M, Clark AR, Saklatvala J: A p38 MAP kinase inhibitor regulates stability of interleukin-1-induced cyclooxygenase-2 mRNA. FEBS Lett. 1998, 439: 75-80. 10.1016/S0014-5793(98)01342-8.
Weichhart T, Costantino G, Poglitsch M, Rosner M, Zeyda M, Stuhlmeier KM, Kolbe T, Stulnig TM, Horl WH, Hengstschlager M, Müller M, Säemann MD: The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity. 2008, 29: 565-577. 10.1016/j.immuni.2008.08.012.
Hay N, Sonenberg N: Upstream and downstream of mTOR. Genes Dev. 2004, 18: 1926-1945. 10.1101/gad.1212704.
Lim GP, Yang F, Chu T, Chen P, Beech W, Teter B, Tran T, Ubeda O, Ashe KH, Frautschy SA, Cole GM: Ibuprofen suppresses plaque pathology and inflammation in a mouse model for Alzheimer's disease. J Neurosci. 2000, 20: 5709-5714.
Scali C, Prosperi C, Vannucchi MG, Pepeu G, Casamenti F: Brain inflammatory reaction in an animal model of neuronal degeneration and its modulation by an anti-inflammatory drug: implication in Alzheimer's disease. Eur J Neurosci. 2000, 12: 1900-1912. 10.1046/j.1460-9568.2000.00075.x.
Teismann P, Tieu K, Choi DK, Wu DC, Naini A, Hunot S, Vila M, Jackson-Lewis V, Przedborski S: Cyclooxygenase-2 is instrumental in Parkinson's disease neurodegeneration. Proc Natl Acad Sci USA. 2003, 100: 5473-5478. 10.1073/pnas.0837397100.
Wang T, Pei Z, Zhang W, Liu B, Langenbach R, Lee C, Wilson B, Reece JM, Miller DS, Hong JS: MPP+-induced COX-2 activation and subsequent dopaminergic neurodegeneration. FASEB J. 2005, 19: 1134-1136. 10.1096/fj.04-2370com.
Manabe Y, Anrather J, Kawano T, Niwa K, Zhou P, Ross ME, Iadecola C: Prostanoids, not reactive oxygen species, mediate COX-2-dependent neurotoxicity. Ann Neurol. 2004, 55: 668-675. 10.1002/ana.20078.
Carlson NG: Neuroprotection of cultured cortical neurons mediated by the cyclooxygenase-2 inhibitor APHS can be reversed by a prostanoid. J Neurosci Res. 2003, 71: 79-88. 10.1002/jnr.10465.
Kim EJ, Kwon KJ, Park JY, Lee SH, Moon CH, Baik EJ: Neuroprotective effects of prostaglandin E2 or cAMP against microglial and neuronal free radical mediated toxicity associated with inflammation. J Neurosci Res. 2002, 70: 97-107. 10.1002/jnr.10373.
Zhang B, Yang L, Konishi Y, Maeda N, Sakanaka M, Tanaka J: Suppressive effects of phosphodiesterase type IV inhibitors on rat cultured microglial cells: comparison with other types of cAMP-elevating agents. Neuropharmacology. 2002, 42: 262-269. 10.1016/S0028-3908(01)00174-5.
Akaike A, Kaneko S, Tamura Y, Nakata N, Shiomi H, Ushikubi F, Narumiya S: Prostaglandin E2 protects cultured cortical neurons against N-methyl-D-aspartate receptor-mediated glutamate cytotoxicity. Brain Res. 1994, 663: 237-243. 10.1016/0006-8993(94)91268-8.
Cazevieille C, Muller A, Meynier F, Dutrait N, Bonne C: Protection by prostaglandins from glutamate toxicity in cortical neurons. Neurochem Int. 1994, 24: 395-398. 10.1016/0197-0186(94)90118-X.
Craft JM, Watterson DM, Van Eldik LJ: Neuroinflammation: a potential therapeutic target. Expert Opin Ther Targets. 2005, 9: 887-900. 10.1517/14728222.9.5.887.
Hoozemans JJ, Veerhuis R, Rozemuller JM, Eikelenboom P: Neuroinflammation and regeneration in the early stages of Alzheimer's disease pathology. Int J Dev Neurosci. 2006, 24: 157-165. 10.1016/j.ijdevneu.2005.11.001.
Hoozemans JJ, O'Banion MK: The role of COX-1 and COX-2 in Alzheimer's disease pathology and the therapeutic potentials of non-steroidal anti-inflammatory drugs. Curr Drug Targets CNS Neurol Disord. 2005, 4: 307-315. 10.2174/1568007054038201.
Minghetti L: Role of inflammation in neurodegenerative diseases. Curr Opin Neurol. 2005, 18: 315-321. 10.1097/01.wco.0000169752.54191.97.
Montine TJ, Woltjer RL, Pan C, Montine KS, Zhang J: Liquid chromatography with tandem mass spectrometry-based proteomic discovery in aging and Alzheimer's disease. NeuroRx. 2006, 3: 336-343. 10.1016/j.nurx.2006.05.002.
Martin BK, Szekely C, Brandt J, Piantadosi S, Breitner JC, Craft S, Evans D, Green R, Mullan M: Cognitive function over time in the Alzheimer's Disease Anti-inflammatory Prevention Trial (ADAPT): results of a randomized, controlled trial of naproxen and celecoxib. Arch Neurol. 2008, 65: 896-905. 10.1001/archneur.65.12.noc80051.
Thal LJ, Ferris SH, Kirby L, Block GA, Lines CR, Yuen E, Assaid C, Nessly ML, Norman BA, Baranak CC, Reines SA: A randomized, double-blind, study of rofecoxib in patients with mild cognitive impairment. Neuropsychopharmacology. 2005, 30: 1204-1215. 10.1038/sj.npp.1300690.
Aisen PS, Schafer KA, Grundman M, Pfeiffer E, Sano M, Davis KL, Farlow MR, Jin S, Thomas RG, Thal LJ: Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. Jama. 2003, 289: 2819-2826. 10.1001/jama.289.21.2819.
Reines SA, Block GA, Morris JC, Liu G, Nessly ML, Lines CR, Norman BA, Baranak CC: Rofecoxib: no effect on Alzheimer's disease in a 1-year, randomized, blinded, controlled study. Neurology. 2004, 62: 66-71.
Narumiya S, Sugimoto Y, Ushikubi F: Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999, 79: 1193-1226.
Slawik H, Volk B, Fiebich B, Hull M: Microglial expression of prostaglandin EP3 receptor in excitotoxic lesions in the rat striatum. Neurochem Int. 2004, 45: 653-660. 10.1016/j.neuint.2004.04.007.
Shie FS, Montine KS, Breyer RM, Montine TJ: Microglial EP2 is critical to neurotoxicity from activated cerebral innate immunity. Glia. 2005, 52: 70-77. 10.1002/glia.20220.
Baker JG: The selectivity of beta-adrenoceptor antagonists at the human beta1, beta2 and beta3 adrenoceptors. Br J Pharmacol. 2005, 144: 317-322. 10.1038/sj.bjp.0706048.
Hieble JP: Recent advances in identification and characterization of beta-adrenoceptor agonists and antagonists. Curr Top Med Chem. 2007, 7: 207-216. 10.2174/156802607779318208.
Sears MR, Lotvall J: Past, present and future--beta2-adrenoceptor agonists in asthma management. Respir Med. 2005, 99: 152-170. 10.1016/j.rmed.2004.07.003.
Caggiano AO, Kraig RP: Prostaglandin E receptor subtypes in cultured rat microglia and their role in reducing lipopolysaccharide-induced interleukin-1beta production. J Neurochem. 1999, 72: 565-575. 10.1046/j.1471-4159.1999.0720565.x.
Colton CA, Chernyshev ON: Inhibition of microglial superoxide anion production by isoproterenol and dexamethasone. Neurochem Int. 1996, 29: 43-53. 10.1016/0197-0186(95)00139-5.
Minghetti L, Nicolini A, Polazzi E, Creminon C, Maclouf J, Levi G: Prostaglandin E2 downregulates inducible nitric oxide synthase expression in microglia by increasing cAMP levels. Adv Exp Med Biol. 1997, 433: 181-184.
Scales WE, Chensue SW, Otterness I, Kunkel SL: Regulation of monokine gene expression: prostaglandin E2 suppresses tumor necrosis factor but not interleukin-1 alpha or beta-mRNA and cell-associated bioactivity. J Leukoc Biol. 1989, 45: 416-421.
Pouw Kraan van der TC, Boeije LC, Smeenk RJ, Wijdenes J, Aarden LA: Prostaglandin-E2 is a potent inhibitor of human interleukin 12 production. J Exp Med. 1995, 181: 775-779. 10.1084/jem.181.2.775.
Levi G, Minghetti L, Aloisi F: Regulation of prostanoid synthesis in microglial cells and effects of prostaglandin E2 on microglial functions. Biochimie. 1998, 80: 899-904. 10.1016/S0300-9084(00)88886-0.
Hasko G, Shanley TP, Egnaczyk G, Nemeth ZH, Salzman AL, Vizi ES, Szabo C: Exogenous and endogenous catecholamines inhibit the production of macrophage inflammatory protein (MIP) 1 alpha via a beta adrenoceptor mediated mechanism. Br J Pharmacol. 1998, 125: 1297-1303. 10.1038/sj.bjp.0702179.
Bondareff W, Mountjoy CQ, Roth M: Loss of neurons of origin of the adrenergic projection to cerebral cortex (nucleus locus ceruleus) in senile dementia. Neurology. 1982, 32: 164-168.
Heneka MT, Galea E, Gavriluyk V, Dumitrescu-Ozimek L, Daeschner J, O'Banion MK, Weinberg G, Klockgether T, Feinstein DL: Noradrenergic depletion potentiates beta-amyloid-induced cortical inflammation: implications for Alzheimer's disease. J Neurosci. 2002, 22: 2434-2442.
Acknowledgements
We thank Ulrike Götzinger-Berger and Brigitte Günter for excellent technical assistance. Antonio Carlos Pinheiro de Oliveira was supported by CAPES (Brasília/Brazil). This work was supported by KNDD (Kompetenznetz Degenerative Demenzen).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
JCMS contributed to the design of the study, carried out western blot analysis, performed the statistical analysis and wrote the manuscript; BLF directed the work of the study, reviewed the data and the manuscript; EH participated in western blot analysis and prostaglandin measurements; ECJ and ACPO provided consultation and reviewed the manuscript; MTH was involved in drafting the manuscript and revised it critically; MH conceived of the study, directed the work and reviewed the manuscript. All authors read and approved the final manuscript.
Johannes CM Schlachetzki, Bernd L Fiebich contributed equally to this work.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Schlachetzki, J.C., Fiebich, B.L., Haake, E. et al. Norepinephrine enhances the LPS-induced expression of COX-2 and secretion of PGE2 in primary rat microglia. J Neuroinflammation 7, 2 (2010). https://doi.org/10.1186/1742-2094-7-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1742-2094-7-2