Abstract
Introduction
Autism is complex neuro-developmental disorder which has a symptomatic diagnosis in patients characterized by disorders in language/communication, behavior, and social interactions. The exact causes for autism are largely unknown, but is has been speculated that immune and inflammatory responses, particularly those of Th2 type, may be involved. Thiazolidinediones (TZDs) are agonists of the peroxisome proliferator activated receptor gamma (PPARγ), a nuclear hormone receptor which modulates insulin sensitivity, and have been shown to induce apoptosis in activated T-lymphocytes and exert anti-inflammatory effects in glial cells. The TZD pioglitazone (Actos) is an FDA-approved PPARγ agonist used to treat type 2 diabetes, with a good safety profile, currently being tested in clinical trials of other neurological diseases including AD and MS. We therefore tested the safety and therapeutic potential of oral pioglitazone in a small cohort of children with diagnosed autism.
Case description
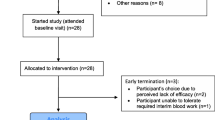
The rationale and risks of taking pioglitazone were explained to the parents, consent was obtained, and treatment was initiated at either 30 or 60 mg per day p.o. A total of 25 children (average age 7.9 ± 0.7 year old) were enrolled. Safety was assessed by measurements of metabolic profiles and blood pressure; effects on behavioral symptoms were assessed by the Aberrant Behavior Checklist (ABC), which measures hyperactivity, inappropriate speech, irritability, lethargy, and stereotypy, done at baseline and after 3–4 months of treatment.
Discussion and evaluation
In a small cohort of autistic children, daily treatment with 30 or 60 mg p.o. pioglitazone for 3–4 months induced apparent clinical improvement without adverse events. There were no adverse effects noted and behavioral measurements revealed a significant decrease in 4 out of 5 subcategories (irritability, lethargy, stereotypy, and hyperactivity). Improved behaviors were inversely correlated with patient age, indicating stronger effects on the younger patients.
Conclusion
Pioglitazone should be considered for further testing of therapeutic potential in autistic patients.
Similar content being viewed by others
Introduction
Autism, the most common of the group of disorders collectively referred to as Autism Spectrum Disorders (ASD), is a complex neurological disease of unknown etiology. The incidence of autism is estimated to be 1 per 166 [1] with a male to female ratio of 4:1. Autism has been found throughout the world in families of all racial, ethnic and social backgrounds. Although accumulating evidence suggests that genetic, environmental, inflammatory, immunological, and metabolic factors play a prominent role in this disease [2–7], the precise causes remain to be determined.
Altered immune responses in children with ASD are well documented. Autoimmune disorders of thyroiditis, colitis, myelin basic protein autoantibodies, and diabetes are prevalent in children with ASD. Stubbs (1976) published that 5 of 13 autistic children had no detectable rubella antibodies despite prior immunization [7]. An additional study showed peripheral mononuclear cells had a decreased proliferative response to mitogenic stimulation compared to normal children [8]. These findings of abnormal T-lymphocyte function have been replicated by other investigators [9, 10]. Inflammatory responses in ASD have also been reported to occur in brain, for example neuroinflammatory processes involving both microglia and astroglia were found on post mortem examination in autistic children with elevated cytokine levels in the cerebral spinal fluid [11, 12]. Children with ASD have increased cytokines of Th2 and Th1 arms of the immune response with Th2 predominant without an increase in IL10 [13].
Peroxisome proliferator-activated receptor gamma (PPARγ) is a nuclear hormone receptor originally characterized by its ability to regulate adipocyte differentiation and gene transcription [14]. PPARγ agonists include fatty acids, non-steroidal anti-inflammatory drugs (NSAIDs), the natural compound 15-deoxy12,14-prostaglandin-J2 (PGJ2), and members of the class of synthetic drugs termed thiazolidinediones (TZDs) which include pioglitazone (Actos) and rosiglitazone (Avandia). TZDs were originally designed as anti-diabetic drugs due to their insulin sensitizing effects, and several are now in clinical use. In addition to insulin sensitizing effects, TZDs also exert anti-inflammatory effects on a variety of cell types, and for this reason some are being considered for treatment of inflammatory diseases including artherosclerosis [15], psoriasis [16, 17], and inflammatory bowel disease [18–21]. TZDs also reduce inflammatory activation of brain glial cells, and increase metabolic activities in glial cells which can lead to increased glucose uptake, lactate production, and mitochondrial function [22, 23]. Furthermore, pioglitazone can cross the BBB, [24] suggesting possible direct effects on brain physiology, which could positively influence possible abnormalities in regional brain glucose utilization [25] or dysregulation of functional activity [26] as reported to occur ASD.
The safety and efficacy of pioglitazone has been established by clinical studies worldwide [27, 28] and since FDA approval, pioglitazone has been prescribed to several million patients. The adverse events associated with TZDs including pioglitazone are generally mild and transient, and those effects returned to baseline upon withdrawal from, or completion of the studies. Two recent studies for the treatment of diabetes in adolescents point to a good safety profile for Actos in younger populations [29, 30]. Studies with PPARγ drugs in animal models of neurological conditions have led to clinical testing of these drugs in Alzheimer's disease (AD) and multiple sclerosis (MS) [31, 32]. These properties of PPARγ agonists make them promising candidates for a therapeutic approach to influence the clinical course of ASD. In this report we discuss initial findings using pioglitazone to treat children with autism, which provides the rationale for design of larger clinical trials.
Case description
Population
The autistic children all were patients of Marvin Boris, MD, Allan Goldblatt, PA, and Michael Elice, MD. Twenty-five children and adolescents participated in this study. The mean age was 7.9 ± 3.5 years, with a range from 3 to 17 years. There were 22 males and 3 females. All of the participants received an independent diagnosis of Autism Spectrums Disorder (ASD) from an independent clinician and/or agency. None of the children had diagnosed Asperger's Disorder (a mild variant of ASD with higher social functioning) or PDDNOS (Pervasive Developmental Disorder – Not Otherwise Specified, a condition with social or behavioral impairments but which do not meet the DSM-IV criteria for ASD). The diagnosis of autism was initially established by a board certified pediatric neurologist, developmental pediatrician, or psychiatrist with experience in ASD. In addition, at the first visit to the offices of the treating physician, the child had to meet the DSM-IV checklist criteria for ASD. All the children had been receiving behavioral and educational therapies. These included speech, occupational, and physical therapy, applied behavioral analysis, and auditory integration therapy. The children had also received various biomedical interventions for at least one year. These included dairy and gluten free diet, metabolic treatment with supplements to known deficiencies such as MTHFR (methylenetetrahydrofolate reductase), treatment with intravenous gamma globulin or secretin, vitamin supplementation, and heavy metal chelation. The children who responded poorly (no noticeable improvements in cognitive, social, behavior, or language skills) for at least one year to biomedical, behavioral, or educational therapies were selected to be treated with pioglitazone as part of the routine health care treatment, based on papers suggesting that ASD includes an auto-immune or inflammatory component [33, 34], and that pioglitazone can reduce T-cell activation and Th2-type cytokine production, both implicated in ASD [35–38]. The rationale and risks of taking pioglitazone were explained to the parents, and parental written consents were obtained for all participants. A retrospective review of their personal medical records was approved by the Internal Review Board of Arizona State University.
Comorbities
The autistic population has well-known auto-immunne comorbidities. In this group of autistic children, 7/25 (28%) had thyroiditis, 8/25 (32%) had colitis, 8/25 (32%) had PANDAS (Pediatric acquired neurological disorder associated with streptococcus), 20/25 (80%) had allergic diseases, and 7/25 (28%) were positive for serum antibodies to myelin basic protein. In addition 2/25 had seizures prior to being treated with pioglitazone.
Treatment
Children were prescribed pioglitazone either 30 mg per day, p.o. for ages 3–5 years old; or 60 mg per day for ages 6–17 years old. These children were followed with monthly complete blood counts, glucose and insulin levels, and serum metabolic assays.
Analysis
The participants' parents completed the Aberrant Behavior Checklist (ABC) prior to the administration of pioglitazone and then at a follow-up assessment, 12 or 16 weeks later. There are five subscales on the ABC, consisting of 58 questions. The subscales are: hyperactivity, inappropriate speech, irritability, lethargy, and stereotypy. Each question was rated on a 4-point scale: 0 = 'not a problem,' 1 = 'the behavior is a problem but slight in degree,' 2 = 'the problem is moderately serious,' and 3 = 'the problem is severe in degree.' 'The ABC has been shown to be a valid and reliable procedure to evaluate treatment efficacy [39–41]. Each of the five subscales was analyzed using paired t-tests. The relationship between age and amount of behavior change was examined using Pearson product correlations
Outcomes
There were no significant abnormalities observed in standard blood analyses in the group of 25 autistic children treated with pioglitazone for up to 4 months (Table 1). Over the course of treatment, there were no elevations in hemoglobin, creatine, BUN (blood urea nitrogen) or insulin levels. There were 2 incidents of slightly and transiently elevated white blood counts and glucose levels, and 3 incidents of slightly and transiently elevated liver enzyme (ALT and AST) levels. All elevations resolved without interventions.
A comparison of the mean scores for ABC subscales between baseline and end of treatment for each of the patients revealed that four of the five ABC subscales decreased significantly following the administration of pioglitazone (Figure 1). These subscales were hyperactivity, irritability, lethargy, and stereotypy. There was no change in inappropriate speech; however, it should be noted that the speech subscale is of limited value in children with autism who lack or have very limited speech.
Of the 25 patients, 76% showed an improvement (defined as >50% decrease in score) in at least one subgroup; while 56% showed an improvement in two or more subgroups, and 40% showed improvements in 3 or more subcategories. If response rate is estimated as those who showed >25% decrease in at least 2 of the 5 subscales, then the percentage is much higher 71%. The majority of patients (52%) showed an improvement (>50%) in the hyperactivity subscale.
Significant inverse correlations (Figure 2) were detected between age and the improvements calculated for irritability (P = 0.03), lethargy (P = 0.02) and hyperactivity (P = 0.007). This indicates a tendency for younger participants to benefit more from pioglitazone than the older participants.
Discussion and evaluation
The current study provides evidence that treatment with the PPARγ agonist pioglitazone (Actos) does not induce any significant adverse effects, and may have a beneficial effect on patterns of aberrant social behavior in children with diagnosed autism. Despite the small sample size (n = 25 total), we observed statistically significant decreases in 4 of the 5 subscales of the ABC after a relatively short (4 months) treatment with pioglitazone. It is yet not known if these improvements are long lasting, or if they will continue after treatment is withdrawn. Although originally approved for treatment of Type 2 diabetes in adults, recent clinical trials of pioglitazone for treatment of diabetes in adolescents suggest this drug will be well tolerated in younger populations [29, 30].
There is increasing evidence for an association of ASD with various immune syndromes. It was reported that 66% of children with autism have a relative with an autoimmune disease [42], and families of children with PDD (Pervasive Development Disorder) have a higher average number of autoimmune diseases than families of healthy children [43]. Recently the occurrence of AITD (Autoimmune Thyroid Disease) in first or second order relatives was concluded to be a risk factor for those ASD children who show regression (the early loss of already established skills of communication or of social interactions) [44]. The possibility therefore exists that pioglitazone influences some aspect of auto-immune nature in ASD children.
It has been suggested that a Th2-like dysfunction may contribute to the causes of ASD. In children with ASD, a preponderance of Th2-like (IL4, IL6, IL10) over Th1-like (IL2, IFNg, IL1β) cytokines has been reported [45–48]. These studies support the idea that a predominance of Th2 cytokines may be a factor in ASD. PPARγ agonists are known to influence T-cell physiology, and although most often they have been shown to reduce Th1-like cytokine (IL1β, TNFa, IL12) production, in several studies they also reduced Th2 responses. In CD4 cells, PGJ2 and the TZD ciglitazone reduced IL4 production [35] and in EAE, the animal model of Multiple Sclerosis, PGJ2 blocked splenic T cell production of IL10 and IL4 [36]. PPARγ agonists also reduce the clinical symptoms in animal models of asthma, a disease which is also thought to be predominantly Th2 type involving IL4, IL5, and IL13 [37]. PPARγ agonists have been shown to reduce IL4, IL5, and IL13 production from Tcells of mice with induced lung inflammation [38, 49]. However, in one study the TZDs increased IL4 and IL10, and stimulated GATA3 expression (a transcription factor which shifts cells towards Th2 phenotype) [50]; although in other studies PPARγ drugs were shown to inhibit GATA3 activity [51, 52]. Nevertheless, taken together these studies demonstrate that PPARγ agonists have the potential to shift the T-cell response from Th2 to Th1, or to reduce Th2 cytokine expression, which may be of therapeutic benefit in ASD.
Despite observing significant improvements in 4 of 5 subscales of the ABC, the open-label nature of this study limits the ability to draw strong conclusions regarding treatment-dependent benefits. In addition, well-known expectancy effects in the parent population make interpretation of the ABC subject to potential bias [53, 54]. The placebo effect in ASD has been reported to be high in some studies where improvement was assessed using the ABC. Improvements occurred in 25% of patients following atomoxetine treatment for 6 weeks, [55]; 34% after 8 week treatment with risperidone [56]; and 37% after 3 weeks treatment with amantadine [54]. In the current study, the number of responders (those showing >50% improvement in at least one subscale) was 76%, considerably higher than the values reported in the above studies.
An additional confound of the current study is the diversity of auto-immune comorbidities that are common in the autistic population. It is possible that pioglitazone effects are, in part or in full, an indirect consequence of reducing symptoms of the autoimmune diseases present in the study population (thyroiditis, colitis, and PANDAS). For example, in autoimmune thyroiditis (AITD), pioglitazone could increase levels of suppressor T-cells that are deficient [57] and as a result reduce circulating levels of Th1 or Th2 cytokines. Similarly, activation of PPARγ can suppress experimentally induced colitis [58] which could also reduce plasma cytokine levels, and in fact several clinical trials of PPARγ agonists for treating colitis are in progress [19, 59]. PANDAS, a pediatric autoimmune neuropsychiatric disorder associated with streptococcal infections is defined by obsessive-compulsive (OCD) and or tic disorders, is thought to be due to the actions of auto-immune antibodies on basal ganglia neurons [60], and is improved by immunomodulatory therapies [61]; anti-inflammatory effects of PPARγ agonists could therefore influence the course of this disease. However, since the precise relationships between autoimmune diseases and the penetrance of autistic symptoms remains to be established, deciphering the relative importance of indirect effect of pioglitazone on behavior will be a formidable task.
The recent increase in type 2 diabetes in children has resulted in an increased interest of researchers to explore the use of anti-diabetic drugs including TZDs in children, therefore providing additional information regarding the safety of TZDs in this population. A recent clinical trial tested the effects of rosiglitazone (2 mg bid increased to 4 mg bid after 8 weeks), a related TZD, in 195 obese type 2 diabetic children (age range 8–17 years), in a 24-week double-blind, randomized, metformin-controlled, parallel group design. The rosiglitazone group gained ~3 kg after 24 weeks with the occurrence of peripheral edema in 1 child [29]. However, no other adverse effects were reported, suggesting that TZDs are well tolerated in children as in adults. More recently [30] pioglitazone (15 mg po escalated to 30 mg po after 4 weeks) was tested as an adjunct therapy for the treatment of type 1 diabetes in a small group of young adolescents (age range 10–17.9 years). After 6 months treatment the pioglitazone subjects showed a small but significant increase in BMI z-score (body mass index standard deviation for age) suggesting treatment-related weight gain. In the 35 subjects who completed the study, there was no evidence of edema, anemia, or of any significant increase in the frequency of hypoglycemia in the treatment group versus the placebo group. However, it is clear that the safety of pioglitazone, and of other TZDs, in the pediatric population requires additional testing.
Conclusion
In view of its established safety profile, the current results provide the rationale for further testing of pioglitazone in autism and other forms of ASD.
Abbreviations
- ABC:
-
Aberrant Behavior Checklist
- AD:
-
Alzheimer's disease
- ASD:
-
Autism Spectrum Disorder
- BBB:
-
Blood brain barrier
- CBC:
-
Complete blood count
- CD:
-
Cluster of differentiation
- IL:
-
Interleukin
- MS:
-
Multiple Sclerosis
- NSAID:
-
Non steroidal anti-inflammatory drug
- PANDAS:
-
Pediatric autoimmune neuropsychiatric disorder associated with streptococcal infections
- PGJ2:
-
15-deoxy-delta12,14-prostaglandin J2
- PDD:
-
pervasive developmental disorder
- PPAR:
-
Peroxisome proliferator activated receptor
- TNF:
-
Tumor necrosis factor
- TZD:
-
thiazolidinedione
References
Polak PE, Kalinin S, Dello RC, Gavrilyuk V, Sharp A, Peters JM, Richardson J, Willson TM, Weinberg G, Feinstein DL: Protective effects of a peroxisome proliferator-activated receptor-beta/delta agonist in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2005, 168: 65-75. 10.1016/j.jneuroim.2005.07.006.
Rapin I, Katzman R: Neurobiology of autism. Ann Neurol. 1998, 43: 7-14. 10.1002/ana.410430106.
Newschaffer CJ, Fallin D, Lee NL: Heritable and nonheritable risk factors for autism spectrum disorders. Epidemiol Rev. 2002, 24: 137-153. 10.1093/epirev/mxf010.
Folstein SE, Rosen-Sheidley B: Genetics of autism: complex aetiology for a heterogeneous disorder. Nat Rev Genet. 2001, 2: 943-955. 10.1038/35103559.
Korvatska E, Van de WJ, Anders TF, Gershwin ME: Genetic and immunologic considerations in autism. Neurobiol Dis. 2002, 9: 107-125. 10.1006/nbdi.2002.0479.
Lipkin WI, Hornig M: Microbiology and immunology of autism spectrum disorders. Novartis Found Symp. 2003, 251: 129-143.
Stubbs EG: Autistic children exhibit undetectable hemagglutination-inhibition antibody titers despite previous rubella vaccination. J Autism Child Schizophr. 1976, 6: 269-274. 10.1007/BF01543467.
Stubbs EG, Crawford ML: Depressed lymphocyte responsiveness in autistic children. J Autism Child Schizophr. 1977, 7: 49-55. 10.1007/BF01531114.
Warren RP, Margaretten NC, Pace NC, Foster A: Immune abnormalities in patients with autism. J Autism Dev Disord. 1986, 16: 189-197. 10.1007/BF01531729.
Denney DR, Frei BW, Gaffney GR: Lymphocyte subsets and interleukin-2 receptors in autistic children. J Autism Dev Disord. 1996, 26: 87-97. 10.1007/BF02276236.
Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA: Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005, 57: 67-81. 10.1002/ana.20315.
Pardo CA, Vargas DL, Zimmerman AW: Immunity, neuroglia and neuroinflammation in autism. Int Rev Psychiatry. 2005, 17: 485-495. 10.1080/02646830500381930.
Molloy CA, Morrow AL, Meinzen-Derr J, Schleifer K, Dienger K, Manning-Court, Altaye M, Wills-Karp M: Elevated cytokine levels in children with autism spectrum disorder. J Neuroimmunol. 2006, 172: 198-205. 10.1016/j.jneuroim.2005.11.007.
Berger J, Wagner JA: Physiological and therapeutic roles of peroxisome proliferator-activated receptors. Diabetes Technol Ther. 2002, 4: 163-174. 10.1089/15209150260007381.
Duval C, Chinetti G, Trottein F, Fruchart JC, Staels B: The role of PPARs in atherosclerosis. Trends Mol Med. 2002, 8: 422-430. 10.1016/S1471-4914(02)02385-7.
Bongartz T, Coras B, Vogt T, Scholmerich J, Muller-Ladner U: Treatment of active psoriatic arthritis with the PPARgamma ligand pioglitazone: an open-label pilot study. Rheumatology (Oxford). 2005, 44: 126-129. 10.1093/rheumatology/keh423.
Gniadecki R, Calverley MJ: Emerging drugs in psoriasis. Expert Opin Emerg Drugs. 2002, 7: 69-90. 10.1517/14728214.7.1.69.
Katayama K, Wada K, Nakajima A, Mizuguchi H, Hayakawa T, Nakagawa S, Kadowaki T, Nagai R, Kamisaki Y, Blumberg RS, Mayumi T: A novel PPAR gamma gene therapy to control inflammation associated with inflammatory bowel disease in a murine model. Gastroenterology. 2003, 124: 1315-1324. 10.1016/S0016-5085(03)00262-2.
Liang HL, Ouyang Q: [A clinical trial of rosiglitazone and 5-aminosalicylate combination for ulcerative colitis]. Zhonghua Nei Ke Za Zhi. 2006, 45: 548-551.
Lewis JD, Lichtenstein GR, Stein RB, Deren JJ, Judge TA, Fogt F, Furth EE, Demissie EJ, Hurd LB, Su CG, Keilbaugh SA, Lazar MA, Wu GD: An open-label trial of the PPAR-gamma ligand rosiglitazone for active ulcerative colitis. Am J Gastroenterol. 2001, 96: 3323-3328.
Wada K, Nakajima A, Blumberg RS: PPARgamma and inflammatory bowel disease: a new therapeutic target for ulcerative colitis and Crohn's disease. Trends Mol Med. 2001, 7: 329-331. 10.1016/S1471-4914(01)02076-7.
Dello Russo C, Gavrilyuk V, Weinberg G, Almeida A, Bolanos JP, Palmer J, Pelligrino D, Galea E, Feinstein DL: Peroxisome proliferator-activated receptor gamma thiazolidinedione agonists increase glucose metabolism in astrocytes. J Biol Chem. 2003, 278: 5828-5836. 10.1074/jbc.M208132200.
Feinstein DL, Spagnolo A, Akar C, Weinberg G, Murphy P, Gavrilyuk V, Dello RC: Receptor-independent actions of PPAR thiazolidinedione agonists: is mitochondrial function the key?. Biochem Pharmacol. 2005, 70: 177-188. 10.1016/j.bcp.2005.03.033.
Maeshiba Y, Kiyota Y, Yamashita K, Yoshimura Y, Motohashi M, Tanayama S: Disposition of the new antidiabetic agent pioglitazone in rats, dogs, and monkeys. Arzneimittelforschung. 1997, 47: 29-35.
Haznedar MM, Buchsbaum MS, Hazlett EA, LiCalzi EM, Cartwright C, Hollander E: Volumetric analysis and three-dimensional glucose metabolic mapping of the striatum and thalamus in patients with autism spectrum disorders. Am J Psychiatry. 2006, 163: 1252-1263. 10.1176/appi.ajp.163.7.1252.
Kennedy DP, Redcay E, Courchesne E: Failing to deactivate: resting functional abnormalities in autism. Proc Natl Acad Sci U S A. 2006, 103: 8275-8280. 10.1073/pnas.0600674103.
Gillies PS, Dunn CJ: Pioglitazone. Drugs. 2000, 60: 333-343. 10.2165/00003495-200060020-00009.
Hanefeld M, Belcher G: Safety profile of pioglitazone. Int J Clin Pract Suppl. 2001, 27-31.
Saenger P, Dabiri G, Jones K, Krebs J, Sun Y, Mudd P, Weston WM, Cobitz AR, Freed MI, Porter LE: Diabetes in childhood - Benefits of rosiglitazone in children with type 2 diabetes mellitus. Program and abstracts of the European Association for the Study of Diabetes 41st Annual Meeting. 2005, 133-
Zdravkovic V, Hamilton JK, Daneman D, Cummings EA: Pioglitazone as adjunctive therapy in adolescents with type 1 diabetes. J Pediatr. 2006, 149: 845-849. 10.1016/j.jpeds.2006.08.049.
Landreth G: PPARgamma agonists as new therapeutic agents for the treatment of Alzheimer's disease. Exp Neurol. 2006, 199: 245-248. 10.1016/j.expneurol.2006.04.006.
Feinstein DL: Therapeutic potential of peroxisome proliferator-activated receptor agonists for neurological disease. Diabetes Technol Ther. 2003, 5: 67-73. 10.1089/152091503763816481.
Gupta S, Aggarwal S, Heads C: Dysregulated immune system in children with autism: beneficial effects of intravenous immune globulin on autistic characteristics. J Autism Dev Disord. 1996, 26: 439-452. 10.1007/BF02172828.
Singh VK: Plasma increase of interleukin-12 and interferon-gamma. Pathological significance in autism. J Neuroimmunol. 1996, 66: 143-145. 10.1016/0165-5728(96)00014-8.
Zingarelli B, Sheehan M, Hake PW, O'Connor M, Denenberg A, Cook JA: Peroxisome proliferator activator receptor-gamma ligands, 15-deoxy-Delta(12,14)-prostaglandin J2 and ciglitazone, reduce systemic inflammation in polymicrobial sepsis by modulation of signal transduction pathways. J Immunol. 2003, 171: 6827-6837.
Diab A, Deng C, Smith JD, Hussain RZ, Phanavanh B, Lovett-Racke AE, Drew PD, Racke MK: Peroxisome proliferator-activated receptor-gamma agonist 15-deoxy-Delta(12,14)-prostaglandin J(2) ameliorates experimental autoimmune encephalomyelitis. J Immunol. 2002, 168: 2508-2515.
O'Byrne PM: Cytokines or their antagonists for the treatment of asthma. Chest. 2006, 130: 244-250. 10.1378/chest.130.1.244.
Mueller C, Weaver V, Vanden Heuvel JP, August A, Cantorna MT: Peroxisome proliferator-activated receptor gamma ligands attenuate immunological symptoms of experimental allergic asthma. Arch Biochem Biophys. 2003, 418: 186-196. 10.1016/j.abb.2003.08.006.
Marteleto MR, Pedromonico MR: Validity of Autism Behavior Checklist (ABC): preliminary study. Rev Bras Psiquiatr. 2005, 27: 295-301.
Wadden NP, Bryson SE, Rodger RS: A closer look at the Autism Behavior Checklist: discriminant validity and factor structure. J Autism Dev Disord. 1991, 21: 529-541. 10.1007/BF02206875.
Volkmar FR, Cicchetti DV, Dykens E, Sparrow SS, Leckman JF, Cohen DJ: An evaluation of the Autism Behavior Checklist. J Autism Dev Disord. 1988, 18: 81-97. 10.1007/BF02211820.
Comi AM, Zimmerman AW, Frye VH, Law PA, Peeden JN: Familial clustering of autoimmune disorders and evaluation of medical risk factors in autism. J Child Neurol. 1999, 14: 388-394.
Sweeten TL, Bowyer SL, Posey DJ, Halberstadt GM, McDougle CJ: Increased prevalence of familial autoimmunity in probands with pervasive developmental disorders. Pediatrics. 2003, 112: e420-10.1542/peds.112.5.e420.
Molloy CA, Morrow AL, Meinzen-Derr J, Dawson G, Bernier R, Dunn M, Hyman SL, McMahon WM, Goudie-Nice J, Hepburn S, Minshew N, Rogers S, Sigman M, Spence MA, Tager-Flusberg H, Volkmar FR, Lord C: Familial autoimmune thyroid disease as a risk factor for regression in children with Autism Spectrum Disorder: a CPEA Study. J Autism Dev Disord. 2006, 36: 317-324. 10.1007/s10803-005-0071-0.
Gupta S, Aggarwal S, Rashanravan B, Lee T: Th1- and Th2-like cytokines in CD4+ and CD8+ T cells in autism. J Neuroimmunol. 1998, 85: 106-109. 10.1016/S0165-5728(98)00021-6.
Singh VK, Warren RP, Odell JD, Cole P: Changes of soluble interleukin-2, interleukin-2 receptor, T8 antigen, and interleukin-1 in the serum of autistic children. Clin Immunol Immunopathol. 1991, 61: 448-455. 10.1016/S0090-1229(05)80015-7.
Warren RP, Yonk LJ, Burger RA, Cole P, Odell JD, Warren WL, White E, Singh VK: Deficiency of suppressor-inducer (CD4+CD45RA+) T cells in autism. Immunol Invest. 1990, 19: 245-251.
Yonk LJ, Warren RP, Burger RA, Cole P, Odell JD, Warren WL, White E, Singh VK: CD4+ helper T cell depression in autism. Immunol Lett. 1990, 25: 341-345. 10.1016/0165-2478(90)90205-5.
Lee KS, Park SJ, Hwang PH, Yi HK, Song CH, Chai OH, Kim JS, Lee MK, Lee YC: PPAR-gamma modulates allergic inflammation through up-regulation of PTEN. FASEB J. 2005, 19: 1033-1035. 10.1096/fj.04-2591hyp.
Saubermann LJ, Nakajima A, Wada K, Zhao S, Terauchi Y, Kadowaki T, Aburatani H, Matsuhashi N, Nagai R, Blumberg RS: Peroxisome proliferator-activated receptor gamma agonist ligands stimulate a Th2 cytokine response and prevent acute colitis. Inflamm Bowel Dis. 2002, 8: 330-339. 10.1097/00054725-200209000-00004.
Zheng W, Flavell RA: The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997, 89: 587-596. 10.1016/S0092-8674(00)80240-8.
Zhu J, Min B, Hu-Li J, Watson CJ, Grinberg A, Wang Q, Killeen N, Urban JF, Guo L, Paul WE: Conditional deletion of Gata3 shows its essential function in T(H)1-T(H)2 responses. Nat Immunol. 2004, 5: 1157-1165. 10.1038/ni1128.
Owley T, Walton L, Salt J, Guter SJ, Winnega M, Leventhal BL, Cook EH: An open-label trial of escitalopram in pervasive developmental disorders. J Am Acad Child Adolesc Psychiatry. 2005, 44: 343-348. 10.1097/01.chi.0000153229.80215.a0.
King BH, Wright DM, Handen BL, Sikich L, Zimmerman AW, McMahon W, Cantwell E, Davanzo PA, Dourish CT, Dykens EM, Hooper SR, Jaselskis CA, Leventhal BL, Levitt J, Lord C, Lubetsky MJ, Myers SM, Ozonoff S, Shah BG, Snape M, Shernoff EW, Williamson K, Cook EH: Double-blind, placebo-controlled study of amantadine hydrochloride in the treatment of children with autistic disorder. J Am Acad Child Adolesc Psychiatry. 2001, 40: 658-665. 10.1097/00004583-200106000-00010.
Arnold LE, Aman MG, Cook AM, Witwer AN, Hall KL, Thompson S, Ramadan Y: Atomoxetine for hyperactivity in autism spectrum disorders: placebo-controlled crossover pilot trial. J Am Acad Child Adolesc Psychiatry. 2006, 45: 1196-1205. 10.1097/01.chi.0000231976.28719.2a.
Shea S, Turgay A, Carroll A, Schulz M, Orlik H, Smith I, Dunbar F: Risperidone in the treatment of disruptive behavioral symptoms in children with autistic and other pervasive developmental disorders. Pediatrics. 2004, 114: e634-e641. 10.1542/peds.2003-0264-F.
Resetkova E, Morita T, Akasu F, Carayon P, Volpe R: In vitro effects of cytokines and human thyroglobulin on the induction of antibody-secreting cells in patients with auto-immune thyroid disease. Clin Invest Med. 1993, 16: 256-264.
Shah Y, Morimura K, Gonzalez F: Expression of Peroxisome Proliferator-Activated Receptor-{gamma} in Macrophage Suppresses Experimentally-Induced Colitis. Am J Physiol Gastrointest Liver Physiol. 2006
Dubuquoy L, Rousseaux C, Thuru X, Peyrin-Biroulet L, Romano O, Chavatte P, Chamaillard M, Desreumaux P: PPARgamma as a new therapeutic target in inflammatory bowel diseases. Gut. 2006, 55: 1341-1349. 10.1136/gut.2006.093484.
Snider LA, Swedo SE: PANDAS: current status and directions for research. Mol Psychiatry. 2004, 9: 900-907. 10.1038/sj.mp.4001542.
Swedo SE, Garvey M, Snider L, Hamilton C, Leonard HL: The PANDAS subgroup: recognition and treatment. CNS Spectr. 2001, 6: 419-6.
Acknowledgements
The authors wish to acknowledge the financial assistance of the Autism Research Institute (San Diego, CA) and dedicate this study to the memory of its founder Dr Bernard Rimland,
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
MB and AG were the primary physicians who treated the patients, and carried out behavioral testing to determine if the medication was helping their patients. CK prepared the first draft of the paper, and analyzed the data. DLF organized and analyzed the data, contributed to the original idea to treat ASD patients, helped write and edit the manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Boris, M., Kaiser, C.C., Goldblatt, A. et al. Effect of pioglitazone treatment on behavioral symptoms in autistic children. J Neuroinflammation 4, 3 (2007). https://doi.org/10.1186/1742-2094-4-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1742-2094-4-3