Abstract
Background
Aggressive behavior in animals is important for survival and reproduction. Identifying the underlying genes and environmental contexts that affect aggressive behavior is important for understanding the evolutionary forces that maintain variation for aggressive behavior in natural populations, and to develop therapeutic interventions to modulate extreme levels of aggressive behavior in humans. While the role of neurotransmitters and a few other molecules in mediating and modulating levels of aggression is well established, it is likely that many additional genetic pathways remain undiscovered. Drosophila melanogaster has recently been established as an excellent model organism for studying the genetic basis of aggressive behavior. Here, we present the results of a screen of 170 Drosophila P-element insertional mutations for quantitative differences in aggressive behavior from their co-isogenic control line.
Results
We identified 59 mutations in 57 genes that affect aggressive behavior, none of which had been previously implicated to affect aggression. Thirty-two of these mutants exhibited increased aggression, while 27 lines were less aggressive than the control. Many of the genes affect the development and function of the nervous system, and are thus plausibly relevant to the execution of complex behaviors. Others affect basic cellular and metabolic processes, or are mutations in computationally predicted genes for which aggressive behavior is the first biological annotation. Most of the mutations had pleiotropic effects on other complex traits. We characterized nine of these mutations in greater detail by assessing transcript levels throughout development, morphological changes in the mushroom bodies, and restoration of control levels of aggression in revertant alleles. All of the P-element insertions affected the tagged genes, and had pleiotropic effects on brain morphology.
Conclusion
This study reveals that many more genes than previously suspected affect aggressive behavior, and that these genes have widespread pleiotropic effects. Given the conservation of aggressive behavior among different animal species, these are novel candidate genes for future study in other animals, including humans.
Similar content being viewed by others
Background
Aggressive behavior in animals is important for survival and reproduction. Aggression is used for self-defense against con-specifics and predators, in acquisition of territory, food and mates, and in defense of progeny. However, aggressive behaviors are energetically expensive, and there is likely an intermediate optimum level of aggression in natural populations from a balance between the energy and risk associated with territory defense and the need to find food and mates. In social organisms such as humans or other primates, an extremely high level of aggression can be disadvantageous or even pathological.
Aggressive behaviors are quantitative traits, with continuous variation in natural populations due to segregating alleles at multiple interacting loci, with effects that are sensitive to developmental and environmental conditions. Identifying the underlying genes and environmental contexts that affect aggressive behavior is necessary if we are to understand the evolutionary forces acting to maintain variation for aggressive behavior in natural populations, and to develop therapeutic interventions to modulate extreme levels of aggressive behavior in humans. Much of the work on the neurobiology and genetics of aggressive behavior to date has used the candidate gene approach to establish the role of neurotransmitters in mediating and modulating levels of aggression. In particular, biogenic amines and genes affecting their biosynthesis and metabolism have been associated with aggressive behavior in mammals [1–7] and invertebrates [8–15]. The neurotransmitters nitric oxide and γ-aminobutyric acid also modulate aggressive behavior in mammals [15–17]. Neuropeptide Y affects aggression in mammals [18–20] and its invertebrate homolog, neuropeptide F, affects aggression in Drosophila [13]. In Drosophila, correct expression of the male-specific transcript of fruitless, a gene in the sex-determination pathway, is required for executing male aggressive behaviors [12, 21–23].
Drosophila exhibit territorial behavior in wild populations [24, 25], and therefore represent an excellent model system for investigating the genetic basis of aggressive behavior. Recent studies have revealed a much more complex genetic architecture of Drosophila aggression than suggested by targeted evaluation of candidate genes in biologically plausible pathways. Many novel loci affecting aggressive behavior have been implicated from widespread correlated responses in gene expression to selection for divergent levels of aggressive behavior [26, 27]. Subsequent evaluation of aggressive behavior of mutations in a sample of these candidate genes revealed that a large number indeed affected aggressive behavior, including mutations in a member of the cytochrome P450 gene family [26]; and genes involved in electron transport, catabolism, nervous system development, G-protein coupled receptor signaling, as well as computationally predicted genes [27, 28]. Analysis of quantitative trait loci (QTLs) affecting variation in aggression between two wild-type strains also identified a complex genetic basis for natural variation in aggressive behavior, characterized by extensive epistasis among QTLs [29]. Complementation tests to mutations at positional candidate genes in the QTL regions also revealed four additional novel loci affecting aggressive behavior [29]. These results motivate a broader screen for mutations affecting Drosophila aggression.
Previously, we developed a highly reproducible and rapid assay to quantify levels of aggression in D. melanogaster males [27]. Here, we employed a modified version of this assay to screen 170 P{GT1} transposable element (P-element) mutant lines that were generated in the same co-isogenic background. All of these lines are viable and fertile as homozygotes; therefore, the mutations are unlikely to be genetic null alleles. This is obviously an essential criterion for evaluating effects of mutations in essential genes on behavioral traits expressed in adults, and the quantitative assay enables detection of mutations with subtle as well as large effects. Further, the exact insertion site of the transposon, and thus the identity of the candidate gene(s) it disrupts, can be readily determined. The same panel of lines has been screened for mutations affecting numbers of sensory bristles [30], resistance to starvation stress [31], sleep [32] and olfactory [33] and locomotor [34] behavior, enabling us to assess pleiotropic mutational effects. We identified 59 mutations in 57 genes that affect aggressive behavior, none of which had been previously implicated to affect aggression. While many of the genes affect the development and function of the nervous system, and are thus plausibly relevant to the execution of complex behaviors, others affect basic cellular and metabolic processes, or computationally predicted genes for which aggressive behavior is the first biological annotation. Most of the mutations had pleiotropic effects on other complex traits. More detailed characterization of nine of the mutations indicated that the P-element insertions affected the tagged genes, and that the mutations had pleiotropic effects on brain morphology.
Methods
Drosophila stocks
Flies were reared on cornmeal/molasses/agar medium under standard culture conditions (25°C, 12:12 hour light/dark cycle). CO2 was used as an anesthetic. All mutant lines are homozygous and contain single P{GT1} transposable element inserts in the w 1118 Canton-S B co-isogenic background, and were constructed as part of the Berkeley Drosophila Gene Disruption Project [35]. Male w 1118 Canton-S B flies were used as the control line.
Quantitative assay for aggressive behavior
Assays were performed on socially experienced, 3–7 day-old male flies. Groups of eight males from the same mutant line were anesthetized 24 hours prior to the assay and placed in vials with food. On the day of the assay, the males were transferred without anesthesia to an empty vial and were deprived of food for 90 minutes, after which they were exposed to a food droplet and given one minute to acclimate to this disturbance. The flies were then observed for an additional one minute, and the total number of aggressive encounters scored as described previously [27]. Behavioral assays were conducted in a behavioral chamber (25°C, 70% humidity) between 8 a.m. and 11 a.m.
The screen was conducted in 34 blocks of 1–7 mutant lines and the contemporaneous control, with 20 replicate vials for each mutant line and the control line per block. P-element insert lines with significantly different levels of aggression than the control were identified using a one-way fixed effect ANOVA model. Post-hoc Tukey tests were used to determine whether aggression levels of mutant lines in a block differed significantly from that of the control after correcting for multiple tests. In addition, a one-way random effects ANOVA was performed on the entire data set, expressing the aggressive behavior of the mutant lines as deviations from their contemporaneous control. The among line (σ L 2) and within line (σ E 2) variance components were computed, and the broad sense mutational heritability estimated as:

Bioinformatics
Gene ontology categories among P-element insert lines associated with increased or decreased levels of aggression were assessed using DAVID [36] and Babelomics [37]. Only gene ontology categories that applied to greater than 5% of the genes queried were considered in these analyses. Human orthologs of the genes tagged by the P-elements were assessed using FlyAtlas [38].
Generation and verification of revertant lines
Genetic revertants were generated using standard crossing schemes, while preserving the co-isogenic background of the parental and revertant strains [32]. PCR products were sequenced to ascertain whether revertants were genetically precise. Primers were chosen to span either the 5' or 3' site of the original insertion. PCR products were run on 2% agarose gels and compared with a DNA ladder to determine whether they were of the appropriate length. Sequencing reactions were run on the PCR products, and the sequence of each P [-] line was compared with that of the control w 1118 Canton-S B to determine whether the excision of the P-element was precise.
RNA extraction and cDNA generation
Samples were collected in triplicate from each of the following developmental stages: embryos aged 12–14 hours after egg laying (AEL); third instar larvae; pupae aged 8–9 days AEL; and male adults aged 3–5 days post-eclosion, with heads and bodies separated. Whole RNA was extracted using Trizol (GIBCO-BRL, Gaithersburg, MD) and purified using standard procedures. cDNA was generated using 500 ng of whole RNA with reagents from Applied Biosystems (Foster City, CA).
Quantitative reverse transcription-PCR
Primers for quantitative reverse transcription PCR (qPCR) were obtained from Sigma (St. Louis, MO) and targeted gene regions common to all transcripts. cDNA was diluted 1:6 for a concentration of 41.7 ng/μl, and 2 μl cDNA were used for each 10 μl qPCR reaction. Each biological replicate was assessed in three technical replicates. An ABI-7900 sequence detector and protocols from Applied Biosystems were used to perform the qPCR with the SYBR Green detection method. Relative mRNA quantities were standardized using the housekeeping gene Glyceraldehyde-3-phosphate dehydrogenase 1 (Gapdh1). Standardization was conducted on Ct values reported by the ABI-7900 software. Since Ct values are relative exponential measures, standardized values were converted to linearized values as described in Livak and Schmittgen [39] for statistical tests. Differences in gene expression level between the P-element insert line and the control line were tested for statistical significance using two-tailed Student's t-tests.
Whole-mount in situ hybridization
cDNA clones for ed (LD11008), sgl (SD09476), emc (LD10532), pbx (RE16319), Syx4 (RE02884), CG13377 (RE15974), CG32572 (AT02481) and CG3638 (LD20542) were ordered from the Berkeley Drosophila Genome Project. Act5c cDNA was obtained using the High Fidelity PCR System (Roche Applied Science, Indianapolis, IN) on Canton-S genomic DNA using the following primers: 5' ATGTGTGACGAAGAAGTTGCTG, 5' CACGTGGCGTTCACGAAGATT. The 1131 bp fragment was cloned into the pCR ® II-TOPO vector (Invitrogen). Digoxigenin-labeled sense and antisense RNA probes were synthesized by in vitro transcription using the DIG RNA labeling kit (Roche Applied Science). The probes were hydrolyzed at 60°C in buffer containing 200 mM Na2CO3 and 200 mM NaHCO3 (X = (Lo - Ld)/(0.11 × Lo × Ld) with Lo = original length of the transcript in kb; Ld = desired 0.2 kb length), precipitated in ethanol and resuspended in RNase-free water. Whole-mount in situ hybridization was performed using a variation of the protocol described by Tautz and Pfeifle [40]. Signal detection was carried out using anti-digoxigenin-AP Fab fragments (Roche Applied Science). Color development was performed in the dark using 0.5 mg/ml NBT (Roche Applied Science) and 0.25 mg/ml BCIP (Roche Applied Science). Embryos were 0–17 h old. Images were obtained using a light microscope (model BX61; Olympus) and Cell^D 2.6 imaging software.
Immunohistochemistry and morphometric analysis
Immunohistochemical labeling of adult Drosophila brains with anti-fasciclin II MAb 1D4 (Developmental Studies Hybridoma Bank; under the auspices of the NICHD and maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA 52242) and morphometric analyses of mushroom body lobes and ellipsoid body were done as previously described [28]. Measurements were taken for each hemisphere of 10 brains per line. Statistical significance was determined using t-tests for differences between mutant lines and the Canton-S B control line.
Results and discussion
Mutations affecting aggressive behavior
We quantified aggressive behavior of P-element insertional mutations that had been generated in a common isogenic background (Canton S B [35]), as well as the control line (Additional file 1). The 170 P-element lines represented insertions in 148 genes. Approximately one-half of these lines were chosen because they represent mutations in genes that exhibited changes in transcript abundance as a correlated response to artificial selection for aggressive behavior [27]. We also included genes if they had previously been shown to have a mutant phenotype for a different behavior, to examine possible pleiotropic effects of mutations on aggression and other behaviors; or if the P-element tagged a computationally predicted gene, to provide potential biological annotations for these sequences.
The broad sense mutational heritability (H M 2) for aggressive behavior was rather high: H M 2 = 0.432. The high mutational heritability could be due to a few mutants of large effect, or many mutants with smaller effects. Analysis of the effects of individual mutations revealed that the latter was the case. A total of 59 (approximately 35%) of the P-element insert lines exhibited levels of aggression that differed significantly from the control; 27 lines were less aggressive, and 32 lines were more aggressive than the control line (Table 1, Figure 1). The absolute values of the standardized mutational effects (a/σ, where a is one-half of the difference in the mean aggression score of the P-element insert line and the control line, and σ is the phenotypic standard deviation of the control line) of the 59 lines with significantly increased or decreased levels of aggression ranged from 0.28 to 2.27, with a mean of 0.77 (Table 1).
The high proportion of mutations associated with alterations in aggressive behavior is likely in part to be because the screen was enriched for mutations in candidate genes previously implicated to affect aggression [27] and with mutations affecting other quantitative traits [30–34]. However, the large mutational target size for aggressive behavior is consistent with a growing body of evidence that large numbers of loci can affect most quantitative traits [27, 30–34, 41–46].
Gene ontology analysis
The genes tagged by the P-element inserts associated with increased or decreased levels of aggression spanned a variety of gene ontology categories [36, 37] (Figure 2). Many of these genes affect early development, including the development of the nervous system, and are involved in transcriptional regulation, signal transduction, and ATP binding. There is a trend towards differential representation of some gene ontology categories between the lines associated with increased versus decreased levels of aggression (Figure 2), although the differences are not significant due to the small numbers of mutations. For example, approximately 42% of the mutations with low levels of aggression are in genes affecting metabolism, but only approximately 26% of mutations with high levels of aggression fall into this category. A plausible interpretation is that dysfunction of metabolic processes can lead to a lower propensity to expend energy on demanding behaviors. Over 24% of mutations with low levels of aggression affect 'localization'; no mutations with high levels of aggression affect localization. The connection between this biological process and aggressive behavior is not intuitively obvious. Nearly all 59 genes tagged by P-elements that were associated with increased or decreased levels of aggression have orthologs that been implicated in human diseases or disorders (Additional file 2), including susceptibility to schizophrenia, diabetes, deafness, and mental retardation [38].
Gene ontologies of candidate genes with mutations associated with aggressive behavior. (A) Biological Process gene ontology categories. (B) Molecular Function gene ontology categories. The x-axis indicates the percentage of genes in each category for mutations increasing (red bars) and decreasing (blue bars) aggressive behavior.
Pleiotropic effects
Many of the P-element lines included in this screen have previously been examined for mutational effects on numbers of sensory bristles [30], resistance to starvation stress [31], olfactory behavior [33], 24-hour sleep [32] and locomotor reactivity (a startle response [34]). Mutational correlations (r M ) between aggressive behavior and male abdominal bristle number (n = 160, r M = 0.09, P = 0.293), male sternopleural bristle number (n = 160, r M = 0.11, P = 0.183), olfactory avoidance behavior (n = 158, r M = 0.01, P = 0.94), starvation resistance (n = 88, r M = 0.09, P = 0.40), and 24-hour sleep (n = 28, r M = 0.20, P = 0.30) were not significantly different from zero. Mutational correlations could be non-significant if mutations specifically affect aggressive behavior, or if there are pleiotropic effects of mutations affecting aggression on other traits, but the effects are not in the same direction. It is the second explanation which is true – the mutations affecting aggressive behavior are highly pleiotropic, but mutations associated with increases (decreases) in aggressive behavior are not consistently associated with increases (decreases) of resistance to bristle number, starvation stress, olfactory behavior, or sleep (Table 1).
The mutational correlation between aggressive behavior and locomotor startle response, although weak, was significantly different from zero and positive (n = 157, r M = 0.29, P = 0.0002; Figure 3). The mutational correlation for the subset of 58 lines with significantly increased and decreased levels of aggression and for which locomotor startle data was available was not significantly different from that estimated from all 157 lines (r M = 0.40, P = 0.0019). Overall, variation in locomotor startle response only explains 8% of the variation in aggressive behavior. This is largely attributable to a few insert lines with decreased levels of both aggression and duration of the locomotor startle response. However, many lines with increased levels of aggression had reduced locomotor startle responses.
Analysis of P-element excision alleles
Nine lines were chosen for more detailed analysis that had large effects on aggressive behavior, and in which the P-element insertion site was located within the gene or in the presumed 5' regulatory region (Figure 4). The P-element insertions in Actin 5C (Act5C), extra macrochaetae (emc), CG32572, and Syntaxin 4 (Syx4) were associated with decreased levels of aggression; while P-element insertions in pxb, echinoid (ed), sugarless (sgl), CG3638, and CG13377 were associated with increased levels of aggression. We attempted to generate precise revertant alleles of each of these P-element tagged genes, in order to map the mutant phenotype to the P-element insertion. The revertant alleles were sequenced, and at least one precise revertant was identified for each line except CG3638. The effects of imprecise excision alleles were also evaluated for the lines in which no or only a single precise revertant allele was generated.
Structure of nine genes in which mutations affect male aggressive behavior. All genes are oriented 5' to 3', with boxes indicating exons and solid lines indicating introns. Dashed lines represent 5' putative promoter regions. Solid triangles indicate the location of the P-element insertion, with the direction of the triangle indicating the orientation of the insertion.
The aggressive behavior of the revertant alleles was quantified, and for seven of the nine lines the behavior of the precise excision alleles also reverted to control levels, thus mapping the mutant phenotype to the P-element insertion in the tagged gene (Figure 5). The exceptions were emc and CG3638. The emc precise revertant allele only showed partial phenotypic reversion. The behavior of one of the CG3638 imprecise revertants differed only marginally from that of the control (P = 0.04). The failure of the behavior of precise excision alleles to revert to the level of the control could indicate that the insertions of the P-elements in these loci do not cause the mutant phenotype. However, the observation of partial phenotypic reversion in conjunction with reduced levels of expression of CG3638 and emc in the respective mutant alleles is consistent with a complex mutation in the precise excision alleles; for example, local hopping of the P-elements elsewhere in emc.
Mean aggression scores of P -element mutations affecting aggressive behavior and revertant alleles. The mean aggression score is given as the deviation from the contemporaneously tested control line for the mutant lines and up to three revertant alleles. Blue bars indicate significantly (P < 0.05) lower levels of aggression than the control; red bars indicate higher levels of aggression than the control; and green bars indicate no significant difference in mean aggression score from the control. Hatched bars indicate imprecise revertant alleles.
Analysis of gene expression
qPCR analyses were used to assess the effect of the P-element insertion on transcript levels of the tagged genes in the nine lines selected for further characterization. Since many of these genes have roles in development, expression was evaluated in embryos, larvae, pupae, and adults. The adult tissues were separated into heads and headless bodies (with the exception of the sgl mutant line, for which insufficient tissue was obtained to conduct qPCR on pupae or embryos, due to poor viability). All mutant lines were associated with alterations in transcript abundance in one or more developmental stages, confirming that the P-element insertions affect the expression of all tagged genes (Figure 6).
Quantitative reverse transcription-PCR analysis of candidate genes affecting aggressive behavior. Levels of mRNA for each gene (white bars) are depicted relative to the level in the co-isogenic control (black bars). mRNA levels were assessed at four developmental time periods: embryos aged 10–12 h after egg laying (E), third instar larvae (L), pupae (P), and adults (heads [H] and headless bodies [B]). Only larvae and adults could be obtained for sgl mutants. Standard errors were obtained using Ct values normalized to an internal control (Gapdh1). The significance of two-tailed Student's t-tests conducted on linearized Ct values are depicted by asterisks (*: P < 0.05; **: P < 0.01; ***: P < 0.001).
There was no consistent pattern of gene expression changes in mutations associated with decreased levels of aggression. Act5C and CG32572 mutants were associated with increased transcript levels – in embryos, pupae, and adult bodies for Act5C, and in embryos and adult heads for CG32572. Transcript levels in emc mutants were decreased throughout development, but increased in adult bodies. Syx4 mutants had reduced levels of gene expression in embryos and adult bodies, but increased expression in pupae. Mutations associated with increased aggression tended to have decreased levels of transcript abundance at one or more developmental stages. Gene expression was reduced in embryos and adult bodies of CG3638 mutants; in larvae, pupae, and adult heads of ed mutants; in pupae and adult bodies of pxb mutants; and in adult heads of sgl mutants. In contrast, there was an increase of transcript abundance in adult heads of CG13377 mutants.
This analysis shows that none of the mutations affecting aggressive behavior are transcriptional null alleles. The effects of all of the mutations on gene expression varied across development, and between adult heads and bodies. Depending on the developmental time point and/or adult tissue assessed,CG3638, ed, pxb and sgl are hypomorphic mutations; Act5C and CG13377 are hypermorphic mutations; and CG32572, emc and Syx4 are both hypomorphs and hypermorphs. All of the mutations showed significant differences in gene expression from the control in adults, but these differences were apparent in heads of only four of the mutations (CG13377, CG32572, ed and sgl). Further, many of the alterations in gene expression between mutant and control lines were of the order of two-fold or less. These results indicate that even subtle mutational effects on transcription can be associated with large changes in behavior. Because the effects of the mutations on gene expression in pre-adult stages are often much larger than observed for adults, we cannot rule out the possibility that changes in gene expression during development affect adult behavior [33].
The lack of a common pattern of gene expression differences among the mutations affecting increased and decreased levels of aggression suggests that there are multiple mechanisms by which this complex behavior can be altered. Finally, the observation that only four of the nine mutations show changes in gene expression in heads of adult flies indicates that only assessing changes in transcript abundance in heads of lines that are genetically divergent for behavioral traits will underestimate the number of transcripts associated with differences in the trait phenotype [26, 47, 48].
We further characterized the patterns of expression of the nine P-element tagged genes affecting aggressive behavior in wild-type embryos. Consistent with previous results [49–53], Act5C, emc and sgl were expressed in multiple tissues, including the ventral nerve cord for Act5C and emc. CG32572, CG13377, ed and Syx4 were expressed in the central nervous system (Figure 7). Expression outside the nervous system was also observed for most of the genes (data not shown).
Expression of candidate genes affecting aggressive behavior in the embryonic nervous system. The insets show the sense control probes. (A) CG32572, stage 17, ventral view: expression in the ventral cord (highlighted with dotted line). (B) CG13377, stage 14, lateral view: expression in the ventral nerve cord (arrow). (C) CG13377, stage 17, ventral view: expression in the ventral nerve cord (highlighted with dotted line). (D) ed, stage 11, lateral view: expression in the procephalic neuroblasts (arrow) and the neuroblasts forming the ventral nerve cord (arrowheads). (E) emc, stage 14, lateral view: expression in the ventral nerve cord (arrow) and the brain (asterisk). (F) emc, stage 17, ventral view: expression in the ventral nerve cord (highlighted with dotted line). (G) Syx4, stage 13, lateral view: expression in the ventral nerve cord (arrow) and the brain (asterisk). (H) Act5C, stage 16, ventral view: expression in the ventral nerve cord (highlighted with dotted line).
Morphometric analysis of central brain neuropils
Mushroom bodies and the ellipsoid body are central brain neuroplils that have been previously implicated in Drosophila aggressive behavior. Disruption of mushroom body output results in near abolishment of aggression [11], and aberrant morphology of the mushroom bodies and ellipsoid body have been observed in hyper-aggressive mutants [28]. Therefore, we measured the length and width of the alpha and beta lobes of the mushroom bodies, and the surface area of the ellipsoid body, standardizing the values to overall brain size as a function of distance between peduncles (Table 2). There were significant quantitative changes in the length or width of one or both lobes of the mushroom bodies in all mutants except emc, further linking mushroom bodies and aggressive behavior. No significant differences in ellipsoid body area were observed for any of the mutations. The most frequently detected difference in mutants relative to control was an increase in the width of the alpha lobe. Only two of the mutations were associated with decreases in size: Syx4 mutants had shorter beta lobes than controls, and sgl mutants had shorter alpha lobes. Increases in beta lobe measurements were only observed for mutations associated with increased levels of aggression. However, there was no overall correlation between any of the quantitative measurements of brain morphology and aggressive behavior, consistent with previous studies [28, 34] showing that there is no simple relationship between aggressive behavior and brain structure.
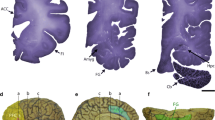
In addition to quantitative alterations in brain morphology, we also observed qualitative morphological defects in both alpha and beta lobes for five of the mutant lines (Figure 8). One of ten brains examined for mutations of CG32572, emc and CG13377 had, respectively, a missing beta lobe, shorter alpha lobe, and an enlarged alpha lobe tip. Two of the ten ed mutant brains had qualitatively thicker beta lobes and thinner alpha lobes than the control, and three of the ten sgl brains had fused beta lobes. Although not completely penetrant, these defects were never observed in the control line.
Gross morphological defects in the mushroom bodies in mutations of candidate genes affecting aggressive behavior. A-H: anti-fasciclin 2 staining of adult brains using the 1D4 monoclonal antibody. Defects are indicated by the white arrows. (A) Canton S B control line. α, alpha lobes of mushroom bodies; β, beta lobes of mushroom bodies. (B) Missing beta lobe in CG32572 mutation. (C) Misrouting of some of the alpha lobe axons leads to thicker beta lobes and thinner alpha lobes in ed mutation. (D) Shorter alpha lobe in emc mutation. (E) Overextension resulting in fusion of the beta lobes in sgl mutation. (F) Alpha lobe tip defect resulting in enlargement of the tip in CG13377 mutation.
Candidate genes affecting aggressive behavior
None of the candidate genes identified in this screen have been previously implicated to affect aggressive behavior. Three of the nine candidate genes characterized in greater detail are computationally predicted genes. CG13377 is predicted to function in binding and metabolism [54]. RNAi-knockdown mutations of CG3638 display reduced phagocytic immune response to Candida albicans cells [55]. All that was previously known about CG32572 is that it is expressed in the testis [38]. Act5C is involved in ATP and protein binding [54], and also has roles in cytokinesis [56, 57] and spermatogenesis [58]. ed has many developmental functions, including the negative regulation of neurogenesis [59], appendage formation [60], and negative regulation of epidermal growth factor signaling [61]. In adults, ed is expressed in ovaries, crop, and male accessory glands [38]. emc is also highly pleiotropic, and functions in peripheral nervous system [62], midgut [63], and spermatid development [64]. pxb mutants have been implicated in olfactory learning and memory [65] and in the smoothened signaling pathway [66]. sgl, like pxb, appears to be involved in smoothened signaling [67], metabolism [54], and biosynthesis [68, 69]. Syx4 has been implicated in synaptic functions [70].
Given that we were able to map the behavioral mutant phenotype to the P-element insertion for seven of the nine mutations characterized in greater detail, and that all of the mutations affected gene expression of the tagged gene, we can predict that the majority of the remaining 48 P-element mutations associated with increased or decreased levels of aggression will also affect the genes into or near which they have inserted. Many of these genes are plausible candidates as they affect the development or the functioning of the nervous system (Guanine nucleotide exchange factor GEF64C, NMDA receptor 1, schizo, tramtrack, Laminin A, longitudinals lacking), and the effects of mutations in neuralized on aggressive behavior have been independently confirmed [28]. Many other genes affect other aspects of development, metabolism or basic cellular processes, or are computationally predicted – these loci would not have been detected had we concentrated on examining aggressive behavior for mutations in only 'plausible' candidate genes.
The general picture emerging from the analysis of quantitative effects of de novo mutations that have been induced in a defined isogenic background is that a large fraction of the genome can potentially affect most quantitative traits, including complex behaviors [30–34]. Consequently, we expect that most genes have pleiotropic effects on multiple traits, and indeed, 55 of the 59 mutations associated with a significant difference in aggressive behavior from the control line had pleiotropic effects on one (19 lines), two (22 lines), three (11 lines) or four (three lines) additional quantitative traits (Table 1). Further, different mutations in the same gene can have a different spectrum of pleiotropic effects [28, 71], and the mutational effects on any one trait can be contingent on genetic background and the environment [28, 33, 72]. Given these complexities, an exhaustive mutational dissection of any complex behavior (or, indeed, any quantitative trait) is not feasible. However, the collection of over 70 mutations affecting aggressive behavior that have been generated in the same isogenic background (this study and [27]) are valuable molecular probes that can be used to gain insight into the key pathways and mechanisms affecting this trait using systems biology approaches [73].
Conclusion
Aggressive behavior is important for survival and reproduction, and is near universal among animals. While the role of neurotransmitters in mediating and modulating levels of aggression is clear, little is known about other genes and pathways affecting aggression. Analysis of aggressive behavior in 170 D. melanogaster P-element mutant lines and their co-isogenic control lines revealed 59 mutations in 57 novel genes affecting aggression. More detailed characterization of nine of the mutations indicated that the P-element insertions affected the tagged genes. Most of the mutations had pleiotropic effects on other complex traits and on morphology of mushroom bodies, central brain neuroplils that have been previously implicated in Drosophila aggressive behavior.
Abbreviations
- AEL:
-
after egg laying
- ANOVA:
-
analysis of variance
- QTL:
-
quantitative trait locus.
References
Brunner HG, Nelen MR, van Zandvoort P, Abeling NG, van Gennip AH, Wolters EC, Kuniper MA, Ropers HH, van Oost BA: X-linked borderline mental retardation with prominent behavioral disturbance: phenotype, genetic localization, and evidence for disturbed monoamine metabolism. Am J Hum Genet. 1993, 52: 1032-1039.
Haller J, Makara GB, Kruk MR: Catecholaminergic involvement in the control of aggression: hormones, the peripheral sympathetic, and central noradrenergic systems. Neurosci Biobehav Rev. 1998, 22: 85-97. 10.1016/S0149-7634(97)00023-7.
Manuck SB, Flory JD, Ferrell RE, Dent KM, Mann JJ, Muldoon MF: Aggression and anger-related traits associated with a polymorphism of the tryptophan hydroxylase gene. Biol Psychiatry. 1999, 45: 603-614. 10.1016/S0006-3223(98)00375-8.
Nelson RJ, Chiavegatto S: Molecular basis of aggression. Trends Neurosci. 2001, 24: 713-719. 10.1016/S0166-2236(00)01996-2.
Han DH, Park DB, Na C, Kee BS, Lee YS: Association of aggressive behavior in Korean male schizophrenic patients with polymorphisms in the serotonin transporter promoter and catecholamine-O-methyltransferase genes. Psychiatr Res. 2004, 129: 29-37. 10.1016/j.psychres.2004.06.013.
de Almeida RM, Ferrari PF, Parmigiani S, Miczek KA: Escalated aggressive behavior: dopamine, serotonin and GABA. Eur J Pharmacol. 526: 51-64. 10.1016/j.ejphar.2005.10.004.
Alia-Klein N, Goldstein RZ, Kriplani A, Logan J, Tomasi D, Williams B, Telang F, Shumay E, Biegon A, Craig IW, Henn F, Wang GJ, Volkow ND, Fowler JS: Brain monoamine oxidase A activity predicts trait aggression. J Neurosci. 2008, 28: 5099-5104. 10.1523/JNEUROSCI.0925-08.2008.
Jacobs ME: Influence of beta-alanine on mating and territorialism in Drosophila melanogaster. Behavior Genetics. 1978, 8: 487-502. 10.1007/BF01067478.
Edwards DH, Kravitz EA: Serotonin, social status, and aggression. Curr Opin Neurobiol. 1997, 7: 812-819. 10.1016/S0959-4388(97)80140-7.
Huber R, Smith K, Delago A, Isaksson K, Kravitz EA: Serotonin and aggressive motivation in crustaceans: altering the decision to retreat. Proc Nat Acad Sci. 1997, 94: 5939-5942. 10.1073/pnas.94.11.5939.
Baier A, Wittek B, Brembs B: Drosophila as a new model organism for the neurobiology of aggression?. J Exp Biol. 2002, 205: 1233-1240.
Certel SJ, Savella MG, Schlegel DC, Kravitz EA: Modulation of Drosophila male behavioral choice. Proc Nat Acad Sci. 2007, 104: 4706-4711. 10.1073/pnas.0700328104.
Dierick HA, Greenspan RJ: Serotonin and neuropeptide F have opposite modulatory effects on fly aggression. Nat Genet. 2007, 39: 678-682. 10.1038/ng2029.
Hoyer SC, Eckart A, Herrel A, Zars T, Fischer SA, Hardie SL, Heisenberg M: Octopamine in male aggression of Drosophila. Curr Biol. 2008, 18: 159-167. 10.1016/j.cub.2007.12.052.
Miczek KA, Fish EW, De Bold JF: Neurosteroids, GABAA receptors, and escalated aggressive behavior. Horm Behav. 2003, 44: 242-257. 10.1016/j.yhbeh.2003.04.002.
Nelson RJ, Demas GE, Huang PL, Fishman MC, Dawson VL, Dawson TM, Snyder SH: Behavioral abnormalities in male mice lacking neuronal nitric oxide synthase. Nature. 1995, 378: 383-386. 10.1038/378383a0.
Rujescu D, Giegling I, Mandelli L, Schneider B, Hartmann AM, Schnabel A, Maurer K, Moller HJ, Serretti A: NOS-I and -III gene variants are differentially associated with facets of suicidal behavior and aggression-related traits. Am J Med Genet B Neuropsychiatr Genet. 2008, 147B: 42-48. 10.1002/ajmg.b.30569.
Gammie SC, Auger AP, Jessen HM, Vanzo RJ, Awad TA, Stevenson SA: Altered gene expression in mice selected for high maternal aggression. Genes Brain Behav. 2007, 6: 432-443. 10.1111/j.1601-183X.2006.00271.x.
Karl T, Herzog H: Behavioral profiling of NPY in aggression and neuropsychiatric diseases. Peptides. 2007, 28: 326-333. 10.1016/j.peptides.2006.07.027.
Karl T, Lin S, Schwarzer C, Sainsbury A, Couzens M, Wittmann W, Boey D, von Horsten S, Herzog H: Y1 receptors regulate aggressive behavior by modulating serotonin pathways. Proc Nat Acad Sci. 2004, 101: 12742-12747. 10.1073/pnas.0404085101.
Chan YB, Kravitz EA: Specific subgroups of FruM neurons control sexually dimorphic patterns of aggression in Drosophila melanogaster. Proc Nat Acad Sci. 2007, 104: 19577-19582. 10.1073/pnas.0709803104.
Lee G, Hall JC: A newly uncovered phenotype associated with the fruitless gene of Drosophila melanogaster : aggression-like head interactions between mutant males. Behav Genet. 2000, 30: 263-275. 10.1023/A:1026541215546.
Vrontou E, Nilsen SP, Demir E, Kravitz EA, Dickson BJ: Fruitless regulates aggression and dominance in Drosophila. Nat Neurosci. 2006, 9: 1469-1471. 10.1038/nn1809.
Hoffmann AA: Heritable variation for territorial success in two Drosophila melanogaster populations. Anim Behav. 1988, 36: 1180-1189. 10.1016/S0003-3472(88)80077-0.
Hoffmann AA: Geographic variation in the territorial success of Drosophila melanogaster males. Behav Genet. 1989, 19: 241-255. 10.1007/BF01065908.
Dierick HA, Greenspan RJ: Molecular analysis of flies selected for aggressive behavior. Nat Genet. 2006, 38: 1023-1031. 10.1038/ng1864.
Edwards AC, Rollmann SM, Morgan TJ, Mackay TF: Quantitative genomics of aggressive behavior in Drosophila melanogaster. PLoS Genet. 2006, 2: e154-10.1371/journal.pgen.0020154.
Rollmann S, Zwarts L, Edwards A, Yamamoto A, Callaerts P, Norga K, Mackay TF, Anholt R: Pleiotropic effects of Drosophila neuralized on complex behaviors and brain structure. Genetics. 2008, 179: 1327-1336. 10.1534/genetics.108.088435.
Edwards AC, Mackay TF: Quantitative trait loci for aggressive behavior in Drosophila melanogaster. Genetics.
Norga KK, Gurganus MC, Dilda CL, Yamamoto A, Lyman RF, Patel PH, Rubin GM, Hoskins RA, Mackay TF, Bellen HJ: Quantitative analysis of bristle number in Drosophila mutants identifies genes involved in neural development. Curr Biol. 2003, 13: 1388-1396. 10.1016/S0960-9822(03)00546-3.
Harbison ST, Yamamoto AH, Fanara JJ, Norga KK, Mackay TF: Quantitative trait loci affecting starvation resistance in Drosophila melanogaster. Genetics. 2004, 166: 1807-1823. 10.1534/genetics.166.4.1807.
Harbison ST, Sehgal A: Quantitative genetic analysis of sleep in Drosophila melanogaster. Genetics. 2008, 178: 2341-2360. 10.1534/genetics.107.081232.
Sambandan D, Yamamoto A, Fanara JJ, Mackay TF, Anholt RR: Dynamic genetic interactions determine odor-guided behavior in Drosophila melanogaster. Genetics. 2006, 174: 1349-1363. 10.1534/genetics.106.060574.
Yamamoto A, Zwarts L, Callaerts P, Norga K, Mackay TF, Anholt RR: Neurogenetic networks for startle-induced locomotion in Drosophila melanogaster. Proc Nat Acad Sci. 2008, 105: 12393-12398. 10.1073/pnas.0804889105.
Bellen HJ, Levis RW, Liao G, He Y, Carlson JW, Tsang G, Evans-Holm M, Hiesinger PR, Schulze KL, Rubin GM, Hoskins RA, Spradling AC: The BDGP gene disruption project: single transposon insertions associated with 40% of Drosophila genes. Genetics. 2004, 167: 761-781. 10.1534/genetics.104.026427.
Dennis G, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA: DAVID: database for annotation, visualization, and integrated discovery. Genome Biol. 2003, 4: P3-10.1186/gb-2003-4-5-p3.
Al-Shahrour F, Minguez P, Tarraga J, Montaner D, Alloza E, Vaquerizas JM, Conde L, Blaschke C, Vera J, Dopazo J: BABELOMICS: a systems biology perspective in the functional annotation of genome-scale experiments. Nucleic Acids Res. 2006, 34: W472-476. 10.1093/nar/gkl172.
Chintapalli VR, Wang J, Dow JA: Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat Genet. 2007, 39: 715-720. 10.1038/ng2049.
Livak KJ, Schmittgen TD: Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001, 25: 402-408. 10.1006/meth.2001.1262.
Tautz D, Pfeifle C: A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals translational control of the segmentation gene hunchback. Chromosoma. 1989, 98: 81-85. 10.1007/BF00291041.
Anholt RR, Lyman RF, Mackay TF: Effects of single P-element insertions on olfactory behavior in Drosophila melanogaster. Genetics. 1996, 143: 293-301.
Lyman RF, Lawrence F, Nuzhdin SV, Mackay TF: Effects of single P-element insertions on bristle number and viability in Drosophila melanogaster. Genetics. 1996, 143: 277-292.
Mackay TF, Heinsohn SL, Lyman RF, Moehring AJ, Morgan TJ, Rollmann SM: Genetics and genomics of Drosophila mating behavior. Proc Nat Acad Sci. 2005, 102 (Suppl 1): 6622-6629. 10.1073/pnas.0501986102.
Morozova TV, Anholt RR, Mackay TF: Transcriptional response to alcohol exposure in Drosophila melanogaster. Genome Biol. 2006, 7: R95-10.1186/gb-2006-7-10-r95.
Morozova TV, Anholt RR, Mackay TF: Phenotypic and transcriptional response to selection for alcohol sensitivity in Drosophila melanogaster. Genome Biol. 2007, 8: R231-10.1186/gb-2007-8-10-r231.
Jordan KW, Carbone MA, Yamamoto A, Morgan TJ, Mackay TF: Quantitative genomics of locomotor behavior in Drosophila melanogaster. Genome Biol. 2007, 8: R172-10.1186/gb-2007-8-8-r172.
Toma DP, White KP, Hirsch J, Greenspan RJ: Identification of genes involved in Drosophila melanogaster geotaxis, a complex behavioral trait. Nat Genet. 2002, 31: 349-353.
Cirelli C, LaVaute TM, Tononi G: Sleep and wakefulness modulate gene expression in Drosophila. J Neurochem. 2005, 94: 1411-1419. 10.1111/j.1471-4159.2005.03291.x.
Burn TC, Vigoreaux JO, Tobin SL: Alternative 5C actin transcripts are localized in different patterns during Drosophila embryogenesis. Dev Biol. 1989, 131: 345-355. 10.1016/S0012-1606(89)80008-9.
Tobin SL, Cook PJ, Burn TC: Transcripts of individual Drosophila actin genes are differentially distributed during embryogenesis. Dev Genet. 1990, 11: 15-26. 10.1002/dvg.1020110104.
Cubas P, Modolell J, Ruiz-Gomez M: The helix-loop-helix extramacrochaetae protein is required for proper specification of many cell types in the Drosophila embryo. Development. 1994, 120: 2555-2566.
Binari RC, Staveley BE, Johnson WA, Godavarti R, Sasisekharan R, Manoukian AS: Genetic evidence that heparin-like glycosaminoglycans are involved in wingless signaling. Development. 1997, 124: 2623-2632.
Haerry TE, Heslip TR, Marsh JL, O'Connor MB: Defects in glucuronate biosynthesis disrupt wingless signaling in Drosophila. Development. 1997, 124: 3055-3064.
Wilson RJ, Goodman JL, Strelets VB: FlyBase: integration and improvements to query tools. Nucleic Acids Res. 2008, 36: D588-593. 10.1093/nar/gkm930.
Stroschein-Stevenson SL, Foley E, O'Farrell PH, Johnson AD: Identification of Drosophila gene products required for phagocytosis of Candida albicans. PLoS Biol. 2006, 4: e4-10.1371/journal.pbio.0040004.
Echard A, Hickson GR, Foley E, O'Farrell PH: Terminal cytokinesis events uncovered after an RNAi screen. Curr Biol. 2004, 14: 1685-1693.26. 10.1016/j.cub.2004.08.063.
Eggert US, Kiger AA, Richter C, Perlman ZE, Perrimon N, Mitchison TJ, Field CM: Parallel chemical genetic and genome-wide RNAi screens identify cytokinesis inhibitors and targets. PLoS Biol. 2004, 2: e379-10.1371/journal.pbio.0020379.
Noguchi T, Miller KG: A role for actin dynamics in individualization during spermatogenesis in Drosophila melanogaster. Development. 2003, 130: 1805-1816. 10.1242/dev.00406.
Ahmed A, Chandra S, Magarinos M, Vaessin H: Echinoid mutants exhibit neurogenic phenotypes and show synergistic interactions with the Notch signaling pathway. Development. 2003, 130: 6295-6304. 10.1242/dev.00796.
Laplante C, Nilson LA: Differential expression of the adhesion molecule Echinoid drives epithelial morphogenesis in Drosophila. Development. 2006, 133: 3255-3264. 10.1242/dev.02492.
Bai J, Chiu W, Wang J, Tzeng T, Perrimon N, Hsu J: The cell adhesion molecule Echinoid defines a new pathway that antagonizes the Drosophila EGF receptor signaling pathway. Development. 2001, 128: 591-601.
Prokopenko SN, He Y, Lu Y, Bellen HJ: Mutations affecting the development of the peripheral nervous system in Drosophila : a molecular screen for novel proteins. Genetics. 2000, 156: 1691-1715.
Saito M, Awasaki T, Hama C: Genetic analyses of essential genes in cytological region 61D1–2 to 61F1–2 of Drosophila melanogaster. Mol Genet Genomics. 2002, 268: 446-454. 10.1007/s00438-002-0770-6.
Castrillon DH, Gonczy P, Alexander S, Rawson R, Eberhart CG, Viswanathan S, DiNardo S, Wasserman SA: Toward a molecular genetic analysis of spermatogenesis in Drosophila melanogaster : characterization of male-sterile mutants generated by single P element mutagenesis. Genetics. 1993, 135: 489-505.20.
Dubnau J, Chiang AS, Grady L, Barditch J, Gossweiler S, McNeil J, Smith P, Buldoc F, Scott R, Certa U, Broger C, Tully T: The staufen/pumilio pathway is involved in Drosophila long-term memory. Curr Biol. 2003, 13: 286-296. 10.1016/S0960-9822(03)00064-2.
Inaki M, Kojima T, Ueda R, Saigo K: Requirements of high levels of Hedgehog signaling activity for medial-region cell fate determination in Drosophila legs: identification of pxb, a putative Hedgehog signaling attenuator gene repressed along the anterior-posterior compartment boundary. Mech Dev. 2002, 116: 3-18. 10.1016/S0925-4773(02)00119-3.
Nybakken K, Perrimon N: Heparan sulfate proteoglycan modulation of developmental signaling in Drosophila. Biochim Biophys Acta. 2002, 1573: 280-291.
Perrimon N, Bernfield M: Specificities of heparan sulphate proteoglycans in developmental processes. Nature. 2000, 404: 725-728. 10.1038/35008000.
Ghabrial A, Luschnig S, Metzstein MM, Krasnow MA: Branching morphogenesis of the Drosophila tracheal system. Annu Rev Cell Dev Biol. 2003, 19: 623-647. 10.1146/annurev.cellbio.19.031403.160043.
Littleton JT: A genomic analysis of membrane trafficking and neurotransmitter release in Drosophila. J Cell Biol. 2000, 150: F77-82. 10.1083/jcb.150.2.F77.
Rollmann SM, Magwire MM, Morgan TJ, Ozsoy ED, Yamamoto A, Mackay TF, Anholt RR: Pleiotropic fitness effects of the Tre1-Gr5a region in Drosophila melanogaster. Nat Genet. 2006, 38: 824-829. 10.1038/ng1823.
Fedorowicz GM, Fry JD, Anholt RR, Mackay TF: Epistatic interactions between smell-impaired loci in Drosophila melanogaster. Genetics. 1998, 148: 1885-1891.
Anholt RR, Dilda CL, Chang S, Fanara JJ, Kulkarni NH: The genetic architecture of odor-guided behavior in Drosophila : epistasis and the transcriptome. Nat Genet. 35: 180-184. 10.1038/ng1240.
Acknowledgements
This work was supported by National Institutes of Health grants F31 MH-074161 to ACE and R01 GM-076083 to TFCM. This is a publication of the W. M. Keck Center for Behavioral Biology.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors' contributions
ACE, TFCM, and PC designed the experiments and wrote the paper. ACE performed all behavioral measurements, bioinformatic analyses, molecular analyses of revertant alleles, and statistical analyses of behavioral and gene expression data. LZ performed the whole mount in situ hybridization analyses, immunohistochemistry and morphometric analyses of central brain neuropils, and statistical analysis of the morphometric measurements. AY created revertant alleles. All authors have read and approved the final manuscript.
Electronic supplementary material
12915_2009_237_MOESM1_ESM.xls
Additional file 1: Mean aggression scores (MAS) of 170 P{GT1} insert lines. This file shows the results of the screen of 170 P-element insert lines for alterations in aggressive behavior, the genes tagged by the P-elements, the mean aggression scores and effects, and gene ontology information. (XLS 57 KB)
12915_2009_237_MOESM2_ESM.xls
Additional file 2: Human diseases associated with P{GT1} insertions in Drosophila genes with aberrant aggressive behavior. This file shows the P-element insert lines with altered aggressive behavior, their mean aggression scores, and diseases in which the human orthologues of the tagged genes have been implicated. (XLS 34 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Edwards, A.C., Zwarts, L., Yamamoto, A. et al. Mutations in many genes affect aggressive behavior in Drosophila melanogaster . BMC Biol 7, 29 (2009). https://doi.org/10.1186/1741-7007-7-29
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1741-7007-7-29