Abstract
A recent review in Allergy, Asthma, and Clinical Immunology suggested that eosinophils play a minor role, if any, in the inflammatory spectrum of asthma and allergic inflammation. The article that dealt with mast cells suggested that the presence of these important cells within the smooth muscle layer in asthmatic airways renders this cell type primal in asthma and an obvious and important target for therapy. This article proposes that in a complex inflammatory milieu characterizing the complex syndromes we call asthma, no single cell phenotype is responsible for the condition and thus should be a sole target for therapeutic strategies. Our reductionist approach to research in asthma and related conditions has provided us with convincing evidence for multiple roles that immune, inflammatory, and structural cell types can play in complex diseases. The next stage in understanding and ameliorating these complex conditions is to move away from the simplistic notion of one cell type being more important than another. Instead, what is needed is to acquire knowledge of intricate and exquisite biological systems that regulate such conditions in both health and disease involving various cell types, mediators, pharmacologically active products, their multifaceted capacities, and their socio-biological networking.
Similar content being viewed by others
Our current understanding of the complex events associated with the immunobiology of inflammation is progressively evolving. Research over the last century has provided an ever-expanding appreciation of the multifactorial and complex nature of the wide spectrum of changes associated with immunity and inflammation. Numerous players and cascades contribute to both up-and downregulation of the potential function and role of various immunologic, structural, and inflammatory cell types and other components in health and disease. The article by Bradding in the previous issue of this journal argued the case for the mast cell being the key cell type in asthma [1]. It was suggested that eosinophils, which are major orchestrators of the pathophysiological changes seen in asthma, could be used as biomarkers of disease phenotype and response to therapy. The author, therefore, proposed that strategies targeted at mast cells, rather than eosinophils, may be a novel therapeutic option for the control of asthma.
The current article is not an attempt to praise the eosinophil and rush to defend its potential role in asthma or to attack or denigrate the role of the mast cell. The aim, instead, is to attract attention to the concept of complexity of systems and to refute the notion that any given disease, and the eventual pathway to its control, may be due to the deleterious action of one prominent cell type. In particular, the author contended that, in contrast to other cell types, including the eosinophils, the presence of mast cells within hypertrophied smooth muscle layers in airway tissues in asthmatic patients [1, 2] is indicative of the importance of this cell type, as a target for therapy.
Eosinophils, Mast Cells, T-Helper 2-Type Response and Allergic Asthma
We owe a debt of gratitude to Paul Ehrlich for first describing both the mast cell and the eosinophil [3]. Early studies consistently identified an association between these two cell types and a number of disease conditions, most of which are now known to be biased toward both innate and adaptive T-helper (Th)2-type response [4]. Th2-type responses are characterized by increases in the levels of interleukin (IL)-4 and other Th2 cytokines (IL-5, IL-9, IL-13, and IL-21), activation and expansion of CD4+ Th2 cells, plasma cells secreting IgE, eosinophils, basophils, and mast cells, all of which can synthesize and release several types of Th2 cytokines [5]. The observed preponderance of eosinophils and mast cells in parasitic helminth infections led to an upsurge in both in vitro and in vivo studies examining the capacity of these cells to influence the inflammatory milieu associated with these infections in favour of the host [6, 7]. It is now known that T cell-dependent recruitment and activation of eosinophils and mast cells are a crucial step toward the control of parasite-induced granulomas in tissues and expulsion of adult worms from the gut [8]. It was during the 1980s that elegant clinical studies pointed to a close statistical correlation between airway tissue damage in asthma and the activation of eosinophils as manifested by secretion of their crystalloid granule-stored cationic proteins [9, 10]. Other studies also identified mast cell hyperplasia as an important component of airway pathology in asthma. Since this discovery, both cell types were subjects of extensive studies to determine their precise roles in the immunopathology of asthma. Mast cells and eosinophils synthesize, store, and release a similar profile of Th2 cytokines. However, whereas mast cells store and release histamine following activation, eosinophils store and release cationic proteins [11]. As previously indicated in the Bradding article, mast cell-derived histamine plays a crucial role in the induction of bronchial hyperresponsiveness during the early phase of asthma [1]. Conversely, the late-phase response, seen in some asthmatics, is associated with activation of eosinophils [12]; direct instillation of major basic protein (MBP), derived from eosinophilic granules, into the lungs of monkeys was shown, like mast cell-derived histamine, to cause bronchospasm and increased smooth muscle responsiveness to methacholine [13].
A major argument advanced by Bradding to support an "executive" role for mast cells in the pathophysiology of asthma is the apparent similarity between the immunopathology of asthma and eosinophilic bronchitis (EB) in spite of the stark differences in physiological derangement between the two conditions [2]. Bradding suggested that a major factor in asthmatic AHR and airway smooth muscle (ASM) dysfunction seen in asthma but not in EB is likely due to the presence of smooth muscle-infiltrating mast cells in asthma, which is absent in EB [1]. This is an excellent argument that confirms the notion that merely counting inflammatory cells may not necessarily indicate a role for such cells in a chronic inflammatory disease; cells playing an effector role must be found at the right place and time during the course of the disease. Interestingly, a similar mechanism has been found for the induction of AHR by eosinophils. Several studies have shown that asthmatic airway tissue, unlike non-asthmatic controls, is characterized by a preponderance of activated eosinophils, releasing MBP, around cholinergic nerve fibres [14, 15]. Following the release of acetylcholine (Ach) from cholinergic nerve endings, Ach binds to M3 receptors on ASM cells; further release of Ach is halted through the activity of M2 receptors on cholinergic nerve endings, limiting ASM constriction [16]. Studies in animal models and humans have shown that eosinophil-derived MBP, found in the vicinity of cholinergic nerve endings in asthmatics, inhibits the ability of M2 muscarinic receptors to halt the release of Ach, leading to airway hyperresponsiveness in asthmatics (AHR) [17, 18]. The role of cholinergic nerve endings and eosinophil-derived MBP in the pathophysiology of EB vis-à-vis asthma is currently unknown. Nonetheless, it is quite instructive to note that the localization of mast cells, in ASM cells or eosinophils, around cholinergic nerve fibres, in different phenotypes of eosinophilic airway diseases, may determine the relative role of each cell type in such conditions.
One Cell, One Disease, One Treatment?
Early studies reporting the preponderance of eosinophils in allergic asthma and the toxicity of their granule-stored mediators indicated the need to focus on understanding eosinophil effector function in vitro and in vivo. This resulted in focused attention on targeting the eosinophil as a major therapeutic strategy for asthma using novel approaches. This included anti-eosinophil strategies, which were particularly relevant since corticosteroids, choice therapies for asthma, were shown to downregulate eosinophil counts in blood, sputum, bronchoalveolar lavage (BAL), and airway tissue. These changes correlated well with symptom improvement and amelioration of disease severity [19]. These studies led to the discovery of IL-5 in the 1980s and identification of the range of its activities, especially its role as the most crucial esoinophil terminal-differentiating cytokine [20, 21]. As a result, major pharmaceutical firms invested widely in the area of IL-5 antagonism with the hope of blocking eosinophil influx into the airway tissue and the subsequent associated inflammatory and damaging sequelae. Animal models, particularly studies in monkeys, optimistically anticipated successful targeting of a single cell phenotype in a complex disease condition [22]. As mentioned in the Bradding review, clinical trials with a humanized anti-IL-5 monoclonal antibody, mepolizumab, were disappointing. Indeed, it was shown that targeting the eosinophil is far more complex than blocking its differentiation at the level of the bone marrow and blood [23].
Following the poor results of mepolizumab, various laboratories sought to understand the reasons behind the apparent failure of this treatment in the management of asthma. To start with, the Leckie and colleagues study was regarded to have been not only well underpowered to appreciate statistical differences in the treatment group but also that the airways of the positive control group were not hyperreactive, [24] rendering the main outcome of airway hyperreactivity (AHR) impossible to assess accurately. Subsequent studies showed similar disappointing results with this antibody, suggesting that eosinophils may not play a significant role in airway hyperresponsiveness. There was also doubt whether the presence of eosinophils in sputum or airway fluids truly reflected those in the airway tissue. Indeed, Flood-Page and colleagues showed that mepolizumab depleted less than 55% of bronchial tissue and bone marrow eosinophils while significantly diminishing blood and BAL fluid eosinophils in treated subjects [25]. Whether this explains the observed lack of effect of anti-IL-5 on AHR remains to be fully addressed. It is interesting that Liu and colleagues later showed a marked reduction in the expression of messenger ribonucleic acid of the surface IL-5 receptor (mIL-5Rα), as well as its intracellular component (mIL-5Rβ), from BAL eosinophils in contrast to circulating blood eosinophils [26]. Further, airway eosinophils were shown not to release eosinophil-derived neurotoxin (EDN) when treated with IL-5 compared with their blood counterparts. This suggests that the function (both survival and mediator release) of BAL eosinophils may be independent of IL-5 [27]. Recent studies have reported that the reduction in blood and sputum eosinophils in mepolizumab-treated subjects had an effective steroid-sparing effect in patients with EB with or without asthma [28].
It is important to note that the development, maturation, and survival of the eosinophil may occur in situ in tissue inflammatory sites. It has been shown that eosinophil progenitors released into the circulation reach tissue sites [29] and can differentiate, in situ [30–32]. Furthermore, eosinophils store and release up to 30 different cytokines, chemokines, and growth factors, [33–35] which may further amplify the inflammatory milieu. As such, in situ production of various eosinophil-activating factors may be important in tissue eosinophil reactions not involving IL-5. More importantly, association studies are notoriously difficult in delineating the role of specific cells or factors in disease since such studies are carried out in patients already diagnosed with asthma. Thus, the use of animal models has extended our understanding of the role of inflammatory cells in the pathophysiology of asthma.
Eosinophils Are Crucial in the Pathophysiology of Asthma: The PHIL Mouse Model
Mouse model sensitization to ovalbumin (OVA) via the intraperitoneal route, followed by intranasal challenge with the same protein, has been used to generate eosinophilic airway inflammation accompanied by AHR and other components of allergic asthma. The advent of genetically modified mouse systems has allowed a carefully planned reductionist approach toward understanding the role of specific factors in the immunopathology of eosinophilic airway inflammation. The use of such models has shown that IL-5-dependent differentiation of eosinophils in the bone marrow and eotaxin-dependent recruitment of eosinophils to lung tissue are very important in the generation of eosinophilic airway inflammation [36–40]. However, a definitive role for eosinophils could not be established until Lee and colleagues developed an eosinophil-deficient transgenic line of mice, the PHIL mouse, using a method involving the developmental ablation of eosinophil peroxidase-expressing progenitor cells through simultaneous activation of the diphtheria toxin A chain protein [41]. Using this model, it was possible to show that the absence of eosinophils resulted in the abrogation of all of the pathophysiologic features of allergic airway inflammation, including ASM hyperreactivity and mucus hypersecretion. However, using this model, it was impossible to show whether the effect of eosinophils was dependent on the release of granule-stored proteins or other factors. A recent study from the same laboratory further confirmed the role of eosinophils in severe asthma; using an allergen-free model that systemically expresses IL-5 in T cells and locally expresses eotaxin-2 in lung epithelial cells, the authors demonstrated that the specific recruitment of eosinophils to the airways resulted in the development of pathological lesions compatible with severe asthma in human [40]. Thus, these studies showed that the eosinophil is sufficient for the genesis of the immunopathological derangements seen in allergic airway inflammation and the expression of the pathophysiological changes associated with severe asthma.
In human studies, eosinophils have also been linked to tissue remodelling, a critical feature of asthma, even in young children. The cytokines thought to be involved, including IL-4, IL-13, [42, 43] and transforming growth factor β (TGF-β), [44] as well as chemokines (eg, RANTES [45]) known to be produced by lymphocytes, are also synthesized, stored and released by eosinophils. These cells may also be involved in airway remodelling through tenascin production; indeed, using an allergen-induced cutaneous model of asthmatic inflammation, it was shown that the release of TGF-β and IL-13 by eosinophils contributes to airway remodelling [46].
Eosinophils and Immune Regulation
Recent studies have shown that eosinophils, in addition to their effector role, may play an immunoregulatory role in the immunopathogenesis of allergic asthma through interaction with T cells. Eosinophils have been shown to influence the function of lymphocytes directly since they express costimulatory molecules essential for interaction with lymphocytes [47] and were shown, at least in mice, to transmigrate to and from lymphoid tissues and to present antigens to lymphocytes, [48, 49] albeit at a lower efficiency than that of professional antigen-presenting cells to naive T cells. This supports the notion that eosinophils can maintain and play a role in immune responses.
Four areas involving the role of the eosinophil in immune system regulation are worth emphasizing. The first relates to the fact that eosinophils naturally home to the thymus during infancy and in the absence of any identifiable "danger signal" [50, 51]. Thus, eosinophils may also be involved earlier in the ontogeny of the immune response, as suggested by studies showing that thymus eosinophils are active participants in MHC class I-restricted deletion of autoreactive T cells during the early neonatal period [52]. Second, eosinophils synthesize, store, and release at least 30 different cytokines, chemokines, and growth factors with the potential to regulate the local (in situ) immune and inflammatory milieu in lymphoid tissue [53]. As such, eosinophil-derived cytokine production may directly influence T-cell selection by dendritic cells and may, therefore, determine the choice between T-cell tolerance or activation. TGF-β, for which the eosinophil is a well-acknowledged source, [54] has also been related to T-lymphocyte subset development [55]. The specific recruitment of eosinophils into lymphoid tissues puts these cells in a position to exert immunomodulatory effects on T cells in eosinophil-associated diseases.
Third, the induction by interferon-γ (IFN-γ) of indoleamine 2,3-dioxygenase (IDO), the rate-limiting enzyme in the oxidative catabolism of tryptophan, may also be a significant and potent mechanism by which dendritic cells induce apoptosis and inhibit proliferation of T-helper cells [56]. Lymphoid-tissue eosinophils, either directly or indirectly, may induce T-cell apoptosis through synthesis and release of IFN-γ, following the ligation of CD28 on eosinophils [57] and subsequent induction of IDO in dendritic cells. Our studies recently showed that eosinophils constitutively express IDO and induce Th1 but not Th2 apoptosis [58]. Eosinophils may, therefore, directly influence T-cell function through tryptophan catabolism via eosinophil constitutive expression of biologically active IDO.
The fourth indication that eosinophils have the capacity to influence T-cell regulation and its inflammatory and damaging sequelae was reports showing that EDN, a cationic protein stored in the crystalloid granules of eosinophils, induced the migration and maturation of dendritic cells [59]. Subsequent studies from the same authors demonstrated that intratracheal instillation of OVA-loaded dendritic cells pretreated with EDN led to the enhancement of an OVA-specific Th2 response in the mouse through direct activation of Toll-like receptor 2 by EDN [60].
Eosinophil as a Marker of Allergic Disease and Asthma Phenotyping
Atopic diseases such as asthma, dermatitis, and rhinitis are classically associated with increased tissue eosinophils [61]. The presence of eosinophils has been correlated with disease severity and bronchial hyperresponsiveness [62]. Despite this association, there is significant heterogeneity among subgroups in asthma and even within individual patients from season to season. Clearly, different inflammatory phenotypes are present in asthmatics [63]. For example, EB is characterized by an increase in airway eosinophils, yet in contrast to asthma, AHR does not appear to be a feature. This raises the question: Why is EB not associated with AHR if eosinophils contribute to AHR? In comparing mild asthma with EB, Brightling and colleagues found that although both groups had eosinophilia, the significant difference in the airway pathology of the asthma patient was the presence of mast cells within the smooth muscle [2]. This mast cell myositis was proposed as the cause of AHR in asthma, which suggested that AHR, a key feature of asthma, involves cells and mediators beyond the eosinophil.
Traditionally, mast cells are responsible for the acute phase of the asthmatic response via IgE-mediated histamine release and smooth muscle stimulation. Mild asthma, by definition, can have AHR and acute periods of bronchospasm, often allergy related. In contrast, patients with mild asthma should not have decreased lung function by spirometry, nor should they show exacerbations requiring hospitalization [64]. Thus, it was important to note that in a subsequent article by many of the same authors interested in mast cell myositis, in moderate to severe asthmatics, management of eosinophils did make a difference in asthma symptoms and outcomes [65]. After a run-in period in which they attempted to gain baseline measurements of control with systemic and inhaled corticosteroids, patients with moderate to severe asthma were randomized to two groups. One group received standard but strict medical therapy based on the guidelines of the British Thoracic Society (BTS). The other group was managed by the same guidelines but with the addition of regular sputum analyses of eosinophilia or nitric oxide (NO) production. The sputum group was closely monitored for direct evidence of eosinophil activity in the airway as a signal to adjust medication accordingly. Unlike the sputum group, the BTS group had only symptoms and lung function to guide therapy, which are the end result of inflammatory damage. There was a very convincing improvement in the outcomes for the eosinophil-controlled group. Sputum eosinophil number and NO production were decreased by 63% and 48%, respectively, compared with the BTS group. The sputum group had lowered AHR, fewer exacerbations, lower prednisone doses, and fewer admissions to hospital while receiving the same inhaled steroid dose. Further, in patients with low eosinophil numbers, the inhaled steroid dose could be lowered in the sputum group.
It appears that because the sputum group provided earlier information about the degree of inflammation reflected by eosinophilia, the asthma in this group could be controlled before eosinophil activity caused damage and subsequent clinical morbidity. Thus, just as the type of animal model is important in the study of eosinophils, depending on the clinical phenotype of the asthma patient, the role of the eosinophil may also vary. Knowing both the inflammatory profile and the clinical categorization of the patient develops a clearer phenotype of the asthma patient and appears to contribute to improved asthma care.
The ultimate goal in asthma therapy will continue to be the development of the most effective anti-inflammatory strategy for individual patients. Thus, the focus may be to correlate the clinical picture of the asthmatic patient with the inflammation in the tissue; such correlations will provide distinct phenotypes of asthma. Eosinophils are extremely sensitive to the effects of glucocorticosteroids. The subpopulation of asthmatics who have a primarily neutrophilic airway inflammation may be better served by an alternative agent to control inflammation than glucocorticosteroids. Underrecognition of ongoing airway inflammation despite clinical remission is a problem for both patients and physicians [66]. Lower airway inflammation can be evaluated safely and in a non-invasive fashion by measuring changes in induced sputum [67]. The use of the latter in evaluating asthmatics has now evolved from the research arena to clinical management [68]. Characterization also depends on the compartment analyzed (sputum, blood, urine). Eosinophils from blood and BAL express different cell surface markers following allergen challenge [69]. Correlating which compartment is the most relevant for clinical response to therapy will also be important.
Paradigm of Convergence
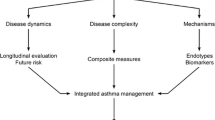
The unified goal of all asthma researchers is to identify therapeutic strategies to reverse the chronic inflammatory response associated with this serious and complex condition. A major and painful lesson acquired through increasing evidence from a wide range of studies is that developing asthma therapy that targets a single inflammatory cell type or a particular cell or inflammatory product will not lead to a significant remission of asthma symptoms. After all, patients do not uniformly present with the same clinical features of the disease or the same pathologic inflammatory profiles. All of this confirms the paradigm that asthma is a complex heterogeneous set of syndromes and that treating the multitude of changes occurring in the asthmatic airways requires targeting the complexity of the inflammatory cell phenotype environment with a view to reducing the clinical manifestation of the condition. We fully agree that conservative reductionist approaches have, hitherto, served us well in understanding the potential functions of various cells and molecules but may not necessarily generate the best therapeutic options in a disease characterized by complex cellular and cytokine dyscrasia. Of note is the fact that the most effective asthma drugs to date, corticosteroids, target multiple cell types involved in the chronic inflammation that characterizes asthma. Our next major challenge is to begin thinking about how we target systems and make sense of the extraordinary amount of data obtained from the complex setting of multiple phenotypes of asthma and tissue and organ environment in an individualized fashion, avoiding the current "shotgun" approach of therapeutic intervention. As well, it is likely that targeting a functional pathway common to all inflammatory cellular infiltrates in asthma may prove to be an excellent future strategy in asthma therapy. In this regard, a major bias of our laboratory proposes that targeting specific elements involved in intracellular mechanisms regulating exocytosis leading to mediator release, a common feature of all immune and inflammatory cells, may be an efficient path to pursue in this regard.
In conclusion, any attempt at ascribing a precise role for any given immune, inflammatory, or structural cell type in asthma will be constantly hampered by the recognition that asthma is not a single clinical entity and should, therefore, not be expected to be associated with or dependent on a single cell-type function. One disease, one cell type, one molecule will never be a viable approach to such a complex condition. Instead, what is needed is a better and more accurate phenotyping of asthma and a greater appreciation of patient-directed, rather than disease-directed, therapies. It is our firm conviction that complex diseases require complex therapeutic approaches.
Note
Experimental work described in this review was supported by the Canadian Institutes of Health Research and the Alberta Heritage Foundation for Medical Research. R.M. is an Alberta Heritage Medical Senior Investigator.
References
Bradding P: Allergy Asthma Clin Immunol. 2008, 4: 84-90. 10.1186/1710-1492-4-2-84.
Brightling CE, Bradding P, Symon FA: Mast-cell infiltration of airway smooth muscle in asthma. N Engl J Med. 2002, 346: 1699-705. 10.1056/NEJMoa012705.
Ehrlich P: Ueber die specifischen granulationen des Blutes. Arch Anat Physiol LPZ. 1879, 3: 571-
Anthony RM, Rutitzky LI, Urban JF: Protective immune mechanisms in health infection. Nat Rev Immunol. 2007, 7: 975-87. 10.1038/nri2199.
Tato CM, Laurence A, O'Shea JJ: Helper T cell differentiation enters a new era: le roi est mort; vive le roi!. J Exp Med. 2006, 203: 809-12. 10.1084/jem.20060522.
Butterworth AE, Vadas MA, Wassom DL: Interactions between human eosinophils and schistosomula of Schistosoma mansoni. II. The mechanism of irreversible eosinophil adherence. J Exp Med. 1979, 150: 1456-71. 10.1084/jem.150.6.1456.
Butterworth AE, David JR: Eosinophil function. N Engl J Med. 1981, 304: 154-6. 10.1056/NEJM198101153040305.
Meeusen EN, Balic A, Bowles V: Cells, cytokines and other molecules associated with rejection of gastrointestinal nematode parasites. Vet Immunol Immunopathol. 2005, 108: 121-5. 10.1016/j.vetimm.2005.07.002.
Filley WV, Holley KE, Kephart GM, Gleich GJ: Identification by immunofluorescence of eosinophil granule major basic protein in lung tissues of patients with bronchial asthma. Lancet. 1982, 2: 11-6. 10.1016/S0140-6736(82)91152-7.
Frigas E, Gleich GJ: The eosinophil and the pathophysiology of asthma. J Allergy Clin Immunol. 1986, 77: 527-37. 10.1016/0091-6749(86)90341-6.
Moqbel R, Coughlin JJ: Differential secretion of cytokines. Sci STKE. 2006, 338: pe26-10.1126/stke.3382006pe26.
Cieslewicz G, Tomkinson A, Adler A: The late, but not early, asthmatic response is dependent on IL-5 and correlates with eosinophil infiltration. J Clin Invest. 1999, 104: 301-8. 10.1172/JCI7010.
Gleich GJ, Adolphson C: Bronchial hyperreactivity and eosinophil granule proteins. Agents Actions Suppl. 1993, 43: 223-30.
Fryer AD, Adamko DJ, Yost BL, Jacoby DB: Effects of inflammatory cells on neuronal M2 muscarinic receptor function in the lung. Life Sci. 1999, 64: 449-55. 10.1016/S0024-3205(98)00587-6.
Fryer AD, Jacoby DB: Function of pulmonary M2 muscarinic receptors in antigen-challenged guinea pigs is restored by heparin and poly-L-glutamate. J Clin Invest. 1992, 90: 2292-8. 10.1172/JCI116116.
Fryer AD, Maclagan J: Muscarinic inhibitory receptors in pulmonary parasympathetic nerves in the guinea-pig. Br J Pharmacol. 1984, 83: 973-8.
Fryer AD, Stein LH, Nie Z: Neuronal eotaxin and the effects of CCR3 antagonist on airway hyperreactivity and M2 receptor dysfunction. J Clin Invest. 2006, 116: 228-36. 10.1172/JCI25423.
Adamko DJ, Yost BL, Gleich GJ: Ovalbumin sensitization changes the inflammatory response to subsequent parainfluenza infection. Eosinophils mediate airway hyperresponsiveness, m(2) muscarinic receptor dysfunction, and antiviral effects. J Exp Med. 1999, 190: 1465-78. 10.1084/jem.190.10.1465.
Wardlaw AJ, Moqbel R, Kay AB: Eosinophils: biology and role in disease. Adv Immunol. 1995, 60: 151-266. full_text.
Clutterbuck E, Shields JG, Gordon J: Recombinant human interleukin 5 is an eosinophil differentiation factor but has no activity in standard human B cell growth factor assays. Eur J Immunol. 1987, 17: 1743-50. 10.1002/eji.1830171210.
Sanderson CJ: Interleukin-5, eosinophils, and disease. Blood. 1992, 79: 3101-9.
Egan RW, Athwahl D, Chou CC: Pulmonary biology of anti-interleukin 5 antibodies. Mem Inst Oswaldo Cruz. 1997, 92 (Suppl 2): 69-73.
Kips JC, O'Connor BJ, Langley SJ: Effect of SCH55700, a humanized anti-human interleukin-5 antibody, in severe persistent asthma: a pilot study. Am J Respir Crit Care Med. 5700, 167: 1655-9. 10.1164/rccm.200206-525OC.
Leckie MJ, ten Brinke A, Khan J: Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet. 2000, 356: 2144-8. 10.1016/S0140-6736(00)03496-6.
Flood-Page PT, Menzies-Gow AN, Kay AB, Robinson DS: Eosinophil's role remains uncertain as anti-interleukin-5 only partially depletes numbers in asthmatic airway. Am J Respir Crit Care Med. 2003, 167: 199-204. 10.1164/rccm.200208-789OC.
Liu LY, Sedgwick JB, Bates ME: Decreased expression of membrane IL-5 receptor alpha on human eosinophils: I. Loss of membrane IL-5 receptor alpha on airway eosinophils and increased soluble IL-5 receptor alpha in the airway after allergen challenge. J Immunol. 2002, 169: 6452-8.
Liu LY, Sedgwick JB, Bates ME: Decreased expression of membrane IL-5 receptor alpha on human eosinophils: II. IL-5 down-modulates its receptor via a proteinase-mediated process. J Immunol. 2002, 169: 6459-66.
Nair P, Pizzichini M, Kjarsgaard M: Abstract in the American Journal of Respiratory and Critical Care Medicine. Am J Respir Crit Care Med. 2008, 177: A568-
Denburg JA: Bone marrow in atopy and asthma: hematopoietic mechanisms in allergic inflammation. Immunol Today. 1999, 20: 111-3. 10.1016/S0167-5699(98)01423-6.
Cameron L, Christodoulopoulos P, Lavigne F: Evidence for local eosinophil differentiation within allergic nasal mucosa: Inhibition with soluble IL-5 receptor. J Immunol. 2000, 164: 1538-45.
Simon HU, Yousefi S, Schranz C: Direct demonstration of delayed eosinophil apoptosis as a mechanism causing tissue eosinophilia. J Immunol. 1997, 158: 3902-8.
Eidelman DH, Minshall E, Dandurand RJ: Evidence for major basic protein immunoreactivity and interleukin 5 gene activation during the late phase response in explanted airways. Am J Respir Cell Mol Biol. 1996, 15: 582-9.
Levi-Schaffer F, Lacy P, Severs NJ: Association of granulocyte-macrophage colony-stimulating factor (GM-CSF) with the crystalloid granules of human eosinophils. Blood. 1995, 85: 2579-86.
Moqbel R, Hamid Q, Ying S: Expression of mRNA and immunoreactivity for the granulocyte/macrophage colony-stimulating factor (GM-CSF) in activated human eosinophils. J Exp Med. 1991, 174: 749-52. 10.1084/jem.174.3.749.
Anwar AR, Moqbel R, Walsh GM: Adhesion to fibronectin prolongs eosinophil survival. J Exp Med. 1993, 177: 839-43. 10.1084/jem.177.3.839.
Justice JP, Borchers MT, Crosby JR: Ablation of eosinophils leads to a reduction of allergen-induced pulmonary pathology. Am J Physiol Lung Cell Mol Physiol. 2003, 284: L169-78.
Shen HH, Ochkur SI, McGarry MP: A causative relationship exists between eosinophils and the development of allergic pulmonary pathologies in the mouse. J Immunol. 2003, 170: 3296-305.
Lee NA, McGarry MP, Larson KA: Expression of IL-5 in thymocytes/T cells leads to the development of a massive eosinophilia, extramedullary eosinophilopoiesis, and unique histopathologies. J Immunol. 1997, 158: 1332-44.
Foster PS, Hogan SP, Ramsay AJ: Interleukin 5 deficiency abolishes eosinophilia, airways hyperreactivity, and lung damage in a mouse asthma model. J Exp Med. 1996, 183: 195-201. 10.1084/jem.183.1.195.
Ochkur SI, Jacobsen EA, Protheroe CA: Coexpression of IL-5 and eotaxin-2 in mice creates an eosinophil-dependent model of respiratory inflammation with characteristics of severe asthma. J Immunol. 2007, 178: 7879-89.
Lee JJ, Dimina D, Macias MP: Defining a link with asthma in mice congenitally deficient in eosinophils. Science. 2004, 305: 1773-6. 10.1126/science.1099472.
Woerly G, Lacy P, Younes AB: IL-13 release by human eosinophils following CD28-dependent activation. J Leukoc Biol. 2002, 72: 769-79.
Schmid-Grendelmeier P, Altznauer F, Fischer B: Eosinophils express functional IL-13 in eosinophilic inflammatory diseases. J Immunol. 2002, 169: 1021-7.
Wong DT, Weller PF, Galli SJ: Human eosinophils express transforming growth factor alpha. J Exp Med. 1990, 172: 673-81. 10.1084/jem.172.3.673.
Velazquez JR, Lacy P, Mahmudi-Azer S: Interleukin-4 and RANTES expression in maturing eosinophils derived from human cord blood CD34+ progenitors. Immunology. 2000, 101: 419-25. 10.1046/j.1365-2567.2000.00104.x.
Phipps S, Ying S, Wangoo A: The relationship between allergen-induced tissue eosinophilia and markers of repair and remodeling in human atopic skin. J Immunol. 2002, 169: 4604-12.
Celestin J, Rotschke O, Falk K: IL-3 induces B7.2 (CD86) expression and costimulatory activity in human eosinophils. J Immunol. 2001, 167: 6097-104.
MacKenzie JR, Mattes J, Dent LA, Foster PS: Eosinophils promote allergic disease of the lung by regulating CD4(+) Th2 lymphocyte function. J Immunol. 2001, 167: 3146-55.
Shi HZ, Humbles A, Gerard C: Lymph node trafficking and antigen presentation by endobronchial eosinophils. J Clin Invest. 2000, 105: 945-53. 10.1172/JCI8945.
Matthews AN, Friend DS, Zimmermann N: Eotaxin is required for the baseline level of tissue eosinophils. Proc Natl Acad Sci USA. 1998, 95: 6273-8. 10.1073/pnas.95.11.6273.
Contreiras EC, Lenzi HL, Meirelles MN: The equine thymus microenvironment: a morphological and immunohistochemical analysis. Dev Comp Immunol. 2004, 28: 251-64. 10.1016/S0145-305X(03)00134-4.
Throsby M, Herbelin A, Pleau JM, Dardenne M: CD11c+ eosinophils in the murine thymus: developmental regulation and recruitment upon MHC class I-restricted thymocyte deletion. J Immunol. 2000, 165: 1965-75.
Lacy P, Moqbel R: Molecular mechanisms in eosinophil activation. Chem Immunol. 2000, 76: 134-55. full_text.
Gharaee-Kermani M, Phan SH: The role of eosinophils in pulmonary fibrosis. Int J Mol Med. 1998, 1: 43-53.
Gorelik L, Flavell RA: Transforming growth factor-beta in T-cell biology. Nat Rev Immunol. 2002, 2: 46-53. 10.1038/nri704.
Fallarino F, Grohmann U, Vacca C: T cell apoptosis by tryptophan catabolism. Cell Death Differ. 2002, 9: 1069-77. 10.1038/sj.cdd.4401073.
Woerly G, Roger N, Loiseau S: Expression of CD28 and CD86 by human eosinophils and role in the secretion of type 1 cytokines (interleukin 2 and interferon gamma): inhibition by immunoglobulin a complexes. J Exp Med. 1999, 190: 487-95. 10.1084/jem.190.4.487.
Odemuyiwa SO, Ghahary A, Li Y: Cutting Edge: Human eosinophils can regulate T-cell subset selection through indoleamine 2, 3-dioxygenase. J Immunol. 2004, 173: 5909-13.
Yang D, Rosenberg HF, Chen Q: Eosinophil-derived neurotoxin (EDN), an antimicrobial protein with chemotactic activities for dendritic cells. Blood. 2003, 102: 3396-403. 10.1182/blood-2003-01-0151.
Yang D, Chen Q, Su SB: Eosinophil-derived neurotoxin acts as an alarmin to activate the TLR2-MyD88 signal pathway in dendritic cells and enhances Th2 immune responses. J Exp Med. 2008, 205: 79-90. 10.1084/jem.20062027.
Gleich GJ, Adolphson CR, Leiferman KM: The biology of the eosinophilic leukocyte. Annu Rev Med. 1993, 44: 85-101. 10.1146/annurev.me.44.020193.000505.
Woodruff PG, Khashayar R, Lazarus SC: Relationship between airway inflammation, hyperresponsiveness, and obstruction in asthma. J Allergy Clin Immunol. 2001, 108: 753-8. 10.1067/mai.2001.119411.
O'Donnell RA, Frew AJ: Is there more than one inflammatory phenotype in asthma?. Thorax. 2002, 57: 566-8. 10.1136/thorax.57.7.566.
Holloway JW, Yang IA, Holgate ST: Interpatient variability in rates of asthma progression: can genetics provide an answer?. J Allergy Clin Immunol. 2008, 121: 573-9. 10.1016/j.jaci.2008.01.007.
Green RH, Brightling CE, McKenna S: Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet. 2002, 360: 1715-21. 10.1016/S0140-6736(02)11679-5.
Toorn van den LM, Overbeek SE, de Jongste JC: Airway inflammation is present during clinical remission of atopic asthma. Am J Respir Crit Care Med. 2001, 164: 2107-13.
Pizzichini E, Pizzichini MM, Efthimiadis A: Indices of airway inflammation in induced sputum: reproducibility and validity of cell and fluid-phase measurements. Am J Respir Crit Care Med. 1996, 154 (2 Pt 1): 308-17.
Jayaram L, Parameswaran K, Sears MR, Hargreave FE: Induced sputum cell counts: their usefulness in clinical practice. Eur Respir J. 2000, 16: 150-8. 10.1034/j.1399-3003.2000.16a27.x.
Mengelers HJ, Maikoe T, Brinkman L: Immunophenotyping of eosinophils recovered from blood and BAL of allergic asthmatics. Am J Respir Crit Care Med. 1994, 149 (2 Pt 1): 345-51.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Moqbel, R., Odemuyiwa, S.O. Allergy, Asthma, and Inflammation: Which Inflammatory Cell Type Is More Important?. All Asth Clin Immun 4, 150 (2008). https://doi.org/10.1186/1710-1492-4-4-150
Published:
DOI: https://doi.org/10.1186/1710-1492-4-4-150