Abstract
Background
When given during the course of puberty, anastrozole (A), an aromatase inhibitor, has been shown to increase the predicted adult height (PAH) of GH-deficient (GHD) boys treated with recombinant human growth hormone (rhGH). Our study questioned whether this treatment could retain some of its effects in non-GHD adolescent boys if started only at the very end of puberty, a time when rhGH treatment is denied to short adolescents who have almost reached their final height.
Objective
To explore the effect on adult height of a combination of rhGH and A, compared with rhGH alone, at the end of puberty in boys with idiopatic short stature (ISS).
Methods
A prospective randomized study comparing rhGH + A and rhGH was conducted in 24 healthy adolescent boys aged 15.2 ± 1.2 yrs with serum testosterone at adult levels and a faltering growth velocity <3.5 cm/yr leading to a predicted adult height (PAH) <2.5 SDS. Treatments were stopped when growth velocity became <10 mm in 6 months or when height was close to 170 cm. A historical group of ISS adolescents (N = 17) matched for puberty and growth was used for comparison.
Results
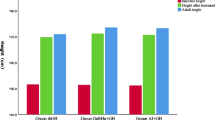
IGF1 levels remained within normal limits in all treated patients. Mean treatment duration was 19 months in the rhGH + A group and 11.5 months in the rhGH group (P = 6.10−4). Adult height reached 168.4 ± 2.6 cm in the rhGH + A group and 164.2 ± 5.6 cm in the rhGH group (P < 0.02). Adult height was 160.1 ± 2.8 cm in the historical controls.
Conclusion
A combination of rhGH and A, started at the very end of puberty, seems to allow boys with ISS to reach a greater adult height than rhGH alone. Larger trials are needed to confirm this preliminary observation.
Similar content being viewed by others
Introduction
Idiopathic short stature (ISS) describes a heterogeneous group of children of unknown etiology [1–4] who become adults of short stature [5–18]. Based on general considerations on the tolerability of short stature by adults [19–30], and on the limited height benefit that is considered to result from years of a costly treatment whose long term safety has been questioned (see Discussion) [31–37], the use of recombinant human growth hormone (rhGH) to increase the height of healthy children with ISS remains debated. The prerequisites for the use of rhGH in ISS set by the FDA are that other diagnoses are excluded, that the presenting height is < −2.25 SDS for age and sex, and that adult stature is expected to be < −2.0 SDS [2]. Several reviews of studies on treatment with rhGH in ISS [1–3, 38–40] concluded that a mean gain in predicted adult height (PAH) of ~5-7 cm can be expected following an average of 5.4 years of treatment. More meaningful information comes from studies that have provided adult height values [12–18, 41–44]. In fact, the different studies showed different rhGH-induced height gains [5, 12–18, 41–52], for reasons that are most clearly discussed in the study by Sotos and al [41]. The potential growth promoting effect of starting rhGH administration at the very end of puberty, months to years after height peak velocity is passed, has not been explored yet. At his particular moment, the fusion of epiphyseal plates of the long bones governs the tempo of growth deceleration; when growth velocity falls under 15 mm per 6 months, cessation of growth is expected to occur within the next two years [53–56].
The use of aromatase inhibitors for promoting growth has been recently reviewed [57–63] and a debate has started in the pediatric endocrinology community regarding the benefit/risk ratio of these drugs [64, 65]. In non- growth hormone deficient (GHD) boys with ISS and/or delayed puberty, aromatase inhibitors effectively delay bone maturation and thereby increase PAH [66–69]. In a Finnish study, 23 boys aged 15.1 years with delayed puberty were randomly allocated to 1 year of letrozole or placebo. Both groups also received testosterone injections for 6 months and were evaluated 18 months after initiation of therapy [66]. A third, nonrandomized group received no treatment. PAH increased by 5.1 cm with letrozole vs 0.3 cm with placebo. The nonrandomized, untreated controls gained 2 cm. In a follow-up study [68], the near-adult height of the letrozole-treated group was 6.9 cm more than the placebo group, positive results that might have been affected by a selection bias at start of treatment [57]. In another 2-year randomized study of 91 Iranian boys with a constitutional delay of growth and puberty, letrozole increased PAH more than placebo [69]. In a Finnish study, 30 boys with ISS aged 9.0–14.5 years were randomly allocated to receive either letrozole or placebo for 2 years [67]. Most participants (81% and 93%, respectively) had not entered puberty at the start of the study, and 44% after 2 years. Height at start was < −2 SDS and mean bone age < 14 years. Letrozole-treated boys showed growth velocities similar to those receiving placebo, and again bone age advanced less with letrozole therapy, thus the PAH increased by 5.9 cm However, when reevaluating the results in 23/30 six years after starting the study, the difference in PAH was no longer statistically significant (166.5 cm in letrozole-treated versus 162.4 cm in placebo-treated) [70]. Adult height data are not available for these trials [57].
The efficacy of anastrozole co-treatment with rhGH has been investigated in GHD boys in two studies from the same US center [71, 72]. An open-label pilot study on 20 patients treated for 1 year did not show an effect on PAH [71]. In a later study, 52 male adolescents treated with rhGH for GH deficiency were randomly allocated to co-treatment with A or placebo for 1–3 years [72]. At entry, serum testosterone was in a 1–3 ng/ml range. PAH increased in the A-treated group by 6.7 cm after 3 years, whereas only 1 cm of PAH gain was observed in the placebo group [72]. The decrease in growth velocity during the course of the study was greater in the placebo group than in the A group at 36 months [61, 72] No adult height data are available to date.
These results paved the way for testing the combination of rhGH + A in adolescents with ISS who are finishing their growth. Indeed, we were not aware of any trial having tested the combination of rhGH and A in ISS during the late stage of near-ending growth, likely because adolescents, families, and most pediatric endocrinologists believe it would be too late for rhGH to allow a significant gain in stature. The current pilot trial questioned this belief in a sample of adolescents with predicted adult short stature. When their growth velocity drops, boys of short stature realize that they will not be able to reach an adult height acceptable for them (see an example in Figure 1).
Pubertal growth and response to rhGH + A treatment in a representative adolescent. Height of a patient showing actual growth (continuous dotted line), age at take off (ATO), age at peak growth velocity (APV), age at rhGH onset, predicted growth trajectory, response to GH administration and final height. HG = height gain. HI = height increase.
Methods
Patients
Based on previous results with aromatase inhibitors, a minimal sample size was calculated to allow detecting a 6 cm increase in final height by adding A to rhGH with an alpha of 0.05 and a power of 0.80. We included 24 adolescents who consulted us between March 2004 and September 2009. They were selected on the following inclusion criteria: a complete or near complete sexual maturation (testes volume >12 ml and adult testosterone levels), a reliable series of height measurements over puberty showing a decelerating growth velocity equal or less than 3.5 cm/yr during the preceding 6 months, leading to a PAH < −2.5 SDS (see our model for calculation in lower section). We chose this height because a well-conducted quality-of-life (QOL) study set 160 cm as the threshold for observing a negative impact of short stature on male adults [19]. Although only a fraction of these adolescents fulfilled the strict definition of ISS at time of entry (because their height was not < −2SDS), they would have fulfilled the definition at adult ages, given the PAH. Although a mix of short stature and mildly advanced puberty would seem a more accurate definition for approximately half of the adolescent boys, we used the generic term “ISS” to define their category for simplification. GH deficiency was excluded by a stimulation test with a GH peak value superior to 15 ng/ml. This cut-off threshold was chosen instead of the usual 5–10 ng/ml value to adjust to the age of the participants and ensure that no studied adolescent had any degree of GH deficiency. Subtle forms of dyschondrosteosis or other chondrodysplasia were excluded by radiographs of the forearm, spine, hand, pelvis and leg. TSH levels were normal. All adolescents were healthy.
The growth trajectory could be modeled in the adolescents from their “Carnet de Santé”, a national pediatric health booklet where height and weight are reported by pediatricians or general practitioners during the period of growth [73]. The pubertal growth spurt trajectory was approximated using the height and age at the two inflection points that mark the onset of acceleration (take-off) and starting deceleration (peak growth velocity), respectively (Figure 1 and Tables 1 and 2) [53, 54]. Height measurements were performed independently at onset and end of visit, using a stadiometer with 0.11% precision (SD/mean). Testes volume was evaluated with a direct measurement of their major and minor axes and calculation of the corresponding ellipsoid.
The same investigators (GK, AR, PB) evaluated bone age at the hand independently according to Greulich and Pyle [74] and the degree of closing of the femoral inferior and tibial superior growth plates according to O'Connor and Roche [75, 76] then averaged their estimations. These evaluations were blind to treatment allocation.
The parents were informed by written material about the uncertainties of rhGH or A benefits at this age, about the results of the rhGH and A safety studies, and knew the position of the national agencies before giving their written informed consent to the trial according to the French rules of bioethics.
A group of historical controls was formed with 17 untreated adolescent males with ISS matched for testosterone, bone age and growth velocity (3 were older brothers of adolescents from the rhGH group, 3 from the rhGH + A group). These subjects have consulted our center for ISS without a strong enough motivation for considering GH treatment. We used the data collected from their Carnet de santé and final height to model their growth trajectory. Knee score was available in only 9 of them. Other missing data include a precise following of growth trajectory at the end of adolescence and serial IGF1 measurements
Randomization and treatment protocol
The randomization procedure was chosen to allow the parallel treatment of participating children under each regimen. Initially, our three-arm protocol randomly allocated 1 subject per group of 3 adolescents (allocation was drawn at pre-inclusion in the trial) to rhGH, rhGH + A, or no treatment. After three years and inclusion of six groups (N = 18), a primary refusal had occurred in 4/6 subjects in the untreated group, who sought rhGH treatment in other centers. Therefore we could not maintain a reasonable rate of accrual for the recruitment of the untreated arm, thus we switched our randomization process to allocation of 2 subjects within groups of 4 to rhGH or rhGH + A treatment. We stopped the inclusions after a total of 12 subjects had been included in each treatment arm.
rhGH was given at an initial dose of approximately 0.07 mg/kg.d then adjusted to growth velocity while maintaining serum IGF1 level close to +1SDS. Anastrozole (A) was given at a daily dose of 2 mg. The doses of both rhGH and A were deliberately chosen to be high, based on the short expected duration of trial, in order to maximize the therapeutic effects.
Patients were seen every 3 months until near-end of growth. Treatment was stopped when growth velocity under rhGH treatment was < 10 mm over 6 months (N = 18) or when the adolescent has reached a height near 170 cm (N = 6).
At each consultation, parents and adolescents were asked to fill out a questionnaire that listed known secondary effects of A, including mood changes and neuro-psychic symptoms (depression, nervousness, dizziness, insomnia, weakness), hot flashes, digestive symptoms (stomach pain, nausea, loss of appetite, constipation, diarrhea, vomiting), skin (rash, acne), joint symptoms (arthralgia, arthritis), back pain, muscle pain, headaches.
Biological parameters
Serum IGF1 levels were measured between 7 and 11 am, 12–16 hours after the previous evening rhGH injection. Values at 6, 9, 12, 15, 18 and 24 months were used for monitoring rhGH treatment and were averaged to calculate individual IGF1 means during rhGH administration Serum IGF1 was measured by immunoradiometric assay after ethanol-acid extraction using DSL-5600 Active reagents (Diagnostic Systems Laboratories, Webster, TX). IGF1 SDS calculations were provided by DSL as reported [77]. Intra- and interseries coefficients of variation were 1.5 and 3.7% at 260 ng/ml, and 2.5 and 3.9% at 760 ng/ml. The sensitivity was 4 ng/ml. FSH and LH were measured as reported by a time-resolved fluorometric assay using Delfia reagents (Perkin Elmer Life Sciences, Courtaboeuf, France). Sensitivity for both assays was 0.01 IU/liter. Serum testosterone was measured every six months with a direct RIA (CisBio International, Gif sur Yvette, France). Sensitivity was 0.01 ng/ml (0.05 nmol/liter).
Growth model, calculation and statistics
As pointed by Garn et al. [78] the bone age evaluated at the hand (wrist and phalanges) at the end of puberty does not predict the complete epiphyseal union of long bones of the leg, thus we did not use the Bayley Pinneau method based on the hand. Instead, we modeled the deceleration of growth velocity in each adolescent to be able to extrapolate adult height, using the age (t) and height (h) of each subject accurately measured during the deceleration period, we modeled. We considered that growth velocity thereafter slows uniformly and is terminated within 2 years from the peak growth velocity. The slope p of the curve is approximated from the recorded values at peak growth velocity (t1,h1) and at rhGH onset(t2,h2) as p = (h2-h1)/(t2-t1)). The derivative between rhGH onset and final h is f'(t) = p - p/(tfin-t2) * (t-t2). Integral is f(t) = p*t - p/(tfin-t2)* (1/2*t2 - t2*t) + K. When t = t2, f(t2) = h2, then k = h2 - {p*t2 + p/2(tfin-t2)*t22}. The growth curve is thus made of real values between t1 and t2, then between t2 and tfin of the function that we determined, then becomes null 2 years after peak growth velocity time. We tested the potential benefit of rhGH + A treatment vs rhGH treatment with the Student’s t test and the chi-square test.
Results
The two randomized groups of adolescent boys had comparable characteristics as shown in Table 1 including the pubertal growth spurt trajectory shown in Table 2. None of the studied adolescents had a constitutional delay of puberty, 18/22 had followed a normal maturation pattern. At inclusion, four could be considered early maturing boys according to Sandberg and al [23], with ages at take-off growth 10–12 yrs, age at peak growth velocity 12.5-13 yrs and full sexual development achieved at age 13–13.5 yrs. rhGH treatment was initiated at 15.2 ± 1.2 yrs in the rhGH group and 15.2 ± 0.8 yrs in the rhGH + A group.
The main parameters of the treatment and height evolution are shown in Table 3. A representative growth chart (Figure 1) illustrates what we observed in many children. The 12 adolescents of the rhGH group stopped growing after 11.5 ± 5 months, while those in the rhGH + A group kept growing for 19 ± 6 months (P = 6.10−4), leading to termination of treatment at 16.2 ± 1.1 yrs in the rhGH group and 16.8 ± 0.6 yrs in the rhGH + A group. During the treatment period, all patients maintained their IGF1 level within 0–1.5 SDS (average 0.60 ± 0.45 SDS). Only 5 of the 66 total IGF1 measures exceeded +2SD on one occasion and not one IGF1 value ever exceeded +2.5 SDS.
Adult height was 164.2 ± 5.6 cm in the rhGH alone group and 168.4 ± 2.6 cm in the rhGH + A group (P = 0.019). The difference between adult height and height at rhGH onset was 7.8 ± 5 cm in the rhGH group, and 12.7 ± 5.6 cm in the rhGH + A group (P = 0.023). Individual height increase was variable. For example, 4/12 (33%) adolescents in the rhGH group showed an increase of height <3 cm (mean 1.85 cm, range 0.6 to 2.6 cm) vs 0% in the rhGH + A group, despite comparable knee score and growth velocity at onset of treatment (P = 0.046).
We found that the increase in height correlated closely with the duration of rhGH treatment (R = −0.82, P = 0.01) (Figure 2). The magnitude of the height gain was also negatively correlated with the knee score when both groups were merged (R = −0.59, P = 0.002), not with bone age at the hand. In fact, the correlation of height increase with knee score was strong in the adolescents treated with rhGH alone (R = −0.86, P = 0.01). We also found that the persisting growth velocity at the time of rhGH onset was another predictor of height increase (R = 0.70, P = 0.0015). The failure to respond to rhGH was associated with a higher knee score and a minimal growth velocity at time of rhGH onset.
Correlation between the duration of rhGH administration and height increase. Black dots figure the children treated with rhGH alone, empty circles those treated with rhGH and anastrozole. The height increase up to final height is closely correlated with the duration of rhGH administration according to the equation Y = 70X + 0.025 (R = 0.82, P = 2×10−7).
We found no significant correlation of height increase with baseline IGF1 or with the increase in IGF1 in response to rhGH, nor with other studied parameters in either group or the two merged groups listed in Additional file 1 and Additional file 2.
At entry in the trial, PAH calculated with our equation is 158.2 ± 2.9 cm in the rhGH group and 157.9 ± 3.7 cm in the rhGH + A group. Therefore the difference between reached adult height and PAH before treatment was 5.9 ± 4.5 cm in the rhGH group and 10.5 ± 5.1 cm in the rhGH + A group (P = 0.019). This difference had the expected high degree of correlation with the increase in height from the onset of rhGH administration (Y = 1.0X + 1.8; R = 0.97, P = 5.10−9), and the same predictors (Additional file 1).
The historical controls were comparable to the participants for age, bone age at the hand and epiphyseal closure at knee, testes volume and testosterone levels at entry into the trial (Tables 1 and 2). They reached a mean final adult height of 160.1 ± 2.8 cm (−2.5 SDS), lower than that of adolescents treated with rhGH (P < 0.05) or with rhGH + A (P < 0.0001) (Table 3). This statistical difference should however be considered questionable given the non-randomized nature of the untreated group. True adult height was close to PAH (calculated to be 160.3 ± 5 cm), which validates our predictive equation.
We detected no significant secondary effects of rhGH or A during the short observation period (Additional file 2). Lipid values, testosterone and gonadotropin values during the rhGH + A treatment are presented at Additional file 3.
Discussion
rhGH studies on ISS have focused on childhood or on the beginning of puberty [2, 7, 12–18, 20, 33, 37–52], with the belief that a younger age favors rhGH effects [37]. Growth-promoting effects of aromatase inhibitors, employed alone [66–70, 79, 80] or in conjunction with rhGH [71, 72], have only been explored in pre-pubertal adolescents with ISS [67, 70, 79] or in adolescents with constitutional delay of puberty [66, 68–70] or GH deficiency [71, 72]. Despite an extensive Pubmed search, we were not able to find a single study that tested the effect of rhGH and/or aromatase inhibitors starting during the latest stage of puberty in adolescents with ISS.
Assessment of Benefit and Cost
Using adult height as a gold-standard outcome in growth studies, the current observation suggests that combination of rhGH and A allows for an additional mean height gain of 4.9 cm versus rhGH alone, with a large variability of individual responses. The main predictor of height gain was the duration of treatment, which was longer by 7.5 months in the rhGH + A group. The other predictors of height gain were the knee score, not the largely used hand bone age, and the remaining growth velocity at rhGH onset.
A weakness of our trial is the lack of a true control group. We could only compare the adult heights of our patients with PAH calculated by an equation especially designed for near-ending and decelerating growth. Since this equation proved capable of predicting adult height accurately in historical controls, we felt comfortable to use it for the treated patients. We found that rhGH allowed a height gain close to 6 cm versus PAH, and that addition of A to rhGH may have increased this gain up to 10.5 cm in average. Comparison with non-randomized controls suggests a gain in final adult height of 4 cm with rhGH alone and 8.3 cm with rhGH + A. In summary, the rhGH + A treatment seems capable of increasing the adult height of adolescent boys with ISS by 8–10 cm. We stress however that the latter values should only be considered indicative until randomized controlled trials in ISS adolescents allow a more reliable estimation of the height gain improvement.
The optimal dose and duration of rhGH treatment are debated in children with ISS. The impression is that despite the significant gain in height, many rhGH-treated children remain short as adults, in the lower level of the normal range. This may simply be that most studies have used rhGH doses of 0.16 to 0.26 mg/kg/week, which may not have been adequate [41]. Several dose–response studies in prepubertal children with ISS have explored a wide range of rhGH doses from 0.20-1.75 mg/kg/week [2, 12, 17, 38–40, 43, 50–52, 77]. The benefit obtained seems dose dependent and mean benefits of 7–8 cm for adult height have been reported with doses of 0.32 to 0.4 mg/kg/week [11, 12, 17] consistently with a recent report from the US [41]. Given that the treatment duration was expected to be short in our trial, we selected a rhGH dose around 0.5 mg/kg/week then guided therapy using growth velocity and IGF1 levels. The total rhGH dose delivered to the adolescents with ISS was 21 mg/kg in the rhGH group and 35 mg/kg in the rhGH + A group, respectively. Although given at a higher weekly dosage, these cumulative doses represent only 23% and 38% of the 91 mg/kg totaled by the majority of children with ISS who are treated with a traditionally recommended dose of 0.05 mg/kg.d for an average of 5.4 yrs [2]. Although this is yet speculative, it is thus possible that our regimen of IGF1-based rhGH dosing may offer a dose-sparing and safer mode of therapy, as discussed by Chen and al [51]. Clearly however, it would be premature to draw conclusions from such a small number of children on trial, who are being presented here to stimulate the study of new rhGH regimens for short stature. The intention of our trial was initially to find a mean to rescue short stature at a time of near finished growth. Notably, our approach of rhGH + A administration at the end of puberty cannot be extrapolated to a large proportion of children with ISS before comparison is performed with the traditional prolonged rhGH treatment at lower classical doses starting at younger ages through larger trials.
The cost to the patients (or insurances), for the purchase of rhGH from distributing pharmacies, may be as much as $88,000 to $100,000 per gram in the USA [41]. If our observations are confirmed in larger series of adolescents with ISS, they might contribute to design a cheaper treatment of ISS without compromising height gain. The estimated cost of rhGH therapy compared with no therapy, in 2011, was $47,000 or more per cm [72], depending on unit cost and height gain. If our results were confirmed, the 8–10 cm gain would translate into a cost of $18,000 per cm in the rhGH + A (vs untreated subjects) group, where no treated subject gained less than 4 cm.
We have not evaluated the effect of the treatment or of perceived height gain on the quality of life (QOL) of the participants. Not unexpectedly, all adolescents faced with a small height simply enjoyed the gain of extra height, and regretted only that the gain could not be greater. Our personal opinion is that QOL questionnaires and methodology are not specific enough to reflect people’s thoughts about their height accurately.
Safety issues
Concerns over long-term safety of rhGH have been recently revisited [81–84]. As summarized, rhGH has “an enviable track record of safety” [83]. Recent results have been reassuring [81, 85]. The current trial being maybe the shortest in duration of published studies, safety issues were only limited to short term tolerance.
The utilization and safety of aromatase inhibitors in male subjects were recently reviewed [86]. Lowering estradiol levels within the male physiological range is associated with an increase in levels of LH, FSH and testosterone [87, 88], as observed in the current study and found to be reversible with cessation of treatment. When treated with letrozole at the beginning of puberty, boys showed lower IGF1 levels than controls [89], an observation that was not replicated here under rhGH treatment. As reported [90], we observed a slight and reversible decrease in HDL-cholesterol, but none of the lipid values recorded during A administration could be considered even borderline-abnormal [91]. Letrozole-treated boys with ISS showed no loss of bone density [92, 93], but some mild vertebral deformities were observed in prepubertal boys with ISS or a delayed onset of puberty treated with letrozole for 2 years [71, 93]; we screened but did not observe any vertebral deformities in the studied adolescents. Again, the short duration of our trial does not allow us to draw any conclusion for mid or long term safety, and off label use of aromatase inhibitors for the treatment of short stature is currently not recommended outside a research setting.
Conclusion
If the current results are confirmed, starting treatment of boys with ISS in late adolescence may have several advantages over the classical regimen of rhGH administration to healthy children predicted to be adults of very short stature. First, it allows more mature adolescents to participate in the decision of attempting rhGH treatment, a clear ethical advantage over treating children unable to participate to the decision. Secondly, the advanced epiphyseal fusion and near-ending growth trajectory allow a more accurate prediction of adult height than when it is attempted in earlier ages. Also, the shorter duration of administration may have a cost/benefit advantage.
In summary, a short administration of rhGH and A seems able to increase adult height in adolescents with ISS in their late stage of puberty, more than does rhGH alone, provided that epiphyseal plates are not completely fused at the knee and growth velocity is still significant at time of starting rhGH administration. However, because of the small size of the current trial, the lack of randomization versus untreated short adolescents and the short-term surveillance, we cannot recommend this off label treatment, which should remain in a clinical research setting. Larger RCTs will be needed to establish the cost/benefit ratio of rhGH and A administration when attempted in the late period of pubertal growth.
Authors’ information
AR is pediatric endocrinologist, AL is professor of pediatric endocrinology, PB is professor of pediatric endocrinology and head of pediatric endocrinology, all three at Bicêtre hospital, Paris Sud University.
References
Ranke MB: Towards a consensus on the definition of idiopathic short stature.Horm Res 1996,45(Suppl 2):64–6.
Cohen P, Rogol AD, Deal CL, Saenger P, Reiter EO, Ross JL, et al.: Consensus statement on the diagnosis and treatment of children with idiopathic short stature: a summary of the Growth Hormone Research Society, the Lawson Wilkins Pediatric Endocrine Society, and the European Society for Paediatric Endocrinology Workshop.J Clin Endocrinol Metab 2008, 93:4210–7. 10.1210/jc.2008-0509
Wit JM, Clayton PE, Rogol AD, Savage MO, Saenger PH, Cohen P: Idiopathic short stature: definition, epidemiology, and diagnostic evaluation.Growth Horm IGF Res 2008,18(2):89–110. 10.1016/j.ghir.2007.11.004
Rosenfeld RG, Hwa V: Toward a molecular basis for idiopathic short stature.J Clin Endocrinol Metab 2004,89(3):1066–7. 10.1210/jc.2004-0092
Wit JM, Kamp GA, Rikken B: Spontaneous growth and response to growth hormone treatment in children with growth hormone deficiency and idiopathic short stature.Pediatr Res 1996, 39:295–302.
Rekers-Mombarg LT, Wit JM, Massa GG, Ranke MB, Buckler JM, Butenandt O, et al.: Spontaneous growth in idiopathic short stature.European Study Group Arch Dis Child 1996,75(3):175–80. 10.1136/adc.75.3.175
Wit JM: Idiopathic short stature: reflections on its definition and spontaneous growth.Horm Res 2007,67(Suppl 1):50–7.
Price DA: Spontaneous adult height in patients with idiopathic short stature.Horm Res 1996,45(Suppl 2):59–63.
Ranke MB, Grauer ML, Kistner K, Blum WF, Wollmann HA: Spontaneous adult height in idiopathic short stature.Horm Res 1995,44(4):152–7. 10.1159/000184616
Leschek EW, Rose SR, Yanovski JA, Troendle JF, Quigley CA, Chipman JJ, et al.: Effect of growth hormone treatment on adult height in peripubertal children with idiopathic short stature: a randomized, double-blind, placebo-controlled trial.J Clin Endocrinol Metab 2004,89(7):3140–8. 10.1210/jc.2003-031457
McCaughey ES, Mulligan J, Voss LD, Betts PR: Randomised trial of growth hormone in short normal girls.Lancet 1998,351(9107):940–4. 10.1016/S0140-6736(05)60604-6
Albertsson-Wikland K, Aronson AS, Gustafsson J, Hagenäs L, Ivarsson SA, Jonsson B, et al.: Dose-dependent effect of growth hormone on final height in children with short stature without growth hormone deficiency.J Clin Endocrinol Metab 2008,93(11):4342–50. 10.1210/jc.2008-0707
López-Siguero JP, García-Garcia E, Carralero I, Martínez-Aedo MJ: Adult height in children with idiopathic short stature treated with growth hormone.J Pediatr Endocrinol Metab 2000,13(9):1595–1602.
Coutant R, Rouleau S, Despert F, Magontier N, Loisel D, Limal JM: Growth and adult height in GH-treated children with nonacquired GH deficiency and idiopathic short stature: the influence of pituitary magnetic resonance imaging findings.J Clin Endocrinol Metab 2001,86(10):4649–54. 10.1210/jcem.86.10.7962
Lopez-Siquero JP, Martínez-Aedo MJ, Moreno-Molina JA: Final height after growth hormone therapy in children with idiopathic short stature and a subnormal growth velocity.Acta Pediatr 1996, 85:113–47.
Hindmarsh PC, Brook CG: Final height of short normal children treated with growth hormone.Lancet 1996,348(9019):13–6. 10.1016/S0140-6736(96)01038-0
Wit JM, Rekers-Mombarg LT, Dutch Growth Hormone Advisory Group: Final height gain by GH therapy in children with idiopathic short stature is dose dependent.J Clin Endocrinol Metab 2002,87(2):604–11. 10.1210/jcem.87.2.8225
Buchlis JG, Irizarry L, Crotzer BC, Shine BJ, Allen L, MacGillivray MH: Comparison of final heights of growth hormone-treated vs. untreated children with idiopathic growth failure.The Journal of Clinical Endocrinology & Metabolism 1998,83(4):1075–9. 10.1210/jcem.83.4.4703
Christensen TL, Djurhuus CB, Clayton P, Christiansen JS: An evaluation of the relationship between adult height and health-related quality of life in the general UK population.Clin Endocrinol (Oxf) 2007, 67:407–12. 10.1111/j.1365-2265.2007.02901.x
Coste J, Pouchot J, Carel JC: Height and health-related quality of life: a nationwide population study.J Clin Endocrinol Metab 2012, 97:3231–9. 10.1210/jc.2012-1543
Theunissen NC, Kamp GA, Koopman HM, Zwinderman KA, Vogels T, Wit JM: Quality of life and self-esteem in children treated for idiopathic short stature.J Pediatr 2002,140(5):507–15. 10.1067/mpd.2002.123766
Radcliffe DJ, Pliskin JS, Silvers JB, Cuttler L: Growth hormone therapy and quality of life in adults and children.Pharmacoeconomics 2004, 22:499–524. 10.2165/00019053-200422080-00003
Sandberg DE, Brook AE, Campos SP: Short stature: a psychosocial burden requiring growth hormone therapy?Pediatrics 1994, 94:832–40.
Bullinger M: Psychological criteria for treating children with idiopathic short stature.Horm Res Paediatr 2011,76(Suppl 3):20–3.
Chaplin JE: Growth-related quality of life.Horm Res Paediatr 2011,76(Suppl 3):51–2.
Dunkel L, Wit JM: Developments in idiopathic short stature.Horm Res Paediatr 2011,76(Suppl 3):1–60.
Noeker M: Psychological functioning in idiopathic short stature.Horm Res Paediatr 2011,76(Suppl 3):52–6.
Rekers-Mombarg LT, Busschbach JJ, Massa GG, Dicke J, Wit JM: Quality of life of young adults with idiopathic short stature: effect of growth hormone treatment.Dutch Growth Hormone Working Group Acta Paediatr 1998,87(8):865–70.
Sandberg DE: Psychosocial aspects of short stature and its management: good deeds require good science.Horm Res Paediatr 2011,76(Suppl 3):37–9.
Sandberg DE, Bukowski WM, Fung CM, Noll RB: Height and social adjustment: are extremes a cause for concern and action?Pediatrics 2004,114(3):744–50. 10.1542/peds.2003-1169-L
Rosenbloom AL: Idiopathic short stature: conundrums of definition and treatment.Int J Pediatr Endocrinol 2009., 470378:
Voss LD: Growth hormone therapy for the short normal child: who needs it and who wants it? The case against growth hormone therapy.J Pediatr 2000,136(1):103–6. 10.1016/S0022-3476(00)90058-1
Ambler GR, Fairchild J, Wilkinson DJ: Debate: idiopathic short stature should be treated with growth hormone.J Paediatr Child Health 2013,49(3):165–9. 10.1111/j.1440-1754.2012.02465.x
Guyda HJ: Four decades of growth hormone therapy for short children: what have we achieved?J Clin Endocrinol Metab 1999,84(12):4307–16. 10.1210/jcem.84.12.6189
Rosenbloom AL: Pediatric endo-cosmetology and the evolution of growth diagnosis and treatment.J Pediatr 2011,158(2):187–93. 10.1016/j.jpeds.2010.10.004
Collett-Solberg PF: Update in growth hormone therapy of children.J Clin Endocrinol Metab 2011, 96:573–9. 10.1210/jc.2010-1131
Ranke MB: Treatment of children and adolescents with idiopathic short stature.Nat Rev Endocrinol 2013, 9:325–34. 10.1038/nrendo.2013.71
Finkelstein BS, Imperiale TF, Speroff T, Marrero U, Radcliffe DJ, Cuttler L: Effect of growth hormone therapy on height in children with idiopathic short stature: a meta-analysis.Arch Pediatr Adolesc Med 2002, 156:230–40. 10.1001/archpedi.156.3.230
Bryant J, Baxter L, Cave CB, Milne R: Recombinant growth hormone for idiopathic short stature in children and adolescents.Cochrane Database Syst Rev 2007, CD004440.
Deodati A, Peschiaroli E, Cianfarani S: Review of growth hormone randomized controlled trials in children with idiopathic short stature.Horm Res Paediatr 2011,76(Suppl 3):40–2.
Sotos JF, Tokar NJ: Growth hormone significantly increases the adult height of children with idiopathic short stature: comparison of subgroups and benefit.Int J Pediatr Endocrinol 2014,2014(1):15. 10.1186/1687-9856-2014-15
Loche S, Cambiaso P, Setzu S, Carta D, Marini R, Borrelli P, et al.: Final height after growth hormone therapy in non-growth-hormone-deficient children with short stature.J Pediatr125:196–2000.
Wit JM, Rekers-Mombarg LT, Cutler GB, Crowe B, Beck TJ, Roberts K, et al.: Growth hormone (GH) treatment to final height in children with idiopathic short stature: evidence for a dose effect.J Pediatr 2005, 146:45–53. 10.1016/j.jpeds.2004.08.055
Hintz RL, Attie KM, Baptista J, Roche A: Effect of growth hormone treatment on adult height of children with idiopathic short stature.Genentech Collaborative Group N Engl J Med 1999, 340:502–7.
Lee PA, Savendahl L, Oliver I, Tauber M, Blankenstein O, Ross J, et al.: Comparison of response to 2-years’ growth hormone treatment in children with isolated growth hormone deficiency, born small for gestational age, idiopathic short stature, or multiple pituitary hormone deficiency: combined results from two large observational studies.Int J Pediatr Endocrinol 2012, 2012:22. 10.1186/1687-9856-2012-22
Dahlgren J: Growth outcomes in individuals with idiopathic short stature treated with growth hormone therapy.Horm Res Paediatr 2011,76(Suppl 3):42–5.
Hopwood NJ, Hintz RL, Gertner JM, Attie KM, Johanson AJ, Baptista J, et al.: Growth response of children with non-growth-hormone deficiency and marked short stature during three years of growth hormone therapy. 1993.J Pediatr 1994, 123:215–22.
Hughes IP, Harris M, Choong CS, Ambler G, Cutfield W, Hofman P, et al.: Growth hormone regimens in Australia: analysis of the first 3 years of treatment for idiopathic growth hormone deficiency and idiopathic short stature.Clin Endocrinol (Oxf) 2012, 77:62–71. 10.1111/j.1365-2265.2011.04230.x
Van Gool SA, Kamp GA, Odink RJ, de Muinck Keizer-Schrama SM, de Waal HA D-v, Oostdijk W, et al.: High-dose GH treatment limited to the prepubertal period in young children with idiopathic short stature does not increase adult height.Eur J Endocrinol 2010, 162:653–60. 10.1530/EJE-09-0880
Cohen P, Germak J, Rogol AD, Weng W, Kappelgaard AM, Rosenfeld RG: Variable degree of growth hormone (GH) and insulin-like growth factor (IGF) sensitivity in children with idiopathic short stature compared with GH-deficient patients: evidence from an IGF-based dosing study of short children.J Clin Endocrinol Metab 2010, 95:2089–98. 10.1210/jc.2009-2139
Cohen P, Weng W, Rogol AD, Rosenfeld RG, Kappelgaard AM, Germak J: Dose-sparing and safety-enhancing effects of an IGF-I-based dosing regimen in short children treated with growth hormone in a 2-year randomized controlled trial: therapeutic and pharmacoeconomic considerations.Clin Endocrinol (Oxf) 2014.
Cohen P, Rogol AD, Weng W, Kappelgaard AM, Rosenfeld RG, Germak J: Efficacy of IGF-based growth hormone (GH) dosing in nonGH-deficient (nonGHD) short stature children with low IGF-I is not related to basal IGF-I levels.Clin Endocrinol (Oxf) 2013, 78:405–14. 10.1111/cen.12014
Roche AF: The final phase of growth in stature.Growth, Genetics and Hormones 1989, 5:4–6.
Roche AF: Growth, Maturation and Body Composition. The Fels Longitudinal Study 1929–1991. Press CU; 1992. 104–106 and 132–138
Tanner JM, Whitehouse RH: Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty.Arch Dis Child 1976, 51:170–9. 10.1136/adc.51.3.170
Tanner JM, Whitehouse RH, Marubini E, Resele LF: The adolescent growth spurt of boys and girls of the Harpenden growth study.Ann Hum Biol 1976, 3:109–26. 10.1080/03014467600001231
Wit JM, Hero M, Nunez SB: Aromatase inhibitors in pediatrics.Nat Rev Endocrinol 2011, 8:135–47. 10.1038/nrendo.2011.161
Dunkel L: Update on the role of aromatase inhibitors in growth disorders.Horm Res 2009,71(Suppl 1):57–63.
Dunkel L: Use of aromatase inhibitors to increase final height.Mol Cell Endocrinol 2006, 254–255:207–16.
Shulman DI, Francis GL, Palmert MR, Eugster EA: Use of aromatase inhibitors in children and adolescents with disorders of growth and adolescent development.Pediatrics 2008, 121:e975–83. 10.1542/peds.2007-2081
Mauras N: Strategies for maximizing growth in puberty in children with short stature.Endocrinol Metab Clin North Am 2009, 38:613–24. 10.1016/j.ecl.2009.06.004
Dunkel L: Off-label use of aromatase inhibitors to promote taller stature: is it safe.Horm Res Paediatr 2010, 74:436–7. 10.1159/000317434
Dunkel L: Treatment of idiopathic short stature: effects of gonadotropin-releasing hormone analogs, aromatase inhibitors and anabolic steroids.Horm Res Paediatr 2011,76(Suppl 3):27–9.
Diaz-Thomas A, Shulman D: Use of aromatase inhibitors in children and adolescents: what’s new?Curr Opin Pediatr 2010, 22:501–7. 10.1097/MOP.0b013e32833ab888
Geffner ME: For debate: Aromatase inhibitors to augment height: have we lost our inhibitions?Pediatr Endocrinol Rev 2008, 5:756–9.
Geffner ME: Aromatase inhibitors to augment height: continued caution and study required.J Clin Res Pediatr Endocrinol 2009, 1:256–61. 10.4274/jcrpe.v1i6.256
Wickman S, Sipilä I, Ankarberg-Lindgren C, Norjavaara E, Dunkel L: A specific aromatase inhibitor and potential increase in adult height in boys with delayed puberty: a randomised controlled trial.Lancet 2001, 357:1743–8. 10.1016/S0140-6736(00)04895-9
Hero M, Norjavaara E, Dunkel L: Inhibition of estrogen biosynthesis with a potent aromatase inhibitor increases predicted adult height in boys with idiopathic short stature: a randomized controlled trial.J Clin Endocrinol Metab 2005, 90:6396–402. 10.1210/jc.2005-1392
Hero M, Wickman S, Dunkel L: Treatment with the aromatase inhibitor letrozole during adolescence increases near-final height in boys with constitutional delay of puberty.Clin Endocrinol (Oxf) 2006, 64:510–3. 10.1111/j.1365-2265.2006.02499.x
Salehpour S, Alipour P, Razzaghy-Azar M, Ardeshirpour L, Shamshiri A, Monfared MF, et al.: A double-blind, placebo-controlled comparison of letrozole to oxandrolone effects upon growth and puberty of children with constitutional delay of puberty and idiopathic short stature.Horm Res Paediatr 2010, 74:428–35. 10.1159/000315482
Hero M, Toiviainen-Salo S, Wickman S, Mäkitie O, Dunkel L: Vertebral morphology in aromatase inhibitor-treated males with idiopathic short stature or constitutional delay of puberty.J Bone Miner Res 2010, 25:1536–43. 10.1002/jbmr.56
Mauras N, Welch S, Rini A, Klein KO: An open label 12-month pilot trial on the effects of the aromatase inhibitor anastrozole in growth hormone (GH)-treated GH deficient adolescent boys.J Pediatr Endocrinol Metab 2004, 17:1597–606.
Mauras N, Gonzalez de Pijem L, Hsiang HY, Desrosiers P, Rapaport R, Schwartz ID, et al.: Anastrozole increases predicted adult height of short adolescent males treated with growth hormone: a randomized, placebo-controlled, multicenter trial for one to three years.J Clin Endocrinol Metab 2008, 93:823–31. 10.1210/jc.2007-1559
Vincelet C, Tabone MD, Berthier M, Bonnefoi MC, Chevallier B, Lemaire JP, et al.: Le carnet de santé de l’enfant est-il informatif? Évaluation dans différentes structures de prévention et de soins.Arch Pediatr 2003,10(5):403–9. 10.1016/S0929-693X(03)00086-1
Greulich WaP S: Radiographic Atlas of Skeletal Development of Hand and Wrist. Stanford University Press; 1959.
O’Connor JE, Bogue C, Spence LD, Last J: A method to establish the relationship between chronological age and stage of union from radiographic assessment of epiphyseal fusion at the knee: an Irish population study.J Anat 2008, 212:198–209. 10.1111/j.1469-7580.2007.00847.x
Roche AF, Wainer H, Thissen D: The Knee Joint as a Biological Indicator. New York: Plenum Press; 1975.
Cohen P, Rogol AD, Howard CP, Bright GM, Kappelgaard AM, Rosenfeld RG: Insulin growth factor-based dosing of growth hormone therapy in children: a randomized, controlled study.J Clin Endocrinol Metab 2007, 92:2480–6. 10.1210/jc.2007-0204
Garn SM, Rohman CG, Apfelbaum B: Complete epiphyseal union of the hand.Am J Phys Anthropol 1961, 19:365–72. 10.1002/ajpa.1330190408
Shams K, Cameo T, Fennoy I, Hassoun AA, Lerner SE, Aranoff GS, et al.: Outcome analysis of aromatase inhibitor therapy to increase adult height in males with predicted short adult stature and/or rapid pubertal progress: a retrospective chart review.J Pediatr Endocrinol Metab 2014,27(7–8):725–30.
Durand-Zaleski I: Developments in idiopathic short stature: cost versus allocation of resources.Horm Res Paediatr 2011,76(Suppl 3):33–5.
Bell J, Parker KL, Swinford RD, Hoffman AR, Maneatis T, Lippe B: Long-term safety of recombinant human growth hormone in children.J Clin Endocrinol Metab 2010, 95:167–77. 10.1210/jc.2009-0178
Quigley CA, Gill AM, Crowe BJ, Robling K, Chipman JJ, Rose SR, et al.: Safety of growth hormone treatment in pediatric patients with idiopathic short stature.J Clin Endocrinol Metab 2005,90(9):5188–96. 10.1210/jc.2004-2543
Rosenfeld RG, Cohen P, Robison LL, Bercu BB, Clayton P, Hoffman AR, et al.: Long-term surveillance of growth hormone therapy.J Clin Endocrinol Metab 2012,97(1):68–72. 10.1210/jc.2011-2294
Kemp SF, Kuntze J, Attie KM, Maneatis T, Butler S, Frane J, et al.: Efficacy and safety results of long-term growth hormone treatment of idiopathic short stature.J Clin Endocrinol Metab 2005,90(9):5247–53. 10.1210/jc.2004-2513
Savendahl L, Maes M, Albertsson-Wikland K, Borgstrom B, Carel JC, Henrard S, et al.: Long-term mortality and causes of death in isolated GHD, ISS, and SGA patients treated with recombinant growth hormone during childhood in Belgium, The Netherlands, and Sweden: preliminary report of 3 countries participating in the EU SAGhE study.J Clin Endocrinol Metab 2012, 97:E213–7. 10.1210/jc.2011-2882
De Ronde W, de Jong FH: Aromatase inhibitors in men: effects and therapeutic options.Reprod Biol Endocrinol 2011, 9:93. 10.1186/1477-7827-9-93
Raven G, de Jong FH, Kaufman JM, de Ronde W: In men, peripheral estradiol levels directly reflect the action of estrogens at the hypothalamo-pituitary level to inhibit gonadotropin secretion.J Clin Endocrinol Metab 2006, 91:3324–8. 10.1210/jc.2006-0462
T’Sjoen GG, Giagulli VA, Delva H, Crabbe P, De Bacquer D, Kaufman JM: Comparative assessment in young and elderly men of the gonadotropin response to aromatase inhibition.J Clin Endocrinol Metab 2005, 90:5717–22. 10.1210/jc.2005-0982
Hero M, Ankarberg-Lindgren C, Taskinen MR, Dunkel L: Blockade of oestrogen biosynthesis in peripubertal boys: effects on lipid metabolism, insulin sensitivity, and body composition.Eur J Endocrinol 2006, 155:453–60. 10.1530/eje.1.02226
Jolliffe CJ, Janssen I: Distribution of lipoproteins by age and gender in adolescents.Circulation 2006, 114:1056–62. 10.1161/CIRCULATIONAHA.106.620864
Wickman S, Kajantie E, Dunkel L: Effects of suppression of estrogen action by the p450 aromatase inhibitor letrozole on bone mineral density and bone turnover in pubertal boys.J Clin Endocrinol Metab 2003, 88:3785–93. 10.1210/jc.2002-021643
Hero M, Makitie O, Kroger H, Nousiainen E, Toiviainen-Salo S, Dunkel L: Impact of aromatase inhibitor therapy on bone turnover, cortical bone growth and vertebral morphology in pre- and peripubertal boys with idiopathic short stature.Horm Res 2009, 71:290–7. 10.1159/000208803
Acknowledgements
We thank Baptiste Ormières, from the Biomathematic team of INSERM U986, who set the growth modeling equation and Gabriel Kalifa, a retired professor of Pediatric Radiology, who took part in the radiological interpretation of bone ages and knee scores. We thank the nurses of the Pediatric Endocrinology Dept for their dedication to clinical research.
Disclosure statement
The present study received no grant from industry. PB and AR have had no personal consultancy fees from any of the pharmaceutical companies selling GH for the past five years. PB’s Inserm laboratory received grants from Pfizer, NovoNordisk and Ipsen for growth related and Type 1 diabetes translational research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
AR and PB designed the trial and collected the data, AR, AL and PB treated the patients and organized the trial, PB did the statistical analysis and wrote the manuscript. All authors read and approved the final manuscript.
An erratum to this article can be found online at http://dx.doi.org/10.1186/s13633-017-0043-0.
Electronic supplementary material
13633_2014_368_MOESM2_ESM.doc
Additional file 2:Incidence of known secondary effects of A over the whole period of treatment in the two studied groups.(DOC 30 KB)
13633_2014_368_MOESM3_ESM.doc
Additional file 3:Serum testosterone, gonadotropin levels and lipid parameters in adolescents treated with rhGH +Anastrazole.(DOC 34 KB)
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Rothenbuhler, A., Linglart, A. & Bougnères, P. A randomized pilot trial of growth hormone with anastrozole versus growth hormone alone, starting at the very end of puberty in adolescents with idiopathic short stature. Int J Pediatr Endocrinol 2015, 4 (2015). https://doi.org/10.1186/1687-9856-2015-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1687-9856-2015-4