Abstract
Background
Ageing is associated with a significant reduction in skeletal muscle carnosine which has been linked with a reduction in the buffering capacity of muscle and in theory, may increase the rate of fatigue during exercise. Supplementing beta-alanine has been shown to significantly increase skeletal muscle carnosine. The purpose of this study, therefore, was to examine the effects of ninety days of beta-alanine supplementation on the physical working capacity at the fatigue threshold (PWCFT) in elderly men and women.
Methods
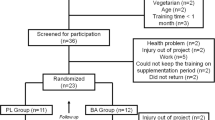
Using a double-blind placebo controlled design, twenty-six men (n = 9) and women (n = 17) (age ± SD = 72.8 ± 11.1 yrs) were randomly assigned to either beta-alanine (BA: 800 mg × 3 per day; n = 12; CarnoSyn™) or Placebo (PL; n = 14) group. Before (pre) and after (post) the supplementation period, participants performed a discontinuous cycle ergometry test to determine the PWCFT.
Results
Significant increases in PWCFT (28.6%) from pre- to post-supplementation were found for the BA treatment group (p < 0.05), but no change was observed with PL treatment. These findings suggest that ninety days of BA supplementation may increase physical working capacity by delaying the onset of neuromuscular fatigue in elderly men and women.
Conclusion
We suggest that BA supplementation, by improving intracellular pH control, improves muscle endurance in the elderly. This, we believe, could have importance in the prevention of falls, and the maintenance of health and independent living in elderly men and women.
Similar content being viewed by others
Background
Carnosine (beta-alanyl-L-histidine), a dipeptide is an efficient hydrogen ion (H+) buffer over the physiological pH range [1, 2]. In muscle, where its concentration is highest, carnosine makes an important contribution to the maintenance of intracellular pH, which is vital for normal muscle function during intense exercise [1]. While the dipeptide is found in both Type I and Type II muscle, its concentration is highest in Type II muscle. Studies in humans and rats have demonstrated an inverse relationship between age and muscle carnosine content [3, 4]. Sarcopenia, the loss in muscle mass with age, is associated with significant reductions in strength, power, and the ability to resist fatigue in elderly men and women [5, 6]. Significant decreases in skeletal muscle and decline in muscle function are clearly evident after the age of fifty [5, 7]. Deterioration of motor coordination, as a result of losses in strength and/or fatigue, is related to an increase in the frequency of falls [6, 8] which repeatedly lead to injury and even deaths among the elderly [9].
A number of investigations have used surface electromyographic (EMG) procedures to identify the power output associated with the onset of neuromuscular fatigue (NMF) during cycle ergometry [10–12]. NMF is typically characterized by an increase in the electrical activity of the working muscles over time [11, 13]. Moritani et al. [11] suggested that the fatigue-induced increase in EMG amplitude is a result of progressive recruitment of additional motor units (MU) and/or an increase in the firing frequency of MUs that have already been recruited. De Vries et al. [13] developed a submaximal discontinuous cycle ergometer test utilizing this concept of progressive muscle activation and introduced the concept of "the physical working capacity at the fatigue threshold" (PWCFT). This utilized the EMG fatigue curves to identify the power output that corresponded to the onset of the NMF threshold. PWCFT represents the highest power output that does not result in a significant increase in the electrical activity of the thigh muscle over time (p > 0.05). PWCFT has been shown to be reliable [10], valid [10] and sensitive to changes in fitness levels and creatine supplementation [14] in older men and women (62 – 73 years). Furthermore, de Vries et al. [10] suggested that PWCFT in elderly men and women may be more appropriate than the assessment of physical work capacity by measurement of maximal oxygen uptake capacity (i.e., V02 max). In addition, de Vries et al. [10] reported PWCFT was a more sensitive measure of monitoring changes, in comparison to fitness tests that depend upon heart rate/power output relationships and/or maximal cardiac output.
Recently, Harris et al. [2] and Hill et al. [15] have shown that beta-alanine (BA) supplementation can significantly increase skeletal muscle carnosine levels, and that the increase is correlated to improvements in exercise performance. In addition, Stout et al. [16, 17] used the PWCFT test to examine the effect of BA supplementation in young (18 to 30 years) men and women, and reported significant increases in PWCFT of 12 to 15% with BA supplementation. Accordingly, Stout et al. [16, 17] suggested that the increase in PWCFT was the result of a BA induced increase in skeletal muscle carnosine, increasing the muscle's capacity to buffer H+ during exercise. However, the effect of BA supplementation on PWCFT in elderly men and women is presently unknown. In theory, increasing skeletal muscle carnosine levels, should improve muscle buffering capacity, leading to a delay in fatigue in certain types of exercise. These adaptations may translate into improved functional capacity during daily living tasks and quality of life. The purpose of this study, therefore, was to examine the effects of ninety days of BA supplementation on PWCFT in elderly men and women.
Methods
Subjects
Twenty-six elderly men and women (Table 1) from independent-living communities in South Florida volunteered to participate in the study. None of the participants had any previous history of BA supplementation and maintained their regular activity and dietary patterns throughout the study. It should be noted; however, that BA is available from meat contained in the diet, although the amount ingested is highly variable according to the meat ingested, and of course quantity. Each participant had a physician's approval to participate in the study, and completed a health history questionnaire prior to partaking. None of the subjects had major orthopedic surgery within the previous year, history of asthma, heart or pulmonary disease, uncontrolled hypertension, and were not taking any medications that would interfere with exercise. All procedures were approved by the Institutional Review Board for Human Subjects. Prior to the initiation of the study, each participant was advised of any possible risks before providing written informed consent.
Experimental design
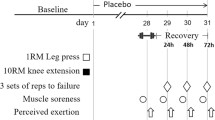
Immediately following pre-testing, the participants were randomly assigned to one of two treatment conditions using a double-blind placebo controlled design: (a) BA: 2.4 grams of beta-alanine in clear gelatin capsule (CarnoSyn™, Natural Alternatives International, San Marcos, CA, USA), n = 12; or (b) PL: 2.4 grams of microcrystalline cellulose in clear gelatin capsule, n = 14. Each capsule contained 800 mg and was identical in appearance. One capsule was ingested three times per day for ninety days with meals, then within 3 to 5 days participants were post-tested. During the course of the study, the participants were asked to maintain their normal dietary and activity pattern and refrain from starting additional nutritional supplements and nonprescription drugs. In addition, the participants were instructed to refrain from exhaustive physical exercise, caffeine, and alcohol consumption for 24 hours prior to testing. Upon each visit to the testing area, the participants were questioned to assure that they had complied with the investigator's instructions and that they followed the supplementation protocol as directed. Subjects also kept a three-day food diary prior to the pre- and post-testing to ensure caloric intake was consistent.
Electromyographic (EMG) measurements
A bipolar (2.54 cm center-to-center) surface electrode (Quinton Quick prep silver-silver chloride) arrangement was placed on the right thigh over the lateral portion of the vastus lateralis muscle, midway between the greater trochanter and the lateral condyle of the femur. The reference electrode was placed over the spinous process of the 7th cervical vertebrae. Interelectrode impedance was kept below 5000 ohms by careful abrasion of the skin. The raw EMG signals were pre-amplified (gain: × 1000) using a differential amplifier (EMG100C, Biopac Systems, Inc., Santa Barbara, CA), sampled at 1000 Hz, and stored on a personal computer for off-line analysis. The EMG signals were later bandpass filtered from 10–500 Hz (2nd order Butterworth filter) and expressed as root mean square (rms) amplitude values (uVrms) by software (AcqKnowledge v3.7, Biopac Systems, Inc., Santa Barbara, CA).
Determination of PWCFT
The PWCFT values were determined using the EMG amplitude values from the vastus lateralis muscle from the methods previously described by de Vries et al. [13]. The initial work rate for each participant was determined by the principal investigator, based on the participant's estimate of his or her physical fitness. The subjects began pedaling (with toe clips) at 50 rpm on a calibrated, electronically-braked cycle ergometer (Corval 400, Quinton Instruments, Seattle, WA). Power output was increased 10 to 20 W for each two-minute stage of the discontinuous protocol. Rest intervals between bouts were sufficiently long to allow resting heart rate to return within 10 bpm of that obtained upon arrival to the laboratory. During each two-minute bout, six 10-second EMG samples were recorded from the vastus lateralis. The PWCFT was determined by averaging the highest power output that resulted in a non-significant (p > 0.05; single-tailed t-test) slope value for the EMG amplitude vs. time relationship and with the lowest power output that resulted in a significant (p ≤ 0.05) slope value [13].
Test-retest reliability for the PWCFT test was determined from 11 participants measured 14 days apart. The intraclass correlation coefficient (ICC) was 0.83 (SEM = 8.4 W). No significant difference (p > 0.05) was noted between the mean PWCFT values from trial 1 (45.9 ± 17.3 W) to trial 2 (48.3 ± 18.1 W). These results were similar to de Vries et al. [13] who reported no significant (p > 0.05) difference from trial 1 (108.8 ± 53.3 W) and trial 2 (109.1 ± 49.4 W) and an ICC of 0.97 in 16 well-trained elderly men and women (67.6 ± 5.6 years). The difference in mean PWCFT values between the current participants versus the de Vries et al. [13] could be due to the mean age difference or trained [13] versus untrained (current study) status of the participants.
Statistical Analysis
Data were analyzed using 2 × 2 [treatment (BA vs. PL) × time (pre- vs. post)] repeated measures analysis of variance (ANOVA). If a significant interaction or main effect occurred, follow-up analyses using dependent samples t-tests were run. Prior to all statistical analyses, the alpha level was set to p = 0.05 to determine statistical significance. Data were analyzed using SPSS version 12.0 (SPSS Inc., Chicago, IL) software.
Results
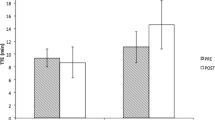
A significant interaction was evident between treatment and time for PWCFT (p = 0.007). A dependent samples t-test demonstrated an increase in PWCFT (28.6%) from pre- to post-supplementation with BA (p < 0.05), but no significant change for the PL group (Figure 1). Furthermore, 67% of the individuals in the BA group demonstrated improvements from pre- to post-PWCFT values, compared to 21.5% of the individuals in the PL group (Figure 2).
The percent change values for the physical working capacity at fatigue threshold (PWCFT) from pre- to post-test for each individual in the BA and placebo treatment groups, respectively. Sixty-seven percent of the individuals in the BA group demonstrated improvements from pre- to post-PWCFT values, compared to 21.5% of the individuals in the placebo group.
Discussion
Data from this study suggest that ninety days of BA supplementation may increase physical working capacity in elderly men and women. These findings may be clinically significant, as a decrease in functional capacity to perform daily living tasks has been associated with an increase in mortality [18], primarily due to increased risk of falls [9]. Further, deVries et al. [13] and Alexander et al. [8] have suggested that falls may be related to fatigue-induced deterioration of motor coordination. Thus, an improved resistance to fatigue, as reported in this study, (Figure 1 and 2) may be important to consider when working with a similar population.
The concept of physical working capacity (PWC), a measure of aerobic power, muscular endurance and efficiency is typically measured by oxygen consumption rate (VO2) during a maximal graded exercise test (GXT) [19]. Recently, several studies have reported on the effects of BA supplementation on PWCFT during incremental cycle ergometry tests in young men and women [16, 17]. Stout et al. [16, 17] reported that 28 days of BA supplementation in a younger population (21–27 years) resulted in a significant increase in PWCFT by 12 – 15%, respectively. In agreement, the current study demonstrated a 28.5% increase in PWCFTafter ninety days of BA supplementation. The two-fold increase in PWCFT in the elderly compared to young men and women in previous studies may be due to differences in initial skeletal muscle carnosine levels or supplementing duration. In support, Tallon et al. [4] reported that in Type II skeletal muscle, carnosine concentration was 47% lower in elderly (70.4 ± 5.0 yrs) compared to younger (23.8 ± 4.6 yrs) men and women, although Kim [20] found normal muscle carnosine levels in elderly Korean subjects with impaired glucose tolerance. Harris et al. [2] demonstrated that 28 days of BA supplementation significantly (60%) increased skeletal muscle carnosine levels while Hill et al. [15] demonstrated a further 20% increase when BA supplementation was continued for an additional 35 days. In light of these reports, the greater change in PWCFT in elderly participants in the present study, compared to previous studies in young men and women, was most likely due to both initial carnosine levels and length of time of BA supplementation.
De Vries et al. [13] suggested the PWCFT test may be more appropriate and sensitive to training effects on PWC in elderly, compared to other methods (i.e. VO2 max) that require maximal effort and may be ill-advised or possibly hazardous in this population. To our knowledge, this is the first study to examine the effects of BA supplementation using the PWCFT test in elderly men and women. However, de Vries et al. [13] did examine the effects of 10 weeks of moderate intensity (70% PWCFT) endurance training three times per week on PWCFT in elderly men and women (67.9 ± 5.3 years). The results showed a significant (p < 0.05), 30% increase in PWCFT as a result of the endurance training. While the results by de Vries et al. [13] support and recommend endurance training as a means to significantly improve physical working capacity, it should be noted that the elderly subjects in the current study were untrained, and the 28.5% increase in PWCFT occurred without any type of additional training during the ninety days of supplementation.
It has been proposed that exercise-induced decreases in intramuscular pH may interfere with the excitation-contraction coupling process of skeletal muscle, which, in turn may lead to decreases in power output and fatigue [21]. Maintaining the intracellular pH during exercise could therefore be important for normal muscle function in the elderly [4]. In order to maintain pH homeostasis, various buffering systems are involved, including active H+ export from muscle [2]. However, the immediate line of defense remains the buffering of H+ by intracellular physico-chemical buffers, principally phosphates and carnosine. Marsh et al. [22] demonstrated that there was a significant delay in the onset of intracellular acidosis during progressive exercise after six weeks of moderate intensity training, resulting in an increased capacity for submaximal work in a similar cohort of elderly individuals. Improving the ability to buffer intramuscular H+ accumulation, therefore, appears to be an important factor for delaying the onset of fatigue and increasing exercise capacity in older men and women.
Based on these results, it is not unreasonable to expect that introducing BA supplementation to increase muscle carnosine levels, prior to starting an exercise program in elderly men and women, would lead to an improvement in the quality of training. This would be especially true if muscle carnosine contents were already reduced with elderly persons. The limiting factor for muscle carnosine synthesis is the availability of BA, obtained either from uracil degradation in the liver or from the release of BA from the ingestion of carnosine (and related dipeptides in meat) [2]. From preliminary observations showing that the level of carnosine is reduced by up to 50% in vegetarian subjects [23], compared to age matched controls, it seems likely that the capacity of the body to produce BA is limited and capable of supporting only a limited capacity to synthesize carnosine. This is overcome in meat eaters through the dietary supply of BA. However, elderly subjects, maintained on a diet with a restricted meat intake would be expected to show a similar reduction in their muscle carnosine content as vegetarians, and to respond beneficially to BA supplementation.
Conclusion
The results of this study suggest that ninety days of BA supplementation may have significantly increased intramuscular carnosine resulting in a 28.5% increase in PWCFT due to a greater H+ buffering capacity. However, future studies are needed to confirm our results while measuring changes in skeletal muscle carnosine content.
References
Harris RC, Marlin DJ, Dunnett M, Snow DH, Hultman E: Muscle buffering capacity and dipeptide content in the thoroughbred horse, greyhound dog and man. Comp Biochem Physiol A. 1990, 97 (2): 249-251. 10.1016/0300-9629(90)90180-Z.
Harris RC, Tallon MJ, Dunnett M, Boobis L, Coakley J, Kim HJ, Fallowfield JL, Hill CA, Sale C, Wise JA: The absorption of orally supplied beta-alanine and its effect on muscle carnosine synthesis in human vastus lateralis. Amino acids. 2006, 30 (3): 279-289. 10.1007/s00726-006-0299-9.
Stuerenburg HJ, Kunze K: Concentrations of free carnosine (a putative membrane-protective antioxidant) in human muscle biopsies and rat muscles. Archives of gerontology and geriatrics. 1999, 29 (2): 107-113. 10.1016/S0167-4943(99)00020-5.
Tallon MJ, Harris RC, Maffulli N, Tarnopolsky MA: Carnosine, taurine and enzyme activities of human skeletal muscle fibres from elderly subjects with osteoarthritis and young moderately active subjects. Biogerontology. 2007, 8 (2): 129-137. 10.1007/s10522-006-9038-6.
Brooks SV, Faulkner JA: Skeletal muscle weakness in old age: underlying mechanisms. Medicine and science in sports and exercise. 1994, 26 (4): 432-439.
Chandler JM, Hadley EC: Exercise to improve physiologic and functional performance in old age. Clinics in geriatric medicine. 1996, 12 (4): 761-784.
Doherty TJ, Vandervoort AA, Taylor AW, Brown WF: Effects of motor unit losses on strength in older men and women. J Appl Physiol. 1993, 74 (2): 868-874. 10.1063/1.354879.
Alexander NB, Schultz AB, Ashton-Miller JA, Gross MM, Giordani B: Muscle strength and rising from a chair in older adults. Muscle & nerve. 1997, 5: S56-59. 10.1002/(SICI)1097-4598(1997)5+<56::AID-MUS14>3.0.CO;2-X.
Kannus P, Parkkari J, Niemi S, Palvanen M: Fall-induced deaths among elderly people. American journal of public health. 2005, 95 (3): 422-424. 10.2105/AJPH.2004.047779.
deVries HA, Brodowicz GR, Robertson LD, Svoboda MD, Schendel JS, Tichy AM, Tichy MW: Estimating physical working capacity and training changes in the elderly at the fatigue threshold (PWCft). Ergonomics. 1989, 32 (8): 967-977. 10.1080/00140138908966858.
Moritani T, Takaishi T, Matsumoto T: Determination of maximal power output at neuromuscular fatigue threshold. J Appl Physiol. 1993, 74 (4): 1729-1734.
Stout J, Eckerson J, Ebersole K, Moore G, Perry S, Housh T, Bull A, Cramer J, Batheja A: Effect of creatine loading on neuromuscular fatigue threshold. J Appl Physiol. 2000, 88 (1): 109-112.
deVries HA, Tichy MW, Housh TJ, Smyth KD, Tichy AM, Housh DJ: A method for estimating physical working capacity at the fatigue threshold (PWCFT). Ergonomics. 1987, 30 (8): 1195-1204. 10.1080/00140138708966008.
Stout JR, Sue Graves B, Cramer JT, Goldstein ER, Costa PB, Smith AE, Walter AA: Effects of creatine supplementation on the onset of neuromuscular fatigue threshold and muscle strength in elderly men and women (64 – 86 years). The journal of nutrition, health & aging. 2007, 11 (6): 459-464.
Hill CA, Harris RC, Kim HJ, Harris BD, Sale C, Boobis LH, Kim CK, Wise JA: Influence of beta-alanine supplementation on skeletal muscle carnosine concentrations and high intensity cycling capacity. Amino acids. 2007, 32 (2): 225-233. 10.1007/s00726-006-0364-4.
Stout JR, Cramer JT, Mielke M, O'Kroy J, Torok DJ, Zoeller RF: Effects of twenty-eight days of beta-alanine and creatine monohydrate supplementation on the physical working capacity at neuromuscular fatigue threshold. Journal of strength and conditioning research/National Strength & Conditioning Association. 2006, 20 (4): 928-931.
Stout JR, Cramer JT, Zoeller RF, Torok D, Costa P, Hoffman JR, Harris RC, O'Kroy J: Effects of beta-alanine supplementation on the onset of neuromuscular fatigue and ventilatory threshold in women. Amino acids. 2007, 32 (3): 381-386. 10.1007/s00726-006-0474-z.
Beltran B, Cuadrado C, Martin ML, Carbajal A, Moreiras O: Activities of daily living in the Spanish elderly. Association with mortality. The journal of nutrition, health & aging. 2001, 5 (4): 259-260.
deVries HA, Housh TJ: Physical Fitness Testing (ch. 14). Physiology of Exercise. 1994, Dubuque, IA: Wm.C. Brown Publishers, 254-282. 5
Kim HJ: Comparison of the carnosine and taurine contents of vastus lateralis of elderly Korean males, with impaired glucose tolerance, and young elite Korean swimmers. Amino acids. 2008
Fitts RH, Holloszy JO: Lactate and contractile force in frog muscle during development of fatigue and recovery. The American journal of physiology. 1976, 231 (2): 430-433.
Marsh GD, Paterson DH, Thompson RT, Cheung PK, MacDermid J, Arnold JM: Metabolic adaptations to endurance training in older individuals. Canadian journal of applied physiology = Revue canadienne de physiologie appliquee. 1993, 18 (4): 366-378.
Harris RC, Jones G, Hill CH, Kendrick IP, Boobis L, Kim CK, Kim HJ, Dang VH, Edge J, Wise JA: The carnosine content of vastus lateralis in vegetarians and omnivores. FASEB J. 2007, 21: 76.20-10.1096/fj.06-6925com.
Acknowledgements
We would like to thank The FAU Well Program, Boca Raton, Florida and Beth Shires and Heather Costa from John Knox Village, Pompano Beach, Florida for assisting us in subject recruitment. Mr. Pablo Costa and Ms. Erica Goldstein are acknowledged for their dedication and invaluable laboratory assistance. We would also like to thank Dr. John Wise and Natural Alternatives International, San Marcos, USA, for donating the Beta-Alanine (CarnoSynTM) and Placebo in randomized and double- blind fashion. The results of the present study do not constitute an endorsement by the American College of Sports Medicine.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors' contributions
JS assisted in study coordination, supervision, protocol development and statistical analysis. BG assisted in study coordination, data management and supervision. AS assisted in data management, statistical analysis and manuscript preparation. MH assisted in supervision, data management and study coordination. JC assisted in protocol development and manuscript preparation. TB assisted in manuscript preparation. RH assisted in protocol development and manuscript preparation.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Stout, J.R., Graves, B.S., Smith, A.E. et al. The effect of beta-alanine supplementation on neuromuscular fatigue in elderly (55–92 Years): a double-blind randomized study. J Int Soc Sports Nutr 5, 21 (2008). https://doi.org/10.1186/1550-2783-5-21
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1550-2783-5-21