Abstract
Regulatory T cells have an important role in limiting immune reactions and are essential regulators of self-tolerance. Among them, CD4+CD25high regulatory T cells are the best-described subset. In this article, we summarize current knowledge on the phenotype, function, and development of CD4+CD25high regulatory T cells. We also review the literature on the role of these T cells in rheumatic diseases and discuss the potential for their use in immunotherapy.
Similar content being viewed by others
Introduction
Tolerance to "self" is a major immune regulatory mechanism that protects the body's own tissues from immune-mediated damages and restricts active immune responses to those against microbial invaders (Figure 1). A classical type of tolerance, called central tolerance, is the mechanism by which "forbidden clones" of lymphocytes that recognize self antigens are eliminated in the thymus during normal lymphocyte development [1–3]. However, some lymphocyte clones with specificities for self antigens are found in animals and humans without autoimmunity [4–8]. In addition, autoimmunity can develop in the absence of defects in central tolerance. These findings initially led to the hypothesis that peripheral tolerancemust prevent auto-aggression by self-reactive T cells that escape thymic deletion. In the 1970s and 1980s, work on peripheral tolerance focused on characterization of specific suppressor T cells, the presumed regulators of immune responses in the periphery [9]. However, attempts to define and isolate suppressor T cells led to conflicting results, disappointment, and near abandonment of the field. With the development of new technologies in the 1990s, compelling evidence was put forward to support the existence of cellular subsets that possess immunosuppressive activities, this time under the name regulatory T cells[10].
Types of regulatory T cells
There are various types of regulatory T cells, including TCRαβ+CD4+, TCRαβ+CD8+, TCRαβ+CD4-CD8-, and TCRγι+ T cells. The majority of recent research has focused on TCRαβ+CD4+ regulatory T cells, of which there are several subtypes with distinct surface phenotypes, cytokine production profiles and mechanisms of immune suppression. Among the subtypes, T cells produced in the thymus and delivered to the periphery as a long-lived lineage of self-antigen-specific lymphocytes are called natural CD4+CD25high regulatory T cells (nTreg). In contrast+, CD4+ T cells that are recruited from circulating lymphocytes and acquire regulatory properties under particular conditions of stimulation are called adaptive Tcells(Figure 2). Two types of adaptive CD4+ regulatory T cells are type 1 regulatory T cells (Tr1) and T helper 3 regulatory cells (Th3). Suppressive effects of Tr1 and Th3 cells are dependent on the production of inhibitory cytokines, IL-10 and TGF-β, respectively [11–18]. A third type of adaptive regulatory T cell is the CD4+CD25high T cell induced in the periphery; these are termed induced regulatory T cells (iTreg). iTreg have similar properties to thymus-generated nTreg. Both cell types are anergic and do not proliferate upon TCR stimulation. Both cell types can inhibit proliferation of CD4+CD25- T cells in a dose dependent manner. Despite their characteristic anergy, CD4+CD25high regulatory T cells cultured with anti-CD3 antibodies (for TCR stimulation) and excess IL-2 (a T cell growth factor), can proliferate and still retain their suppressive activities. CD4+CD25high regulatory T cells (nTreg and iTreg) are the subject of this review.
Development of CD4+CD25high regulatory T cells
NTreg arise during normal lymphocyte ontogeny in the thymus [18, 19], and this is thought to be the exclusive site of nTreg development in children [20]. NTreg represent 5–10% of CD4+CD8- thymocytes in humans, mice, and rats. It seems likely that nTreg are positively selected through high-affinity recognition of self peptides presented by thymic stromal cells. This event, possibly together with signals from thymic dendritic cells, stimulates production of anti-apoptotic molecules to protect against negative selection. Recent data also indicate that CD4+CD25high regulatory T cells have a reciprocal developmental relationship in with Th17 cells, inflammatory T helper cells that produce IL-17 [21].
Many aspects of nTreg development in the thymus, such as their site of development, their interaction with thymic epithelial cells, and their selection are still poorly understood [22, 23]. Despite these uncertainties, it is clear that the transcription factor forkhead box P3 (Foxp3) plays a major role in the ontogeny and function of nTreg [23–29]. FoxP3 is preferentially and stably expressed in peripheral nTreg, even after proliferation [23, 27]. However, the signals that induce the stable up-regulation of Foxp3 and committed regulatory function in nTreg are not known. Furthermore, recent research shows that much of the nTreg transcriptional signature is not ascribable to Foxp3. It seems that a complex regulatory mechanism upstream of Foxp3 determines nTreg lineage and is distinct from elements downstream of Foxp3 that are essential for the cell's regulatory properties [30]. After their thymic selection, nTreg populate peripheral tissues. They are believed to be long-lived and may repeatedly proliferate in the periphery upon encountering specific self antigens [31–33]. However, their potential for continuous cell division is limited, which is thought to be associated with their diminished telomerase activity compared to CD4+CD25- T cells [34, 35].
The total number of CD4+CD25high regulatory T cells in human peripheral blood increases with age, despite thymic involution [36]. The likely explanation is the thymus-independent generation of CD4+CD25high iTreg. Several lines of evidences have suggested that induction of iTreg requires FoxP3. When a Foxp3 gene is transduced into CD4+CD25- T cells, these cells acquire CD25 surface expression and other phenotypic characteristics of nTreg. These transduced CD4+CD25high iTreg are able to inhibit proliferation and cytokine production in the effector T cells and the development of some experimental autoimmune diseases in animals [37]. Murine and human studies show that several cytokines are also required for generation of extra-thymic CD4+CD25high iTreg. Essential stimuli include TGF-β [17, 38–41], IFN-γ [42], anti-CD3/CD28 antibodies or antigen specific stimulation [43, 44], IL-4/IL-13 [45, 46], and thrombospondin-CD47 interaction [46]. Murine studies also show that tolerogenic conditions and homeostatic proliferation during lymphopenia induce the development of CD4+CD25high Foxp3+ iTreg in vivo [47–51].
Phenotype of CD4+CD25high regulatory T cells
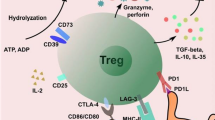
No specific marker for CD4+CD25high regulatory T cells is yet known (Figure 3). Foxp3 has been considered the most reliable marker [23], but is intracellular and cannot be used for isolation or in vivo tracking of CD4+CD25high regulatory T cells. In addition, activation of CD4+CD25- T cells can transiently up-regulate FoxP3 expression in human cells, although this is not the case in mice [41, 52, 53]. Hence, FoxP3 alone may not be a specific marker for human CD4+CD25high regulatory T cells [53].
Another molecule associated with CD4+CD25high regulatory T cells is CD25, the α chain of the IL-2 receptor, Both nTreg and iTreg constitutively express CD25 and suppressive activity is optimal in CD4+ T cells expressing the highest levels of CD25 (approximately 2–4% of human peripheral blood CD4+ T cells). However, CD25 by itself has limitations as a marker for CD4+CD25high regulatory T cells, as it is also up-regulated in activated effector T cells. The recent discovery of low expression of CD127 (IL-7 receptor α) on CD4+CD25high regulatory T cells provides further delineation of this population [54–56]. However, some regulatory CD4+ T cells that are Foxp3+CD127low express little-to-no CD25 [56].
Several other molecules associated with CD4+CD25high regulatory T cells have been descsribed. In humans, these cells constitutively express intracellular cytotoxic T-lymphocyte antigen 4 (CTLA-4) and glucocorticoid-induced tumor-necrosis-factor-receptor-related protein (GITR). Upon activation, they also express membrane-bound TGF-β and HLA-DR [57]. Other surface markers reportedly expressed on human CD4+CD25high regulatory T cells include CD69, CD45RA/CD45RO, CD134 (OX40), CD95, and programmed cell death-ligand 1 (PD-L1). CD4+CD25high regulatory T cells also express chemokine receptors to direct their migration to different tissues. Current data suggest that signals from various chemokines and integrin ligands determine which membrane chemokine receptors and integrins are expressed on CD4+CD25high regulatory T cells. Similar to effector T cells, CD62L (also known as L-selectin) and CCR7 are important lymph node homing molecules for CD4+CD25high regulatory T cells [58]. The majority of CD4+CD25high regulatory T cells express CCR4 and CCR8 [59], but other chemokine receptors and integrin molecules, such CD103, are also present. The expression level of integrins dictates the direction of cell migration. For example, CD4+CD25high CD103- regulatory T cells preferentially migrate to lymph nodes, whereas CD4+CD25high CD103+ regulatory T cells efficiently migrate into inflammatory sites [58]. Most human CD4+CD25high regulatory T cells are believed to be in a late stage of differentiation. This notion is supported by their expression of activation/memory markers, as indicated above [60].
The absence of specific markers makes it difficult to isolate pure populations of CD4+CD25high regulatory T cells, to further characterize their phenotype. At least a small number of non-regulatory activated effector T cells usually contaminate isolated CD4+CD25high regulatory T cells, due to the overlapping expression of CD25. Thus, strategies to expand CD4+CD25high regulatory T cells for higher yield and purity have been sought. Use of IL-2, a T cell growth factor that induces proliferation of CD4+CD25high regulatory T cells in vitro, was considered. However, IL-2 also favors the expansion of non-regulatory effector T cells. Another candidate is the immunosuppressive drug rapamycin (sirolimus), used for the prevention of organ transplant rejection as well as resistant graft versus host disease (GVHD) [61–63]. Human peripheral blood CD4+CD25high regulatory T cells cultured in the presence of rapamycin survive and vigorously expand for at least 3 weeks, while effector T cells are inhibited from proliferation. This phenomenon is thought to result from differential intracellular signaling in CD4+CD25high regulatory T cells compared to CD4+CD25- effector T cells in response to rapamycin, which blocks progression from G1 into S phase in activated effectors [64]. The rapamycin-expanded CD4+CD25high regulatory T cells are suppressive and have the same phenotype as freshly isolated blood CD4+CD25high regulatory T cells. Thus, in vitro rapamycin may allow the generation of highly efficient CD4+CD25high regulatory T cells and better characterization of their functions for potential clinical use [65, 66].
CD4+CD25high regulatory T cell function
A key characteristic of CD4+CD25high regulatory T cells is their in vitro anergy. In contrast to CD4+CD25- T cells, which proliferate upon receiving T cell receptor (TCR) stimulation, CD4+CD25high regulatory T cells are unresponsive to this proliferative signal and do not produce IL-2. However, CD4+CD25high regulatory T cells cultured with anti-CD3 antibodies for TCR stimulation and excessexogenous IL-2 overcome anergy and proliferate; blocking IL-2 inhibits this phenomemon [67]. The anergic state of CD4+CD25high regulatory T cells can also be overcome by anti-CD28 costimulation or interaction with mature dendritic cells [68–70]. Interestingly, recent studies suggest that CD4+CD25high regulatory T cells are not anergic in vivo, but have a high turnover rate [71, 72].
The second cardinal feature of CD4+CD25high regulatory T cells is their ability to suppress immune responses [72, 73]. Suppression occurs when CD4+CD25high regulatory T cells are activated with antigens recognized by their specific TCR, but can be maintained without further TCR stimulation [74]. Thus, suppressive activity is antigen-nonspecific. However, CD4+CD25high regulatory T cells that share the same antigenic specificity with effector cells are more suppressive. Similarly, allogeneic CD4+CD25high regulatory T cells are suppressive, but autologous CD4+CD25high regulatory T cells are more potent suppressors. Some studies suggest that CD4+CD25high regulatory T cells inhibit proliferation of effector CD4+CD25- T cells and CD8+ T cells by arresting the proliferation of these cells at G1-S interphase of the cell cycle [75]. Interestingly, the addition of exogenous IL-2 does not overcome the suppression, suggesting unresponsiveness at the level of the IL-2 receptor [72]
Contact-dependent suppression by CD4+CD25high regulatory T cells has been reported to occur via CTLA-4 signaling: CTLA-4 blockade leads to diminished suppression of effector T cell proliferation by CD4+CD25high regulatory T cells [76, 77]. Recent studies have suggested that multiple CTLA-4 associated pathways could mediate suppression by CD4+CD25high regulatory T cells. Preferential engagement of CTLA-4, instead of CD28, with CD80/CD86 may provide a negative proliferative signal [78]. Alternatively, CTLA-4 on CD4+CD25high regulatory T cells may signal dendritic cells to produce the immunosuppressive cytokines, IL-10 and TGF-β [79]. In a novel mechanism, suggested by results of Fallarino et al., CTLA-4 signals dendritic cells to produce high levels of the enzyme indoleamine, which in turn breaks down tryptophan, an amino acid important for T cell proliferation [80], and consequentially inhibits the proliferation of effector T cells.
While the main targets of suppression by CD4+CD25high regulatory T cells are innate and adaptive immune cells [81], these regulatory T cells also participate in immune responses against infectious agents [82], malignant cells [83], and allogeneic organ and stem-cell grafts [84]. Although CD4+CD25high regulatory T cells regulate both Th1 and Th2 immune responses, Th2 cells may partially escape this suppressive activity via their ability to respond to growth factors other than IL-2, such as IL-4, IL-7, and IL-9 [85]. In contrast, the proliferation of Th1 cells is only restored by the administration of IL-15 [85]. In mice, the depletion of CD4+CD25high regulatory T cells prevents antigen-induced Th2 differentiation by increasing the differentiation of Th1 cells [86, 87]. Under appropriate conditions, CD4+CD25high regulatory T cells are able to confer suppressive capacity on CD4+CD25- T cells, converting them to either Th3 or Tr1 cells [88, 89].
CD4+CD25high regulatory T cells and autoimmunity
Several autoimmune disorders have been linked to physical and genetic alterations in thymus that disrupt the development of nTreg. Thymectomized neonatal mice are deficient in CD4+CD25high regulatory T cells and develop multi-organ autoimmune disease, which can be overcome by the adoptive transfer of CD25+ thymocytes from normal mice [90, 91]. Children with thymic hypoplasia as a result of the 22.q2 deletion syndrome display impaired CD4+CD25high regulatory T cell generation and have an increased risk of developing an autoimmune disorder [92]. Mutations in Foxp3 result in the scurfy phenotype in mice. Foxp3 mutant "scurfy" mice and Foxp3-null mice lack CD4+CD25high regulatory T cells and die of a lymphoproliferative-wasting disease, likely due to uncontrolled expansion of effector T lymphocytes. Adoptive transfer of CD4+CD25high regulatory T cells into neonatal Foxp3-null or scurfy mice protects them temporarily from disease [92, 93].
Human patients with Foxp3 gene mutations develop IPEX syndrome, a potentially fatal disorder, characterized by immune dysregulation, polyendocrinopathy, and enteropathy (Table 1) [94–96]. IPEX CD4+CD25high regulatory T cells are less suppressive, although their surface phenotype and levels in peripheral blood remain normal [97]. Consequently, it is suggested that functional insufficiency rather than defective differentiation of CD4+CD25high regulatory T cells may occur in these patients. Allogeneic bone marrow transplantation in IPEX subjects is effective in correcting Foxp3 associated dysfunctions [98], and clinical recovery accompanies regeneration of functionally competent CD4+CD25high regulatory T cells [99].
In addition to IPEX, many more common polygenic autoimmune disorders, including multiple sclerosis, type 1 diabetes, are hypothesized to have abnormalities in CD4+CD25high regulatory T cell function [100–105]. Below, we consider this hypothesis and discuss findings from studies of these cells in rheumatic diseases. Across the spectrum of autoimmune diseases, it is not yet clear whether changes in these cells are primary or secondary to disease.
CD4+CD25high regulatory T cells in rheumatic diseases
In rheumatic diseases, most studies have focused on CD4+CD25high regulatory T cells, while the roles of other regulatory T cell types remain unclear (Table 2). Early attempts to characterize CD4+CD25high regulatory T cells were flawed due to use of high surface expression of CD25 as the single cell marker and the resulting inclusion of variable numbers of activated T effector cells over the course of disease. In addition, levels and/or activity of CD4+CD25high regulatory T cells are influenced by different immunosuppressive treatments. Therefore, future studies that employ a better combination of markers (e.g. CD4, CD25, and CD127) and consider medication status and disease severity in the analysis will be important. Nonetheless, current studies of CD4+CD25high regulatory T cells in rheumatic diseases provide the scientific foundation for further research.
Juvenile idiopathic arthritis (JIA)
Research on CD4+CD25high regulatory T cells in juvenile idiopathic arthritis (JIA) has revealed distinct abnormalities in function and distribution in various disease subtypes. De Kleer et al. found reduced numbers of circulating CD4+CD25high regulatory T cells in extended oligoarticular JIA, compared to persistent oligoarticular JIA [106]. The numbers of CD4+CD25high Foxp3+ regulatory T cells in the synovial fluid of inflamed joints were comparable, but more CD4+CD25intermediate Foxp3+ regulatory T cells were present in persistent vs. extended oligoarticular JIA. Synovial fluid CD4+CD25high regulatory T cells had more potent in vitro suppressive effects compared to their peripheral blood counterparts, suggesting possible functional enhancement of these cells in the joints. In addition, CD4+CD25high regulatory T cells more easily suppress peripheral blood CD4+CD25- T effector cells than T effectors from synovial fluid, consistent with in vitro findings on the effects of IL-1 and IL-6 on susceptibility to suppression [107]. The authors conclude that CD4+CD25high regulatory T cells cannot prevent disease development, but synovial CD4+CD25high regulatory T cells may contribute to reversal of ongoing inflammation in persistent oligoarticular JIA [106, 108].
In another study of synovial CD4+CD25high regulatory T cells in persistent and extended oligoarticular JIA, Massa et al. demonstrated that certain epitopes of human HSP increase the frequency of CD4+CD25high regulatory T cells and induce Foxp3 expression [109]. Reactivity of CD4+CD25high regulatory T cells to these human HSP epitopes appears to influence regulation of inflammation in oligoarticular JIA [109].
In systemic JIA, circulating CD4+CD25high regulatory T cell frequency was reported to be lower than healthy controls [110]. Studies from our laboratory showed that circulating CD4+CD25high CD127lo/- regulatory T cell numbers are normal, but their in vitro suppressive function is lower than that of healthy controls (unpublished data). This defect in CD4+CD25high regulatory T cell-mediated suppression does not appear to result from a deficiency of CD45RA+ naïve cells, the more suppressive subset of CD4+CD25high CD127lo/- regulatory T cells (unpublished data). In contrast, we find reduced levels of circulating CD4+CD25high regulatory T cells in polyarticular JIA (unpublished data).
Ruprecht et al. [111] also investigated CD4+CD25high regulatory T cells in synovial fluid of patients with JIA. They found that CD4+CD25high regulatory T cells expressing surface CD27 exhibit a higher level of Foxp3 and have stronger suppressive activity. They concluded that, used in conjunction with CD25, CD27 is a useful marker to distinguish regulatory from effector T cells in inflamed tissues. However, others have disputed the specificity of CD27 as a CD4+CD25high regulatory T cell marker [112].
Another important issue is how various JIA treatments affect CD4+CD25high regulatory T cell distribution and function. It was reported that methotrexate and corticosteroids do not influence the frequency or activity of these cells in JIA [106, 110]. De Kleer et al. observed normalization of levels of circulating CD4+CD25high regulatory T cell after autologous stem cell transplantation (ASCT), perhaps from the preferential homeostatic expansion of CD4+CD25high regulatory T cells during the lymphopenic phase of immune reconstitution. They postulated that ASCT reprograms auto-reactive T cells and restores the immune regulatory network of CD4+CD25high regulatory T cells [110].
Rheumatoid arthritis (RA)
Reported data on frequency and activity of CD4+CD25high regulatory T cells in rheumatoid arthritis (RA) are conflicting. Liu et al. found the quantities and functional properties of CD4+CD25high regulatory T cells in peripheral blood of RA patients to be comparable to healthy control subjects [113, 114], while Cao et al. reported a decreased frequency of CD4+CD25high regulatory T cells in peripheral blood of RA subjects [114]. Some studies found that treatment with methotrexate, hydroxychloroquine, anti-TNF-α, and systemic/intra-articular steroids does not influence the abundance or suppressive function of CD4+CD25high regulatory T cells [115–118], while others reported increased levels and suppressive function with TNF-α blockade [117, 118].
Nevertheless, there is a consensus that synovial fluid in inflamed joints is enriched in CD4+CD25high regulatory T cells [113, 114, 119]. These synovial CD4+CD25high regulatory T cells express increased levels of inflammation-related chemokine receptors, such as CCR4, CCR5, and CXCR4 [120]. Like findings in JIA, evidence for the increased resistance of RA synovial T effector cells to suppression by CD4+CD25high regulatory T cells has been reported [116]. Behrens et al. linked CD4+CD25high regulatory T cell dysfunction in RA to a disturbance in the homeostatic relationship between CD4+CD25high regulatory T cells and Th1 cells in the synovium. CD4+CD25high regulatory T cells from RA subjects are capable of suppressing the production of IFN-γ by synovial membrane Th1 lymphocytes [121]. However, the ratio of CD4+CD25high regulatory T cells to IFN-γ producing cells is lower in the synovial membrane than in synovial fluid or blood. The authors suggest that the local imbalance between Th1 and CD4+CD25high regulatory T cells may be responsible for repeated rheumatic flares and could be a target for future treatments [121].
Systemic lupus erythematosus (SLE)
Findings that central tolerance remains intact in murine models of SLE suggest a critical breakdown of peripheral tolerance in this disease [122–124]. Consistent with this possibility, most studies in human SLE indicate that CD4+CD25high regulatory T cell distribution is altered in association with active disease. Numbers of circulating CD4+CD25high regulatory T cells decrease in patients with active SLE [125–127] while clinical remission is associated with elevated or normal CD4+CD25high regulatory T cell frequency [128–131]. A single study reported that disease activity in SLE correlates positively with the numbers of CD4+CD25high regulatory T cells [131].
In a study of CD4+CD25high regulatory T cell function, Vallencia et al. claimed that a reversible defect occurs in patients with SLE. CD4+CD25high regulatory T cells from active but not inactive SLE patients were deficient in in vitro suppressive activity and had decreased Foxp3 mRNA and protein [132, 133]. Opposite findings of increased Foxp3 expression in active disease were reported in one study of pediatric SLE [133]. Yan et al. found no difference in Foxp3 expression in CD4+CD25high regulatory T cells of SLE patients [134]. However, decreased suppressive function of CD4+CD25high regulatory T cells appeared to be a consequence of inhibition by IFN-activated autologous antigen presenting cells. These cells could also inhibit the function of CD4+CD25high regulatory T cells from healthy control subjects [135].
Other rheumatic diseases
The work on CD4+CD25high regulatory T cells in other rheumatic diseases is limited to date. In primary Sjogren syndrome, Gottenberg et al. reported an increase in circulating CD4+CD25high regulatory T cells, and no change in levels with methotrexate or corticosteroid treatment [136]. However, a more recent report argues that the numbers of circulating CD4+CD25high regulatory T cells in patients with Sjogren syndrome decrease [137].
In Kawasaki disease, Furuno et al. found that during the active phase of the disease, the number of circulating CD4+CD25high regulatory T cells is reduced compared to patients with infectious causes of febrile illness, whose CD4+CD25high regulatory T cell numbers are higher than in healthy subjects. In defervesce phase of the disease, the number of CD4+CD25high regulatory T cells in patients with Kawasaki disease increases to/or above normal levels, while CD4+CD25high regulatory T cells in patients with infectious febrile disease decrease to normal levels [138].
In spondyloarthropathy, a single study by Cao et al. found normal levels of circulating CD4+CD25high regulatory T cells, but a higher proportion of CD4+CD25high regulatory T cells in synovial fluid of inflamed joints than in peripheral blood [114].
In sarcoidosis, Miyara et al. showed an increase in frequency of CD4+CD25high regulatory T cells in sarcoid granulomas, bronchoalveolar lavage fluid (BALF), and peripheral blood of patients with active disease. The cells reportedly exhibit powerful anti-proliferative activity, but cannot completely inhibit TNF-α production. The authors conclude that although sarcoidosis is associated with global CD4+CD25high regulatory T cell amplification, the cells are functionally insufficient to control local inflammation [139]. In contrast, Idali et al. [140] found decreased frequency of Foxp3+ cells among BALF and blood CD4+ cells in sarcoidosis patients.
Mechanistic issues
Current data indicate that reduced numbers of circulating CD4+CD25high regulatory T cells is not a general finding in rheumatic diseases, while reduced function is more commonly found. Several hypothetical defects in CD4+CD25high regulatory T cell function that could lead to autoimmunity have been proposed [141]. However, data pointing to a secondary effect on CD4+CD25high regulatory T cells in autoimmune disorders have also emerged. The example of SLE is illustrative. Compromised function could result from direct interaction between SLE-associated auto-antigens and their cognate ligands on CD4+CD25high regulatory T cells [142]. Alternatively, endogenous stimulants in SLE may activate antigen presenting cells to produce alpha-interferon and related factors that inhibit CD4+CD25high regulatory T cell activity [134]. Pro-inflammatory factors associated with autoimmunity, such as IL-1, IL-6, and TNF-α, also can inhibit CD4+CD25high regulatory T cell function [143–145]. The resolution of this issue is central to a full understanding of autoimmunity.
Increased suppressive potency of CD4+CD25high regulatory T cells at sites of inflammation has been reported in several diseases. The relative importance of circulating versus tissue CD4+CD25high regulatory T cells requires more study. One attractive possibility is that tissue CD4+CD25high regulatory T cells may be more antigen-specific, and consequentially more suppressive [106, 116] while circulating CD4+CD25high regulatory T cells may be recruited to different tissues in response to inflammatory conditions [146], and non-specifically augment suppression. The occasionally reported reduction in numbers of CD4+CD25high regulatory T cells in the circulation may result from their recruitment to sites of inflammation. However, expansion of tissue localized and circulating CD4+CD25high regulatory T cells may occur during autoimmune-associated inflammation [116]. Thus, CD4+CD25high regulatory T cells may be actively recruited or be generated de novo at sites of inflammation (or both). It is anticipated that the development of new technologies that allow in vivo tracking of circulating CD4+CD25high regulatory T cells will advance our current understanding of migratory and suppressive potentials of different subsets of CD4+CD25high regulatory T cells. Finally, the potent suppressive activity of CD4+CD25high regulatory T cells at inflammatory sites is usually insufficient to control inflammation. One probable explanation is that the presence of inflammatory cytokines at these sites makes effector T cells more resistant to suppression. In addition, the recently reported induction of highly inflammatory Th17 cells from CD4+CD25high regulatory T cells that are not terminally differentiated [147] suggests that the latter may, under certain conditions, potentiate rather than suppress inflammation.
CD4+CD25high regulatory T cells as a treatment in autoimmune and rheumatic diseases
There is a need to carefully control the size of the CD4+CD25high regulatory T cell population in vivo to achieve a balance between the necessity to suppress auto-reactivity and the ability to allow appropriate responses to foreign and tumor antigens. Little is known of the mechanisms of this control; however, the alterations in distribution and function of CD4+CD25high regulatory T cells in autoimmune and rheumatic diseases suggest a role for the therapeutic use of these cells. In mice with collage-induced arthritis, depletion of CD4+CD25high regulatory T cells causes rapid progression, and the transfer of isolated and ex vivo-proliferated CD4+CD25high regulatory T cells can reverse early joint damage [148]. Administration of CD4+CD25high regulatory T cell also yields improvement in murine models of colitis, autoimmune encephalomyelitis, diabetes, and allogeneic transplantion [149–152].
Human research has shown that some established therapies may promote CD4+CD25high regulatory T cell development and survival in vivo. For instance, monoclonal antibody to CD20 (rituximab), which depletes B cells, leads to a selective increase in CD4+CD25high regulatory T cells [153]. Polyclonal antibody therapies, such as anti-lymphocyte serum (ALS) and anti-thymocyte globulin (ATG), have been shown to preferentially deplete T effector cells, and induce CD4+CD25high regulatory T cells [154, 155]. As described above, rapamycin preferentially expands CD4+CD25high regulatory T cells. Therefore, a major therapeutic effect of rapamycin may be the induction of tolerogenic CD4+CD25high regulatory T cells in vivo.
Besides these established therapies, recent research has focused on cytokine related therapies to modulate CD4+CD25high regulatory T cell function. Among candidate cytokines are growth factors in the IL-2 family. These cytokines signal via STAT5, the homeostatic pathway that regulates CD4+CD25high regulatory T cell function. Several studies have reported that these cytokines enhance immune regulation by CD4+CD25high regulatory T cells. For instance, IL-7 and IL-15 are involved in the preservation of optimal suppressive function by CD4+CD25high regulatory T cells [156]. In addition, IL-15 administration alone induces de novo generation of CD4+CD25high regulatory T cells [157]. The newly identified IL-35 has been shown to trigger CD4+CD25high regulatory T cell expansion and subsequent immune suppression [158]. However, the specificity of these cytokines for CD4+CD25high regulatory T cells needs to be further examined to avoid undesirable expansion of effector T cells.
In contrast to T cell growth factors, pro-inflammatory cytokines have been shown to inhibit function of CD4+CD25high regulatory T cells, possibly via promotion of Th17 development [159]. Therefore, anti-TNF-α, anti-IL1, anti-IL6, and anti-IL-21 therapies may affect inflammation not only by direct inhibition of the pro-inflammatory cytokines but also by reestablishment of immune regulation by CD4+CD25high regulatory T cells. On the other hand, short term treatment with high dose CTLA-4Ig (abatacept), which has been shown to have anti-inflammatory properties in arthritis, leads to a precipitous loss of CD4+CD25high regulatory T cells and, in some animal models, exacerbation of autoimmunity [160].
Direct transfusion of CD4+CD25high regulatory T cell in humans is starting to be explored as a therapy. We are aware of two early trials in patients post stem cell transplantation (SCT). In patients with allogeneic SCT, Matthias Edinger and his team from the Department of Hematology and Oncology at the University Hospital of Regensburg, Germany are conducting a phase I clinical trial (safety and feasibility) using CD4+CD25high regulatory T cells-enriched lymphocyte products (personal communication). Patients with a high risk of relapse after allogeneic SCT are preemptively treated with donor T cells enriched with 50–60% of CD4+CD25high regulatory T cells, in order to reduce GVHD. Eight patients have been treated so far without complications. A trial using third party cord blood CD4+CD25high regulatory T cell in patients with SCT has been recently initiated at the University of Minnesota (Dr. B. Balazar, personal communication). We are not aware of any established clinical trials in autoimmune diseases, although CD4+CD25high regulatory T cell therapy will possibly be initiated in type 1 diabetes in the near future.
Despite encouraging data from animal models and early human trials, a number of issues must be resolved for optimal use of CD4+CD25high regulatory T cells as a therapy [161, 162]. Firstly, there are likely to be differences in the specific role of CD4+CD25high regulatory T cells in particular diseases, and these must be elucidated. Secondly, CD4+CD25high regulatory T cell-specific surface markers remain elusive, which hampers the isolation of pure populations of CD4+CD25high regulatory T cells. Third, the use of autologous CD4+CD25high regulatory T cell clones for particular auto-antigens would increase the effectiveness and decrease potential side effects of "bystander" suppression. This will require techniques for identifying and expanding antigen specific clones of CD4+CD25high regulatory T cells. Recent successes with CD4+CD25high regulatory T cell expansion using rapamycin are promising in this regard [163, 164]. Lastly, the fate of transfused CD4+CD25high regulatory T cells in vivo is not fully known. In the unlikely event that CD4+CD25high regulatory T cells expand into tumor/effector cells or simply become broadly immunosuppressive, there needs to be a way to eliminate them from the body. Future therapies may require the use of "designer" CD4+CD25high regulatory T cells that have been modified by gene transfer to selectively express preferred proteins including antigen specific TCR, homing receptors, cytokines, and "suicide" genes [161, 162]. Nevertheless, the manipulation of CD4+CD25high regulatory T cell function shows great promise as a novel therapeutic option in autoimmune and rheumatic diseases.
References
Kappler JW, Roehm N, Marrack P: T cell tolerance by clonal elimination in the thymus. Cell. 1987, 49: 273-280.
Kisielow P, Bluthmann H, Staerz UD, Steinmetz M, von Boehmer H: Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988, 333: 742-746.
Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, Mathis D: Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002, 298: 1395-1401.
Villoslada P, Abel K, Heald N, Goertsches R, Hauser SL, Genain CP: Frequency, heterogeneity and encephalitogenicity of T cells specific for myelin oligodendrocyte glycoprotein in naive outbred primates. Eur J Immunol. 2001, 31: 2942-2950.
Tiller T, Tsuiji M, Yurasov S, Velinzon K, Nussenzweig MC, Wardemann H: Autoreactivity in human IgG+ memory B cells. Immunity. 2007, 26: 205-213.
Koelsch K, Zheng NY, Zhang Q, Duty A, Helms C, Mathias MD, Jared M, Smith K, Capra JD, Wilson PC: Mature B cells class switched to IgD are autoreactive in healthy individuals. J Clin Invest. 2007, 117: 1558-1565.
Lohse AW, Dinkelmann M, Kimmig M, Herkel J, Meyer zum Buschenfelde KH: Estimation of the frequency of self-reactive T cells in health and inflammatory diseases by limiting dilution analysis and single cell cloning. J Autoimmun. 1996, 9: 667-675.
Fredrikson S, Soderstrom M, Hillert J, Sun JB, Kall TB, Link H: Multiple sclerosis: occurrence of myelin basic protein peptide-reactive T cells in healthy family members. Acta Neurol Scand. 1994, 89: 184-189.
Tada T, Takemori T: Selective roles of thymus-derived lymphocytes in the antibody response. I. Differential suppressive effect of carrier-primed T cells on hapten-specific IgM and IgG antibody responses. J Exp Med. 1974, 140: 239-252.
Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M: Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995, 155: 1151-1164.
Roncarolo MG, Bacchetta R, Bordignon C, Narula S, Levings MK: Type 1 T regulatory cells. Immunol Rev. 2001, 182: 68-79.
Levings MK, Gregori S, Tresoldi E, Cazzaniga S, Bonini C, Roncarolo MG: Differentiation of Tr1 cells by immature dendritic cells requires IL-10 but not CD25+CD4+ Tr cells. Blood. 2005, 105: 1162-1169.
Cottrez F, Groux H: Specialization in tolerance: innate CD(4+)CD(25+) versus acquired TR1 and TH3 regulatory T cells. Transplantation. 2004, 77: S12-15.
Weiner HL: Oral tolerance: immune mechanisms and the generation of Th3-type TGF-beta-secreting regulatory cells. Microbes Infect. 2001, 3: 947-954.
Lan RY, Ansari AA, Lian ZX, Gershwin ME: Regulatory T cells: development, function and role in autoimmunity. Autoimmun Rev. 2005, 4: 351-363.
Weiner HL: Induction and mechanism of action of transforming growth factor-beta-secreting Th3 regulatory cells. Immunol Rev. 2001, 182: 207-214.
Zheng SG, Gray JD, Ohtsuka K, Yamagiwa S, Horwitz DA: Generation ex vivo of TGF-beta-producing regulatory T cells from CD4+CD25- precursors. J Immunol. 2002, 169: 4183-4189.
Seddon B, Mason D: The third function of the thymus. Immunol Today. 2000, 21: 95-99.
Cupedo T, Nagasawa M, Weijer K, Blom B, Spits H: Development and activation of regulatory T cells in the human fetus. Eur J Immunol. 2005, 35: 383-390.
Yagi H, Nomura T, Nakamura K, Yamazaki S, Kitawaki T, Hori S, Maeda M, Onodera M, Uchiyama T, Fujii S, Sakaguchi S: Crucial role of FOXP3 in the development and function of human CD25+CD4+ regulatory T cells. Int Immunol. 2004, 16: 1643-1656.
Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B: TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006, 24: 179-189.
Bensinger SJ, Bandeira A, Jordan MS, Caton AJ, Laufer TM: Major histocompatibility complex class II-positive cortical epithelium mediates the selection of CD4(+)25(+) immunoregulatory T cells. J Exp Med. 2001, 194: 427-438.
Wing K, Larsson P, Sandstrom K, Lundin SB, Suri-Payer E, Rudin A: CD4+ CD25+ FOXP3+ regulatory T cells from human thymus and cord blood suppress antigen-specific T cell responses. Immunology. 2005, 115: 516-525.
Sakaguchi S: Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005, 6: 345-352.
von Boehmer H: Mechanisms of suppression by suppressor T cells. Nat Immunol. 2005, 6: 338-344.
Picca CC, Caton AJ: The role of self-peptides in the development of CD4+ CD25+ regulatory T cells. Curr Opin Immunol. 2005, 17: 131-136.
Fontenot JD, Gavin MA, Rudensky AY: Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003, 4: 330-336.
Hori S, Nomura T, Sakaguchi S: Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003, 299: 1057-1061.
Khattri R, Cox T, Yasayko SA, Ramsdell F: An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003, 4: 337-342.
Hill JA, Feuerer M, Tash K, Haxhinasto S, Perez J, Melamed R, Mathis D, Benoist C: Foxp3 transcription-factor-dependent and -independent regulation of the regulatory T cell transcriptional signature. Immunity. 2007, 27: 786-800.
Hsieh CS, Liang Y, Tyznik AJ, Self SG, Liggitt D, Rudensky AY: Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity. 2004, 21: 267-277.
Gavin MA, Clarke SR, Negrou E, Gallegos A, Rudensky A: Homeostasis and anergy of CD4(+)CD25(+) suppressor T cells in vivo. Nat Immunol. 2002, 3: 33-41.
Fisson S, Darrasse-Jeze G, Litvinova E, Septier F, Klatzmann D, Liblau R, Salomon BL: Continuous activation of autoreactive CD4+ CD25+ regulatory T cells in the steady state. J Exp Med. 2003, 198: 737-746.
Allsopp RC, Chang E, Kashefi-Aazam M, Rogaev EI, Piatyszek MA, Shay JW, Harley CB: Telomere shortening is associated with cell division in vitro and in vivo. Exp Cell Res. 1995, 220: 194-200.
Pan C, Xue BH, Ellis TM, Peace DJ, Diaz MO: Changes in telomerase activity and telomere length during human T lymphocyte senescence. Exp Cell Res. 1997, 231: 346-353.
Gregg R, Smith CM, Clark FJ, Dunnion D, Khan N, Chakraverty R, Nayak L, Moss PA: The number of human peripheral blood CD4+ CD25high regulatory T cells increases with age. Clin Exp Immunol. 2005, 140: 540-546.
Mays LE, Chen YH: Maintaining immunological tolerance with Foxp3. Cell Res. 2007, 17: 904-918.
Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM: Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003, 198: 1875-1886.
Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF: Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25- T cells through Foxp3 induction and down-regulation of Smad7. J Immunol. 2004, 172: 5149-5153.
Peng Y, Laouar Y, Li MO, Green EA, Flavell RA: TGF-beta regulates in vivo expansion of Foxp3-expressing CD4+CD25+ regulatory T cells responsible for protection against diabetes. Proc Natl Acad Sci USA. 2004, 101: 4572-4577.
Selvaraj RK, Geiger TL: A kinetic and dynamic analysis of Foxp3 induced in T cells by TGF-beta. J Immunol. 2007, 179: 11-following 1390
Wang Z, Hong J, Sun W, Xu G, Li N, Chen X, Liu A, Xu L, Sun B, Zhang JZ: Role of IFN-gamma in induction of Foxp3 and conversion of CD4+ CD25- T cells to CD4+ Tregs. J Clin Invest. 2006, 116: 2434-2441.
Walker MR, Carson BD, Nepom GT, Ziegler SF, Buckner JH: De novo generation of antigen-specific CD4+CD25+ regulatory T cells from human CD4+CD25- cells. Proc Natl Acad Sci USA. 2005, 102: 4103-4108.
Walker MR, Kasprowicz DJ, Gersuk VH, Benard A, Van Landeghen M, Buckner JH, Ziegler SF: Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25- T cells. J Clin Invest. 2003, 112: 1437-1443.
Skapenko A, Kalden JR, Lipsky PE, Schulze-Koops H: The IL-4 receptor alpha-chain-binding cytokines, IL-4 and IL-13, induce forkhead box P3-expressing CD25+CD4+ regulatory T cells from CD25-CD4+ precursors. J Immunol. 2005, 175: 6107-6116.
Grimbert P, Bouguermouh S, Baba N, Nakajima T, Allakhverdi Z, Braun D, Saito H, Rubio M, Delespesse G, Sarfati M: Thrombospondin/CD47 interaction: a pathway to generate regulatory T cells from human CD4+ CD25- T cells in response to inflammation. J Immunol. 2006, 177: 3534-3541.
Apostolou I, von Boehmer H: In vivo instruction of suppressor commitment in naive T cells. J Exp Med. 2004, 199: 1401-1408.
Knoechel B, Lohr J, Kahn E, Bluestone JA, Abbas AK: Sequential development of interleukin 2-dependent effector and regulatory T cells in response to endogenous systemic antigen. J Exp Med. 2005, 202: 1375-1386.
Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H: Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005, 6: 1219-1227.
Curotto de Lafaille MA, Lino AC, Kutchukhidze N, Lafaille JJ: CD25- T cells generate CD25+Foxp3+ regulatory T cells by peripheral expansion. J Immunol. 2004, 173: 7259-7268.
Thorstenson KM, Khoruts A: Generation of anergic and potentially immunoregulatory CD25+CD4 T cells in vivo after induction of peripheral tolerance with intravenous or oral antigen. J Immunol. 2001, 167: 188-195.
Allan SE, Passerini L, Bacchetta R, Crellin N, Dai M, Orban PC, Ziegler SF, Roncarolo MG, Levings MK: The role of 2 FOXP3 isoforms in the generation of human CD4+ Tregs. J Clin Invest. 2005, 115: 3276-3284.
Gavin MA, Torgerson TR, Houston E, DeRoos P, Ho WY, Stray-Pedersen A, Ocheltree EL, Greenberg PD, Ochs HD, Rudensky AY: Single-cell analysis of normal and FOXP3-mutant human T cells: FOXP3 expression without regulatory T cell development. Proc Natl Acad Sci USA. 2006, 103: 6659-6664.
Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas de St Groth B, Clayberger C, Soper DM, Ziegler SF, Bluestone JA: CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006, 203: 1701-1711.
Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, Solomon M, Selby W, Alexander SI, Nanan R, Kelleher A, Fazekas de St Groth B: Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006, 203: 1693-1700.
Zhang B, Zhang X, Tang FL, Zhu LP, Liu Y, Lipsky PE: Clinical significance of increased CD4+CD25-Foxp3+ T cells in patients with new-onset systemic lupus erythematosus. Ann Rheum Dis. 2008, 67: 1037-1040.
Yi H, Zhen Y, Jiang L, Zheng J, Zhao Y: The phenotypic characterization of naturally occurring regulatory CD4+CD25+ T cells. Cell Mol Immunol. 2006, 3: 189-195.
Wei S, Kryczek I, Zou W: Regulatory T-cell compartmentalization and trafficking. Blood. 2006, 108: 426-431.
Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W: Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004, 10: 942-949.
Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA: CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001, 167: 1245-1253.
Hamdy AF, Bakr MA, Ghoneim MA: Long-term efficacy and safety of a calcineurin inhibitor-free regimen in live-donor renal transplant recipients. J Am Soc Nephrol. 2008, 19: 1225-1232.
Zimmerman MA, Trotter JF, Wachs M, Bak T, Campsen J, Skibba A, Kam I: Sirolimus-based immunosuppression following liver transplantation for hepatocellular carcinoma. Liver Transpl. 2008, 14: 633-638.
Perez-Simon JA, Sanchez-Abarca I, Diez-Campelo M, Caballero D, San Miguel J: Chronic graft-versus-host disease: Pathogenesis and clinical management. Drugs. 2006, 66: 1041-1057.
Abraham RT, Wiederrecht GJ: Immunopharmacology of rapamycin. Annu Rev Immunol. 1996, 14: 483-510.
Strauss L, Whiteside TL, Knights A, Bergmann C, Knuth A, Zippelius A: Selective survival of naturally occurring human CD4+CD25+Foxp3+ regulatory T cells cultured with rapamycin. J Immunol. 2007, 178: 320-329.
Keever-Taylor CA, Browning MB, Johnson BD, Truitt RL, Bredeson CN, Behn B, Tsao A: Rapamycin enriches for CD4(+) CD25(+) CD27(+) Foxp3(+) regulatory T cells in ex vivo-expanded CD25-enriched products from healthy donors and patients with multiple sclerosis. Cytotherapy. 2007, 9: 144-157.
Turka LA, Walsh PT: IL-2 signaling and CD4+ CD25+ Foxp3+ regulatory T cells. Front Biosci. 2008, 13: 1440-1446.
Hombach AA, Kofler D, Hombach A, Rappl G, Abken H: Effective proliferation of human regulatory T cells requires a strong costimulatory CD28 signal that cannot be substituted by IL-2. J Immunol. 2007, 179: 7924-7931.
Oberg HH, Wesch D, Lenke J, Kabelitz D: An optimized method for the functional analysis of human regulatory T cells. Scand J Immunol. 2006, 64: 353-360.
Ahn JS, Krishnadas DK, Agrawal B: Dendritic cells partially abrogate the regulatory activity of CD4+CD25+ T cells present in the human peripheral blood. Int Immunol. 2007, 19: 227-237.
Fehervari Z, Sakaguchi S: CD4+ Tregs and immune control. J Clin Invest. 2004, 114: 1209-1217.
Annunziato F, Cosmi L, Liotta F, Lazzeri E, Manetti R, Vanini V, Romagnani P, Maggi E, Romagnani S: Phenotype, localization, and mechanism of suppression of CD4(+)CD25(+) human thymocytes. J Exp Med. 2002, 196: 379-387.
Jonuleit H, Schmitt E, Kakirman H, Stassen M, Knop J, Enk AH: Infectious tolerance: human CD25(+) regulatory T cells convey suppressor activity to conventional CD4(+) T helper cells. J Exp Med. 2002, 196: 255-260.
Takahashi T, Kuniyasu Y, Toda M, Sakaguchi N, Itoh M, Iwata M, Shimizu J, Sakaguchi S: Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998, 10: 1969-1980.
de la Rosa M, Rutz S, Dorninger H, Scheffold A: Interleukin-2 is essential for CD4+CD25+ regulatory T cell function. Eur J Immunol. 2004, 34: 2480-2488.
Tang Q, Boden EK, Henriksen KJ, Bour-Jordan H, Bi M, Bluestone JA: Distinct roles of CTLA-4 and TGF-beta in CD4+CD25+ regulatory T cell function. Eur J Immunol. 2004, 34: 2996-3005.
Read S, Greenwald R, Izcue A, Robinson N, Mandelbrot D, Francisco L, Sharpe AH, Powrie F: Blockade of CTLA-4 on CD4+CD25+ regulatory T cells abrogates their function in vivo. J Immunol. 2006, 177: 4376-4383.
Paust S, Lu L, McCarty N, Cantor H: Engagement of B7 on effector T cells by regulatory T cells prevents autoimmune disease. Proc Natl Acad Sci USA. 2004, 101: 10398-10403.
Chen W, Jin W, Wahl SM: Engagement of cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) induces transforming growth factor beta (TGF-beta) production by murine CD4(+) T cells. J Exp Med. 1998, 188: 1849-1857.
Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, Belladonna ML, Fioretti MC, Alegre ML, Puccetti P: Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003, 4: 1206-1212.
Stassen M, Fondel S, Bopp T, Richter C, Muller C, Kubach J, Becker C, Knop J, Enk AH, Schmitt S, Schmitt E, Jonuleit H: Human CD25+ regulatory T cells: two subsets defined by the integrins alpha 4 beta 7 or alpha 4 beta 1 confer distinct suppressive properties upon CD4+ T helper cells. Eur J Immunol. 2004, 34: 1303-1311.
Raghavan S, Suri-Payer E, Holmgren J: Antigen-specific in vitro suppression of murine Helicobacter pylori-reactive immunopathological T cells by CD4CD25 regulatory T cells. Scand J Immunol. 2004, 60: 82-88.
Johnson BD, Jing W, Orentas RJ: CD25+ regulatory T cell inhibition enhances vaccine-induced immunity to neuroblastoma. J Immunother. 2007, 30: 203-214.
Xia G, He J, Zhang Z, Leventhal JR: Targeting acute allograft rejection by immunotherapy with ex vivo-expanded natural CD4+ CD25+ regulatory T cells. Transplantation. 2006, 82: 1749-1755.
Cosmi L, Liotta F, Angeli R, Mazzinghi B, Santarlasci V, Manetti R, Lasagni L, Vanini V, Romagnani P, Maggi E, Annunziato F, Romagnani S: Th2 cells are less susceptible than Th1 cells to the suppressive activity of CD25+ regulatory thymocytes because of their responsiveness to different cytokines. Blood. 2004, 103: 3117-3121.
Bopp T, Palmetshofer A, Serfling E, Heib V, Schmitt S, Richter C, Klein M, Schild H, Schmitt E, Stassen M: NFATc2 and NFATc3 transcription factors play a crucial role in suppression of CD4+ T lymphocytes by CD4+ CD25+ regulatory T cells. J Exp Med. 2005, 201: 181-187.
Suto A, Nakajima H, Kagami SI, Suzuki K, Saito Y, Iwamoto I: Role of CD4(+) CD25(+) regulatory T cells in T helper 2 cell-mediated allergic inflammation in the airways. Am J Respir Crit Care Med. 2001, 164: 680-687.
Dieckmann D, Bruett CH, Ploettner H, Lutz MB, Schuler G: Human CD4(+)CD25(+) regulatory, contact-dependent T cells induce interleukin 10-producing, contact-independent type 1-like regulatory T cells [corrected]. J Exp Med. 2002, 196: 247-253.
Dieckmann D, Plottner H, Berchtold S, Berger T, Schuler G: Ex vivo isolation and characterization of CD4(+)CD25(+) T cells with regulatory properties from human blood. J Exp Med. 2001, 193: 1303-1310.
Godfrey VL, Wilkinson JE, Russell LB: X-linked lymphoreticular disease in the scurfy (sf) mutant mouse. Am J Pathol. 1991, 138: 1379-1387.
Smyk-Pearson SK, Bakke AC, Held PK, Wildin RS: Rescue of the autoimmune scurfy mouse by partial bone marrow transplantation or by injection with T-enriched splenocytes. Clin Exp Immunol. 2003, 133: 193-199.
Sullivan KE, McDonald-McGinn D, Zackai EH: CD4(+) CD25(+) T-cell production in healthy humans and in patients with thymic hypoplasia. Clin Diagn Lab Immunol. 2002, 9: 1129-1131.
Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, Otsuka F, Sakaguchi S: Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J Immunol. 1999, 162: 5317-5326.
Asano M, Toda M, Sakaguchi N, Sakaguchi S: Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J Exp Med. 1996, 184: 387-396.
Gambineri E, Torgerson TR, Ochs HD: Immune dysregulation, polyendocrinopathy, enteropathy, and X-linked inheritance (IPEX), a syndrome of systemic autoimmunity caused by mutations of FOXP3, a critical regulator of T-cell homeostasis. Curr Opin Rheumatol. 2003, 15: 430-435.
Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD: The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001, 27: 20-21.
Bacchetta R, Passerini L, Gambineri E, Dai M, Allan SE, Perroni L, Dagna-Bricarelli F, Sartirana C, Matthes-Martin S, Lawitschka A, Azzari C, Ziegler SF, Levings MK, Roncarolo MG: Defective regulatory and effector T cell functions in patients with FOXP3 mutations. J Clin Invest. 2006, 116: 1713-1722.
Zhan H, Sinclair J, Adams S, Cale CM, Murch S, Perroni L, Davies G, Amrolia P, Qasim W: Immune reconstitution and recovery of FOXP3 (forkhead box P3)-expressing T cells after transplantation for IPEX (immune dysregulation, polyendocrinopathy, enteropathy, X-linked) syndrome. Pediatrics. 2008, 121: e998-1002.
Wildin RS, Smyk-Pearson S, Filipovich AH: Clinical and molecular features of the immunodysregulation, polyendocrinopathy, enteropathy, X linked (IPEX) syndrome. J Med Genet. 2002, 39: 537-545.
Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA: Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004, 199: 971-979.
Putnam AL, Vendrame F, Dotta F, Gottlieb PA: CD4+CD25high regulatory T cells in human autoimmune diabetes. J Autoimmun. 2005, 24: 55-62.
Brusko T, Atkinson M: Treg in type 1 diabetes. Cell Biochem Biophys. 2007, 48: 165-175.
Brusko T, Wasserfall C, McGrail K, Schatz R, Viener HL, Schatz D, Haller M, Rockell J, Gottlieb P, Clare-Salzler M, Atkinson M: No alterations in the frequency of FOXP3+ regulatory T-cells in type 1 diabetes. Diabetes. 2007, 56: 604-612.
Brusko TM, Wasserfall CH, Clare-Salzler MJ, Schatz DA, Atkinson MA: Functional defects and the influence of age on the frequency of CD4+ CD25+ T-cells in type 1 diabetes. Diabetes. 2005, 54: 1407-1414.
Kukreja A, Cost G, Marker J, Zhang C, Sun Z, Lin-Su K, Ten S, Sanz M, Exley M, Wilson B, Porcelli S, Maclaren N: Multiple immuno-regulatory defects in type-1 diabetes. J Clin Invest. 2002, 109: 131-140.
de Kleer IM, Wedderburn LR, Taams LS, Patel A, Varsani H, Klein M, de Jager W, Pugayung G, Giannoni F, Rijkers G, Albani S, Kuis W, Prakken B: CD4+CD25bright regulatory T cells actively regulate inflammation in the joints of patients with the remitting form of juvenile idiopathic arthritis. J Immunol. 2004, 172: 6435-6443.
Pasare C, Medzhitov R: Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003, 299: 1033-1036.
Cao D, Malmstrom V, Baecher-Allan C, Hafler D, Klareskog L, Trollmo C: Isolation and functional characterization of regulatory CD25brightCD4+ T cells from the target organ of patients with rheumatoid arthritis. Eur J Immunol. 2003, 33: 215-223.
Massa M, Passalia M, Manzoni SM, Campanelli R, Ciardelli L, Yung GP, Kamphuis S, Pistorio A, Meli V, Sette A, Prakken B, Martini A, Albani S: Differential recognition of heat-shock protein dnaJ-derived epitopes by effector and Treg cells leads to modulation of inflammation in juvenile idiopathic arthritis. Arthritis Rheum. 2007, 56: 1648-1657.
de Kleer I, Vastert B, Klein M, Teklenburg G, Arkesteijn G, Yung GP, Albani S, Kuis W, Wulffraat N, Prakken B: Autologous stem cell transplantation for autoimmunity induces immunologic self-tolerance by reprogramming autoreactive T cells and restoring the CD4+CD25+ immune regulatory network. Blood. 2006, 107: 1696-1702.
Ruprecht CR, Gattorno M, Ferlito F, Gregorio A, Martini A, Lanzavecchia A, Sallusto F: Coexpression of CD25 and CD27 identifies FoxP3+ regulatory T cells in inflamed synovia. J Exp Med. 2005, 201: 1793-1803.
Duggleby RC, Shaw TN, Jarvis LB, Kaur G, Gaston JS: CD27 expression discriminates between regulatory and non-regulatory cells after expansion of human peripheral blood CD4+ CD25+ cells. Immunology. 2007, 121: 129-139.
Liu MF, Wang CR, Fung LL, Lin LH, Tsai CN: The presence of cytokine-suppressive CD4+CD25+ T cells in the peripheral blood and synovial fluid of patients with rheumatoid arthritis. Scand J Immunol. 2005, 62: 312-317.
Cao D, van Vollenhoven R, Klareskog L, Trollmo C, Malmstrom V: CD25brightCD4+ regulatory T cells are enriched in inflamed joints of patients with chronic rheumatic disease. Arthritis Res Ther. 2004, 6: R335-346.
Dombrecht EJ, Aerts NE, Schuerwegh AJ, Hagendorens MM, Ebo DG, Van Offel JF, Bridts CH, Stevens WJ, De Clerck LS: Influence of anti-tumor necrosis factor therapy (Adalimumab) on regulatory T cells and dendritic cells in rheumatoid arthritis. Clin Exp Rheumatol. 2006, 24: 31-37.
van Amelsfort JM, Jacobs KM, Bijlsma JW, Lafeber FP, Taams LS: CD4(+)CD25(+) regulatory T cells in rheumatoid arthritis: differences in the presence, phenotype, and function between peripheral blood and synovial fluid. Arthritis Rheum. 2004, 50: 2775-2785.
Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA, Mauri C: Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J Exp Med. 2004, 200: 277-285.
Vigna-Perez M, Abud-Mendoza C, Portillo-Salazar H, Alvarado-Sanchez B, Cuevas-Orta E, Moreno-Valdes R, Baranda L, Paredes-Saharopulos O, Gonzalez-Amaro R: Immune effects of therapy with Adalimumab in patients with rheumatoid arthritis. Clin Exp Immunol. 2005, 141: 372-380.
Mottonen M, Heikkinen J, Mustonen L, Isomaki P, Luukkainen R, Lassila O: CD4+ CD25+ T cells with the phenotypic and functional characteristics of regulatory T cells are enriched in the synovial fluid of patients with rheumatoid arthritis. Clin Exp Immunol. 2005, 140: 360-367.
Jiao Z, Wang W, Jia R, Li J, You H, Chen L, Wang Y: Accumulation of FoxP3-expressing CD4+CD25+ T cells with distinct chemokine receptors in synovial fluid of patients with active rheumatoid arthritis. Scand J Rheumatol. 2007, 36: 428-433.
Behrens F, Himsel A, Rehart S, Stanczyk J, Beutel B, Zimmermann SY, Koehl U, Moller B, Gay S, Kaltwasser JP, Pfeilschifter JM, Radeke HH: Imbalance in distribution of functional autologous regulatory T cells in rheumatoid arthritis. Ann Rheum Dis. 2007, 66: 1151-1156.
Wu HY, Staines NA: A deficiency of CD4+CD25+ T cells permits the development of spontaneous lupus-like disease in mice, and can be reversed by induction of mucosal tolerance to histone peptide autoantigen. Lupus. 2004, 13: 192-200.
Rubio CF, Kench J, Russell DM, Yawger R, Nemazee D: Analysis of central B cell tolerance in autoimmune-prone MRL/lpr mice bearing autoantibody transgenes. J Immunol. 1996, 157: 65-71.
Fatenejad S, Peng SL, Disorbo O, Craft J: Central T cell tolerance in lupus-prone mice: influence of autoimmune background and the lpr mutation. J Immunol. 1998, 161: 6427-6432.
Crispin JC, Martinez A, Alcocer-Varela J: Quantification of regulatory T cells in patients with systemic lupus erythematosus. J Autoimmun. 2003, 21: 273-276.
Liu MF, Wang CR, Fung LL, Wu CR: Decreased CD4+CD25+ T cells in peripheral blood of patients with systemic lupus erythematosus. Scand J Immunol. 2004, 59: 198-202.
Mellor-Pita S, Citores MJ, Castejon R, Tutor-Ureta P, Yebra-Bango M, Andreu JL, Vargas JA: Decrease of regulatory T cells in patients with systemic lupus erythematosus. Ann Rheum Dis. 2006, 65: 553-554.
Crispin JC, Vargas MI, Alcocer-Varela J: Immunoregulatory T cells in autoimmunity. Autoimmun Rev. 2004, 3: 45-51.
Azab NA, Bassyouni IH, Emad Y, Abd El-Wahab GA, Hamdy G, Mashahit MA: CD4+CD25+ regulatory T cells (TREG) in systemic lupus erythematosus (SLE) patients: the possible influence of treatment with corticosteroids. Clin Immunol. 2008, 127: 151-157.
Lin SC, Chen KH, Lin CH, Kuo CC, Ling QD, Chan CH: The quantitative analysis of peripheral blood FOXP3-expressing T cells in systemic lupus erythematosus and rheumatoid arthritis patients. Eur J Clin Invest. 2007, 37: 987-996.
Cepika AM, Marinic I, Morovic-Vergles J, Soldo-Juresa D, Gagro A: Effect of steroids on the frequency of regulatory T cells and expression of FOXP3 in a patient with systemic lupus erythematosus: a two-year follow-up. Lupus. 2007, 16: 374-377.
Valencia X, Yarboro C, Illei G, Lipsky PE: Deficient CD4+CD25high T regulatory cell function in patients with active systemic lupus erythematosus. J Immunol. 2007, 178: 2579-2588.
Lee JH, Wang LC, Lin YT, Yang YH, Lin DT, Chiang BL: Inverse correlation between CD4+ regulatory T-cell population and autoantibody levels in paediatric patients with systemic lupus erythematosus. Immunology. 2006, 117: 280-286.
Yan B, Ye S, Chen G, Kuang M, Shen N, Chen S: Dysfunctional CD4+, CD25+ regulatory T cells in untreated active systemic lupus erythematosus secondary to interferon-alpha-producing antigen-presenting cells. Arthritis Rheum. 2008, 58: 801-812.
Barath S, Soltesz P, Kiss E, Aleksza M, Zeher M, Szegedi G, Sipka S: The severity of systemic lupus erythematosus negatively correlates with the increasing number of CD4+CD25(high)FoxP3+ regulatory T cells during repeated plasmapheresis treatments of patients. Autoimmunity. 2007, 40: 521-528.
Gottenberg JE, Lavie F, Abbed K, Gasnault J, Le Nevot E, Delfraissy JF, Taoufik Y, Mariette X: CD4 CD25high regulatory T cells are not impaired in patients with primary Sjogren's syndrome. J Autoimmun. 2005, 24: 235-242.
Li X, Li X, Qian L, Wang G, Zhang H, Wang X, Chen K, Zhai Z, Li Q, Wang Y, Harris DC: T regulatory cells are markedly diminished in diseased salivary glands of patients with primary Sjogren's syndrome. J Rheumatol. 2007, 34: 2438-2445.
Furuno K, Yuge T, Kusuhara K, Takada H, Nishio H, Khajoee V, Ohno T, Hara T: CD25+CD4+ regulatory T cells in patients with Kawasaki disease. J Pediatr. 2004, 145: 385-390.
Miyara M, Amoura Z, Parizot C, Badoual C, Dorgham K, Trad S, Kambouchner M, Valeyre D, Chapelon-Abric C, Debre P, Piette JC, Gorochov G: The immune paradox of sarcoidosis and regulatory T cells. J Exp Med. 2006, 203: 359-370.
Idali F, Wahlstrom J, Muller-Suur C, Eklund A, Grunewald J: Analysis of regulatory T cell associated forkhead box P3 expression in the lungs of patients with sarcoidosis. Clin Exp Immunol. 2008, 152: 127-137.
Brusko TM, Putnam AL, Bluestone JA: Human regulatory T cells: role in autoimmune disease and therapeutic opportunities. Immunol Rev. 2008, 223: 371-390.
Mudd PA, Teague BN, Farris AD: Regulatory T cells and systemic lupus erythematosus. Scand J Immunol. 2006, 64: 211-218.
O'Sullivan BJ, Thomas HE, Pai S, Santamaria P, Iwakura Y, Steptoe RJ, Kay TW, Thomas R: IL-1 beta breaks tolerance through expansion of CD25+ effector T cells. J Immunol. 2006, 176: 7278-7287.
Wan S, Xia C, Morel L: IL-6 produced by dendritic cells from lupus-prone mice inhibits CD4+CD25+ T cell regulatory functions. J Immunol. 2007, 178: 271-279.
Stoop JN, Woltman AM, Biesta PJ, Kusters JG, Kuipers EJ, Janssen HL, Molen van der RG: Tumor necrosis factor alpha inhibits the suppressive effect of regulatory T cells on the hepatitis B virus-specific immune response. Hepatology. 2007, 46: 699-705.
Hasegawa H, Inoue A, Muraoka M, Yamanouchi J, Miyazaki T, Yasukawa M: Therapy for pneumonitis and sialadenitis by accumulation of CCR2-expressing CD4+CD25+ regulatory T cells in MRL/lpr mice. Arthritis Res Ther. 2007, 9: R15-
Yang L, Anderson DE, Baecher-Allan C, Hastings WD, Bettelli E, Oukka M, Kuchroo VK, Hafler DA: IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008, 454: 350-352.
Liu H, Hu B, Xu D, Liew FY: CD4+CD25+ regulatory T cells cure murine colitis: the role of IL-10, TGF-beta, and CTLA4. J Immunol. 2003, 171: 5012-5017.
Kohm AP, Carpentier PA, Anger HA, Miller SD: Cutting edge: CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J Immunol. 2002, 169: 4712-4716.
Tarbell KV, Yamazaki S, Olson K, Toy P, Steinman RM: CD25+ CD4+ T cells, expanded with dendritic cells presenting a single autoantigenic peptide, suppress autoimmune diabetes. J Exp Med. 2004, 199: 1467-1477.
Nagahama K, Nishimura E, Sakaguchi S: Induction of tolerance by adoptive transfer of Treg cells. Methods Mol Biol. 2007, 380: 431-442.
Nishimura E, Sakihama T, Setoguchi R, Tanaka K, Sakaguchi S: Induction of antigen-specific immunologic tolerance by in vivo and in vitro antigen-specific expansion of naturally arising Foxp3+CD25+CD4+ regulatory T cells. Int Immunol. 2004, 16: 1189-1201.
Noris M, Casiraghi F, Todeschini M, Cravedi P, Cugini D, Monteferrante G, Aiello S, Cassis L, Gotti E, Gaspari F, Cattaneo D, Perico N, Remuzzi G: Regulatory T cells and T cell depletion: role of immunosuppressive drugs. J Am Soc Nephrol. 2007, 18: 1007-1018.
Minamimura K, Gao W, Maki T: CD4+ regulatory T cells are spared from deletion by antilymphocyte serum, a polyclonal anti-T cell antibody. J Immunol. 2006, 176: 4125-4132.
Lopez M, Clarkson MR, Albin M, Sayegh MH, Najafian N: A novel mechanism of action for anti-thymocyte globulin: induction of CD4+CD25+Foxp3+ regulatory T cells. J Am Soc Nephrol. 2006, 17: 2844-2853.
Yates J, Rovis F, Mitchell P, Afzali B, Tsang JY, Garin M, Lechler RI, Lombardi G, Garden OA: The maintenance of human CD4+ CD25+ regulatory T cell function: IL-2, IL-4, IL-7 and IL-15 preserve optimal suppressive potency in vitro. Int Immunol. 2007, 19: 785-799.
Imamichi H, Sereti I, Lane HC: IL-15 acts as a potent inducer of CD4(+)CD25(hi) cells expressing FOXP3. Eur J Immunol. 2008, 38: 1621-1630.
Niedbala W, Wei XQ, Cai B, Hueber AJ, Leung BP, McInnes IB, Liew FY: IL-35 is a novel cytokine with therapeutic effects against collagen-induced arthritis through the expansion of regulatory T cells and suppression of Th17 cells. Eur J Immunol. 2007, 37: 3021-3029.
Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK: IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007, 448: 484-487.
Karandikar NJ, Vanderlugt CL, Walunas TL, Miller SD, Bluestone JA: CTLA-4: a negative regulator of autoimmune disease. J Exp Med. 1996, 184: 783-788.
Bluestone JA, Thomson AW, Shevach EM, Weiner HL: What does the future hold for cell-based tolerogenic therapy?. Nat Rev Immunol. 2007, 7: 650-654.
Barrett AJ: Manipulating regulatory T cells. Cytotherapy. 2007, 9: 109-110.
Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo MG: Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J Immunol. 2006, 177: 8338-8347.
Horibe EK, Sacks J, Unadkat J, Raimondi G, Wang Z, Ikeguchi R, Marsteller D, Ferreira LM, Thomson AW, Lee WP, Feili-Hariri M: Rapamycin-conditioned, alloantigen-pulsed dendritic cells promote indefinite survival of vascularized skin allografts in association with T regulatory cell expansion. Transpl Immunol. 2008, 18: 307-318.
Acknowledgements
This work is supported by the American College of Rheumatology REF award to Diana Milojevic.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
DM has formulated the concept and design of the manuscript and has written the review. KDN critically revised the initial manuscript and created the figures. DW has been involved in revising the manuscript. EDM has made critical contributions to the concept, design, and revision of the manuscript. All authors read and approved the final manuscript.
Diana Milojevic, Khoa D Nguyen contributed equally to this work.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Milojevic, D., Nguyen, K.D., Wara, D. et al. Regulatory T cells and their role in rheumatic diseases: a potential target for novel therapeutic development. Pediatr Rheumatol 6, 20 (2008). https://doi.org/10.1186/1546-0096-6-20
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1546-0096-6-20