Abstract
Background
The deafness-associated gene mutation profile varies greatly among regions and races. Due to the multi-ethnic coalition of over one thousand years, non-syndromic deafness (NSD) patients of Uyghur ethnicity may exhibit a unique deafness-associated gene mutation spectrum as compared to Han Chinese deaf population.
Methods
In order to characterize nine loci of four deafness-associated genes of Uyghur NSD patients in comparison with Chinese Han deaf population, NSD patients (n = 350) were enrolled, including Uyghur (n = 199) and Han Chinese (n = 151). Following the history taking, blood samples were collected for DNA extraction. DNA microarray was performed on nine loci of four deafness-associated genes, including 35delG, 176-191del16, 235delC, 299-300delAT, 538C > T, 1555A > G, 1494C > T, 2168A > G, and IVS7-2A > G. The samples that showed the absence of both wild and mutant probe signals were tested for further DNA sequencing analysis.
Results
The mutations in the nine loci of prevalent deafness-associated genes were detected in 13.06% of Uyghur NSD patients and 32.45% of Han Chinese patients (P < 0.05), respectively. GJB2 mutation was detected in 9.05% of Uyghur patients and 16.56% of Han Chinese patients (P > 0.05), respectively. 235delC was the hotspot mutation region in NSD patients of the two ethnicities, whereas 35delG was the mutation hotspot in Uyghur patients. 187delG mutation was detected for the first time in Uyghur NSD patients and considered as an unreported pathological variant of GJB2. SLC26A4 mutation was found in 2.01% of Uyghur patients and 14.57% of Han Chinese patients (P < 0.05), respectively. The frequencies of mtDNA 12S rRNA mutation in Uyghur and Han Chinese patients were 2.01% and 2.65% (P > 0.05), respectively. The NSD patients exhibited a low frequency of GJB3 mutation regardless of ethnicity.
Conclusion
Prevalent deafness-associated gene mutations in the nine loci studied were less frequently detected in Uyghur NSD patients than in Han Chinese patients. GJB2 was the most common mutant gene in the two ethnicities, whilst the two ethnicities differed substantially in hotspot mutations. A low-frequency SLC26A4 mutation was detected in Uyghur NSD patients. Uyghur NSD patients differed significantly from Han Chinese patients in gene mutation profile.
Similar content being viewed by others
Background
Deafness is a worldwide prevalent disease that seriously impairs human quality of life. The global incidence of congenital deafness is 1-3 per 1,000 newborns. The epidemiological studies show that one half of deafness at childhood is associated with genetic factors [1, 2]. Non-syndromic deafness (NSD) is the most common neurosensory deafness, and accounts for 70% of inherited hearing impairment [3]. Until now, more than 200 genes have been associated with NSD, including 36 autosomal recessive genes, 24 autosomal dominant genes, and two X-linked genes (http://hereditaryhearingloss.org/, updated in October 2010).
The deafness-associated gene mutation spectrum as well as dominant gene profile vary greatly among regions and races [4, 5]. 35de1G, 167de1T, and 235de1C are reported to be the most prevalent mutant genes among Caucasian, Jewish, and Asian populations, respectively [6–8]. The epidemiological studies demonstrate that a large proportion of NSD can be caused by a relative small number of mutant genes, including GJB2, SLC26A4, and mtDNA 12S rRNA [9–13]. A positive result is very likely to be obtained by scanning the hotspot mutations of these genes alone. Microarray chip technology is a high-throughput and high-efficiency tool for the detection of gene mutations. CapitalBio Corporation (Beijing, China) develops a microarray chip that is designated to detect mutation hotspots of inherited deafness in Chinese population, based on the large-scale epidemiological study data across 28 provinces and municipalities from Chinese People's Liberation Army General Hospital [14–16]. This microarray chip allows the simultaneous detection of nine loci of four deafness-associated genes, namely, 35delG, 176-191del16, 235delC and 299-300delAT for GJB2 gene, 538C > T for GJB3 gene, 1555A > G and 1494C > T for mtDNA 12S rRNA gene, and 2168A > G (H723R) and IVS7-2A > G for SLC26A4 gene.
Xinjiang is located on the northwest border of China, where central and western Asian cultures converge. It is a region populated by a variety of ethnicities but mainly by Uyghur ethnicity. The deafness-associated gene mutation spectrum and dominant mutation profile may be unique in Uyghur population residing in Xinjiang due to the multi-ethnic coalition over one thousand years. In this study, we use the aforementioned microarray chip to examine and analyze nine loci of four deafness-associated genes among Uyghur and Han Chinese NSD patients residing in Xinjiang, in aiming to characterize nine loci of four deafness-associated genes of Uyghur NSD patients in comparison with Han Chinese patients.
Materials and methods
Patients and DNA samples
The study protocol was approved by the Institutional Review Board at the First Affiliated Hospital of Xinjiang Medical University. The subjects, or their legal guardians in the case of subjects under 18 years, volunteered to give informed consent. Subjects were enrolled from the representative deaf populations registered with Xinjiang local institutes, including Kashi Municipal Special Education School, Kashi Municipal Disabled People's Association, Changji Municipal Special Education School, Urumqi Lingual Training School, and Otorhinolaryngology Outpatient Clinic, the First Affiliated Hospital of Xinjiang Medical University. Parents were inquired for a full history, including family history, pregnancy/labor process, age of onset/diagnosis, and previous history of infection, head trauma and medication with aminoglycosides. Deaf patients received routine physical and otorhinolaryngoloical examination, as well as pure tone audiometry. Brainstem auditory evoked potential test was performed in uncooperative children. Only biologically unrelated NSD patients (n = 350) who exhibited bilateral moderate or severe sensorineural hearing impairment on pure tone audiometry or brainstem auditory evoked potential test were included. Parents of NSD children were excluded from this study. The ethnicity was determined based on the institutional household records. Healthy volunteers of both Uyghur (n = 103) and Han Chinese ethnicities (n = 70) who resided in Xinjiang and exhibited normal hearing were enrolled as control subjects. Peripheral venous blood samples (3-5 ml) were collected from both NSD patients and control subjects.
Mutational analysis with PCR
Genomic DNA was extracted from peripheral blood leukocytes using a commercial available DNA isolation kit (Tiangen Biotech Corporation, Beijing, China). The concentration and purity of DNA were determined using an ultraviolet spectrometry (ActGene Inc, Taipei, Taiwan). Polymerase chained reaction, chip hybridization, and chip scan were conducted as recommended by the manufacturer of the microarray kit (CapitalBio Corporation, Beijing, China) for the simultaneous detection of nine hotspot mutations in four most prevalent NSD-associated genes, including GJB2 (35delG, 176-191del16, 235delC, and 299-300delAT), GJB3 (538C > T), SLC26A4 (IVS7-2A > G and 2168A > G), and mitochondrial 12S rRNA (1555A > G and 1494C > T). The test results were determined based on the fluorescent hybridization signal and the distribution of microarray probe. In the case of the deletion at any locus, the DNA sample was further sequenced by using an ABI 3730XL analyzer (ABI, Foster, USA).
Statistical analysis
The statistical analysis was performed using SPSS 17.0 (SPSS Inc., Chicago, USA). The intergroup difference in frequency was compared using the two-tailed chi-square test or Fisher's exact probability test. A P-value less than 0.05 was considered statistically significant.
Results
In this study, the NSD population (n = 350) included 199 Uyghur patients (86 males and 113 females), aged 12.7 ± 5.3 years (range, 1-35), and 151 Han Chinese patients (86 males and 65 females), aged 10.9 ± 5.4 years (1-26). Out of 350 patients, 33 patients had a family history of deafness, whose siblings, parents, or other close relatives were also deaf, including 22 Uyghur and 11 Han Chinese subjects. The remaining 317 cases were determined to be sporadic.
Prevalent deafness-associated gene mutations in NSD patients and control subjects
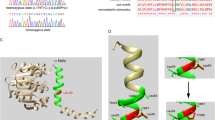
Out of 199 Uyghur patients, 22 (11.1%) patients exhibited mutations in one allele or more. The deletion of both mutant and wild probe signal was detected in four (2.0%) Uyghur patients. This suggested that the possible presence of new mutant locus failed the effective binding of hybridization prime to the DNA sequence of the test sample. The further gene sequencing identified 187delG/187delG for GJB2 in three patients (Figure 1A), and 1503G > A for mtDNA 12S rRNA in one patient (Figure 1B). The homogenous mutations identified in these four patients were, therefore, included as positive test results (Table 1). The frequencies of prevalent deafness-associated gene mutations in the nine loci were 13.06%, 1.94%, 32.45% and 4.28% in Uyghur patients, Uyghur control subjects, Han Chinese patients, and Han Chinese control subjects, respectively (Table 2). The mutation frequency was significantly lower in Uyghur patients than in Han Chinese patients (χ2 = 19.162, P < 0.05). Moreover, the mutations were also more frequently detected in the patient population than in control subjects for either ethnicity (χ2 = 9.983, P < 0.05; χ2 = 21.086, P < 0.05).
Multination of deafness-associated gene loci in NSD patients and control subjects
The frequencies of mutations for GJB2, SLC26A4, mtDNA 12S rRNA and GJB3 in the two ethnicities were shown in Table 3. The mutant genotypes of the four deafness-associated genes in Uyghur patients, Uyghur control subjects, Han Chinese patients, and Han Chinese control subjects were presented in Table 4.
The frequency of GJB2 multination was 9.05% (18/199) in Uyghur patients, involving three mutations, namely, 235delC, 35delG, and 187delG. The allele frequencies were 3.52% (14/398), 2.26% (9/398) and 1.51% (6/398) in Uyghur patients, respectively. 235delC showed the highest frequency, whilst 187delG was identified for the first time in Uyghur NSD patients, and considered as an unreported pathological mutation of GJB2. The GJB2 mutation frequency in Han Chinese patients was 16.56% (25/151), also involving three mutations, namely, 235delC, 299-300delAT, and 176-191del16. The allele frequencies were 9.93% (30/302), 2.32% (7/302) and 0.33% (1/302) in Han Chinese patients, respectively. The mutation frequencies of GJB2 were 1.94% (2/103) and 4.28% (3/70) in Uyghur and Han Chinese control subjects (χ2 = 4.495, P > 0. 05), respectively. The allele frequency of 235delC was significantly higher in Han Chinese patients than in Uyghur patients (χ2 = 12.000, P < 0. 05), whereas that of 35delG was significantly higher in Uyghur patients than in Han Chinese patients (Fisher's exact probability test, P < 0. 05).
The frequencies of SLC26A4 mutation, including 2168A > G (H723R) and IVS7-2A > G, were 2.01% (4/199) in Uyghur patients and 14.57% (22/151) in Han Chinese patients, respectively. Of note, IVS7-2A > G mutant alleles accounted for 83.33% (25/30) of SLC26A4 mutation in Han Chinese patients. SLC26A4 mutation was less frequently found in Uyghur patients than in Han Chinese patients (χ2 = 19.694, P < 0.05).
The frequencies of mtDNA 12S rRNA mutations, including 1555A > G and 1494C > T, were 2.01% (4/199) in Uyghur patients and 2.65% (4/151) in Han Chinese patients, respectively. The homozygous mutation of 1503 G > A was an unreported gene mutation. The mutation frequencies of 538C > T for GJB3 were 0.0% (0/199) in Uyghur patients and 1.32% (2/151) in Han Chinese patients. The mutation frequencies of mtDNA 12S rRNA and GJB3 were relatively low in both Uyghur and Han Chinese patients (Fisher's exact probability test, P > 0. 05).
Mutation profiles of NSD patients with a definitive family history of deafness
Among Uyghur patients (n = 22) with a family history of deafness, the gene mutation in any of the nine loci studied was found in three (3/22, 13.64%) patients. In contrast, out of eleven Han Chinese patients with a family history of deafness, six patients (6/11, 54.55%) were found to carry the gene mutation in any of the nine loci. Most of these mutations were homozygous, homogenous, and double or compound heterozygous. For patients with a family history of deafness, the frequencies of gene mutations in the nine loci studied were significantly higher in Han Chinese patients than in Uyghur patients (Fisher's exact probability test, P < 0. 05).
Discussion
In our study, the mutation frequencies of the nine loci for the four prevalent deafness-associated genes are 13.06% in Uyghur patients, 1.94% in Uyghur control subjects, 32.45% in Han Chinese patients, and 4.28% in Han Chinese control subjects, respectively. The mutation frequencies are significantly higher in NSD patients than in control subjects for either ethnicity, supporting the profound role of prevalent deafness-associated genetic testing in the etiological diagnosis of NSD patients. Moreover, the mutation frequency is significantly lower in Uyghur patients than in Han Chinese patients, requiring the further analysis of deafness-associated genes and mutation hotspots for the two ethnicities.
GJB2 gene encodes CX-26, a connexin associated with the formation of gap junction and osmolality. As Cx-26 is highly expressed on human cochlear hairy cells, GJB2 gene mutation is believed to be closely associated with NSD. GJB2 mutation prevails among deaf people across regions and races [17]. It is reported that GJB2 mutation accounts for over 50% of autosomal recessive inherited deafness cases [2, 8]. Dai et al [18] reported that the frequency of GJB2 mutation was 4.0-30.4% in Chinese NSD patients. In our study, the mutation frequencies of GJB2 are 9.05% Uyghur patients and 16.56% for Han Chinese patients, in consistence with the report of Dai et al. In contrast to SLC26A4, mtDNA 12S rRNA and GJB3, GJB2 exhibits the highest mutation frequency in the two ethnicities, justifying that GJB2 remains the primary deafness-associated gene in both Uyghur and Han Chinese NSD patients. The mutant loci and allele frequencies differ significantly between the two ethnicities, whereas the overall mutation frequency of GJB2 mutation is comparable. Among the three mutations of GJB2 found in Uyghur patients, 235delC shows the highest allele frequency, and 35delG follows 235delC in allele frequency. 235delC is reported to be the major mutation among Eastern Asians [19–21], whereas 35delG is the hotspot mutation in Caucasians. Homozygous or heterozygous 35de1G mutation is also found in Western Asians, an extended family of Caucasian [22, 23]. Both 235delC and 35delG are simultaneously identified as the predominant mutations in Uyghur NSD patients, which is probably attributed to the ethnic origin of Uyghur people. Located close to Central Asia, Kashi is an important stop of the ancient Silk Road. Uyghur people have mixed 30% of Caucasian kinship through the migration and cross-ethnic marriage, rendering themselves the Caucasian and Mongolian characters simultaneously [24]. Among the three mutations detected in Han Chinese NSD patients, 235delC has the highest allele frequency, consistent with the fact that 235delC is the major mutation in Eastern Asian population. In our study, 187delG is found in Uyghur NSD patients for the first time and considered as an unreported pathogenetic mutation of GJB2 (http://davinci.crg.es/deafness/, updated in January 2011). 187delG mutation results in the frameshift mutation of codon and the subsequent production of inactive connexin. Mutant proteins may cause the dysregulation of pH, decrease the permeability of gap junction, and consequently lead to hearing impairment. The allele frequency of 187delG is 1.51% (6/398), manifesting as homozygous mutations in the three Uyghur patients. Two of the three patients were borne by the couples in consanguineous marriages who have normal hearing otherwise. It suggests that all these parents are potential carriers. It is likely that 187delG is a common mutation among Uyghur NSD patients.
As a major sensorineural deafness-associated gene that is discovered following the identification of GJB2, SLC26A4 mutation is associated with the dilation of vestibular aqueduct in NSD [25] and accounts for 4-15% of inherited deafness cases [1, 26]. In Western deaf population, L236P, T416P, E384G, and IVS8+1G > A are common mutant loci of SLC26A4[21], whereas 2168A > G is the primary mutant locus in Japanese and Korean deaf patients [27, 28]. IVS7-2 A > G is reported to be the major mutant locus of SLC26A4 in Han Chinese deaf population as well [15, 29]. In our study, the two locus mutations of SLC26A4, namely, IVS7-2A > G and 2168A > G (H723R), are less frequently found in Uyghur NSD patients, whereas IVS7-2A > G is the primary mutation in Uyghur patients. Dai et al. [15] reported the complete absence of IVS7-2A > G mutation in 60 Uyghur patients. Li et al. [30] reported that IVS7-2A > G, a major mutant locus of SLC26A4 exhibited a relatively low frequency among Northwestern Chinese ethnicities, including Uyghur people. This finding shows a low carrier frequency of the two SLC26A4 locus mutations in Uyghur population. The mutation frequency of SLC26A4 is 14.57% (22/151) in Han Chinese NSD patients, whilst IVS7-2A > G mutant alleles account for 83.33% (25/30) among SLC26A4 mutation. This is consistent with the report that IVS7-2A > G is the primary mutation of SLC26A4 in Han Chinese NSD patients. The frequency of SLC26A4 mutation differs significantly between the two ethnicities. The low-frequency SLC26A4 mutation in Uyghur NSD patients is inconsistent with the fact that SLC26A4 is one of the major sensorineural deafness-associated genes. It can be inferred that Uyghur NDS patients differ from Mongolian ethnicity in the hotspot mutation of SLC26A4. Whether Uyghur patients share some mutation hotspots in SLC26A4 with Caucasian population remains yet to be investigated.
As a matrilineal mitochondrial gene, mtDNA 12S rRNA is associated with aminoglycoside-induced deafness [31, 32]. 1555A > G and 1494C > T are the most important and common mutations. 1555A > G is more frequently detected, but shows a variable prevalence probably due to the ethnic variation in the susceptibility to aminoglycoside toxicity [33]. Both Spanish and Japanese severe progressive deaf populations show a relatively low-frequency 1555A > G mutation [34, 35], in comparison with a frequency of 2.9-13.0% reported by Li et al. [11]. The mutation frequency of 1494C > T is even lower than that of 1555A > G. 1494C > T mutation is detected in only one Han Chinese patient in contrast to the 'zero' reported by Li et al. [11]. The mutation frequency of mtDNA 12S rRNA is statistically comparable between the two ethnicities. The unreported 1503G > A homogenous mutation that is found in one Uyghur NSD patient requires the further analysis to delineate whether it is a pathogenetic mutation.
GJB3 is a gene associated with NSD, and is initially cloned by Xia et al. [36]. GJB3 mutation is associated with the high-frequency hearing impairment in an autosomal dominant or recessive inheritance pattern [37]. GJB3 mutation is rarely detected in European and North American populations [38]. 538C > T mutation of GJB3 is detected in two of Han Chinese patients, but not in Uyghur patients or control subjects, showing a insignificant difference in the frequency of GJB3 mutation between the two ethnicities though.
The mutation frequencies of the nine loci in patients with a definitive family history of deafness are 13.64% for Uyghur and 54.55% for Han Chinese, respectively. The low mutation frequency in Uyghur NSD patients suggests that some mutations occurring at other loci or in other deafness-associated genes may be implicated in this ethnic group.
Conclusion
Our study show that Uyghur NSD patients differ significantly from Han Chinese patients in deafness-associated gene mutation spectrum. Therefore, the further gene sequencing analysis and pedigree analysis are required to examine the mutation profile and determine the predominant mutations in Uyghur NSD patients.
References
Morton CC, Nance WE: Newborn hearing screening-a silent revolution. N Engl J Med. 2006, 354: 2151-2164. 10.1056/NEJMra050700.
Smith RJ, Bale JF, White KR: Sensorineural hearing loss in children. Lancet. 2005, 365: 879-890. 10.1016/S0140-6736(05)71047-3.
Resendes BL, Williamson RE, Morton CC: At the speed of sound: gene discovery in the auditory system. Am J Hum Genet. 2001, 69: 923-935. 10.1086/324122.
Liu XZ, Xia XJ, Ke XM, Ouyang XM, Du LL, Liu YH, Angeli S, Telischi FF, Nance WE, Balkany T, Xu LR: The prevalence of connexin 26 (GJB2) mutations in the Chinese population. Hum Genet. 2002, 111: 394-397. 10.1007/s00439-002-0811-6.
Usami S, Wagatsuma M, Fukuoka H, Suzuki H, Tsukada K, Nishio S, Takumi Y, Abe S: The responsible genes in Japanese deafness patients and clinical application using Invader assay. Acta Otolaryngol. 2008, 128: 446-454. 10.1080/00016480701785046.
Ohtsuka A, Yuge I, Kimura S, Namba A, Abe S, Van Laer L, Van Camp G, Usami S: GJB2 deafness gene shows a specific spectrum of mutations in Japan, including a frequent founder mutation. Hum Genet. 2003, 112: 329-333.
Snoeckx RL, Huygen PL, Feldmann D, Marlin S, Denoyelle F, Waligora J, Mueller-Malesinska M, Pollak A, Ploski R, Murgia A, Orzan E, Castorina P, Ambrosetti U, Nowakowska-Szyrwinska E, Bal J, Wiszniewski W, Janecke AR, Nekahm-Heis D, Seeman P, Bendova O, Kenna MA, Frangulov A, Rehm HL, Tekin M, Incesulu A, Dahl HH, du Sart D, Jenkins L, Lucas D, Bitner-Glindzicz M, Avraham KB: GJB2 mutations and degree of hearing loss: a multicenter study. Am J Hum Genet. 2005, 77: 945-957. 10.1086/497996.
Kenneson A, Van Naarden Braun K, Boyle C: GJB2 (connexin 26) variants and nonsyndromic sensorineural hearing loss: a HuGE review. Genet Med. 2002, 4: 258-274. 10.1097/00125817-200207000-00004.
Dai P, Liu X, Han D, Qian Y, Huang D, Yuan H, Li W, Yu F, Zhang R, Lin H, He Y, Yu Y, Sun Q, Qin H, Li R, Zhang X, Kang D, Cao J, Young WY, Guan MX: Extremely low penetrance of deafness associated with the mitochondrial 12S rRNA mutation in 16 Chinese families: implication for early detection and prevention of deafness. Biochem Biophys Res Commun. 2006, 340: 194-199. 10.1016/j.bbrc.2005.11.156.
Zhao H, Li R, Wang Q, Yan Q, Deng JH, Han D, Bai Y, Young WY, Guan MX: Maternally inherited aminoglycoside-induced and nonsyndromic deafness is associated with the novel C1494T mutation in the mitochondrial 12S rRNA gene in a large Chinese family. Am J Hum Genet. 2004, 74: 139-152. 10.1086/381133.
Li Z, Li R, Chen J, Liao Z, Zhu Y, Qian Y, Xiong S, Heman-Ackah S, Wu J, Choo DI, Guan MX: Mutational analysis of the mitochondrial 12S rRNA gene in Chinese pediatric subjects with aminoglycoside-induced and non-syndromic hearing loss. Hum Genet. 2005, 117: 9-15. 10.1007/s00439-005-1276-1.
Abe S, Yamaguchi T, Usami S: Application of deafness diagnostic screening panel based on deafness mutation/gene database using invader assay. Genet Test. 2007, 11: 333-340. 10.1089/gte.2007.0002.
Dai P, Yu F, Han B, Wu H, Yuan YY, Li Q, Wang GJ, Liu X, He J, Huang DL, Kang DY, Zhang X, Yuan HJ, Leejun CW, Han DY: Features of nationwide distribution and frequency of a common gap junction beta-2 gene mutation in China. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2007, 42: 804-808.
Wang QJ, Zhao YL, Rao SQ, Guo YF, Yuan H, Zong L, Guan J, Xu BC, Wang DY, Han MK, Lan L, Zhai SQ, Shen Y: A distinct spectrum of SLC26A4 mutations in patients with enlarged vestibular aqueduct in China. Clin Genet. 2007, 72: 245-254. 10.1111/j.1399-0004.2007.00862.x.
Dai P, Li Q, Huang D, Yuan Y, Kang D, Miller DT, Shao H, Zhu Q, He J, Yu F, Liu X, Han B, Yuan H, Platt OS, Han D, Wu BL: SLC26A4 c.919-2A > G varies among Chinese ethnic groups as a cause of hearing loss. Genet Med. 2008, 10: 586-592. 10.1097/GIM.0b013e31817d2ef1.
Li CX, Pan Q, Guo YG, Li Y, Gao HF, Zhang D, Hu H, Xing WL, Mitchelson K, Xia K, Dai P, Cheng J: Construction of a multiplex allele-specific PCR-based universal array (ASPUA) and its application to hearing loss screening. Hum Mutat. 2008, 29: 306-314. 10.1002/humu.20622.
Abe S, Usami S, Shinkawa H, Kelley PM, Kimberling WJ: Prevalent connexin 26 gene (GJB2) mutations in Japanese. J Med Genet. 2000, 37: 41-43. 10.1136/jmg.37.1.41.
Dai P, Yu F, Han B, Liu X, Wang G, Li Q, Yuan Y, Liu X, Huang D, Kang D, Zhang X, Yuan H, Yao K, Hao J, He J, He Y, Wang Y, Ye Q, Yu Y, Lin H, Liu L, Deng W, Zhu X, You Y, Cui J, Hou N, Xu X, Zhang J, Tang L, Song R: GJB2 mutation spectrum in 2,063 Chinese patients with nonsyndromic hearing impairment. J Transl Med. 2009, 7: 26-10.1186/1479-5876-7-26.
Yan D, Park HJ, Ouyang XM, Pandya A, Doi K, Erdenetungalag R, Du LL, Matsushiro N, Nance WE, Griffith AJ, Liu XZ: Evidence of a founder effect for the 235delC mutation of GJB2 (connexin 26) in east Asians. Hum Genet. 2003, 114: 44-50. 10.1007/s00439-003-1018-1.
Xiao ZA, Xie DH: GJB2 (Cx26) gene mutations in Chinese patients with congenital sensorineural deafness and a report of one novel mutation. Chin Med J (Engl). 2004, 117: 1797-1801.
Dai P, Yu F, Han B, Yuan Y, Li Q, Wang G, Liu X, He J, Huang D, Kang D, Zhang X, Yuan H, Schmitt E, Han D, Wong LJ: The prevalence of the 235delC GJB2 mutation in a Chinese deaf population. Genet Med. 2007, 9: 283-289. 10.1097/GIM.0b013e31804d2371.
Gasparini P, Rabionet R, Barbujani G, Melçhionda S, Petersen M, Brøndum-Nielsen K, Metspalu A, Oitmaa E, Pisano M, Fortina P, Zelante L, Estivill X: High carrier frequency of the 35delG deafness mutation in European populations. Genetic Analysis Consortium of GJB2 35delG. Eur J Hum Genet. 2000, 8: 19-23. 10.1038/sj.ejhg.5200406.
Abidi O, Boulouiz R, Nahili H, Ridal M, Alami MN, Tlili A, Rouba H, Masmoudi S, Chafik A, Hassar M, Barakat A: GJB2 (connexin 26) gene mutations in Moroccan patients with autosomal recessive non-syndromic hearing loss and carrier frequency of the common GJB2-35delG mutation. Int J Pediatr Otorhinolaryngol. 2007, 71: 1239-1245. 10.1016/j.ijporl.2007.04.019.
Li Q, Dai P, Huang DL, Zhang J, Wang GJ, Zhu QW, Liu X, Han DY: Prevalence of GJB2 mutations in Uigur and Han ethnic populations with deafness in Xinjiang region of China. Zhonghua Yi Xue Za Zhi. 2007, 87: 2977-2981.
Hilgert N, Smith RJ, Van Camp G: Forty-six genes causing nonsyndromic hearing impairment: which ones should be analyzed in DNA diagnostics?. Mutat Res. 2009, 681: 189-196. 10.1016/j.mrrev.2008.08.002.
Dai P, Yuan Y, Huang D, Zhu X, Yu F, Kang D, Yuan H, Wu B, Han D, Wong LJ: Molecular etiology of hearing impairment in Inner Mongolia: mutations in SLC26A4 gene and relevant phenotype analysis. J Transl Med. 2008, 6: 74-10.1186/1479-5876-6-74.
Park HJ, Shaukat S, Liu XZ, Hahn SH, Naz S, Ghosh M, Kim HN, Moon SK, Abe S, Tukamoto K, Riazuddin S, Kabra M, Erdenetungalag R, Radnaabazar J, Khan S, Pandya A, Usami SI, Nance WE, Wilcox ER, Riazuddin S, Griffith AJ: Origins and frequencies of SLC26A4 (PDS) mutations in east and south Asians: global implications for the epidemiology of deafness. J Med Genet. 2003, 40: 242-248. 10.1136/jmg.40.4.242.
Tsukamoto K, Suzuki H, Harada D, Namba A, Abe S, Usami S: Distribution and frequencies of PDS (SLC26A4) mutations in Pendred syndrome and nonsyndromic hearing loss associated with enlarged vestibular aqueduct: a unique spectrum of mutations in Japanese. Eur J Hum Genet. 2003, 11: 916-922. 10.1038/sj.ejhg.5201073.
Guo YF, Liu XW, Guan J, Han MK, Wang DY, Zhao YL, Rao SQ, Wang QJ: GJB2, SLC26A4 and mitochondrial DNA A1555G mutations in prelingual deafness in Northern Chinese subjects. Acta Otolaryngol. 2008, 128: 297-303. 10.1080/00016480701767382.
Li Q, Dai P, Huang DL, Yuan YY, Zhu QW, Han B, Liu X, Yu F, Kang DY, Zhang X, Xue DD, Jin ZC: Frequency of SLC26A4 IVS7-2A > G mutation in patients with severe to profound hearing loss from different area and ethnic group in China. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2007, 42: 893-897.
Fischel-Ghodsian N: Genetic factors in aminoglycoside toxicity. Pharmacogenomics. 2005, 6: 27-36. 10.1517/14622416.6.1.27.
Tang X, Yang L, Zhu Y, Liao Z, Wang J, Qian Y, Tao Z, Hu L, Wu G, Lan J, Wang X, Ji J, Wu J, Ji Y, Feng J, Chen J, Li Z, Zhang X, Lu J, Guan MX: Very low penetrance of hearing loss in seven Han Chinese pedigrees carrying the deafness-associated 12S rRNA A1555G mutation. Gene. 2007, 393: 11-19. 10.1016/j.gene.2007.01.001.
Prezant TR, Agapian JV, Bohlman MC, Bu X, Oztas S, Qiu WQ, Arnos KS, Cortopassi GA, Jaber L, Rotter JI, Shohat M, Fischel-Ghodsian N: Mitochondrial ribosomal RNA mutation associated with both antibiotic-induced and non-syndromic deafness. Nat Genet. 1993, 4: 289-294. 10.1038/ng0793-289.
Estivill X, Govea N, Barceló E, Badenas C, Romero E, Moral L, Scozzri R, D'Urbano L, Zeviani M, Torroni A: Familial progressive sensorineural deafness is mainly due to the mtDNA A1555G mutation and is enhanced by treatment of aminoglycosides. Am J Hum Genet. 1998, 62: 27-35. 10.1086/301676.
Usami S, Abe S, Akita J, Namba A, Shinkawa H, Ishii M, Iwasaki S, Hoshino T, Ito J, Doi K, Kubo T, Nakagawa T, Komiyama S, Tono T, Komune S: Prevalence of mitochondrial gene mutations among hearing impaired patients. J Med Genet. 2000, 37: 38-40. 10.1136/jmg.37.1.38.
Xia JH, Liu CY, Tang BS, Pan Q, Huang L, Dai HP, Zhang BR, Xie W, Hu DX, Zheng D, Shi XL, Wang DA, Xia K, Yu KP, Liao XD, Feng Y, Yang YF, Xiao JY, Xie DH, Huang JZ: Mutations in the gene encoding gap junction protein beta-3 associated with autosomal dominant hearing impairment. Nat Genet. 1998, 20: 370-373. 10.1038/3845.
Liu XZ, Xia XJ, Xu LR, Pandya A, Liang CY, Blanton SH, Brown SD, Steel KP, Nance WE: Mutations in connexin31 underlie recessive as well as dominant non-syndromic hearing loss. Hum Mol Genet. 2000, 9: 63-67. 10.1093/hmg/9.1.63.
Frei K, Ramsebner R, Hamader G, Lucas T, Schoefer C, Baumgartner WD, Wachtler FJ, Kirschhofer K: Lack of association between Connexin 31 (GJB3) alterations and sensorineural deafness in Austria. Hear Res. 2004, 194: 81-86. 10.1016/j.heares.2004.03.007.
Acknowledgements
This study was supported by Young Scientists Fellowship Program of the First Affiliated Hospital of Xinjiang Medical University (200963) to Dr. Yu Chen. The authors also wished to thank Dr. Harry H. Qin (Institute of Liver Studies, King's College, London, UK) for proofreading the English manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
YC, HZ, and JS carried out the molecular genetic studies, participated in the collection of blood samples, and drafted the manuscript. MT, PK, and BH participated in the collection of blood samples. CH, HLL, and QS carried out the mutational analysis with PCR. DJ participated in the sequence alignment. YC and HZ participated in the design of the study, performed the statistical analysis, and drafted the manuscript. HZ and HLL reviewed and interpreted the results, drafted and revised the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Chen, Y., Tudi, M., Sun, J. et al. Genetic mutations in non-syndromic deafness patients of uyghur and han chinese ethnicities in xinjiang, China: a comparative study. J Transl Med 9, 154 (2011). https://doi.org/10.1186/1479-5876-9-154
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1479-5876-9-154