Abstract
Background
Bone-destructive disease treatments include bisphosphonates and antibodies against the osteoclast differentiator, RANKL (aRANKL); however, osteonecrosis of the jaw (ONJ) is a frequent side-effect. Current models fail to explain the restriction of bisphosphonate (BP)-related and denosumab (anti-RANKL antibody)-related ONJ to jaws. Msx-1 is exclusively expressed in craniofacial structures and pivotal to cranial neural crest (CNC)-derived periodontal tissue remodeling. We hypothesised that Msx-1 expression might be impaired in bisphosphonate-related ONJ. The study aim was to elucidate Msx-1 and RANKL-associated signal transduction (BMP-2/4, RANKL) in ONJ-altered and healthy periodontal tissue.
Methods
Twenty ONJ and twenty non-BP exposed periodontal samples were processed for RT-PCR and immunohistochemistry. An automated staining-based alkaline phosphatase-anti-alkaline phosphatase method was used to measure the stained cells:total cell-number ratio (labelling index, Bonferroni adjustment). Real-time RT-PCR was performed on ONJ-affected and healthy jaw periodontal samples (n = 20 each) to quantitatively compare Msx-1, BMP-2, RANKL, and GAPDH mRNA levels.
Results
Semi-quantitative assessment of the ratio of stained cells showed decreased Msx-1 and RANKL and increased BMP-2/4 (all p < 0.05) expression in ONJ-adjacent periodontal tissue. ONJ tissue also exhibited decreased relative gene expression for Msx-1 (p < 0.03) and RANKL (p < 0.03) and increased BMP-2/4 expression (p < 0.02) compared to control.
Conclusions
These results explain the sclerotic and osteopetrotic changes of periodontal tissue following BP application and substantiate clinical findings of BP-related impaired remodeling specific to periodontal tissue. RANKL suppression substantiated the clinical finding of impaired bone remodelling in BP- and aRANKL-induced ONJ-affected bone structures. Msx-1 suppression in ONJ-adjacent periodontal tissue suggested a bisphosphonate-related impairment in cellular differentiation that occurred exclusively jaw remodelling. Further research on developmental biology-related unique features of jaw bone structures will help to elucidate pathologies restricted to maxillofacial tissue.
Similar content being viewed by others
Introduction
Numerous attempts have targeted explaining the etiology of the restriction of amino-bisphosphonate (BP)-associated osteonecrosis of the jaw (BONJ) to the jaws, but an accepted model of formal pathology has been lacking [1, 2]. Existing hypotheses have focused on accumulation of BP in the jaw or BP-specific tissue toxicity as a factor [3]. However, denusomab (humanized anti-RANKL antibody, Prolia, Amgen, USA) also has been demonstrated to cause osteonecrosis specifically of the jaw (ONJ) [4–6]. Thus, any hypothesized etiology of BONJ requires incorporation of these findings [1].
Potential factors to consider include the unique biological features of the alveolar bone of the jaw. Impairment of cranial neural crest (CNC)-specific RANKL-associated cell signaling as an underlying mechanism of ONJ is an attractive hypothesis because CNC-derived periodontal progenitor cells are involved in remodeling of both hard and soft jaw tissues [7–9]. Impairment of CNC cell plasticity affects remodeling of jaw bone and periodontal structures [7–9]. In addition, the transcription factor Msx-1 mediates the innate cellular plasticity of CNC and is expressed exclusively in CNC-derived bone and bone progenitor structures including oral periost and periodontal ligamentum (PDL) throughout adolescence [10, 11]. Within the jaw, Msx-1 is expressed with the highest concentration in the PDL [9, 11–13] and is co-expressed with RANKL on CNC-derived osteoblast and chondroblast progenitors [14–16]. Because of the restriction of Msx-1 to the adult jaw and its co-expression with RANKL, a BP- and denusomab-related loss of RANKL and Msx-1 expression might explain the BP- and denosumab-related impairment of hard and soft tissue remodeling that is restricted to the jaw bone in ONJ [4, 14]. Thus, the aim of this study was to compare Msx-1, BMP-2/4, and RANKL expression at the protein and mRNA levels in samples of BONJ-related oral mucoperiosteal tissue compared to healthy oral periodontal tissue to test the hypothesized impairment of jaw-specific Msx-1-RANKL-associated cell signaling in periodontal progenitor cells.
Materials and methods
Patients and Material Harvesting
This study included oral mucoperiosteal specimens from 40 patients. Of these, 20 were from periodontal soft tissue adjacent to clinically and histologically confirmed BONJ of 20 consecutively treated patients undergoing radical sequestrotomy, taken as part of the tissue samples provided for routine histopathological diagnostics. The study was approved by the ethical committee of the University of Erlangen-Nuremberg. All patients gave their informed consent to participation. Additional criteria for specimen inclusion were intravenous application of either pamidronate or zoledronate for at least 12 months and clinical evidence of an exposed jaw bone for at least 8 weeks. Any former radiotherapy was excluded. Details about patient data, surgical treatment, and the follow-up period were previously documented [17]. Controls were 20 alveolar mucoperiosteal specimens, harvested during intraoral surgery in patients negative for BP history and presenting no clinical signs of intraoral inflammatory processes or periodontitis. The 40 specimens measured on average 5 × 3 × 3 mm and were immediately separated into two equal parts. One part was immediately flash frozen at -80°C in liquid nitrogen. Mature bone pieces were detached from the other part, and the periodontal soft tissue was immersed in RNA-preserving reagent (RNALater, Qiagen, Hilden, Germany) for 24 h at 4°C and then frozen and stored at -80°C.
Immunohistochemical Staining
Tissue samples were processed for immunohistochemistry as previously described[18]. Antibodies and dilutions were as follows: Msx-1, polyclonal rabbit-IgG anti-human Msx-1 antibody (anti-Msx-1; M0944-100G, Sigma-Aldrich, Taufkirchen, Germany; dilution 1:100); BMP-2/4, polyclonal rabbit-IgG (anti-human BMP-2/4, sc-9003, Santa Cruz Biotechnology, Santa Cruz, CA, USA; dilution: 1:100); and RANKL, polyclonal goat-anti-human RANKL antibody (sc-7628, Santa Cruz, dilution 1:100). Secondary antibody was used according to the staining kit [biotinylated polyclonal, goat-anti-rabbit IgG (Msx-1, BMP-2/4) and rabbit-anti-goat (RANKL) (E 0466, DAKO, dilution 1:100)]. Visualization was performed using Fast Red solution, and localized by biotin-associated activation of the staining kit (ChemMate-Kit, Dako) followed by incubation in hematoxylin for nuclear counterstaining. Two consecutive tissue samples were processed per immunohistochemical staining, one for experimental staining and the other as a negative control (replacement of primary antibody incubation with incubation with istotype-IgG of the primary antibody). A known positive staining sample was also included in each series as a positive control.
Semiquantitative Immunohistochemical Analysis
Sections were examined qualitatively under a bright-field microscope (Axioskop, Zeiss, Jena, Germany) at 100-400× magnification for number and localization of stained osteoblast progenitors and fibroblasts. In healthy periodontal samples, subepithelial tissue was observed, including connective, submucous, and periosteal structures. Mature bone tissue, including osteocytes, was excluded from any analysis. In BONJ samples, soft tissue adjacent to the necrotic zone was identified, and three visual fields per section for each sample were digitized at 200× magnification using a CCD camera (Axiocam 5, Zeiss, Jena, Germany) and the program AxioVision (AxioVison, Zeiss, Jena, Germany). For this purpose, randomized systematic subsampling was performed as previously described [18]. Semiquantitative analysis of cytoplasmic expression of Msx-1, BMP-2/4, and RANKL was performed by determining the labeling index as the ratio of positively stained cells to the total number of cells per visual field.
Quantitative mRNA Analysis and Real-time Reverse Transcriptase Polymerase Chain Reaction (RTqPCR)
Frozen tissues were agitated (Mixer Mill, Qiagen, Hilden, Germany) in lysis buffer (RNeasy Kit, Qiagen, Hilden, Germany), and whole RNA from tissues was extracted using the RNeasy Kit according to the manufacturer's protocol. Quantitative measurement of mRNA in each probe was performed using a commercial microfluid Lab-on-a-Chip technology (Agilent RNA 6000 Pico Kit and the Agilent 2100 Bioanalyzer, Agilent, Waldbronn, Germany). The cDNAs from total RNA were synthesized using the High Capacity cDNA Archive Kit (Cat. 4322171; Applied Biosystems, Foster City, CA, USA) according to the manufacturer's protocol. Real-time RT qPCR analyses were done using QuantiTect Primer Assay (200) [Hs_BMP2_1_SGQuantiTect Primer Assay (200) (Cat. GT00012544) for BMP-2; Hs_MSX1_SG QuantiTect Primer Assay (200) (Cat. GT00224350) for Msx-1; and Hs_TNFSF11_va.1_SG QuantiTect Primer Assay (200) (Cat. QT01011381) for RANKL]. For normalization, GAPDH was used [Hs_GAPDH_1_SG QuantiTect Primer Assay (200) (Cat. QT00079247), Qiagen)]. The QuantiTect TM SYBR Green PCR kit (Cat. 204143; Qiagen) was used for PCR amplification. The relative quantification of mRNA was performed with the ABI Prism 7300 Sequence Detection System (Applied Biosystems). In total, 40 ng of cDNA was used for each PCR reaction in a total volume of 25 μl. Each PCR run included a 15-min activation time at 95°C, followed by a three-step cycle: denaturing at 94°C for 15 s, annealing at 55°C for 30 s, and extension at 72°C for 34 s. Formation during PCR of undesired side products that contribute to fluorescence was assessed by melting curve analysis after PCR. Msx-1, BMP-2, and RANKL mRNA quantities were analyzed in duplicate, normalized against GAPDH as an internal control, and expressed in relation to mRNA isolated from healthy periodontal tissue as a calibrator. Relative gene expression was determined using the ΔΔCt method. RNA isolated from healthy oral periodontal tissue (pool of 20 patients) was used as controls.
Statistical Analysis
To analyze the immunohistochemical cytoplasmic staining and the spatial pattern of expression, the labeling index of positively stained cells per visual field was assessed. Comparing the relative gene expression, addressed by the real-time RT-PCR, the median gene expression for Msx-1, BMP-2, and RANKL in the pool of healthy oral mucoperiosteum was set as 1. Gene expression in both groups was stated as relative expression compared to healthy mucoperiosteal expression. Multiple measurements per group of investigation were aggregated prior to analysis. Descriptive analysis of labeling index and relative gene expression data were performed using the median (ME) and the interquartile range (IQR). Graphical representations use diagrams representing ME, IQR, minimum, and maximum. Confirmatory comparisons were made between treatment and control groups using generalized estimating equations with "treatment modality" and "subject id" as independent factors for appropriate analysis of repeated measurements per individual. Multiple p values were adjusted according to Bonferroni by multiplying each p value obtained by the number of confirmatory tests performed (n = 10). Two-sided adjusted p values of p ≤ 0.05 were considered to be significant. All calculations were made using SPSS 18.0 for Windows (SPSS Inc, Chicago, IL, USA).
Results
Immunohistochemistry
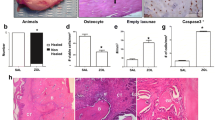
All examined BONJ samples had multinucleated cells and a thickened epithelial layer above necrotic tissueareas between vital zones (Figures 1b, 2b, 3b). Observation consistently showed necrotic lesions of partial confluency. Empty osteocyte lacunae were detected. The mucoperiosteal soft tissue presented variable thickness including inflammatory infiltrates within the connective tissue layers. Capillaries were seen in BONJ-related mucoperiosteal specimens and healthy jaw connective tissue.
Msx-1 expression was reduced in ONJ-related periodontal tissue. (a) The Msx-1 staining was accentuated in periosteal cells, attached to the mineralized bone matrix. The bone trabeculae interconnecting fibrous tissue presented nuclear and cytoplasmic Msx-1 staining. (b) In the BONJ group, staining of periosteal cells was rare, and cytoplasmic staining was decreased, as was the cellular density of Msx-1-expressing fibroblasts in the fibrous and inflammatory tissue surrounding the bone matrix. (c) Relative cellular expression (labeling index) for Msx-1 was significantly reduced (Controls-ME: 34.29, IQR 24.0 vs. BONJ-ME: 14.03, IQR: 6.0; p < 0.05) in ONJ-related oral mucoperiosteum. (d) Relative gene expression for Msx-1 was suppressed 6.8-fold at the mRNA level in ONJ-related periosteum samples (Controls-ME: 1.00, IQR 0.25 vs. BONJ-ME: 0.15, IQR: 0.31; p < 0.03). Horizontal bars indicate median (ME), and error bars indicate interquartile range (IQR).
In control jaw periodontal tissue, Msx-1 expression was localized in the nucleus and cytoplasm of osteoblasts, fibroblasts, and progenitors within the connective tissue layer (Figure 1a). In the BONJ-related tissue, a reduced cellular density of Msx-1 expressing osteoblasts, fibroblasts, and progenitor cells was noted (Figure 1b). BMP-2/4 expression was found in osteoblast progenitors of adjacent periosteal tissue in both healthy jaw bone (Figure 2a) and the BONJ samples (Figure 2b).
BMP-2/4 expression was increased at the protein and mRNA levels in BP-altered oral mucoperiosteum. (a) Rarely, there was pronounced BMP-2/4 staining in healthy jaw periosteum. (b) BMP-2/4-expressing osteocytes showed higher cellular density in the BONJ group. (c) The labeling index of BMP-2/4-expressing osteoblasts and osteocytes was significantly increased compared to control (Controls-ME: 22.06, IQR 25.0 vs. BONJ-ME: 53.97, IQR: 25.0; p < 0.05). (d) Relative BMP-2 gene expression at the mRNA level was elevated 8.9-fold in ONJ samples (Controls-ME: 1.14, IQR 1.07 vs. BONJ-ME: 8.9, IQR: 6.1; p < 0.02) related to healthy samples. Horizontal bars indicate median (ME), and error bars indicate interquartile range (IQR).
RANKL expression was present throughout the soft tissue in normal jaw samples (Figure 3a), including periosteal and subepithelial tissue; however, in BONJ samples, RANKL expression was present sparsely in the endosteal and periosteal tissue at the site of bone resorption (Figure 3b).
RANKL was suppressed in ONJ-adjacent soft tissue. (a, b) Spatial distribution of RANKL-expressing cells in the soft tissue areas of BONJ samples (b) was non-homogeneous compared to normal jaw periodontal samples (a). A local high concentration of RANKL-expressing multinucleated cells was detected only at zones of tissue resorption in BONJ samples. (c) The relative cellular expression (labeling index) of RANKL-positive cells was significantly lower in ONJ samples (Controls-ME: 59.38, IQR 21.0 vs. BONJ-ME: 23.25, IQR: 12.0; p < 0.05). (d) A 2.94-fold suppression of RANKL mRNA was detected in ONJ-related bone samples (Controls-ME: 1.00, IQR 0.13 vs. BONJ-ME: 0.34, IQR: 0.44; p < 0.03). Horizontal bars indicate median (ME), and error bars indicate interquartile range (IQR).
The labeling index of Msx-1-expressing (Figure 1c) and RANKL-expressing (Figure 3c) cells was significantly diminished compared to normal bone. The labeling index of BMP-2/4-expressing osteoblasts and osteocytes (Figure 2c) was significantly increased compared to control.
PCR
The patterns for mRNA expression reflected those for protein expression. Msx-1 mRNA levels were significantly suppressed 6.8-fold in BONJ samples compared to control periodontal tissue (Figure 1d). BMP-2/4 mRNA expression was significantly higher by about 8.9-fold in BONJ tissue than in normal jaw mucoperiosteal tissue (Figure 2d), while RANKL mRNA expression was significantly suppressed 2.9-fold in BONJ samples relative to control (Figure 3d).
Discussion
This study identified a significantly diminished expression of Msx-1, a cellular plasticity and proliferation-mediating transcription factor, in BONJ-affected jaw periodontal tissue at the protein and mRNA levels. Significantly elevated expression of BMP-2/4 in the BONJ-related periodontal and periosteal tissue revealed an increased osseous differentiation stimulation in progenitors of osteoblastic lineage in BP-compromised jaw mucoperiosteal tissue. As with Msx-1 expression, RANKL expression in the jaw bone overlying mucoperiosteal tissue was significantly reduced, suggesting suppressed osteoclast activation by osteoblasts [19].
BP-related Msx-1 loss in the PDL can explain the sclerotic, periapical hypermineralized thin lines around dental roots of BP-altered PDL tissue, which is known for having the highest endogenous Msx-1 expression in the jaw [9, 12, 13, 20]. In addition, Msx-1 is critically involved in cellular plasticity and differentiation. Within the PDL, a balanced progenitor cell differentiation towards fibrous soft tissue takes place between dental and bone hard tissue. The clinical observation of sclerotic remodeling of the PDL is substantiated by the experimental finding of BP-induced osteogenetic cell recruitment and trans-differentiation of progenitor cells within the PDL [21]. Because Msx-1 has been reported to prevent terminal differentiation and to stimulate proliferation of progenitors, loss of Msx-1 in the presence of BMP-2 is likely to be associated with poor cell proliferation and also with overwhelming mineralization in periodontal tissue [22, 23].
The significantly increased expression of BMP-2/4 identified here at the cellular and mRNA levels in BONJ-affected jaw periosteum is consistent with the clinical and radiologic observation of the osteopetrotic aspect of ONJ-related jaw bone: BMP-2/4 is an essential osteoinductive factor and induces terminal osseous differentiation through DLX5 signaling in the absence of Msx-1 [24]; [25]. Increased terminal osseus differentiation and reduced proliferation of progenitor cells within the periodontal tissue might explain sclerosis and osteopetrosis of the alveolar bone and the reduced periodontal soft tissue proliferation. The immunohistochemical and molecular results in this study are consistent with those found in osteopetrotic bone [26], and BONJ has been described as local osteopetrosis [24, 27].
The finding of BP-related RANKL suppression in periodontal progenitor cells in vivo is described here for the first time and indicates the relevance of BP effects on cellular differentiation in explaining the etiology of BONJ. The significantly reduced expression of RANKL in ONJ-adjacent periodontal tissue at the protein and mRNA levels demonstrates the effect of BP action on soft-tissue remodeling. Suppression of RANKL has been described as the main action of BP, preventing osteoclast activation and bone resorption in malignancies and osteoporosis [28–31]. This suggestion finds strong support from clinical findings of ONJ onset following application of the anti-RANKL denosumab without any BP involvement [4, 6]. The concerted regulation of RANKL and Msx-1 identified here connects jaw-specific and common bone remodeling mechanisms, but the details remain to be elucidated at the cellular and subcellular levels.
Conclusion
These findings help to explain some of the molecular underpinnings of the restriction of BONJ to the jaw bone. Jaw restricted osteopetrosis implicated in BONJ can be explained by loss of Msx-1. Msx-1, known to be a key regulator of cellular plasticity and constitutively expressed in CNC-derived jaw hard and soft tissue progenitor cells, could be of relevance in jaw-restricted diseases associated with impaired bone and soft tissue remodeling [32–34]. Addressing the Msx-1-RANKL-associated signaling could help to elucidate mechanisms of CNC-related jaw bone and periodontal-tissue-specific homeostasis [7–9]. In agreement with leading international experts in the field of ONJ, we found that targeting the unique features of the jaw bone is a promising approach to elucidating the underlying pathologic mechanisms of ONJ [35]. Of note, BP and aRANKL had differential impacts on proliferation, vascularisation, and surface marker expression [36, 37]. This suggests that BP and aRANKL effects on Msx- and RANKL-related interactions in CNC- and MsC-derived osteoblasts, osteoclasts, and bone structures should be investigated in more detail in the future.
References
Reid IR: Osteonecrosis of the jaw: who gets it, and why?. Bone. 2009, 44: 4-10. 10.1016/j.bone.2008.09.012.
Ruggiero SL, Drew SJ: Osteonecrosis of the jaws and bisphosphonate therapy. J Dent Res. 2007, 86: 1013-1021. 10.1177/154405910708601101.
Agis H, Blei J, Watzek G, Gruber R: Is zoledronate toxic to human periodontal fibroblasts?. J Dent Res. 89: 40-45.
Taylor KH, Middlefell LS, Mizen KD: Osteonecrosis of the jaws induced by anti-RANK ligand therapy. Br J Oral Maxillofac Surg. 2010, 48 (3): 221-3. 10.1016/j.bjoms.2009.08.030.
Henry D, vonMoos R, Vadhan-Raj S: A double-blind, randomized study of denosumab versus zoledronic acid for the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. Eur J Can Suppl. 2009, 7 (3): 12-10.1016/S1359-6349(09)72055-5.
Stopeck A, Body J, Fujiwara Y: Denosumab versus zoledronic acid for the treatment of breast cancer patients with bone metastases: rusults of a randomized phase 3 study. Eur J Can Suppl. 2007, 5 (3): 31-
Trainor PA: Specification and patterning of neural crest cells during craniofacial development. Brain Behav Evol. 2005, 66: 266-280. 10.1159/000088130.
Luan X, Dangaria S, Ito Y, Walker CG, Jin T, Schmidt MK, Galang MT, Druzinsky R: Neural crest lineage segregation: a blueprint for periodontal regeneration. J Dent Res. 2009, 88: 781-791. 10.1177/0022034509340641.
Chung IH, Yamaza T, Zhao H, Choung PH, Shi S, Chai Y: Stem cell property of postmigratory cranial neural crest cells and their utility in alveolar bone regeneration and tooth development. Stem Cells. 2009, 27: 866-877. 10.1002/stem.2.
Blin-Wakkach C, Lezot F, Ghoul-Mazgar S, Hotton D, Monteiro S, Teillaud C, Pibouin L, Orestes-Cardoso S, Papagerakis P, Macdougall M: Endogenous Msx1 antisense transcript: in vivo and in vitro evidences, structure, and potential involvement in skeleton development in mammals. Proc Natl Acad Sci USA. 2001, 98: 7336-7341. 10.1073/pnas.131497098.
Orestes-Cardoso S, Nefussi JR, Lezot F, Oboeuf M, Pereira M, Mesbah M, Robert B, Berdal A: Msx1 is a regulator of bone formation during development and postnatal growth: in vivo investigations in a transgenic mouse model. Connect Tissue Res. 2002, 43: 153-160.
Babajko S, Petit S, Fernandes I, Meary F, LeBihan J, Pibouin L, Berdal A: Msx1 expression regulation by its own antisense RNA: consequence on tooth development and bone regeneration. Cells Tissues Organs. 2009, 189: 115-121. 10.1159/000151748.
Ruhin-Poncet B, Ghoul-Mazgar S, Hotton D, Capron F, Jaafoura MH, Goubin G, Berdal A: Msx and dlx homeogene expression in epithelial odontogenic tumors. J Histochem Cytochem. 2009, 57: 69-78. 10.1369/jhc.2008.951707.
Houpis CH, Tosios KI, Papavasileiou D, Christopoulos PG, Koutlas IG, Sklavounou A, Alexandridis C: Parathyroid hormone-related peptide (PTHrP), parathyroid hormone/parathyroid hormone-related peptide receptor 1 (PTHR1), and MSX1 protein are expressed in central and peripheral giant cell granulomas of the jaws. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010, 109 (3): 415-24. 10.1016/j.tripleo.2009.09.026.
Idowu BD, Thomas G, Frow R, Diss TC, Flanagan AM: Mutations in SH3BP2, the cherubism gene, were not detected in central or peripheral giant cell tumours of the jaw. Br J Oral Maxillofac Surg. 2008, 46: 229-230. 10.1016/j.bjoms.2007.04.014.
Miah SM, Hatani T, Qu X, Yamamura H, Sada K: Point mutations of 3BP2 identified in human-inherited disease cherubism result in the loss of function. Genes Cells. 2004, 9: 993-1004. 10.1111/j.1365-2443.2004.00784.x.
Stockmann P, Vairaktaris E, Wehrhan F, Seiss M, Schwarz S, Spriewald B, Neukam FW, Nkenke E: Osteotomy and primary wound closure in bisphosphonate-associated osteonecrosis of the jaw: a prospective clinical study with 12 months follow-up. Support Care Cancer. 2010, 18 (4): 449-60. 10.1007/s00520-009-0688-1.
Wehrhan F, Rodel F, Grabenbauer GG, Amann K, Bruckl W, Schultze-Mosgau S: Transforming growth factor beta 1 dependent regulation of Tenascin-C in radiation impaired wound healing. RadiotherOncol. 2004, 72: 297-303. 10.1016/j.radonc.2004.07.011.
Boyce BF, Xing L: Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys. 2008, 473 (2): 139-46. 10.1016/j.abb.2008.03.018.
Groetz KA, Al-Nawas B: Persisting alveolar sockets-a radiologic symptom of BP-ONJ?. J Oral Maxillofac Surg. 2006, 64: 1571-1572. 10.1016/j.joms.2006.05.041.
Lekic P, Rubbino I, Krasnoshtein F, Cheifetz S, McCulloch CA, Tenenbaum H: Bisphosphonate modulates proliferation and differentiation of rat periodontal ligament cells during wound healing. Anat Rec. 1997, 247: 329-340. 10.1002/(SICI)1097-0185(199703)247:3<329::AID-AR4>3.0.CO;2-P.
Newberry EP, Boudreaux JM, Towler DA: Stimulus-selective inhibition of rat osteocalcin promoter induction and protein-DNA interactions by the homeodomain repressor Msx2. JBiolChem. 1997, 272: 29607-29613.
Dodig M, Kronenberg MS, Bedalov A, Kream BE, Gronowicz G, Clark SH, Mack K, Liu YH, Maxon R, Pan ZZ: Identification of a TAAT-containing motif required for high level expression of the COL1A1 promoter in differentiated osteoblasts of transgenic mice. JBiolChem. 1996, 271: 16422-16429.
Marx RE, Sawatari Y, Fortin M, Broumand V: Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention, and treatment. J Oral Maxillofac Surg. 2005, 63: 1567-1575. 10.1016/j.joms.2005.07.010.
Ryoo HM, Lee MH, Kim YJ: Critical molecular switches involved in BMP-2-induced osteogenic differentiation of mesenchymal cells. Gene. 2006, 366: 51-57. 10.1016/j.gene.2005.10.011.
Cohen MM: The new bone biology: pathologic, molecular, and clinical correlates. Am J Med Genet A. 2006, 140: 2646-2706.
Favia G, Pilolli GP, Maiorano E: Histologic and histomorphometric features of bisphosphonate-related osteonecrosis of the jaws: An analysis of 31 cases with confocal laser scanning microscopy. Bone. 2009, 45 (3): 406-13. 10.1016/j.bone.2009.05.008.
Nishida S, Tsubaki M, Hoshino M, Namimatsu A, Uji H, Yoshioka S, Tanimori Y, Yanae M, Iwaki M, Irimajiri K: Nitrogen-containing bisphosphonate, YM529/ONO-5920 (a novel minodronic acid), inhibits RANKL expression in a cultured bone marrow stromal cell line ST2. Biochem Biophys Res Commun. 2005, 328: 91-97. 10.1016/j.bbrc.2004.12.145.
Viereck V, Emons G, Lauck V, Frosch KH, Blaschke S, Grundker C, Hofbauer LC: Bisphosphonates pamidronate and zoledronic acid stimulate osteoprotegerin production by primary human osteoblasts. BiochemBiophysResCommun. 2002, 291: 680-686.
McGonigle JS, Giachelli CM, Scatena M: Osteoprotegerin and RANKL differentially regulate angiogenesis and endothelial cell function. Angiogenesis. 2009, 12: 35-46. 10.1007/s10456-008-9127-z.
Buckle CH, Neville-Webbe HL, Croucher PI, Lawson MA: Targeting RANK/RANKL in the treatment of solid tumours and myeloma. Curr Pharm Des. 16: 1272-1283. 10.2174/138161210791034021.
Lezot F, Coudert A, Petit S, Vi-Fane B, Hotton D, Davideau JL, Kato S, Descroix V, Pibouin L, Berdal A: Does Vitamin D play a role on Msx1 homeoprotein expression involving an endogenous antisense mRNA?. J Steroid Biochem Mol Biol. 2004, 89-90: 413-417. 10.1016/j.jsbmb.2004.03.116.
Galle S, Yanze N, Seipel K: The homeobox gene Msx in development and transdifferentiation of jellyfish striated muscle. Int J Dev Biol. 2005, 49: 961-967. 10.1387/ijdb.052009sg.
Gersch RP, Lombardo F, McGovern SC, Hadjiargyrou M: Reactivation of Hox gene expression during bone regeneration. J Orthop Res. 2005, 23: 882-890. 10.1016/j.orthres.2005.02.005.
Khosla S, Burr D, Cauley J, Dempster DW, Ebeling PR, Felsenberg D, Gagel RF, Gilsanz V, Guise T, Koka S: Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007, 22: 1479-1491. 10.1359/jbmr.0707onj.
Stefanik D, Sarin J, Lam T, Levin L, Leboy PS, Akintoye SO: Disparate osteogenic response of mandible and iliac crest bone marrow stromal cells to pamidronate. Oral Dis. 2008, 14: 465-471. 10.1111/j.1601-0825.2007.01402.x.
Matsubara T, Suardita K, Ishii M, Sugiyama M, Igarashi A, Oda R, Nishimura M, Saito M, Nakagawa K, Yamanaka K: Alveolar bone marrow as a cell source for regenerative medicine: differences between alveolar and iliac bone marrow stromal cells. J Bone Miner Res. 2005, 20: 399-409. 10.1359/JBMR.041117.
Acknowledgements
The authors thank Heidemarie Heider, Andrea Kosel, and Miriam Ramming for technical assistance with the immunohistochemistry autostainer. In addition, we thank Andrea Krautheim-Zenk for help with mRNA processing and RT-PCR.
This study was funded by the ELAN-Fonds of the University of Erlangen-Nuremberg.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
There are no competing interests of the authors to be declared.
This study was funded by the ELAN-Fonds of the University of Erlangen-Nuremberg, Germany.
Authors' contributions
FW was responsible for the application of grant support (ELAN-Fonds, university of Erlangen), the conduction of study, built the hypothesis, established and conducted the methods and analytic procedures and wrote the manuscript. PH built the hypothesis and did the interpretation of the data. JR established the m-RNA analysis and RT-PCR and wrote the manuscript, section RT-PCR. PS and KS did the immunohistochemistry analysis.
FN interpreted the data and wrote the manuscript, section discussion. EN interpreted the data and conducted the study by harvesting samples. KA established immunohistochemistry, analysed the tissue samples, interpreted the data and was responsible for the histopatholgical analysis of ONJ- and control tissue samples. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Wehrhan, F., Hyckel, P., Ries, J. et al. Expression of Msx-1 is suppressed in bisphosphonate associated osteonecrosis related jaw tissue-etiopathology considerations respecting jaw developmental biology-related unique features. J Transl Med 8, 96 (2010). https://doi.org/10.1186/1479-5876-8-96
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1479-5876-8-96