Abstract
Autism spectrum disorders (ASD) are a group of neurodevelopmental conditions whose incidence is reaching epidemic proportions, afflicting approximately 1 in 166 children. Autistic disorder, or autism is the most common form of ASD. Although several neurophysiological alterations have been associated with autism, immune abnormalities and neural hypoperfusion appear to be broadly consistent. These appear to be causative since correlation of altered inflammatory responses, and hypoperfusion with symptology is reported. Mesenchymal stem cells (MSC) are in late phases of clinical development for treatment of graft versus host disease and Crohn's Disease, two conditions of immune dysregulation. Cord blood CD34+ cells are known to be potent angiogenic stimulators, having demonstrated positive effects in not only peripheral ischemia, but also in models of cerebral ischemia. Additionally, anecdotal clinical cases have reported responses in autistic children receiving cord blood CD34+ cells. We propose the combined use of MSC and cord blood CD34+cells may be useful in the treatment of autism.
Similar content being viewed by others
Background
Autism spectrum disorders (ASD) are reaching epidemic proportions, believed to affect approximately 1 in 166 children. Autism, Asperger's syndrome, Rett's disorder, and childhood disintegrae disorder are all encompassed by the term ASD. Autism is the most prevalent ASD, characterized by abnormalities in social interaction, impaired verbal and nonverbal communication, and repetitive, obsessive behavior. Autism may vary in severity from mild to disabling and is believed to arise from genetic and environmental factors. While symptomology of autism may be noted by caregivers around 12–18 months [1], definitive diagnosis generally occurs around 24–36 months, however in some cases diagnosis may be made into adulthood [2]. Determination of autism is performed using the DSM-IV-TR, or other questionnaires and tests. Children with autism appear withdrawn, self-occupied, and distant. Inflexibility in terms of learning from experiences and modifying patterns to integrate into new environments is characteristic of autism. Depending on degree of severity, some children with autism may develop into independent adults with full time employment and self sufficiency; however this is seldom the case.
Current treatments for autism can divided into behavioral, nutritional and medical approaches, although no clear golden standard approach exists. Behavioral interventions usually include activities designed to encourage social interaction, communication, awareness of self, and increase attention. Nutritional interventions aim to restrict allergy-associated dietary components, as well as to supplement minerals or vitamins that may be lacking. Medical interventions usually treat specific activities associated with autism. For example, serotonin reuptake inhibitors (SSRI's) such as fluoxetine, fluvoxamine, sertraline, and clomipramine, are used for treatment of anxiety and depression. Some studies have shown that SSRI's also have the added benefit of increasing social interaction and inhibiting repetitive behavior. Typical antipsychotic drugs such as thioridazine, fluphenazine, chlorpromazine, and haloperidol have been showed to decrease behavioral abnormalities in autism. Atypical antipsychotics such as risperidone, olanzapine and ziprasidone have also demonstrated beneficial effect at ameliorating behavioral problems. Autism associated seizures are mainly treated by administration of anticonvulsants such as carbamazepine, lamotrigine, topiramate, and valproic acid. Attention deficient/hyperactivity is treated by agents such as methylphenidate (Ritalin®).
Currently, numerous clinical trials are being conducted with interventions ranging from hyperbaric oxygen, to administration of zinc, to drugs exhibiting anti-inflammatory properties. Unfortunately, no clear understanding of autism's pathogenic mechanisms exists, and as a result numerous strategies are being attempted with varying degrees of success. In this paper we examine two pathologies associated with autism – hypoperfusion to the brain and immune dysregulation – and propose a novel treatment: the administration of CD34+ umbilical cord cells and mesenchymal cells.
Hypoperfusion of brain in autism
Children with autism have been consistently shown to have impaired, or subnormal CNS circulation, as well as resulting hypoxia. Defects include basal hypoperfusion [3], and decreased perfusion in response to stimuli that under normal circumstances upregulates perfusion [4]. In numerous studies the areas affected by hypoperfusion seem to correlate with regions of the brain that are responsible for functionalities that are abnormal in autism. For example, specific temporal lobe areas associated with face recognition [5], social interaction [6], and language comprehension [7], have been demonstrated to be hypoperfused in autistic but not control children.
The question of cause versus effect is important. If temporal lobe ischemia is not causative but only a symptom of an underlying process, then targeting this pathology may be non-productive from the therapeutic perspective. However this appears not to be the case. It is evident that the degree of hypoperfusion and resulting hypoxia correlates with the severity of autism symptoms. For example, statistically significant inverse correlation has been demonstrated between extent of hypoxia and IQ [8]. Supporting a causative effect of hypoperfusion to autism development, Bachavelier et al reviewed numerous experimental reports of primate and other animal studies in which damage causing hypoperfusion of temporal areas was associated with onset of autism-like disorders [9]. It is also known that after removal or damage of the amygdala, hippocampus, or other temporal structures induces either permanent or transient autistic-like characteristics such as unexpressive faces, little eye contact, and motor stereotypies occurs. Clinically, temporal lobe damage by viral and other means has been implicated in development of autism both in adults [10], and children [11–14].
Evidence suggests that hypoperfusion and resulting hypoxia is intimately associated with autism, however the next important question is whether reversion of this hypoxia can positively influence autism. In autism the associated hypoxia is not predominantly apoptotic or necrotic to temporal neurons but associated with altered function [15]. Hypoperfusion may contribute to defects not only by induction of hypoxia but also allowing for abnormal metabolite or neurotransmitter accumulation. This is one of the reasons why glutamate toxicity has been implicated in autism [16] and a clinical trial at reversing this using the inhibitor of glutamate toxicity, Riluzole, is currently in progress [17]. Conceptually the augmentation of perfusion through stimulation of angiogenesis should allow for metabolite clearance and restoration of functionality. Although not well defined, cell death may also be occurring in various CNS components of autistic children. If this were the case, it is possible that neural regeneration can be stimulated through entry of neuronal progenitor cells into cell cycle and subsequent differentiation. Ample evidence of neural regeneration exists in areas ranging from stroke [18], to subarachinoidal hemorrhage [19, 20], to neural damage as a result of congenital errors of metabolism [21]. Theoretically, it is conceivable that reversing hypoxia may lead to activation of self-repair mechanisms. Such neural proliferation is seen after reperfusion in numerous animal models of cerebral ischemia [22–24]. The concept of increasing oxygen to the autistic brain through various means such as hyperbaric medicine is currently being tested in 2 independent clinical trials in the US [25, 26]. However, to our knowledge, the use of cell therapy to stimulate angiogenesis has not been widely-used for the treatment of autism.
Immune deregulation in autism
The fundamental interplay between the nervous system and the immune system cannot be understated. Philosophically, the characteristics of self/nonself recognition, specificity, and memory are only shared by the immune system and the nervous system. Physically, every immune organ is innervated and bi-directional communication between neural and immune system cells has been established in numerous physiological systems. In autism, several immunological abnormalities have been detected both in the peripheral and the central nervous systems.
Astroglial cells, or astrocytes, surround various portions of the cerebral endothelium and play a critical role in regulating perfusion [27, 28], and blood brain barrier function [29]. Astrocytes are capable of mediating several immunological/inflammatory effects. Expression of various toll like receptors (TLR) on astrocytes endows the ability to recognize not only bacterial and viral signals but also endogenous "danger" signals such as heat shock proteins, fibrinogen degradation products, and free DNA [30]. Physiologically, astrocytes play an important protective role against infection, generating inflammatory cytokines such as TNF-alpha, IL-1beta, and IL-6 [31]. Through secretion of various chemokines such as CXCL10, CCL2 and BAFF, astrocytes play an important role in shaping adaptive immune responses in the CNS [32]. Astrocytes have antigen presenting capabilities and have been demonstrated to activate T and B cell responses against exogenous and endogenous antigens [33, 34]. Although astrocytes play a critical role against CNS infection, these cells also have potential to cause damage to the host when functioning in an aberrant manner. For example, various neurological diseases are associated with astrocyte overproduction of inflammatory agents, which causes neural malfunction or death. In amyotrophic lateral sclerosis (ALS), astrocyte secretion of a soluble neurotoxic substance has been demonstrated to be involved in disease progression [35, 36]. Astrocyte hyperactivation has been demonstrated in this disease by imaging, as well as autopsy studies [37–39]. In multiple sclerosis, astrocytes play a key role in maintaining autoreactive responses and pathological plaque formation [40, 41]. In stroke, activated astrocytes contribute to opening of the blood brain barrier [42], as well as secrete various neurotoxic substances that contribute to post infarct neural damage [43, 44].
Vargas et al compared brain autopsy samples from 11 autistic children with 7 age-matched controls. They demonstrated an active neuroinflammatory process in the cerebral cortex, white matter, and notably in cerebellum of autistic patients both by immunohistochemistry and morphology. Importantly, astrocyte production of inflammatory cytokines was observed, including production of cytokines known to affect various neuronal functions such as TNF-alpha and MCP-1. CSF samples from living autism patients but not controls also displayed upregulated inflammatory cytokines as demonstrated by ELISA [45]. The potent effects of inflammatory cytokines on neurological function cannot be underestimated. For example, patients receiving systemic IFN-gamma therapy for cancer, even though theoretically the protein should not cross the blood brain barrier, report numerous cognitive and neurological abnormalities [46, 47]. In fact, IFN-gamma, one of the products of activated astrocytes [46], has been detected at elevated levels in the plasma of children with autism [48, 49]. Mechanistically, inflammatory mediators mediate alteration of neurological function through a wide variety of different pathways, either directly altering neuron activity or indirectly. For example, the common neurotoxin used in models of Parkinson's Disease, MPTP is believed to mediate its activity through activation of IFN-gamma production, leading to direct killing of dopaminergic neurons in the substantia nigra. This is evidenced by reduced MPTP neuronal toxicity in IFN-gamma knockout mice or by addition of blocking antibodies to IFN-gamma [50]. In terms of indirect effects of IFN-gamma, it is known that this cytokine activates the enzyme 2,3-indolaminedeoxygenase, leading to generation of small molecule neurotoxins such as the kynurenine metabolites 3OH-kynurenine and quinolinic acid which have been implicated in dementias associated with chronic inflammatory states [51, 52].
T cell and B cell abnormalities have been reported systemically in autistic children. These have included systemic T cell lymphopenia, weak proliferative responses to mitogens, and deranged cytokine production [53, 54]. At face value, lymphopenia would suggest general immune deficiency and as a result little inflammation, however, recent studies have demonstrated that almost all autoimmune diseases are associated with a state of generalized lymphopenia (reviewed by Marleau and Sarvetnick [55]). Autoimmune-like pathophysiology appears to be prevalent in autism and several lines of reasoning suggest it may be causative. Firstly, numerous types of autoantibodies have been detected in children with autism but not in healthy or mentally challenged controls. These include antibodies to myelin basic protein [56], brain extracts [57, 58], Purkinje cells and gliadin extracted peptides [59], neutrophic factors [60, 61], and neuron-axon filament and glial fibrillary acidic protein [61]. Secondly, family members of autistic children have a higher predisposition towards autoimmunity compared to control populations [62, 63]. Hinting at genetic mechanisms are observations that specific HLA haplotypes seem to associate with autism [64, 65]. Another genetic characteristic associated with autism is a null allele for the complement component C4b [66]. Both HLA haplotypes as well as complement component gene polymorphisms have been strongly associated with autoimmunity [67–69]. It is known that autoimmune animals have altered cognitive ability and several neurological abnormalities [70]. Thirdly, autism has been associated with a peculiar autoimmune-like syndrome that is still relatively undefined. Mucosal lesions in the form of chronic ileocolonic lymphoid nodular hyperplasia characterized by lymphocyte infiltration, complement deposition, and cytokine production have been described uniquely to children with autism but not healthy controls or cerebral palsy patients [71]. This inflammatory condition is associated not only with lesions on the intestinal wall, but also in the upper GI tract. Although several characteristics of this condition are shared with Crohn's Disease, one unique aspect is eosinophilic infiltrate, which seems to be associated with dietary habits of the patient [72]. Systemic manifestation of the immune deregulation/chronic inflammatory condition are observed through elevated levels of inflammatory cytokines such as IFN-gamma [73], IL-12 [74], and TNF-alpha [75]. Indication that a relevant inflammatory response is ongoing is provided by observation that the macrophage product neopterin is observed elevated in children with autism [76]. Inhibited production of anti-inflammatory cytokines such as IL-10 [77] and TGF-beta [78] has also been observed in children with autism, thus suggesting not only augmentation of inflammatory processes but also deficiency of natural feedback inhibitor mechanisms.
The systemic effects of a chronic inflammatory process in the periphery may result in production of soluble factors such as quinilonic acid, which have neurotoxin activity. Ability of cellular immune deregulation to affect neural function can occur independent of cell trafficking, as was demonstrated in animal studies in which T cell depletion was associated with cognitive loss of function that was reversible through T cell repletion [79]. Localized inflammation and pathological astrocyte activation has been directly demonstrated to be associated with pathogenesis in autism. Clinical trials of inflammatory drugs have demonstrated varying degrees of success. For example, in an open labeled study of the anti-inflammatory PPAR-gamma agonist pioglitozone in 25 children, 75% reported responses on the aberrant behavior checklist [80]. Other interventions aimed at reducing inflammation such as intravenous immunoglobulin administration reported inconsistent results, however a minor subset did respond significantly [81, 82]. Clinical trials are currently using drugs off-label for treatment of autism through inhibiting inflammation such as minocycline [83], n-acetylcysteine [84], or ascorbic acid and zinc [85]. Despite the desire to correct immune deregulation/chronic inflammation in autism, to date, no approach has been successful.
Treatment of hypoperfusion defect by umbilical cord blood CD34+ stem cell administration
Therapeutic angiogenesis, the induction of new blood vessels from preexisting arteries for overcoming ischemia, has been experimentally demonstrated in peripheral artery disease [86], myocardial ischemia [87], and stroke [88]. Angiogenesis is induced through the formation of collateral vessels and has been observed in hypoperfused tissues. This process is believed to be coordinated by the oxygen sensing transcription factor hypoxia inducible factor-1 (HIF-1). During conditions of low oxygen tension, various components of the transcription factor dimerize and coordinately translocate into the nucleus causing upregulation of numerous cytokines and proteins associated with angiogenesis such as SDF-1, VEGF, FGF, and matrix metalloproteases [89]. The potency of tissue ischemia stimulating angiogenesis is seen in patients after myocardial infarction in which bone marrow angiogenic stem cells mobilize into systemic circulating in response to ischemia induced chemotactic factors [90]. The angiogenic response has also been demonstrated to occur after cerebral ischemia in the form of stroke and is believed to be fundamental in neurological recovery [91]. For example, in models of middle cerebral artery occlusion, endogenous angiogenesis occurs which is also involved in triggering migration of neural stem cells into damaged area that participate in neuroregeneration [92]. The association between neural angiogenesis and neurogenesis after brain damage is not only temporally-linked but also connected by common mediators, for example, SDF-1 secreted in response to hypoxia also induces migration of neural progenitors [92]. Angiogenic factors such as VEGF and angiopoietin have been implicated in post ischemia neurogenesis [93].
While recovery after cerebral ischemia occurs to some extent without intervention, this recovery is can be limited. Methods to enhance angiogenesis and as a result neurogenesis are numerous and have utilized approaches that upregulate endogenous production of reparative factors, as well as administration of exogenous agents. For example, administration of exogenous cytokines such as FGF-2 [94], erythropoietin [95], and G-CSF [96], has been performed clinically to accelerate healing with varying degrees of success.
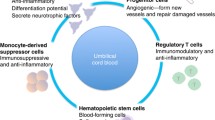
A promising method of increasing angiogenesis in situations of ischemia is administration of cells with potential to produce angiogenic factors and the capacity to differentiate into endothelial cells themselves. Accordingly, the use of CD34+ stem cells has been previously proposed as an alternative to growth factor administration [97]. Therapeutic administration of bone marrow derived CD34+ cells has produced promising results in the treatment of end-stage myocardial ischemia [98], as well as a type of advanced peripheral artery disease called critical limb ischemia [99]. Additionally, autologous peripheral blood CD34+ cells have also been used clinically with induction of therapeutic angiogenesis [100]. Of angiogenesis stimulating cell sources, cord blood seems to possess CD34+ cells with highest activity in terms of proliferation, cytokine production, as well as endothelial differentiation [101, 102].
Cord blood has been used successfully for stimulation of angiogenesis in various models of ischemia. In one report, the CD34+, CD11b+ fraction, which is approximately less than half of the CD34+ fraction of cord blood was demonstrated to possess the ability to differentiate into endothelial cells [102]. In another report, VEGF-R3+, CD34+ cells demonstrated the ability to differentiate into endothelial cells and were able to be expanded 40-fold expansion. The concentration of this potential endothelial progenitor fraction in cord blood CD34+ cells is approximately tenfold higher as compared to bone marrow CD34+ cells (1.9% +/- 0.8% compared to 0.2% +/- 0.1%) [103]. Administration of cord blood CD34+ cells into immune compromised mice that underwent middle cerebral artery ligation reduced neurological deficits and induce neuroregeneration, in part through secretion of angiogenic factors [104]. Several studies have confirmed that systemic administration of cord blood cells is sufficient to induce neuroregeneration [105–107]. Given the potency of cord blood CD34+ cells to induce angiogenesis in areas of cerebral hypoperfusion, we propose that this cell type may be particularly useful for the treatment of autism, in which ischemia is milder than stroke induced ischemia, and as a result the level of angiogenesis needed is theoretically lower. However at face value, several considerations have to be dealt with. Firstly, cord blood contains a relatively low number of CD34+ cells for clinical use. Secondly, very few patients have access to autologous cord blood; therefore allogeneic cord blood CD34+ cells are needed if this therapy is to be made available for widespread use. There is a belief that allogeneic cord blood cells can not be used without immune suppression to avoid host versus graft destruction of the cells.
Numerous laboratories are currently attempting to expand cord blood CD34+ cells, achieving varying degrees of success. Expansion methods typically involve administration of cytokines, and or feeder cell layers [108–110]. The authors have developed a CD34+ expansion protocol that yields up to 60-fold expansion with limited cell differentiation. This expansion method involves numerous growth factors and conditioned medium, however is performed under serum free conditions (manuscript in preparation). Currently over 100 patients have been treated by one of the authors (FS) with expanded CD34+ cells under local ethical approval with varying degrees of success. Since other groups are also generating CD34+ expansion technologies, we do not anticipate number of CD34+ cells to be a problem.
Safety concerns regarding allogeneic CD34+ cells are divided into fears of graft versus host reactions, as well as host versus graft. The authors of the current paper have recently published a detailed rationale for why administration of cord blood cells is feasible in absence of immune suppression [111]. Essentially, GVHD occurs in the context of lymphopenia caused by bone marrow ablation. Administration of cord blood has been reported in over 500 patients without a single one suffering GVHD if no immune suppression was used [112–115]. Although host versus graft may conceptually cause immune mediated clearing of cord blood cells, efficacy of cord blood cells in absence of immune suppression has also been reported [116–118]. Accordingly, we believe that systemic administration of expanded cord blood derived CD34+ cells may be a potent tool for generation of neoangiogenesis in the autistic brain.
Immune modulation by mesenchymal stem cells
The treatment of immune deregulation in autism is expected to not only cause amelioration of intestinal and systemic symptomology, but also to profoundly influence neurological function. Reports exist of temporary neurological improvement by decreasing intestinal inflammation through either antibiotic administration [119] or dietary changes [120]. Although, as previously discussed, some anti-inflammatory treatments have yielded beneficial effects, no clinical agent has been developed that can profoundly suppress inflammation at the level of the fundamental immune abnormality. We believe mesenchymal stem cell administration may be used for this purpose. This cell type, in allogeneic form, is currently in Phase III clinical studies for Crohn's disease and Phase II results have demonstrated profound improvement [121].
Mesenchymal stem cells are classically defined as "formative pluripotential blast cells found inter alia in bone marrow, blood, dermis and periosteum that are capable of differentiating into any of the specific types of mesenchymal or connective tissues. These cells are routinely generated by culture of bone marrow in various culture media and collection of the adherent cell population. This expansion technique is sometimes used in combination with selection procedures for markers described above to generate a pure population of stem cells. An important characteristic of mesenchymal stem cells is their ability to constitutively secrete immune inhibitory factors such as IL-10 and TGF-b while maintaining ability to present antigens to T cells [122, 123]. This is believed to further allow inhibition of immunity in an antigen specific manner, as well as to allow the use of such cells in an allogeneic fashion without fear of immune-mediated rejection. Antigen-specific immune suppression is believed to allow these cells to shut off autoimmune processes. Further understanding of the immune inhibitory effects of mesenchymal stem cells comes from the fact that during T cell activation, two general signals are required for the T cell in order to mount a productive immune response, the first signal is recognition of antigen, and the second is recognition of costimulatory or coinhibitory signals. Mesenchymal cells present antigens to T cells but provide a coinhibitory signal instead of a co-stimulatory signal, thus specifically inhibiting T cells that recognize them, and other cells expressing similar antigens. Supporting this concept, it was demonstrated in a murine model that mesenchymal stem cell transplantation leads to permanent donor-specific immunotolerance in allogeneic hosts and results in long-term allogeneic skin graft acceptance [124]. Other studies have shown that mesenchymal stem cells are inherently immunosuppressive through production of PGE-2, interleukin-10 and expression of the tryptophan catabolizing enzyme indoleamine 2,3,-dioxygenase as well as Galectin-1 [125, 126].
These stem cells also have the ability to non-specifically modulate the immune response through the suppression of dendritic cell maturation and antigen presenting abilities [127, 128]. Immune suppressive activity is not dependent on prolonged culture of mesenchymal stem cells since functional induction of allogeneic T cell apoptosis was also demonstrated using freshly isolated, irradiated, mesenchymal stem cells [129]. Others have also demonstrated that mesenchymal stem cells have the ability to preferentially induce expansion of antigen specific T regulatory cells with the CD4+ CD25+ phenotype [130]. Supporting the potential clinical utility of such cells, it was previously demonstrated that administration of mesenchymal stem cells inhibits antigen specific T cell responses in the murine model of multiple sclerosis, experimental autoimmune encephalomyelitis, leading to prevention and/or regression of pathology [131]. Safety of infusing mesenchymal stem cells was illustrated in studies administering 1–2.2 × 106 cells/kg in order to enhance engraftment of autologous bone marrow cell. No adverse events were associated with infusion, although level of engraftment remained to be analyzed in randomized trials [132]. The ability of mesenchymal stem cells on one hand to suppress pathological immune responses but on the other hand to stimulate hematopoiesis leads to the possibility that these cells may also be useful for treatment of the defect in T cell numbers associated with autism.
Practical clinical entry
We propose a Phase I/II open labeled study investigating combination of cord blood expanded CD34+ cells together with mesenchymal stem cells for the treatment of autism. Such a trial would utilize several classical instruments of autism assessment such as the Aberrant Behavior Checklist and the Vineland Adaptive Behavior Scale (VABS) for assessment of symptomatic effect. Objective measurements of temporal lobe hypoperfusion, intestinal lymphoid hypertrophy, immunological markers and markers of hypoxia will be included. In order to initiate such an investigation, specific inclusion/exclusion criteria will be developed taking into account a population most likely to benefit from such an intervention. Criteria of particular interest would include defined hypoxia areas, as well as frank clinical manifestations of inflammatory intestinal disease. Markers of inflammatory processes may be used as part of the inclusion criteria, for example, elevation of C-reactive protein, or serum levels of TNF-alpha, IL-1, or IL-6 in order to specifically identify patients in whom the anti-inflammatory aspects of stem cell therapy would benefit [133, 134]. More stringent criteria would include restricting the study to only patients in which T cell abnormalities are present such as ex vivo hypersecretion of interferon gamma upon anti-CD3/CD28 stimulation [135], as well as deficient production of immune inhibitory cytokines such as IL-10 [77] and TGF-beta [78].
One of the authors (FS) has utilized both CD34+ and mesenchymal stem cells clinically for treatment of various diseases. In some case reports, the combination of CD34+ and mesenchymal stem cells was noted to induce synergistic effects in neurological diseases, although the number of patients are far too low to draw any conclusions. We propose to conduct this study based on the previous experiences of our group in this field, as well as numerous other groups that have generated anecdotal evidence of stem cell therapy for autism but have not published in conventional journals. We believe that through development of a potent clinical study with appropriate endpoints, much will be learned about the pathophysiology of autism regardless of trial outcome.
References
Mitchell S, Brian J, Zwaigenbaum L, Roberts W, Szatmari P, Smith I, Bryson S: Early language and communication development of infants later diagnosed with autism spectrum disorder. J Dev Behav Pediatr. 2006, 27: S69-78.
Filipek PA, Accardo PJ, Baranek GT, Cook EH, Dawson G, Gordon B, Gravel JS, Johnson CP, Kallen RJ, Levy SE: The screening and diagnosis of autistic spectrum disorders. J Autism Dev Disord. 1999, 29: 439-484.
Ryu YH, Lee JD, Yoon PH, Kim DI, Lee HB, Shin YJ: Perfusion impairments in infantile autism on technetium-99m ethyl cysteinate dimer brain single-photon emission tomography: comparison with findings on magnetic resonance imaging. Eur J Nucl Med. 1999, 26: 253-259.
Bruneau N, Dourneau MC, Garreau B, Pourcelot L, Lelord G: Blood flow response to auditory stimulations in normal, mentally retarded, and autistic children: a preliminary transcranial Doppler ultrasonographic study of the middle cerebral arteries. Biol Psychiatry. 1992, 32: 691-699.
Pierce K, Haist F, Sedaghat F, Courchesne E: The brain response to personally familiar faces in autism: findings of fusiform activity and beyond. Brain. 2004, 127: 2703-2716.
Critchley HD, Daly EM, Bullmore ET, Williams SC, Van Amelsvoort T, Robertson DM, Rowe A, Phillips M, McAlonan G, Howlin P: The functional neuroanatomy of social behaviour: changes in cerebral blood flow when people with autistic disorder process facial expressions. Brain. 2000, 123 (Pt 11): 2203-2212.
Boddaert N, Chabane N, Belin P, Bourgeois M, Royer V, Barthelemy C, Mouren-Simeoni MC, Philippe A, Brunelle F, Samson Y: Perception of complex sounds in autism: abnormal auditory cortical processing in children. Am J Psychiatry. 2004, 161: 2117-2120.
Hashimoto T, Sasaki M, Fukumizu M, Hanaoka S, Sugai K, Matsuda H: Single-photon emission computed tomography of the brain in autism: effect of the developmental level. Pediatr Neurol. 2000, 23: 416-420.
Bachevalier J: Medial temporal lobe structures and autism: a review of clinical and experimental findings. Neuropsychologia. 1994, 32: 627-648.
Gillberg IC: Autistic syndrome with onset at age 31 years: herpes encephalitis as a possible model for childhood autism. Dev Med Child Neurol. 1991, 33: 920-924.
Lipkin WI, Hornig M: Microbiology and immunology of autism spectrum disorders. Novartis Found Symp. 2003, 251: 129-143. discussion 144–128, 281–197
Gillberg C: Onset at age 14 of a typical autistic syndrome. A case report of a girl with herpes simplex encephalitis. J Autism Dev Disord. 1986, 16: 369-375.
Hoon AH, Reiss AL: The mesial-temporal lobe and autism: case report and review. Dev Med Child Neurol. 1992, 34: 252-259.
Taylor DC, Neville BG, Cross JH: Autistic spectrum disorders in childhood epilepsy surgery candidates. Eur Child Adolesc Psychiatry. 1999, 8: 189-192.
Zilbovicius M, Meresse I, Chabane N, Brunelle F, Samson Y, Boddaert N: Autism, the superior temporal sulcus and social perception. Trends Neurosci. 2006, 29: 359-366.
Cubells JF: Targeting the glutamate system in the treatment of autistic spectrum disorders. Curr Psychiatry Rep. 2007, 9: 131-
[http://www.clinicaltrials.gov/ct/show/NCT00251303?order=53]
Lindsey BW, Tropepe V: A comparative framework for understanding the biological principles of adult neurogenesis. Prog Neurobiol. 2006, 80: 281-307.
Tang T, Li XQ, Wu H, Luo JK, Zhang HX, Luo TL: Activation of endogenous neural stem cells in experimental intracerebral hemorrhagic rat brains. Chin Med J (Engl). 2004, 117: 1342-1347.
Sgubin D, Aztiria E, Perin A, Longatti P, Leanza G: Activation of endogenous neural stem cells in the adult human brain following subarachnoid hemorrhage. J Neurosci Res. 2007
Styczynski J, Cheung YK, Garvin J, Savage DG, Billote GB, Harrison L, Skerrett D, Wolownik K, Wischhover C, Hawks R: Outcomes of unrelated cord blood transplantation in pediatric recipients. Bone Marrow Transplant. 2004, 34: 129-136.
Yan YP, Sailor KA, Vemuganti R, Dempsey RJ: Insulin-like growth factor-1 is an endogenous mediator of focal ischemia-induced neural progenitor proliferation. Eur J Neurosci. 2006, 24: 45-54.
Takagi Y, Nozaki K, Takahashi J, Yodoi J, Ishikawa M, Hashimoto N: Proliferation of neuronal precursor cells in the dentate gyrus is accelerated after transient forebrain ischemia in mice. Brain Res. 1999, 831: 283-287.
Dempsey RJ, Sailor KA, Bowen KK, Tureyen K, Vemuganti R: Stroke-induced progenitor cell proliferation in adult spontaneously hypertensive rat brain: effect of exogenous IGF-1 and GDNF. J Neurochem. 2003, 87: 586-597.
[http://www.clinicaltrials.gov/ct/show/NCT00404846?order=11]
Filosa JA, Bonev AD, Straub SV, Meredith AL, Wilkerson MK, Aldrich RW, Nelson MT: Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat Neurosci. 2006, 9: 1397-1403.
Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, Nedergaard M: Astrocyte-mediated control of cerebral blood flow. Nat Neurosci. 2006, 9: 260-267.
Kim JH, Park JA, Lee SW, Kim WJ, Yu YS, Kim KW: Blood-neural barrier: intercellular communication at glio-vascular interface. J Biochem Mol Biol. 2006, 39: 339-345.
Konat GW, Kielian T, Marriott I: The role of Toll-like receptors in CNS response to microbial challenge. J Neurochem. 2006, 99: 1-12.
Wen LL, Chiu CT, Huang YN, Chang CF, Wang JY: Rapid glia expression and release of proinflammatory cytokines in experimental Klebsiella pneumoniae meningoencephalitis. Exp Neurol. 2007, 205: 270-278.
Farina C, Aloisi F, Meinl E: Astrocytes are active players in cerebral innate immunity. Trends Immunol. 2007, 28: 138-145.
Carpentier PA, Begolka WS, Olson JK, Elhofy A, Karpus WJ, Miller SD: Differential activation of astrocytes by innate and adaptive immune stimuli. Glia. 2005, 49: 360-374.
Constantinescu CS, Tani M, Ransohoff RM, Wysocka M, Hilliard B, Fujioka T, Murphy S, Tighe PJ, Sarma JD, Trinchieri G: Astrocytes as antigen-presenting cells: expression of IL-12/IL-23. J Neurochem. 2005, 95: 331-340.
Holden C: Neuroscience. Astrocytes secrete substance that kills motor neurons in ALS. Science. 2007, 316: 353-
Nagai M, Re DB, Nagata T, Chalazonitis A, Jessell TM, Wichterle H, Przedborski S: Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci. 2007, 10: 615-622.
Johansson A, Engler H, Blomquist G, Scott B, Wall A, Aquilonius SM, Langstrom B, Askmark H: Evidence for astrocytosis in ALS demonstrated by [11C](L)-deprenyl-D2 PET. J Neurol Sci. 2007, 255: 17-22.
Yokota O, Tsuchiya K, Oda T, Ishihara T, de Silva R, Lees AJ, Arai T, Uchihara T, Ishizu H, Kuroda S: Amyotrophic lateral sclerosis with dementia: an autopsy case showing many Bunina bodies, tau-positive neuronal and astrocytic plaque-like pathologies, and pallido-nigral degeneration. Acta Neuropathol (Berl). 2006, 112: 633-645.
Schiffer D, Cordera S, Cavalla P, Migheli A: Reactive astrogliosis of the spinal cord in amyotrophic lateral sclerosis. J Neurol Sci. 1996, 139 (Suppl): 27-33.
Bologa L, Deugnier MA, Joubert R, Bisconte JC: Myelin basic protein stimulates the proliferation of astrocytes: possible explanation for multiple sclerosis plaque formation. Brain Res. 1985, 346: 199-203.
Petzold A, Brassat D, Mas P, Rejdak K, Keir G, Giovannoni G, Thompson EJ, Clanet M: Treatment response in relation to inflammatory and axonal surrogate marker in multiple sclerosis. Mult Scler. 2004, 10: 281-283.
Pekny M, Nilsson M: Astrocyte activation and reactive gliosis. Glia. 2005, 50: 427-434.
Dietrich PY, Walker PR, Saas P: Death receptors on reactive astrocytes: a key role in the fine tuning of brain inflammation?. Neurology. 2003, 60: 548-554.
Shie FS, Neely MD, Maezawa I, Wu H, Olson SJ, Jurgens G, Montine KS, Montine TJ: Oxidized low-density lipoprotein is present in astrocytes surrounding cerebral infarcts and stimulates astrocyte interleukin-6 secretion. Am J Pathol. 2004, 164: 1173-1181.
Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA: Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005, 57: 67-81.
Huang D, Han Y, Rani MR, Glabinski A, Trebst C, Sorensen T, Tani M, Wang J, Chien P, O'Bryan S: Chemokines and chemokine receptors in inflammation of the nervous system: manifold roles and exquisite regulation. Immunol Rev. 2000, 177: 52-67.
Loftis JM, Hauser P: The phenomenology and treatment of interferon-induced depression. J Affect Disord. 2004, 82: 175-190.
Stubbs G: Interferonemia and autism. J Autism Dev Disord. 1995, 25: 71-73.
Sweeten TL, Posey DJ, Shankar S, McDougle CJ: High nitric oxide production in autistic disorder: a possible role for interferon-gamma. Biol Psychiatry. 2004, 55: 434-437.
Mount MP, Lira A, Grimes D, Smith PD, Faucher S, Slack R, Anisman H, Hayley S, Park DS: Involvement of interferon-gamma in microglial-mediated loss of dopaminergic neurons. J Neurosci. 2007, 27: 3328-3337.
Sardar AM, Reynolds GP: Frontal cortex indoleamine-2,3-dioxygenase activity is increased in HIV-1-associated dementia. Neurosci Lett. 1995, 187: 9-12.
Brown RR, Ozaki Y, Datta SP, Borden EC, Sondel PM, Malone DG: Implications of interferon-induced tryptophan catabolism in cancer, auto-immune diseases and AIDS. Adv Exp Med Biol. 1991, 294: 425-435.
Cohly HH, Panja A: Immunological findings in autism. Int Rev Neurobiol. 2005, 71: 317-341.
Yonk LJ, Warren RP, Burger RA, Cole P, Odell JD, Warren WL, White E, Singh VK: CD4+ helper T cell depression in autism. Immunol Lett. 1990, 25: 341-345.
Marleau AM, Sarvetnick N: T cell homeostasis in tolerance and immunity. J Leukoc Biol. 2005, 78: 575-584.
Singh VK, Warren RP, Odell JD, Warren WL, Cole P: Antibodies to myelin basic protein in children with autistic behavior. Brain Behav Immun. 1993, 7: 97-103.
Silva SC, Correia C, Fesel C, Barreto M, Coutinho AM, Marques C, Miguel TS, Ataide A, Bento C, Borges L: Autoantibody repertoires to brain tissue in autism nuclear families. J Neuroimmunol. 2004, 152: 176-182.
Singer HS, Morris CM, Williams PN, Yoon DY, Hong JJ, Zimmerman AW: Antibrain antibodies in children with autism and their unaffected siblings. J Neuroimmunol. 2006, 178: 149-155.
Vojdani A, O'Bryan T, Green JA, McCandless J, Woeller KN, Vojdani E, Nourian AA, Cooper EL: Immune response to dietary proteins, gliadin and cerebellar peptides in children with autism. Nutr Neurosci. 2004, 7: 151-161.
Connolly AM, Chez M, Streif EM, Keeling RM, Golumbek PT, Kwon JM, Riviello JJ, Robinson RG, Neuman RJ, Deuel RM: Brain-derived neurotrophic factor and autoantibodies to neural antigens in sera of children with autistic spectrum disorders, Landau-Kleffner syndrome, and epilepsy. Biol Psychiatry. 2006, 59: 354-363.
Kozlovskaia GV, Kliushnik TP, Goriunova AV, Turkova IL, Kalinina MA, Sergienko NS: [Nerve growth factor auto-antibodies in children with various forms of mental dysontogenesis and in schizophrenia high risk group]. Zh Nevrol Psikhiatr Im S S Korsakova. 2000, 100: 50-52.
Sweeten TL, Bowyer SL, Posey DJ, Halberstadt GM, McDougle CJ: Increased prevalence of familial autoimmunity in probands with pervasive developmental disorders. Pediatrics. 2003, 112: e420-
Comi AM, Zimmerman AW, Frye VH, Law PA, Peeden JN: Familial clustering of autoimmune disorders and evaluation of medical risk factors in autism. J Child Neurol. 1999, 14: 388-394.
Warren RP, Odell JD, Warren WL, Burger RA, Maciulis A, Daniels WW, Torres AR: Strong association of the third hypervariable region of HLA-DR beta 1 with autism. J Neuroimmunol. 1996, 67: 97-102.
Daniels WW, Warren RP, Odell JD, Maciulis A, Burger RA, Warren WL, Torres AR: Increased frequency of the extended or ancestral haplotype B44-SC30-DR4 in autism. Neuropsychobiology. 1995, 32: 120-123.
Warren RP, Singh VK, Cole P, Odell JD, Pingree CB, Warren WL, White E: Increased frequency of the null allele at the complement C4b locus in autism. Clin Exp Immunol. 1991, 83: 438-440.
Muller-Hilke B, Mitchison NA: The role of HLA promoters in autoimmunity. Curr Pharm Des. 2006, 12: 3743-3752.
Moulds JM: Ethnic diversity of class III genes in autoimmune disease. Front Biosci. 2001, 6: D986-991.
Yu CY, Chung EK, Yang Y, Blanchong CA, Jacobsen N, Saxena K, Yang Z, Miller W, Varga L, Fust G: Dancing with complement C4 and the RP-C4-CYP21-TNX (RCCX) modules of the major histocompatibility complex. Prog Nucleic Acid Res Mol Biol. 2003, 75: 217-292.
Sakic B, Szechtman H, Denburg JA: Neurobehavioral alterations in autoimmune mice. Neurosci Biobehav Rev. 1997, 21: 327-340.
Wakefield AJ, Murch SH, Anthony A, Linnell J, Casson DM, Malik M, Berelowitz M, Dhillon AP, Thomson MA, Harvey P: Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet. 1998, 351: 637-641.
Ashwood P, Anthony A, Pellicer AA, Torrente F, Walker-Smith JA, Wakefield AJ: Intestinal lymphocyte populations in children with regressive autism: evidence for extensive mucosal immunopathology. J Clin Immunol. 2003, 23: 504-517.
Croonenberghs J, Bosmans E, Deboutte D, Kenis G, Maes M: Activation of the inflammatory response system in autism. Neuropsychobiology. 2002, 45: 1-6.
Singh VK: Plasma increase of interleukin-12 and interferon-gamma. Pathological significance in autism. J Neuroimmunol. 1996, 66: 143-145.
Ashwood P, Van de Water J: Is autism an autoimmune disease?. Autoimmun Rev. 2004, 3: 557-562.
Sweeten TL, Posey DJ, McDougle CJ: High blood monocyte counts and neopterin levels in children with autistic disorder. Am J Psychiatry. 2003, 160: 1691-1693.
Ashwood P, Anthony A, Torrente F, Wakefield AJ: Spontaneous mucosal lymphocyte cytokine profiles in children with autism and gastrointestinal symptoms: mucosal immune activation and reduced counter regulatory interleukin-10. J Clin Immunol. 2004, 24: 664-673.
Okada K, Hashimoto K, Iwata Y, Nakamura K, Tsujii M, Tsuchiya KJ, Sekine Y, Suda S, Suzuki K, Sugihara G: Decreased serum levels of transforming growth factor-beta1 in patients with autism. Prog Neuropsychopharmacol Biol Psychiatry. 2007, 31: 187-190.
Kipnis J, Cohen H, Cardon M, Ziv Y, Schwartz M: T cell deficiency leads to cognitive dysfunction: implications for therapeutic vaccination for schizophrenia and other psychiatric conditions. Proc Natl Acad Sci USA. 2004, 101: 8180-8185.
Boris M, Kaiser CC, Goldblatt A, Elice MW, Edelson SM, Adams JB, Feinstein DL: Effect of pioglitazone treatment on behavioral symptoms in autistic children. J Neuroinflammation. 2007, 4: 3-
Plioplys AV: Intravenous immunoglobulin treatment of children with autism. J Child Neurol. 1998, 13: 79-82.
DelGiudice-Asch G, Simon L, Schmeidler J, Cunningham-Rundles C, Hollander E: Brief report: a pilot open clinical trial of intravenous immunoglobulin in childhood autism. J Autism Dev Disord. 1999, 29: 157-160.
[http://www.clinicaltrials.gov/ct/show/NCT00453180?order=31]
[http://www.clinicaltrials.gov/ct/show/NCT00325572?order=40]
Schirmer SH, Royen NV: Stimulation of collateral artery growth: a potential treatment for peripheral artery disease. Expert Rev Cardiovasc Ther. 2004, 2: 581-588.
Tse HF, Yiu KH, Lau CP: Bone marrow stem cell therapy for myocardial angiogenesis. Curr Vasc Pharmacol. 2007, 5: 103-112.
Wei L, Keogh CL, Whitaker VR, Theus MH, Yu SP: Angiogenesis and stem cell transplantation as potential treatments of cerebral ischemic stroke. Pathophysiology. 2005, 12: 47-62.
Ke Q, Costa M: Hypoxia-inducible factor-1 (HIF-1). Mol Pharmacol. 2006, 70: 1469-1480.
Shintani S, Murohara T, Ikeda H, Ueno T, Honma T, Katoh A, Sasaki K, Shimada T, Oike Y, Imaizumi T: Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation. 2001, 103: 2776-2779.
Chopp M, Zhang ZG, Jiang Q: Neurogenesis, angiogenesis, and MRI indices of functional recovery from stroke. Stroke. 2007, 38: 827-831.
Ohab JJ, Fleming S, Blesch A, Carmichael ST: A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006, 26: 13007-13016.
Zhang Z, Chopp M: Vascular endothelial growth factor and angiopoietins in focal cerebral ischemia. Trends Cardiovasc Med. 2002, 12: 62-66.
Bogousslavsky J, Victor SJ, Salinas EO, Pallay A, Donnan GA, Fieschi C, Kaste M, Orgogozo JM, Chamorro A, Desmet A: Fiblast (trafermin) in acute stroke: results of the European-Australian phase II/III safety and efficacy trial. Cerebrovasc Dis. 2002, 14: 239-251.
Ehrenreich H, Timner W, Siren AL: A novel role for an established player: anemia drug erythropoietin for the treatment of cerebral hypoxia/ischemia. Transfus Apher Sci. 2004, 31: 39-44.
Schabitz WR, Schneider A: Developing granulocyte-colony stimulating factor for the treatment of stroke: current status of clinical trials. Stroke. 2006, 37: 1654-author reply 1655
Cairns K, Finklestein SP: Growth factors and stem cells as treatments for stroke recovery. Phys Med Rehabil Clin N Am. 2003, 14: S135-142.
Oakley RE, Al msherqi Z, Lim SK, Lee SH, Ho KT, Sutandar A, Lee CN, Lim YT: Transplantation of autologous bone marrow-derived cells into the myocardium of patients undergoing coronary bypass. Heart Surg Forum. 2005, 8: E348-350.
Kolvenbach R, Kreissig C, Ludwig E, Cagiannos C: Stem cell use in critical limb ischemia. J Cardiovasc Surg (Torino). 2007, 48: 39-44.
Archundia A, Aceves JL, Lopez-Hernandez M, Alvarado M, Rodriguez E, Diaz Quiroz G, Paez A, Rojas FM, Montano LF: Direct cardiac injection of G-CSF mobilized bone-marrow stem-cells improves ventricular function in old myocardial infarction. Life Sci. 2005, 78: 279-283.
Theunissen K, Verfaillie CM: A multifactorial analysis of umbilical cord blood, adult bone marrow and mobilized peripheral blood progenitors using the improved ML-IC assay. Exp Hematol. 2005, 33: 165-172.
Hildbrand P, Cirulli V, Prinsen RC, Smith KA, Torbett BE, Salomon DR, Crisa L: The role of angiopoietins in the development of endothelial cells from cord blood CD34+ progenitors. Blood. 2004, 104: 2010-2019.
Salven P, Mustjoki S, Alitalo R, Alitalo K, Rafii S: VEGFR-3 and CD133 identify a population of CD34+ lymphatic/vascular endothelial precursor cells. Blood. 2003, 101: 168-172.
Taguchi A, Soma T, Tanaka H, Kanda T, Nishimura H, Yoshikawa H, Tsukamoto Y, Iso H, Fujimori Y, Stern DM: Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest. 2004, 114: 330-338.
Newman MB, Willing AE, Manresa JJ, Sanberg CD, Sanberg PR: Cytokines produced by cultured human umbilical cord blood (HUCB) cells: implications for brain repair. Exp Neurol. 2006, 199: 201-208.
Chen SH, Chang FM, Tsai YC, Huang KF, Lin CL, Lin MT: Infusion of human umbilical cord blood cells protect against cerebral ischemia and damage during heatstroke in the rat. Exp Neurol. 2006, 199: 67-76.
Peterson DA: Umbilical cord blood cells and brain stroke injury: bringing in fresh blood to address an old problem. J Clin Invest. 2004, 114: 312-314.
Galan I, DeLeon JA, Diaz L, Hong JS, Khalek N, Munoz-Fernandez MA, Santolaya-Forgas J: Effect of a bone marrow microenvironment on the ex-vivo expansion of umbilical cord blood progenitor cells. Int J Lab Hematol. 2007, 29: 58-63.
Tanaka H, Matsumura I, Itoh K, Hatsuyama A, Shikamura M, Satoh Y, Heike T, Nakahata T, Kanakura Y: HOX decoy peptide enhances the ex vivo expansion of human umbilical cord blood CD34+ hematopoietic stem cells/hematopoietic progenitor cells. Stem Cells. 2006, 24: 2592-2602.
Mohamed AA, Ibrahim AM, El-Masry MW, Mansour IM, Khroshied MA, Gouda HM, Riad RM: Ex vivo expansion of stem cells: defining optimum conditions using various cytokines. Lab Hematol. 2006, 12: 86-93.
Riordan NH, Chan K, Marleau AM, Ichim TE: Cord blood in regenerative medicine: do we need immune suppression?. J Transl Med. 2007, 5: 8-
Bhattacharya N: Spontaneous transient rise of CD34 cells in peripheral blood after 72 hours in patients suffering from advanced malignancy with anemia: effect and prognostic implications of treatment with placental umbilical cord whole blood transfusion. Eur J Gynaecol Oncol. 2006, 27: 286-290.
Bhattacharya N: Placental umbilical cord whole blood transfusion: a safe and genuine blood substitute for patients of the under-resourced world at emergency. J Am Coll Surg. 2005, 200: 557-563.
Halbrecht J: Fresh and stored placental blood. Lancet. 1939, 2: 1263-
Hassall O, Bedu-Addo G, Adarkwa M, Danso K, Bates I: Umbilical-cord blood for transfusion in children with severe anaemia in under-resourced countries. Lancet. 2003, 361: 678-679.
Valbonesi M, Giannini G, Migliori F, Dalla Costa R, Dejana AM: Cord blood (CB) stem cells for wound repair. Preliminary report of 2 cases. Transfus Apher Sci. 2004, 30: 153-156.
Kang KS, Kim SW, Oh YH, Yu JW, Kim KY, Park HK, Song CH, Han H: A 37-year-old spinal cord-injured female patient, transplanted of multipotent stem cells from human UC blood, with improved sensory perception and mobility, both functionally and morphologically: a case study. Cytotherapy. 2005, 7: 368-373.
Kim SW, Han H, Chae GT, Lee SH, Bo S, Yoon JH, Lee YS, Lee KS, Park HK, Kang KS: Successful stem cell therapy using umbilical cord blood-derived multipotent stem cells for Buerger's disease and ischemic limb disease animal model. Stem Cells. 2006, 24: 1620-1626.
Sandler RH, Finegold SM, Bolte ER, Buchanan CP, Maxwell AP, Vaisanen ML, Nelson MN, Wexler HM: Short-term benefit from oral vancomycin treatment of regressive-onset autism. J Child Neurol. 2000, 15: 429-435.
Lucarelli S, Frediani T, Zingoni AM, Ferruzzi F, Giardini O, Quintieri F, Barbato M, D'Eufemia P, Cardi E: Food allergy and infantile autism. Panminerva Med. 1995, 37: 137-141.
Liu J, Lu XF, Wan L, Li YP, Li SF, Zeng LY, Zeng YZ, Cheng LH, Lu YR, Cheng JQ: Suppression of human peripheral blood lymphocyte proliferation by immortalized mesenchymal stem cells derived from bone marrow of Banna Minipig inbred-line. Transplant Proc. 2004, 36: 3272-3275.
Togel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C: Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005, 289: F31-42.
Deng W, Han Q, Liao L, Li C, Ge W, Zhao Z, You S, Deng H, Zhao RC: Allogeneic bone marrow-derived flk-1+Sca-1- mesenchymal stem cells leads to stable mixed chimerism and donor-specific tolerance. Exp Hematol. 2004, 32: 861-867.
Kadri T, Lataillade JJ, Doucet C, Marie A, Ernou I, Bourin P, Joubert-Caron R, Caron M, Lutomski D: Proteomic study of Galectin-1 expression in human mesenchymal stem cells. Stem Cells Dev. 2005, 14: 204-212.
Ryan JM, Barry FP, Murphy JM, Mahon BP: Mesenchymal stem cells avoid allogeneic rejection. J Inflamm (Lond). 2005, 2: 8-
Beyth S, Borovsky Z, Mevorach D, Liebergall M, Gazit Z, Aslan H, Galun E, Rachmilewitz J: Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005, 105: 2214-2219.
Aggarwal S, Pittenger MF: Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005, 105: 1815-1822.
Plumas J, Chaperot L, Richard MJ, Molens JP, Bensa JC, Favrot MC: Mesenchymal stem cells induce apoptosis of activated T cells. Leukemia. 2005, 19: 1597-1604.
Maccario R, Podesta M, Moretta A, Cometa A, Comoli P, Montagna D, Daudt L, Ibatici A, Piaggio G, Pozzi S: Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4+ T-cell subsets expressing a regulatory/suppressive phenotype. Haematologica. 2005, 90: 516-525.
Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, Giunti D, Ceravolo A, Cazzanti F, Frassoni F: Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005, 106: 1755-1761.
Koc ON, Gerson SL, Cooper BW, Dyhouse SM, Haynesworth SE, Caplan AI, Lazarus HM: Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol. 2000, 18: 307-316.
Molloy CA, Morrow AL, Meinzen-Derr J, Schleifer K, Dienger K, Manning-Courtney P, Altaye M, Wills-Karp M: Elevated cytokine levels in children with autism spectrum disorder. J Neuroimmunol. 2006, 172: 198-205.
Jyonouchi H, Sun S, Le H: Proinflammatory and regulatory cytokine production associated with innate and adaptive immune responses in children with autism spectrum disorders and developmental regression. J Neuroimmunol. 2001, 120: 170-179.
Ashwood P, Wakefield AJ: Immune activation of peripheral blood and mucosal CD3+ lymphocyte cytokine profiles in children with autism and gastrointestinal symptoms. J Neuroimmunol. 2006, 173: 126-134.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Ichim, T.E., Solano, F., Glenn, E. et al. Stem Cell Therapy for Autism. J Transl Med 5, 30 (2007). https://doi.org/10.1186/1479-5876-5-30
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1479-5876-5-30