Abstract
Human metastatic cutaneous melanoma has gained a well deserved reputation for its immune responsiveness. The reason(s) remain(s) unknown. We attempted previously to characterize several variables that may affect the relationship between tumor and host immune cells but, taken one at the time, none yielded a convincing explanation. With explorative purposes, high-throughput technology was applied here to portray transcriptional characteristics unique to metastatic cutaneous melanoma that may or may not be relevant to its immunogenic potential. Several functional signatures could be identified descriptive of immune or other biological functions. In addition, the transcriptional profile of metastatic melanoma was compared with that of primary renal cell cancers (RCC) identifying several genes co-coordinately expressed by the two tumor types. Since RCC is another immune responsive tumor, commonalities between RCC and melanoma may help untangle the enigma of their potential immune responsiveness. This purely descriptive study provides, therefore, a map for the investigation of metastatic melanoma in future clinical trials and at the same time may invite consideration of novel therapeutic targets.
Similar content being viewed by others
Background
Human metastatic cutaneous melanoma relative to other common solid tumors shares with renal cell cancer (RCC) the well deserved reputation of being responsive to immune manipulation [1, 2]. However, the reason(s) for this phenomenon remain(s) largely unknown [3]. Possibly, metastatic cutaneous melanoma is endowed compared to other tumors with a wealth of "tumor rejection" antigens of unique immunogenic potential. Indeed, the ease in which tumor infiltrating lymphocytes recognizing autologous tumor cells can be isolated from melanoma metastases suggests an extraordinary ability of melanoma cells to elicit cognitive T cell responses [4]. In addition, the broad repertoire of melanoma-associated antigens so far discovered largely outnumbers that of other tumors suggesting a stronger immunogenicity of this cancer [5–7]. This explanation, however, contrasts with the paucity of RCC-specific antigens described and the relative difficulty of expanding tumor infiltrating lymphocytes from RCC that can recognize autologous cancer cells. Yet, RCC is somehow also responsive to immune therapy [2, 8]. suggesting that explanations other than solely the identity of T cell epitopes should be considered.
We have previously shown that the microenvironment of a subgroup of melanoma metastases expresses at the transcriptional level an array of biologically active factors that may influence both the innate and the adaptive arm of the immune system [9]. We have also observed that subcutaneous melanoma metastases likely to respond to immunotherapy have a different genetic profile than those unlikely to respond to therapy [10]. This genetic profile differs particularly in expression of immunologically relevant genes suggesting that melanoma metastases that respond to therapy are conditioned to respond even before therapy by an immunologically active environment. These pilot studies encouraged us to collect a large series of melanoma metastases and analyze their genetic profile to search for molecular signatures specific for this tumor entity compared with other less immunogenic cancers. The lack of clinical information limited this study to a descriptive analysis of the molecular signatures characteristic of melanoma that could serve as a map for future studies on this subject. In addition, the application of high-throughput technology to identify transcriptional characteristics unique to metastatic cutaneous melanoma may define novel targets which can be employed for further analysis. . Several signatures were identified descriptive of immune or other biological functions that might be relevant to immune responsiveness. Furthermore, a comparison of the transcriptional profile of metastatic melanoma with that of a library of available primary RCC identified several genes co-coordinately expressed by the two tumor types. Since RCC represents another immune responsive human tumor it is possible that commonalities with melanoma may reveal, in the future, the secret of immune responsiveness. This purely descriptive study provides, therefore, a map of markers for the investigation of metastatic melanoma in novel clinical trials and may invite consideration of novel therapeutic targets.
Results and Discussion
Differences between the transcriptional profile of melanoma metastases and other solid tumors
We first identified genes differentially expressed between 69 melanoma samples and 87 samples obtained from available primary or metastatic solid tumors (Table I). RCC samples were excluded from the statistical comparison because this tumors share immune responsiveness with metastatic melanoma and, therefore, were considered separately from non-immunogenic tumors. Differential expression was defined significant at a p2-value ≤ 0.001 (unpaired two-tailed Student t test). This test identified 4,658 cDNA clones differentially expressed between melanoma metastases and tumors of other histology (see Additional file 1). Non parametric Wilcoxon test yielded comparable results in terms of number and identity of differentially expressed genes (data not shown). Permutation analysis strongly supported the significance of these findings. Approximately half of the differentially expressed clones (2,044) were up-regulated in melanoma metastases relative to other tumors and the remaining 2,614 clones were down-regulated. Up-regulation was defined as a positive value after subtracting the average ratio of other tumors from that of melanoma lesions (Figure 1). Down-regulation was considered a negative value resulting from the same formula. A large proportion of the genes down-regulated in melanoma relative to other tumor were lineage specific and reflected its unique ontogeny from the neuroectoderm while the tumors studied were mostly of epithelial origin. We have previously described the weight that ontogeny may play in balancing the transcriptional profile of RCC [11]. Unfortunately, for this type of analysis to be conclusive availability of matched normal tissues is required which is not as readily achievable in the case of melanoma due to the scattered distribution of normal epithelial melanocytes within the skin layers. The complete list of the 4,658 genes differentially expressed by melanomas is available at http://www.societymelanomaresearch.org
Eisen's clustering based on 2,044 genes up-regulated in metastatic melanoma lesions compared with all other tumors. Signatures include growth regulation (maroon vertical bar); a signature of genes similarly expressed by melanoma and RCC (blue vertical bar) including a sub-cluster of genes predominantly expressed by RCC (double vertical blue bar and blue arrow); an immunological signature (orange vertical bar); a signature specific for genes predominantly expressed by cutaneous and subcutaneous melanoma metastases (green vertical bar); gene related to blood contamination in fine needle aspirates (FNA; red arrow) and a signature specific for melanoma differentiation antigens (MDA; blue arrow). Genes were identified by a two-tailed Student's t test comparing all melanoma lesions with other tumors (with the exception of RCC) applying as cut off of significance a p2-value < 0.001. Up-regulation was defined as a positive value after subtracting the average of other tumor samples Cy5/Cy3 ratios from that of melanoma samples.
Signature-specific genes
Several signatures representing genes preferentially expressed by melanomas were identified that could be partially linked to specific gene functions. Those signatures were segregated according to unsupervised gene rearrangement based on the Eisen's clustering method. The first cluster (cluster a, Figure 1) included 76 clones of which 63 were named corresponding to 53 genes. A subset of genes in this cluster were commonly up-regulated in melanoma and RCC including enolase 2 (neuronal γ-enolase) which is a previously described serum marker of RCC also associated with renal carcinogenesis [11–13]. Differential expression of enolase-2 between melanoma and other cancers with the exception of RCC reached a significance of 5 × 10-7 and 1 × 10-6 for two clones representing this gene (Student's t-test p2 value). Overall, this cluster was enriched of genes associated with active cellular metabolism and included only few genes of previous known relevance to melanoma with the exception of a member of the melanoma antigen family D (MAGED2, Table 2 and Figure 2). Cluster b included 91 clones (72 named representing 65 distinct genes) predominantly associated with growth regulation and apoptosis. Among the genes included in this cluster was BNIP3L (BCL2/Adenovirus E1B interacting protein like-3, t-test p2-value = 2 × 10-7 for both cDNA clones representing this gene) that we have previously reported to be associated with the immune responsiveness of melanoma metastases [10]. Two large and related clusters (cluster c and d) included 262 and 613 clones, respectively (112 and 296 named corresponding respectively to 110 and 289 genes). These clusters were characterized by a high density of unnamed clones and by relatively low Cy5/Cy3 ratios. However, it should be noted that these clusters may be of particular interest because the gene expression profile was similar between melanoma and RCC tumors suggesting that some of these genes may conceal the enigma of immune responsiveness. In particular, a relatively sizable sub-cluster was noted with genes predominantly up-regulated in RCC but also expressed by melanoma lesions compared with other tumors (Blue arrow and double vertical bar, Figure 1). Genes concomitantly up-regulated in melanoma and RCC will be separately discussed later, however, it is important to note that this cluster included JAK-1 (t-test p2-value = 2 × 10-5) that was previously also reported in association with melanoma immune responsiveness to interleukin-2-based immunotherapy [10]. JAK-1 was recently linked to the apoptotic role that interleukin-24 (melanoma differentiation associated gene-7: MDA 7) may exert on melanoma cells [14]. The following cluster (cluster e) included 208 clones (151 named representing 143 different genes) predominantly associated with immune function. This immune signature was underlined by the high prevalence of expression of these genes in samples obtained from lymph node metastases whether from melanoma or colorectal primaries. Cluster f integrated 129 clones (91 named representing 87 distinct genes) including a mixture of genes with disparate functions difficult to categorize into a predominant pattern. This group also included APPBP1 (amyloid β precursor protein; t-test p2-value = 1 × 10-4) which was previously reported in association with melanoma immune responsiveness [10]. APPBP1 is a recently discovered epidermal growth factor that regulates dendrite motility and melanin release in epidermal melanocytes and melanoma cells [15]. It is possible that some of its functions may have an indirect effect in modulating the immunological profile of subcutaneous metastases. Cluster g included genes preferentially up-regulated in subcutaneous melanoma metastases known to be more responsive to immunotherapy with interleukin-2 [16]. This cluster included 201 clones (142 named representing 132 genes). Among the genes representative of this cluster were two classic melanoma associated genes (PRAME and tyrosine-related protein-1; TRP-1). In a small proportion, this cluster included a group of genes only over-expressed in fine needle aspirates (FNA) and generally expressed by circulating cells revealing blood contamination of FNA material (red arrow, Figure 1). Cluster h included 131 clones (102 named representing 99 genes). Cluster I included 222 clones (171 named identifying 155 genes) with most of the melanoma differentiation antigens (MDA) clustering in close proximity with the exception of the TRP-1 already discussed in cluster g. Interestingly, this cluster was also highly enriched of genes associated with ribosomal function and active translation. Furthermore, it included the melanocyte master regulator MITF (t-test p2-value for two respective cDNA clones = 2 × 10-15 and 8 × 10-14) which has been shown to modulate lineage survival and melanoma cell viability through interaction with the anti-apoptotic protein BCL2 [17]. MITF was coordinately expressed with several genes associated with calcium and other solute metabolism including cytochrome p450 (t-test p2-value for two respective cDNA clones = 3 × 10-12 and 7 × 10-13) solute carrier family 7 (t-test p2-value for two respective cDNA clones = 2 × 10-10 and 2 × 10-6), G protein coupled receptor 56 (t-test p2-value = 2 × 10-7) and calpain 3 (t-test p2-value = 6 × 10-15), a calcium-regulated gene found to be highly expressed in melanoma cells [18]. Finally, cluster J included 88 clones of which the 62 named identified 58 genes.
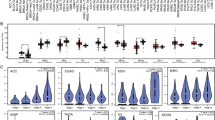
Eisen's clustering of genes already reported to be preferentially expressed by melanomas. The analysis was performed on 180 cancer samples as described in the Results section and ordered according to Table 1. In particular, renal cell cancer (RCC, orange), melanoma (blue), Epithelial Ovarian Cancer (EOC, yellow), Esophageal Cancer (green), Primary Colorectal Cancer (CRC, dark brown) and lymph nodal metastases of CRC (light brown) are shown. Melanoma samples are further subdivided in cutaneous metastases (CM, light blue) from frozen sections (continuous line) or fine needle aspirates (FNA, dashed line) and lymph nodal metastases (LN, darker blue line). Below is the distance among the various genes based on Eisen's clustering.
Genes previously recognized to be associated with melanoma
Genes previously described to be preferentially expressed by melanoma lesions were confirmed to be so at a very high level of significance (Figure 2). Exceptions included AIM-1, CXCL-1 (GRO-α), D2S448 and MAGEA1 which are all significantly more expressed by tumors other than melanomas. Interestingly, different types of melanoma associated genes displayed a different pattern of expression with MDA (tyrosinase, gp100/PMel17 and MART-1/MelanA) being co-coordinately expressed in close proximity to each other in cluster I and MAGE family genes preferentially expressed in cluster d(Table II). Cluster g included a number of genes whose expression had been previously associated with melanoma including preferentially expressed antigen in melanoma (PRAME) and the tyrosine-related protein-1 (TRP-1). When the melanoma associated genes were studied alone, PRAME clustered close to the other MDA believe to be involved in the pigmentation process (tyrosinase, MART-1/Melan and gp100/PMel17). This is of particular interest because PRAME has been also reported to be highly expressed in other cancers of ectodermic origin such as medulloblastoma and neuroblastoma suggesting a link between ectoderm and pigmentation [19, 20]. The coordinated expression of MDA suggests that their down-regulation or loss of expression during melanoma progression may be related to a central regulatory pathway not as yet identified. Indeed in previous studies [21–25], we noted that loss of expression of MART-1/Melan A paralleled that of gp100/PMel17 (SILV) in melanoma metastases while genes of the MAGE family manifested an independent behavior [25]. This finding may have important repercussions in the design of antigen-specific immunization protocols and at the same time may complicate the interpretation of tumor antigen loss variant analysis by broadening loss of expression to antigens other than those targeted by a given therapy.
Immunological Signature
The large majority of genes associated with immune function were included in cluster e. These genes appeared up-regulated in lymph node metastases of melanoma as well as those from colorectal primaries suggesting that their expression results from lymphoid cell infiltration (Figure 3). The same genes were up-regulated in a significant proportion of subcutaneous melanoma metastases suggesting that a strong and active infiltrate of immune cells is present in these tissues. In fact, most of the genes included in cluster e were significantly up-regulated in 10 cutaneous/subcutaneous melanoma lesions compared to 70 primary cancers of other histology (Table III shows a selection of the most significantly up-regulated genes in cutaneous/subcutaneous melanomas). Of interest is the observation that several of the genes up-regulated in these lesions are clustered in specific chromosomal locations with a high predominance of genes located in position 6p21.3, 11p11.2, 19p13 and 19q13. Among the immunologically-related genes specifically up-regulated in subcutaneous melanoma metastases, some are of particular interest because of their known relationship with effector T cell function. In particular, we find interesting that NK4, an anti-angiogenic factor released by natural killer cells [26], was constitutively expressed by cutaneous melanomas. We found this gene to be associated with regression of a melanoma metastasis during interleukin-2 therapy [27] and to be one of the genes most frequently up-regulated during activation of antigen-specific T cells in vitro [28, 29]. Of interest was also the constitutive expression of CD27 a co-stimulatory member of the TNF receptor family strongly associated with cell activation [30, 31]. The expression of CX3CR1 a gene constitutively expressed by natural killer cells that makes them sensitive to chemo-attraction by CXCL12 and CXC3L1 [32] may be an explanation for a preferential localization of these effector cells in melanoma lesions. In particular, this finding suggests that the microenvironment of melanoma metastases is rich of fractalkine (CX3CL1) which is a potent chemo-attractant released by endothelial cells stimulated by interferons [33]. Overall, the presence of these and other (KLRG1; killer cell lectin like receptor subfamily G, member 1 and KLRK1; killer cell lectin like receptor subfamily K, member 1 and the interleukin-21 receptor) natural killer cell-related genes suggests potent chemo-attraction toward natural killer cells by the tumor micro-environment of subcutaneous and cutaneous melanoma metastases. This is also emphasized by the high expression of interleukin-21 receptor which is usually expressed by natural killer cells and stimulates their cytolytic activity upon ligation with interleukin-21 produced by activated T cells [34, 35]. The constitutive expression of interferon regulatory factor (IRF)-7 implicated in the amplification of the innate immune response [36] through interactions with the NF-κB pathway [37] may lead to the activation of various types of type I interferons [38]. More puzzling is the constitutive expression of interleukin-16, a pleiotropic cytokine with predominant chemo-attractant activity for CD4+ T cells [39] and CD4+ eosinophils [40]; a relationship between this cytokine and melanoma metastases has never been observed before. In summary, the immunological signature portrayed by subcutaneous melanoma metastases is that of an active innate immune response centered on natural killer cells. More broadly, the preferential expression of genes with immune function in melanoma lesions compared with other tumors suggests that this cancer is constitutively immunologically active and this status may predispose metastatic melanoma to respond to general or antigen-specific immune manipulation.
A second and smaller group of immunologically-related genes was identified and included genes that segregated separately in clusters f to j. These genes had an expression profile opposite to the immune-related genes seen included in cluster e and appeared over-expressed in subcutaneous compared to lymph node metastases. Two granzyme-related genes were found strongly up-regulated in cluster e including granzyme A and M. This observation contrasted with the increased expression of cathepsin F and L in cluster f to j suggesting an opposite regulation of these genes involved in cell death or survival.
Subcutaneous metastases-associated genes
It has been reported that subcutaneous metastases of melanoma are more responsive to immunotherapy with interleukin-2 than lymph nodal and visceral metastases [16]. Therefore, we identified genes differentially expressed in the former compared with the latter. Since no material from visceral metastases was available, we limited the comparison to subcutaneous versus lymph nodal metastases. Overall, clusters g - j appeared to demonstrate a preferential expression of genes in subcutaneous metastases independent of the technique used for biopsy (excision versus FNA). In particular, cluster g contained a small node of 47 clones highly expressed in subcutaneous metastases that included PRAME and TRP-1. This cluster also included the renal tumor antigen RAGE which has been previously shown to be highly expressed by melanomas [41] and melanophilin and the s100 protein often associated with clinical parameters in melanoma [42]. Interestingly, closely linked to PRAME was the pattern of expression of the serine/threonine-specific protein kinase B-RAF. This gene is mutated in approximately 70 % of melanomas and it is often over-expressed [43]. Although several of these genes had been associated with melanoma their co-ordinate expression has never been previously appreciated. Overall, the identity of the genes over-expressed in subcutaneous metastases did not offer an obvious explanation for the increased immune responsiveness of these lesions and more extensive understanding of their relationship will be necessary in the future.
Genes differential expressed by melanoma and RCC samples compared with other solid tumors
We then pooled together melanoma and RCC samples data to identify genes commonly expressed by these tumors and not by tumors of other histology. Significance was assessed by a two-tailed unpaired student's t test and identified 4,221 genes at a cut off p2-value of ≤ 0.001. The data set was then filtered using the Cluster Program (Stanford, CA) selecting genes that were expressed in at least 80 % of the experiments and for which a Cy5/Cy3 log2 ratio ≥ 2 was present in at least one experiment. Two-thousand eight hundred and forty-three genes resulted from this filter. Because of the predominance of melanoma lesions (69 melanoma lesions compared to 14 RCC) the genes identified strongly represent differences between melanomas and other tumors. Therefore, we identified among them those that were not differentially expressed between melanoma and RCC samples to identify those genes that are truly uniquely expressed by the two immune responsive cancers. Two-thousand three hundred and fifty-eight genes were expressed similarly between the two types of cancer (at a t test p2-value > 0.05). A significant number of genes commonly expressed by melanoma and RCC and not by other tumors had no known function (681 genes). The remaining 1, 677 genes were further analyzed by separating those up-regulated from those down-regulated in melanoma and RCC compared with other tumors. The genes up-regulated in RCC and melanoma were considered those with a median LogRatio above 0.3 in either RCC or melanomas (a selection of these genes is shown in Table IV). This analysis selected 199 genes. A proportion of genes appeared to be specifically related to lymph nodal and immune infiltration as they were particularly up-regulated in melanoma metastases to lymph nodes and in lymph node metastases obtained from patients with CRC (orange vertical bar, Figure 4). These genes included annotations related to immunological function. A set of genes was specifically expressed in cutaneous metastases of melanoma and in RCC and not other tumor samples (dark blue vertical bar, Figure 4). These genes included microphtalmia transcription factor MITF [17, 44]. that has been shown previously to exert a central role in the regulation of transcriptional activity of melanoma cells. Similarly, enolase-2 (previously known to be up-regulated in RCC) [11] was found to be over-expressed in common between the two histologies. This is somewhat surprising since immunohistochemical analysis has used lack of staining for enolase-2 as a reliable method to differentiate malignant melanoma (enolase-2 negative) from Merkel cell carcinoma [45]. It is possible, that although identifiable at the transcriptional level, enolase-2 is not processed into a protein in melanomas. On the other hand, enolase-2 has been shown to be expressed in approximately 90 % of canine oral melanomas [46]. In addition, the macrophage migration inhibiting factor (MIF) which is a modulator of cell cycle progression and angiogenesis in melanoma [47, 48]. was found co-expressed by melanoma and RCC lesions. MIF has modulatory properties on natural killer cell mediated lysis of cancer cells contributing, therefore, to an immune privileged microenvironment in uveal melanoma [49]. Two genes coding for adhesion molecules; L1 cell adhesion molecule (LCAM) and melanoma cell adhesion molecule (MCAM) were also up-regulated in both lesions and may play an important role in mediating migration of immune cells to the tumor deposits [50]. Finally, it is remarkable that serologically defined colon cancer antigen 8 was specifically expressed by melanoma and RCC while was completely absent in colon cancers underlying the need for a better nomenclature of newly identified genes.
This is a descriptive study where genes specifically expressed by melanoma metastases were identified comparing a large collection of samples from patients with metastatic cutaneous melanoma with other primary tumors and lymph nodal metastases. A limitation of the study is the lack of other samples including visceral metastases of melanoma and metastases from tumors of other histology. Nevertheless, we considered useful to compile a list of genes characteristically expressed by subcutaneous and lymph nodal lesions of melanoma for reference purposes and we are willing to provide full information about these genes upon request. In spite of the limitations of this study, few general conclusions could be drawn.
Materials and Methods
Tissue procurement
Fourteen primary renal cell carcinoma (RCC) specimens were collected at the Department of Urology of The Johannes Gutenberg-University, Mainz, Germany; one primary melanoma, three in transit metastases, seven cutaneous metastases, thirty-five lymph nodal metastases and two visceral metastases of cutaneous melanoma were collected at the Department of Surgical Sciences, University of Padua, Italy; twenty-one fine needle aspirates of cutaneous melanoma metastases were obtained at the Surgery Branch, National Cancer Institute, National Institutes of Health, Bethesda, MD; seventeen primary epithelial ovarian cancer (EOC) specimens were obtained at the Department of Gynecologic Oncology, MD Anderson Cancer Center, TX; three primary sarcoma, one primary endometrial cancer, one primary laryngeal cancer, two primary breast cancers and one primary colon adeno-carcinoma were obtained from the Tissue Network (Philadelphia, PA); twelve primary carcinomas of the esophageal junction were obtained from the NCI (Division of Cancer Treatment and Diagnosis); thirty-five primary, 16 lymph node metastases and one hepatic metastasis from colorectal adeno-carcinomas were obtained from the Department of Pathology of the University of Pisa, Italy. Specimens were collected as the result of routine operative procedures and portions were frozen for subsequent analysis while the remnant tissue was used for pathological confirmation. Tissue procurement followed standard ethical procedure according to institutional policy. A summary of the specimens studied is presented in Table 1 with their order reflecting their distribution in figures where supervised analyses are shown.
RNA preparation, amplification and labeling
Total RNA was extracted from frozen material using Trizol reagent according to manufacturer's instructions (Invitrogen, CA) and amplified into anti-sense RNA (aRNA) as previously described [10, 27, 51, 52]. Although the quantity of starting total RNA was in most cases sufficient for cDNA array hybridization, we have shown repeatedly that the fidelity of aRNA hybridization is at least equal and likely superior to total RNA for transcriptional profiling due to lack of contaminant ribosomal and transfer RNA [51, 53]. Therefore, we used aRNA to increase consistency of results particularly when low quality total RNA was documented by Agilent Bioanalyzer 2000 (Agilent Technologies, Palo Alto, CA). After amplification the quality of aRNA was tested with the Agilent Bioanalyzer as previously described [52].
Total RNA from peripheral blood mononuclear cells pooled from six normal donor was extracted and amplified to serve as constant reference as previously described [10, 27, 51, 52]. Test and reference RNA were labeled with Cy5 (red) and Cy3 (green) and co-hybridized to a costum-made17.5 K cDNA micro-array http://nciarray.nci.nih.gov/gal_files/index.shtml. Micro-arrays were printed at the Immunogenetics Section, DTM, CC, NIH with a configuration of 32 × 24 × 23 and contained 17,500 elements. Clones used for printing included a combination of the Research Genetics RG_HsKG_031901 8 k clone set and 9,000 clones selected from the RG_Hs_seq_ver_070700 40 k clone set. The 17,500 spots included 12,072 uniquely named genes, 875 duplicated genes and about 4,000 expression sequence tags.
Data analysis
All statistical analyses were performed using the log2-based ratios normalizing the medial log2 ratio value across the array equal to zero. Validation and reproducibility were performed using our internal reference concordance system as previously described [54]. Unsupervised clustering was performed according to the Eisen's Pearson correlation method [55] and visualized with Tree-View software (Stanford University, CA). Genomic portraits were depicted according to the central method for display using a normalization factor as suggested by Ross et al. [56]. Details about different tests are discussed in the respective results section. Identification of tumor-specific genes was performed using un-paired 2-tailed Student's t test. The same analyses were performed using un-paired Wilcoxon's non-parametric assessment and provided the same conclusions (not shown). Details of each analysis are presented in the results section.
References
Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, Abrams J, Sznol M, Parkinson D, Hawkins M, Paradise C, Kunkel L, Rosenberg SA: High-dose recombinant interleukin-2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1998, 17: 2105-2116.
Atkins MB, Sparano J, Fisher RI, Weiss GR, Margolin KA, Fink KI, Rubinstein L, Louie A, Mier JW, Gucalp R: Randomized phase II trial of high-dose interleukin-2 either alone or in combination with interferon alfa-2b in advanced renal cell carcinoma. J Clin Oncol. 1993, 11: 661-670.
Wang E, Marincola FM: cDNA microarrays and the enigma of melanoma immune responsiveness. Cancer J Sci Am. 2001, 7: 16-23.
Kawakami Y, Zakut R, Topalian SL, Stotter H, Rosenberg SA: Shared human melanoma antigens. Recognition by tumor-infiltrating lymphocytes in HLA-A2.1-transfected melanomas. J Immunol. 1992, 148: 638-643.
Kawakami Y, Rosenberg SA: T-cell recognition of self peptides as tumor rejection antigens. Immunol Res. 1996, 15: 179-190.
Kawakami Y: Identification of human tumor antigens recognized by T-cells and their use for immunotherapy. Int J Hematol. 2003, 77: 427-434.
Paschen A, Eichmuller S, Schadendorf D: Identification of tumor antigens and T-cell epitopes, and its clinical application. Cancer Immunol Immunother. 2004, 53: 196-203. 10.1007/s00262-003-0479-3.
Yang JC, Topalian SL, Parkinson D, Schwartzentruber DJ, Weber JS, Ettinghausen SE, White DE, Steinberg SM, Cole DJ, Kim HI: A randomized comparison of high dose and low dose intravenous interleukin-2 for the treatment of metastatic renal cell carcinoma. J Clin Oncol. 1994, 12: 1572-6.
Marincola FM, Wang E, Herlyn M, Seliger B, Ferrone S: Tumors as elusive targets of T cell-directed immunotherapy. Trends Immunol. 2003, 24: 334-341. 10.1016/S1471-4906(03)00116-9.
Wang E, Miller LD, Ohnmacht GA, Mocellin S, Petersen D, Zhao Y, Simon R, Powell JI, Asaki E, Alexander HR, Duray PH, Herlyn M, Restifo NP, Liu ET, Rosenberg SA, Marincola FM: Prospective molecular profiling of subcutaneous melanoma metastases suggests classifiers of immune responsiveness. Cancer Res. 2002, 62: 3581-3586.
Wang E, Lichtenfels R, Bukur J, Ngalame Y, Panelli MC, Seliger B, Marincola FM: Ontogeny and oncogenesis balance the transcriptional profile of renal cell cancer. Cancer Res. 2004,
Takashi M, Sakata T, Inaguma Y, Kato K: Elevated concentrations of gamma-enolase in renal cell tumors in rats: similarity to renal cell carcinoma in man. Urol Res. 1996, 24: 375-379. 10.1007/BF00389796.
Yaman O, Baltaci S, Arikan N, Ozdiler E, Gogus O, Muftuoglu YZ: Serum neuron specific enolase: can it be a tumour marker for renal cell carcinoma?. Int Urol Nephrol. 1996, 28: 207-210.
Gopalkrishnan RV, Sauane M, Fisher PB: Cytokine and tumor cell apoptosis inducing activity of mda-7/IL-24. Int Immunopharmacol. 2004, 4: 635-647. 10.1016/j.intimp.2004.01.015.
Quast T, Wehner S, Kirfel G, Jaeger K, De Luca M, Herzog V: sAPP as a regulator of dendrite motility and melanin release in epidermal melanocytes and melanoma cells. FASEB J. 2003, 17: 1739-1741.
Phan GQ, Attia P, Steinberg SM, White DE, Rosenberg SA: Factors associated with response to high-dose interleukin-2 in patients with metastatic melanoma. J Clin Oncol. 2001, 19: 3477-3482.
Powell DJ, Rosenberg SA: Phenotypic and functional maturation of tumor antigen-reactive CD8+ T lymphocytes in patients undergoing multiple course peptide vaccination. J Immunother. 2004, 27: 36-47. 10.1097/00002371-200401000-00004.
Weeraratna AT, Becker D, Carr KM, Duray PH, Rosenblatt KP, Yang S, Chen Y, Bittner M, Strausberg RL, Riggins GJ, Wagner U, Kallioniemi OP, Trent JM, Morin PJ, Meltzer PS: Generation and analysis of melanoma SAGE libraries: SAGE advice on the melanoma transcriptome. Oncogene. 2004, 23: 2264-2274. 10.1038/sj.onc.1207337.
Boon K, Edwards JB, Siu IM, Olschner D, Eberhart CG, Marra MA, Strausberg RL, Riggins GJ: Comparison of medulloblastoma and normal neural transcriptomes identifies a restricted set of activated genes. Oncogene. 2003, 22: 7687-7694. 10.1038/sj.onc.1207043.
Oberthuer A, Hero B, Spitz R, Berthold F, Fischer M: The tumor-associated antigen PRAME is universally expressed in high-stage neuroblastoma and associated with poor outcome. Clin Cancer Res. 2004, 10: 4307-4313.
Marincola FM, Hijazi YM, Fetsch P, Salgaller ML, Rivoltini L, Cormier J, Simonis TB, Duray PH, Herlyn M, Kawakami Y, Rosenberg SA: Analysis of expression of the melanoma associated antigens MART-1 and gp100 in metastatic melanoma cell lines and in in situ lesions. J Immunother. 1996, 19: 192-205.
Cormier JN, Panelli MC, Hackett JA, Bettinotti MP, Mixon A, Wunderlich J, Parker LL, Restifo NP, Ferrone S, Marincola FM: Natural variation of the expression of HLA and endogenous antigen modulates CTL recognition in an in vitro melanoma model. Int J Cancer. 1999, 80: 781-790. 10.1002/(SICI)1097-0215(19990301)80:5<781::AID-IJC24>3.0.CO;2-A.
Cormier JN, Hijazi YM, Abati A, Fetsch P, Bettinotti M, Steinberg SM, Rosenberg SA, Marincola FM: Heterogeneous expression of melanoma-associated antigens (MAA) and HLA-A2 in metastatic melanoma in vivo. Int J Cancer. 1998, 75: 517-524. 10.1002/(SICI)1097-0215(19980209)75:4<517::AID-IJC5>3.3.CO;2-M.
Cormier JN, Abati A, Fetsch P, Hijazi YM, Rosenberg SA, Marincola FM, Topalian SL: Comparative analysis of the in vivo expression of tyrosinase, MART-1/Melan-A, and gp100 in metastatic melanoma lesions: implications for immunotherapy. J Immunother. 1998, 21: 27-31.
Ohnmacht GA, Wang E, Mocellin S, Abati A, Filie A, Fetsch P, Riker AI, Kammula US, Rosenberg SA, Marincola FM: Short term kinetics of tumor antigen expression in response to vaccination. J Immunol. 2001, 167: 1809-1820.
Matsumoto K, Nakamura T: NK4 (HGF-antagonist/angiogenesis inhibitor) in cancer biology and therapeutics. Cancer Sci. 2003, 94: 321-327.
Panelli MC, Wang E, Phan G, Puhlmann M, Miller L, Ohnmacht GA, Klein HG, Marincola FM: Genetic profiling of peripharal mononuclear cells and melanoma metastases in response to systemic interleukin-2 administration. Genome Biol. 2002, 3: RESEARCH0035-10.1186/gb-2002-3-7-research0035.
Monsurro' V, Wang E, Panelli MC, Nagorsen D, Jin P, Katia Z, Smith K, Ngalame Y, Even J, Marincola FM: Active-specific immunization against cancer: is the problem at the receiving end?. Sem Cancer Biol. 2003, 13: 473-480. 10.1016/j.semcancer.2003.09.011.
Monsurro VV, Wang E, Yamano Y, Migueles SA, Panelli MC, Smith K, Nagorsen D, Connors M, Jacobson S, Marincola FM: Quiescent phenotype of tumor-specific CD8+ T cells following immunization. Blood. 2004, 104: 1970-1978. 10.1182/blood-2004-02-0525.
Zheng T, Zhu Z, Wang Z, Homer RJ, Ma B, Riese RJ, Chapman HA, Shapiro SD, Elias JA: Inducible targeting of IL-13 to the adult lung causes matrix metalloproteinase- and cathepsin-dependent emphysema. J Clin Invest. 2000, 106: 1081-1093.
Croft M: Costimulation of T cells by OX40, 4-1BB, and CD27. Cytokine Growth Factor Rev. 2003, 14: 265-273. 10.1016/S1359-6101(03)00025-X.
Robertson MJ: Role of chemokines in the biology of natural killer cells. J Leukoc Biol. 2002, 71: 173-183.
Umehara H, Bloom ET, Okazaki T, Nagano Y, Yoshie O, Imai T: Fractalkine in vascular biology: from basic research to clinical disease. Arterioscler Thromb Vasc Biol. 2004, 24: 34-40. 10.1161/01.ATV.0000095360.62479.1F.
Collins M, Whitters MJ, Young DA: IL-21 and IL-21 receptor: a new cytokine pathway modulates innate and adaptive immunity. Immunol Res. 2003, 28: 131-140. 10.1385/IR:28:2:131.
Parrish-Novak J, Foster DC, Holly RD, Clegg CH: Interleukin-21 and the IL-21 receptor: novel effectors of NK and T cell responses. J Leukoc Biol. 2002, 72: 856-863.
Zhang L, Pagano JS: Structure and function of IRF-7. J Interferon Cytokine Res. 2002, 22: 95-101. 10.1089/107999002753452700.
Hiscott J, Grandvaux N, Sharma S, Tenoever BR, Servant MJ, Lin R: Convergence of the NF-kappaB and interferon signaling pathways in the regulation of antiviral defense and apoptosis. Ann N Y Acad Sci. 2003, 1010: 237-248. 10.1196/annals.1299.042.
Levy DE, Marie I, Smith E, Prakash A: Enhancement and diversification of IFN induction by IRF-7-mediated positive feedback. J Interferon Cytokine Res. 2002, 22: 87-93. 10.1089/107999002753452692.
Conti P, Kempuraj D, Kandere K, Di Gioacchino M, Reale M, Barbacane RC, Castellani ML, Mortari U, Boucher W, Letourneau R, Theoharides TC: Interleukin-16 network in inflammation and allergy. Allergy Asthma Proc. 2002, 23: 103-108.
Okubo Y, Tsukadaira A, Takashi S, Kubo K, Koyama S: Chemotaxis of human CD4+ eosinophils. Int Arch Allergy Immunol. 2001, 125 (Suppl 1): 19-21. 10.1159/000053847.
Eichmuller S, Usener D, Jochim A, Schadendorf D: mRNA expression of tumor-associated antigens in melanoma tissues and cell lines. Exp Dermatol. 2002, 11: 292-301. 10.1034/j.1600-0625.2002.110402.x.
Harpio R, Einarsson R: S100 proteins as cancer biomarkers with focus on S100B in malignant melanoma. Clin Biochem. 2004, 37: 512-518. 10.1016/j.clinbiochem.2004.05.012.
Karasarides M, Chiloeches A, Hayward R, Niculescu-Duvaz D, Scanlon I, Friedlos F, Ogilvie L, Hedley D, Martin J, Marshall CJ, Springer CJ, Marais R: B-RAF is a therapeutic target in melanoma. Oncogene. 2004, 23: 6292-6298. 10.1038/sj.onc.1207785.
Steingrimsson E, Copeland NG, Jenkins NA: Melanocytes and the Microphthalmia Transcription Factor Network. Annu Rev Genet. 2004
Kontochristopoulos GJ, Stavropoulos PG, Krasagakis K, Goerdt S, Zouboulis CC: Differentiation between merkel cell carcinoma and malignant melanoma: An immunohistochemical study. Dermatology. 2000, 201: 123-126. 10.1159/000018454.
Ramos-Vara JA, Beissenherz ME, Miller MA, Johnson GC, Pace LW, Fard A, Kottler SJ: Retrospective study of 338 canine oral melanomas with clinical, histologic, and immunohistochemical review of 129 cases. Vet Pathol. 2000, 37: 597-608. 10.1354/vp.37-6-597.
Shimizu T, Abe R, Nakamura H, Ohkawara A, Suzuki M, Nishihira J: High expression of macrophage migration inhibitory factor in human melanoma cells and its role in tumor cell growth and angiogenesis. Biochem Biophys Res Commun. 1999, 264: 751-758. 10.1006/bbrc.1999.1584.
Rumpler G, Becker B, Hafner C, McClelland M, Stolz W, Landthaler M, Schmitt R, Bosserhoff A, Vogt T: Identification of differentially expressed genes in models of melanoma progression by cDNA array analysis: SPARC, MIF and a novel cathepsin protease characterize aggressive phenotypes. Exp Dermatol. 2003, 12: 761-771. 10.1111/j.0906-6705.2003.00082.x.
Repp AC, Mayhew ES, Apte S, Niederkorn JY: Human uveal melanoma cells produce macrophage migration-inhibitory factor to prevent lysis by NK cells. J Immunol. 2000, 165: 710-715.
Nyormoi O, Bar-Eli M: Transcriptional regulation of metastasis-related genes in human melanoma. Clin Exp Metastasis. 2003, 20: 251-263. 10.1023/A:1022991302172.
Wang E, Miller L, Ohnmacht GA, Liu E, Marincola FM: High fidelity mRNA amplification for gene profiling using cDNA microarrays. Nature Biotech. 2000, 17: 457-459.
Wang E, Marincola FM: Amplification of small quantities of mRNA for transcript analysis. In DNA arrays – A Molecular Cloning Manual. Edited by: Bowtell D, Sambrook J. 2002, Cold Springs Harbor, NY: Cold Spring Harbor Laboratory Press, 204-213.
Feldman AL, Costouros NG, Wang E, Qian M, Marincola FM, Alexander HR, Libutti SK: Advantages of mRNA amplification for microarray analysis. Biotechniques. 2002, 33: 906-914.
Jin P, Zhao Y, Ngalame Y, Panelli MC, Nagorsen D, Monsurro V, Smith K, Hu N, Su H, Taylor PR, Marincola FM, Wang E: Selection and validation of endogenous reference genes using a high throughput approach. BMC Genomics. 2004, 13: 55-10.1186/1471-2164-5-55.
Eisen MB, Spellman PT, Brown PO, Botstein D: Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci U S A. 1998, 95: 14863-14868. 10.1073/pnas.95.25.14863.
Ross DT, Scherf U, Eisen MB, Perou CM, Rees C, Spellman P, Iyer V, Jeffrey SS, Van de Rijn M, Waltham M, Pergamenschikov A, Lee JC, Lashkari D, Shalon D, Myers TG, Weinstein JN, Botstein D, Brown PO: Systematic variation in gene expression patterns in human cancer cell lines. Nature Genetics. 2000, 24: 227-235. 10.1038/73432.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
12967_2004_48_MOESM1_ESM.XLS
Additional file 1: AVE Ratio = average Log2 CY5/Cy3 ratio between test and reference sample. The t test p2-value refers to a two-tailed unpaired analysis between the samples mentioned below. RCC = renal cell cancer; MEL = melanoma; Other = tumors other than RCC and melanoma. (XLS 1 MB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Wang, E., Panelli, M.C., Zavaglia, K. et al. Melanoma-restricted genes. J Transl Med 2, 34 (2004). https://doi.org/10.1186/1479-5876-2-34
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1479-5876-2-34