Abstract
Background
Chronic hepatitis C affects approximately 170 million people worldwide, and thus being one of the main causes of chronic liver disease. About 20% of patients with chronic hepatitis C will develop cirrhosis over 20 years, and present an increased risk of developing hepatic complications. Sustained virological response (SVR) is associated with a better prognosis compared to untreated patients and treatment failures.
The objective of this analysis was to compare treatment costs and outcomes of pegylated interferon-alfa-2a versus pegylated interferon-alfa-2b, both associated with ribavirin, in the therapeutic scheme of 24 weeks and 48 week for hepatitis C genotypes 2/3 and genotype 1, respectively, under the Brazilian Public Health System (SUS) scenario.
Methods
To project disease progression, a Markov model was built based on clinical stages of chronic disease. A Delphi panel was conducted to evaluate medical resources related to each stage, followed by costing of related materials, services, procedures and pharmaceutical products. The evaluation was made from a public payer perspective. The source used for costing was government reimbursement procedures list (SAI/SIH–SUS). Drug acquisition costs were obtained from the Brazilian Official Gazette and “Banco de Preços em Saúde” (government official source). It was assumed a mean patient weight of 70 kg. Costs were reported in 2011 Brazilian Reais (US$1 ≈ $Brz1.80). A systematic review followed by a meta-analysis of the 7 identified randomized controlled trials (RCTs) which compared pegylated interferons, was conducted for obtaining relative efficacy of both drugs: for genotype 2/3, mean rate of SVR was 79.2% for peginterferon-alfa-2a and 73.8% for peginterferon-alfa-2b. For genotype 1, SVR mean rate was 42.09% versus 33.44% (peginterferon-alfa-2a and peginterferon-alfa-2b respectively). Time horizon considered was lifetime. Discount rate for costs and outcomes was 5%, according to Brazilian guidelines for Health Technology Assessment (HTA).
Results
Analysis showed that peginterferon-alfa-2a is a dominant therapy compared to peginterferon-alfa-2b for genotype 1 ($Brz 4,345 savings and 0.10 LY/0.25 QALY gains) as well for genotype 2/3 ($Brz 8,001 savings and 0.16 LY/0.39 QALY gains). Projections indicated that for each 1000 patients treated with peginterferon-alfa-2a instead of peginterferon-alfa-2b, the amount of resources saved would be of $Brz 4.3 million for genotypes 2/3 and up to $Brz 8 million for genotype 1.
Conclusion
These findings suggest that treatment with peginterferon-alfa-2a is more effective and less costly when compared to peginterferon-alfa-2b under SUS perspective in Brazil.
Similar content being viewed by others
Background
Hepatitis C virus (HCV) identification is relatively recent (1989) [1] and many efforts were made in the last years to optimize its pharmacological treatment and disease detection rates.
HCV infection becomes chronic in approximately 75%–85% of cases, significantly raising healthcare costs, especially those resulting from hospital bills. According to Wong et al., infection with hepatitis C virus is the leading cause in approximately 30% of liver transplantation, 40% of cases of decompensated cirrhosis and 60% of hepatocellular carcinoma [2]. Sustained virological response (SVR) in chronic hepatitis C is associated with better prognosis, since observed reduction in clinical events, mainly liver failure is observed when SVR is achieved [3].
In 2010, Brazilian Ministry of Health published information on anti-HCV (antibody to the hepatitis C virus, its presence indicates an active or chronic hepatitis C infection) prevalence in Brazilian capital cities; estimated anti-HCV prevalence was 2.1% in North Region, 0.7% in Northeast Region, 1.3% in Central-West Region, 1.3% in Southeast Region and 1.2% in South Region [4]. A total of 60,908 cases of hepatitis C were confirmed and registered in Brazil from 1999 to 2009 according to 2010 Viral Hepatitis Epidemiological Bulletin [5].
According to Brazilian Ministry of Health, in 2011, expenses with Hepatitis C achieved around $Brz 17.7 million [6]. Moreover, disease’s chronic nature and its successive stages, such as cirrhosis, hepatocellular carcinoma or liver transplantation and negative issues produced by disease, such as absenteeism, early retirement, loss of productivity, aggravate social and economic burden related to HCV infection. In this scenario, the adoption of cost-effective strategies would beneficiate health policy makers, society and patients.
In the Public Healthcare System of Brazil (SUS), according to the therapeutic guidelines and clinical protocols for hepatitis C [7], patients with genotype 1 aged from 18 to 70 years old, with platelet counts > 90,000/mm3 (non cirrhotic) or > 75,000/mm3 (cirrhotic) after qualitative PCR (Polymerase Chain Reaction), should be treated with pegylated interferon alfa-2a or 2b weekly and ribavirin 15 mg/kg daily for 48-72-week treatment period. Patients with genotypes 2 or 3, in the absence of low SVR predictors – METAVIR score ≥ F3 or clinical manifestations of cirrhotic fibrosis or viral load > 600,000 UI/mL –, should be treated with conventional interferon alfa-2a or 2b, 3 times a week and ribavirin 15 mg/kg daily for 24-week treatment period; in the presence of low SVR predictors, patients should be treated with pegylated interferon alfa-2a or 2b weekly and ribavirin 15 mg/kg daily for 24-48-week treatment period. The guideline doesn’t mention any differences between efficacies of both pegylated interferons. However, literature has been discussing the clinical superiority of one of them, with the tendency to peginterferon-alfa-2a [8–13]. Economic assessment comparing peginterferon-alfa-2a and peginterferon-alfa-2b in Chronic Hepatitis C indicates that the first one was considered cost-effective or even dominant in several countries like: USA, UK, Spain, Poland and Mexico [14–20].
The objective of this analysis was to assess the cost-effectiveness ratio of peginterferon-alfa-2a versus peginterferon-alfa-2b both plus ribavirin (RBV), in a 24-week therapeutic schedule in the treatment of patients with chronic hepatitis C, genotypes 2 and 3 or 48-week schedule for genotype 1, from the perspective of the Public Health Care System in Brazil. In order to obtain efficacy data for the analysis, our aim was also to conduct a systematic review of RCTs and perform a meta-analysis of the results.
Methods
We used a Markov model to simulate the progression of chronic hepatitis C in a hypothetical cohort of patients with initial age of 45 years old presenting antibodies against hepatitis C virus (anti-HCV) or HCV RNA positivity in individuals known to be previously negative. The model predicted long-term clinical benefits (Life Years - LYs and Quality Adjusted Life Years - QALYs) and associated costs with each intervention, providing the incremental cost-effectiveness ratio (ICER) of peginterferon-alfa-2a versus peginterferon-alfa-2b, both associated with ribavirin. A one-way sensitivity analysis was also conducted in order to assess the impact of uncertainty on the cost-effectiveness ratio.
Model description
A Markov model was used as it allows representing the relevant stages of the natural history of the disease over time, as well as estimates for probability of progression between the several health states related to the chronic infection with hepatitis C virus.
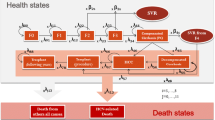
Each scenario was comprised by 7 mutually excluding clinical stages, each of which was defined by a patient’s clinical condition: sustained virological response, chronic hepatitis C, compensated cirrhosis, decompensated cirrhosis, hepatocellular carcinoma, liver transplantation and death, enabling the comparison of final outcomes between therapeutic alternatives.
Associated with each of the clinical stages, quality-of-life values and costs were attributed. Costs associated to Markov stages intended to reflect local medical practice, and resource utilization was determined by a Delphi panel. The panel included prescribing professionals from the public healthcare system. All costs and outcomes were discounted at a 5% rate, according to the Brazilian Guidelines for Health Technology Assessment (HTA) [21].
Diagram of transition between stages
Following, we present diagrams of transition for treatment of chronic infection with hepatitis C virus in mutually excluding stages (Figure 1). Consequently, each patient will correspond to one of the stages at a given time.
The model was similar to others described in literature [22–24]. Patients start at the chronic hepatitis C stage, undergo a 24-weeks treatment with peginterferon-alfa-2a plus ribavirin or peginterferon-alfa-2b plus ribavirin if genotypes 2/3 and 48-weeks treatment with the same drugs if genotype 1. No extension on treatment schedule was considered.
The time horizon of the analysis accounted for patient’s lifetime and was divided into equal increments of time, known as Markov cycles. Each cycle comprises one year and defines possible pathway for each patient in the model based on the natural history of the infection with hepatitis C virus. The probability for a patient to migrate between two stages during a cycle is called probability of transition between stages. Following 24 or 48 weeks of treatment, patients may show positive response to treatment or recurrence every year.
After the treatment cycle, patients can either respond to treatment, which means moving to sustained virological response stage, remain at the chronic stage or progress to compensated cirrhosis. When at the compensated cirrhosis, patients can remain on the stage or progress either to hepatocellular carcinoma or decompensated cirrhosis. Patients who develop decompensated cirrhosis could remain on the stage, develop hepatocellular carcinoma, receive a liver transplantation or have an increased risk disease-related mortality. Finally, patients at the liver transplantation stage or hepatocellular carcinoma can remain on the stage or die.
Transition probabilities
We reviewed clinical and pharmacoeconomic literature aiming to identify studies reporting probability of clinical events which were relevant for the model. These data are presented in the Table 1[25–34].
The transition probabilities were employed as model parameters for each of the stages of chronic hepatitis C, compensated cirrhosis, decompensated cirrhosis, hepatocellular carcinoma, liver transplantation, post-transplantation and death. All patients had their risk of death adjusted by the Brazilian Agency for Geography and Statistics (IBGE) life table, which provides mortality rates for the Brazilian population [35].
Quality of life
Relevant data concerning quality-of-life values (utilities) were extracted from the international literature, due to the lack of local data [24, 29, 36]. The utilities weights assigned for each stage were: 0.90 for sustained virological response, 0.82 for chronic hepatitis C, 0.78 for compensated cirrhosis, 0.65 for decompensated cirrhosis, 0.25 for hepatocellular carcinoma, 0.5 for liver transplant at the 1st year, 0.7 for the subsequent years after liver transplant and finally 0 for death.
Cost data
This study was performed using a public payer’s. Annual medical resources related to each clinical stage of chronic hepatitis C were collected through a Delphi panel, which included experts in infectious diseases and hepatology with 10 to 24-year experience in medical practice. Source used for costing was government reimbursement procedures list (SAI/SIH–SUS) [37]. The aggregated annual costs of managing each clinical stage are shown in Table 2.
Since in Brazil peginterferon-alfa drugs are acquired in a centralized purchasing process, their acquisition costs were obtained from purchase invoices published in the Brazilian Official Gazette [38–40]. Ribavirin acquisition cost was taken from “Banco de Preços em Saúde” (BPS), the government official source. Peginterferon-alfa 2a is given at a dosage of 180 μg per week irrespectively of patient weight and has only one presentation. However, for peginterferon-alfa 2b, dosage is based on patient’s weight (1.5 μg/kg/week) and has three distinct forms of presentation with different prices per μg. As specified in the peginterferon-alfa 2b SPC (summary of product characteristics), we have considered that unused portion of drug was wasted. Therefore, it was assumed a mean patient weight of 70 kg (which means 105 μg of peginterferon-alfa 2b) and the weighted average per μg available at BPS was used. For ribavirin, recommended dosage is 15 mg/kg/day. Costs were reported in 2011 Brazilian Reais (US$1 ≈ $Brz1.80) (Table 3).
Efficacy data
Efficacy data for pegylated interferons, both combined with ribavirin, used in this cost-effectiveness study were derived from a meta-analysis conducted in order to assess relative treatment efficacy of peginterferon-alfa-2a compared to peginterferon-alfa-2b both plus ribavirin. Before carrying out the meta-analysis, a systematic review of literature was performed.
An extensive search through main medical information databases (MEDLINE, Cochrane Library, Embase and Lilacs) and specialized websites was conducted on the second trimester of 2010. We aim to identify RCTs which evaluated treatment efficacy of peginterferon-alfa-2a versus peginterferon-alfa-2b both plus ribavirin for HCV treatment in patients not co-infected with HIV (Table 4).
After removing duplicates, all studies tittles were screened and potentially relevant references were selected by two independent reviewers. Abstract and full text of the potential articles were also analyzed and the selection of studies was made. Included studies were those which evaluate treatment efficacy of peginterferon alfa-2a versus peginterferon alfa-2b both plus ribavirin for hepatitis C treatment in naïve patients or nonresponders to other therapies not co-infected with HIV (Table 5).
To summarize all information gathered in our systematic review a meta-analysis was performed and according to heterogeneity test, a fixed or a random-effect model was considered.
Sensitivity analysis
In order to assess the uncertainty of the results, a one-way sensitivity analysis was performed. The parameters peginterferon-alfa-2a cost, peginterferon-alfa-2b cost, state-transition probabilities; medical costs and utility values were varied over the range of 15% (up and down); patient weight was varied from 50 to 90 kg; SVR was varied according to lower and upper limit values obtained from meta-analysis and starting age was varied from 40 to 50 years old. Sensitivity analysis within same conditions was conducted for cost-effectiveness analysis of genotype 1 and genotype 2/3.
Results
Meta-analysis
We identified seven papers out of 623 found (databases citation without duplicates plus manual searches) which met our inclusion criteria described above: 1) Ascione, 2009 [41] 2) Rumi, 2009 [42] 3) McHutchison, 2009 [43] 4) Yenice, 2006b [44] 5) Scotto, 2008 [45] 6) Berak, 2007 [46] and 7) Kolakowska, 2008 [47]. Not all papers evaluated all patients’ genotypes, therefore, for genotype 2/3, four papers were included in the meta-analysis and for genotype 1, six papers were evaluated. All studies were prospective randomized controlled trials (Figure 2).
For genotypes 2/3 peginterferon-alfa-2a showed higher SVR as compared to peginterferon-alfa-2b: 79.2% versus 73.8% (RR = 1.11, IC 95% 1.01 – 1.22, assuming a fixed-effect model) (Figure 3); for genotype 1 chronic HCV infection patients, peginterferon-alfa-2a showed higher SVR as compared to standard dose of peginterferon-alfa-2b as well: 42.09% versus 33.44% (RR = 1.10, IC 95% 1.01 – 1.20, assuming a fixed-effect framework) (Figure 4). These findings suggest that peginterferon-alfa-2a is associated with a higher clinical response than peginterferon-alfa-2b.
Forest Plot of studies comparing the effect of treatment with peginterferon-alfa-2a vs standard-dose peginterferon-alfa-2b for genotype 1: Peginterferon-alfa-2a showed higher SVR as compared to standard dose of peginterferon-alfa-2b as well: 42.09% versus 33.44% (RR = 1.10, IC 95% 1.01 – 1.20, assuming a fixed-effect framework).
It is important to mention that only standard-dose peginterferon-alfa-2b data were included in the meta-analysis. Therefore, the effect of the low-dose: 1.0 μg/kg of peginterferon-alfa-2b, analyzed in McHutchison [43], 2009 was excluded from the analysis in order to avoid a possible negative impact over the results.
On the other hand, including peginterferon-alfa-2b lower dose appear not to influence the results. A recently published meta-analysis [8] (Awad, 2010) that did not exclude the effects of low-dose treatment showed similar results of efficacy of peginterferon-alfa-2a vs peginterferon-alfa-2b presented in this study. For genotype 1, peginterferon-alfa-2a showed higher sustained virological response when compared to peginterferon-alfa-2b: 42.1% versus 33.3% (RR = 1.11, IC 95% 1.02 – 1.20, assuming a fixed-effect model).
Cost-effectiveness analysis
Our model predicted the costs and outcomes of each alternative by combining the treatment expenses with the costs related to each future stage of the disease progression.
Genotypes 2/3
Overall cost for treatment with peginterferon-alfa-2a was $Brz 13,120, while for peginterferon-alfa-2b, total cost was $Brz 17,465. Treatment with peginterferon-alfa-2a resulted in 15.21 LYs and 14.57 QALYs, while treatment with peginterferon-alfa-2b resulted in 15.11 LYs and 14.32 QALYs. Clinical and economic results are presented in Table 6 for 24 weeks of treatment with peginterferon-alfa-2a or peginterferon-alfa-2b, both in association with a ribavirin.
In this setting, peginterferon-alfa-2a is a dominant therapy compared to peginterferon-alfa-2b, i.e. less costly ($Brz 4,345 savings) and more effective (0.10 LY and 0.25 QALY gains).
Genotype 1
Overall cost for the treatment with peginterferon alfa-2a was $Brz 31,185, while for peginterferon alfa-2b, total cost was $Brz 39,186. Peginterferon-alfa-2a therapy resulted in 14.51 LY and Clinical and economic result is presented in Table 7 for 48 weeks of treatment with peginterferon alfa-2a or peginterferon alfa-2b, both in association with a ribavirin.
Peginterferon-alfa-2a is a dominant therapy also for this genotype, with lower related costs ($Brz 8,001 savings) and higher effectiveness (0.16 LY and 0.39 QALY gains).
Sensitivity analysis
By observing the results from the one-way sensitivity analysis, in Tables 8 and 9, peginterferon-alfa-2a was a dominant therapy in all scenarios: the drug yielded gains in LYs and QALYs although incurred lower costs as opposed to peginterferon-alfa-2b. For genotype 2/3, variables with highest impact were patient weight, sustained virological response and utility values. For genotype 1, variables with highest impact were sustained patient weight, utility values and peginterferon-alfa-2b acquisition cost.
Even considering a hypothetical scenario for genotype 2/3 with 50 kg patients, where peginterferon-alfa-2a cost was simultaneously increased by 15% and peginterferon-alfa-2b cost was simultaneously decreased by 15%, peginterferon-alfa-2a would still be very cost-effective, resulting in a low incremental cost-effectiveness ratio of $Brz 894 per QALY.
Discussion
The results obtained from the meta-analysis are in line with other six recently published meta-analyses (Awad, 2010 [8]; Alavian, 2010 [9]; Zhao, 2010 [10]; Xiao, 2010 [11]; Singal, 2011 [12] and Cheinquer, 2010 [13]) which concluded that peginterferon-alfa-2a has a higher SVR as compared to peginterferon-alfa-2b, with similar safety profile.
This work applied a Markov model to represent the treatment course of the disease, as well as to estimate the cost-effectiveness ratio of the therapeutic schedule with peginterferon-alfa-2a associated with ribavirin, when compared to peginterferon-alfa-2b also associated with ribavirin, aiming to evaluate which drug would represent more advantages to the Brazilian public healthcare system.
The model evaluated 2 different treatment regimens according to patients genotype: patients diagnosed with genotypes 2 and 3 would be eligible to receive 24 weeks of treatment, while patients with genotype 1 would receive 48 weeks of treatment.
It is important to note that analysis presents limitations regarding the usage of data from other countries to adapt transition probabilities and evaluate quality of life. The lack of Brazilian data should be supplied with more studies on this field, which would enable more reliable and accurate local economic analysis.
The extrapolation of results obtained in the analysis revealed that therapy using peginterferon-alfa-2a presented favorable results relative to health life years – LYs and QALYs, being dominant compared to peginterferon-alfa-2b, for a 24-week or 48-week treatment period.
Based on our results, the projections indicate that for each 1000 patients treated with peginterferon-alfa-2a instead of peginterferon-alfa-2b, the amount of resources saved by the SUS can be of up to $Brz 4.3 million for genotypes 2/3 and up to $Brz 8 million for genotype 1, and that would allow the treatment of approximately 145 and 108 more patients, respectively.
Finally, it is important to stress that this study, which enhances the available information on health economics field in Brazil, intends to support medical and health-care professionals, by providing adequate information in decision-making on hepatitis C, a chronic disease which requires special attention to be provided to patients and to the management of resources used in its treatment. Specially, with the introduction of new protease inhibitor drugs, telaprevir and boceprevir, the triple therapy (association of telaprevir or boceprevir with pegylated interferon and ribavirin) has been emerging as standard of care for chronic hepatitis C. The introduction of those new drugs significantly changes the landscape and costs of HCV management. In this new scenario, multiple factors should be considered. Adoption of cost-effective strategies would help decision makers to ensure appropriate usage of resources and proper treatment of patients.
Conclusions
Our findings suggest that treatment with peginterferon-alfa-2a is associated with higher clinical responses and outcomes than peginterferon-alfa-2b. The pharmacoeconomic analysis assessed peginterferon-alfa-2a as a dominant therapy (more effective and less costly) as compared to peginterferon-alfa-2b under SUS perspective in Brazil.
References
Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, et al.: Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science 1989, 244(4902):359–362. 10.1126/science.2523562
Wong JB, McQuillan GM, McHutchison JG, Poynard T: Estimating future hepatitis C morbidity, mortality and costs in the United States. Am J Public Health 2000, 90(10):1562–1569.
Veldt BJ, Heathcote EJ, Wedemeyer H, et al.: Sustained virologic response and clinical outcomes in patients with chronic hepatitis C and advanced fibrosis. Ann Intern Med 2007, 147(10):677–684. 10.7326/0003-4819-147-10-200711200-00003
Brazilian Ministry of Health: Relatório técnico do estudo de prevalência de base populacional das infecções pelos vírus das hepatites A, B e C nas capitais do Brasil: dados preliminares. Secretary for the Vigilance on Health, Department of STD, AIDS and Viral Hepatitis, Recife, Brazil. Ministry of Health, 2010 Feb in: Protocolo Clínico e Diretrizes Terapêuticas para Hepatite Viral C e Coinfecções. Secretary for the Vigilance on Health, Department of STD, AIDS and Viral Hepatitis, Brasilia, Brazil. Ministry of Health, June 2011
Brazilian Ministry of Health: Boletim epidemiológico: hepatites virais. Secretary for the Vigilance on Health, Department of STD, AIDS and Viral Hepatitis, Brazilia, Brazil. Ministry of Health, 2010 in: Protocolo Clínico e Diretrizes Terapêuticas para Hepatite Viral C e Coinfecções. Secretary for the Vigilance on Health, Department of STD, AIDS and Viral Hepatitis, Brasilia, Brazil. Ministry of Health, June 2011
Brazilian Ministry of Health News: Ministério amplia acesso a medicamento para hepatite C. 2011.
Brazilian Ministry of Health: Protocolo Clínico e Diretrizes Terapêuticas para Hepatite Viral C e Coinfecções. Secretary for the Vigilance on Health, Department of STD, AIDS and Viral Hepatitis, Brasilia, Brazil. Ministry of Health, June 2011
Awad T, Thorlund K, Hauser G, et al.: Peginterferon alfa-2a is associated with higher sustained virological response than peginterferon alfa-2b in chronic hepatitis C: systematic review of randomized trials. Hepatology 2010, 51(4):1176–1184. 10.1002/hep.23504
Alavian SM, Behnava B, Tabatabaei SV: The comparative efficacy and safety of peginterferon alfa-2s vs. 2b for the treatment of chronic HCV infection: A meta-analysis. Hepat Mon 2010, 10(2):121–131.
Zhao S, Liu E, Chen P, et al.: A comparison of peginterferon α-2a and α-2b for treatment-naive patients with chronic hepatitis C virus: A meta-analysis of randomized trials. Clin Ther 2010, 32(9):1565–77. 10.1016/j.clinthera.2010.08.009
Nan Xiao N, Shi S, Zhuang H: A meta-analysis that compares the use of either peginterferon-α2a or peginterferon-α2b plus ribavirin for HCV infection. Hepatic Medicine: Evidence and Research 2010, 2: 99–109.
Singal AK, Jampana SC, Anand BS: Peginterferon alfa-2a is superior to peginterferon alfa-2b in the treatment of naïve patients with hepatitis C virus infection: meta-analysis of randomized controlled trials. Dig Dis Sci 2011, 56(8):2221–6. 10.1007/s10620-011-1765-0
Cheinquer H, Barros FMR, Borges LG, et al.: Systematic review and meta-analysis of published randomized controlled trials comparing the efficacy of peginterferon-alpha-2a versus peginterferon alpha-2b both plus ribavirin in chronic hepatitis C patients. Value in Health 2010, 13(no. 3):A69.
Mar J, et al.: Cost-effectiveness analysis of pegylated interferons combined with ribavirin in the treatment of hepatitis C. Value in Health 2003, 6(6):759.
Orlewska E: The cost-effectiveness analysis of combination of peginterferon alfa-2a and ribavirin versus combination of peginterferon alfa-2b and ribavirin in chronic hepatitis in Poland. Value in Health 2004, 7(3):356.
Klaskala W, et al.: Cost-effectiveness modeling of treatment approaches to hepatitis C: a managed care perspective. Value in Health 2004, 7(3):355–356.
Latimer NR, Lewis GJ: A cost-utility analysis of peginterferon alfa-2a (40kD) versus peginterferon alfa-2b (12kD) for the treatment of chronic hepatitis C (CHC) in the UK. Value in Health 2005, 8(6):A58.
Lewis GJ, et al.: A comparison of cost-effectiveness of pginterferon alfa-2a (40kD) (Pegasys) plus ribavirin (Copegus) versus interferon alfa-2b plus ribavirin as first treatment of chronic hepatitis C in the UK. Value in Health 2004, 7(6):763–764.
Escudero GS, Idrovo J, Rivas R, Rodriguez JR, Alba IAR, Zapata L, et al.: Cost-effectiveness of peg-IFN alfa 2a or 2b plus ribavirin in the treatment of chronic hepatitis C in Mexico. Value in Health 2009, 12(3):A59.
Ventayol-Bosch P, et al.: Cost-effectiveness analysis of treatment of chronic hepatitis C patients with peginterferon alfa-2a or peginterferon alfa-2b both plus ribavirin in Spain. Value in Health 2010, 13(7):A438.
Vianna CMM, Caetano R: Diretrizes Metodológicas para Estudos de Avaliação Econômica de Tecnologias para o Ministério da Saúde. 2009.
Tatsch FF, Sette Júnior H, Vianna D: Pharmacoeconomics applied to chronic hepatitis C. Braz J Infect Dis 2006, 10(1):51–54. 10.1590/S1413-86702006000100010
Paraná R, Sette H, Pessoa M, Bueno RLP, et al.: Custo-efetividade de peginterferon alfa-2a (40 KD) associado a ribavirina no tratamento de pacientes com hepatite crônica C genótipos 2 e 3. 2006.
Sullivan SD, Green J, Patel KK, et al.: Comparison of the cost-effectiveness of peginterferon alfa-2a (40KD) (PEGASYS®) plus ribavirin (COPEGUS®) versus interferon alfa-2b plus ribavirin in chronic hepatitis C. Value in Health 2003, 6(3):262.
Yano M, Kumada H, Kage M, Ikeda K, Shimamatsu K, Inoue O, Hashimoto E, et al.: The long-term pathological evolution of chronic hepatitis C. Hepatology 1996, 23: 1334–1340. 10.1002/hep.510230607
Kim WR, Poterucha JJ, Hermans JE, Therneau TM, et al.: Cost-effectiveness of 6 and 12 months of interferon-α therapy for chronic hepatitis C. Ann Intern Med 1997, 127: 866–874. 10.7326/0003-4819-127-10-199711150-00002
Fattovich G, Giustina G, Degos F: Morbidity and mortality in compensated cirrhosis type C: a retrospective follow up study of 384 patients. Gastroenterology 1997, 112(2):463–72. 10.1053/gast.1997.v112.pm9024300
Gines P, Quintero E, Arroyo V, et al.: Compensated cirrhosis: natural history and prognostic factors. Hepatology 1987, 7(no. 1):122–128. 10.1002/hep.1840070124
Tsukuma H, Hiyama T, Tanaka S, et al.: Risk factors for hepatocellular carcinoma among patients with chronic liver disease. N Engl J Med 1993, 328(25):1797–801. 10.1056/NEJM199306243282501
Colombo M, de Franchis R, Del Ninno E, Sangiovanni , et al.: Hepatocellular carcinoma in Italian patients with cirrhosis. N Engl J Med 1991, 328: 64–65.
Bennett WG, Inoue Y, Beck JR, et al.: Estimates of the cost-effectiveness of a single course of interferon-alpha2b in patients with histologically mild chronic hepatitis C. Ann Intern Med 1997, 127: 918–920. 10.7326/0003-4819-127-10-199711150-00011
Ascher NL, Lake JR, Emond J, et al.: Liver transplantation for hepatitis C virus-related cirrhosis. Hepatology 1994, 20: 245–275. 10.1016/S0168-8278(05)80065-0
Detre KM, Belle SH, Lombardero M: Liver transplantation for chronic viral hepatitis. Viral Hepatitis Rev 1996, 2: 219–228.
Kilpe VE, Krakauer H, Wren RE: An analysis of liver transplant experience from 37 transplant centers as reported to Medicare. Transplantation 1993, 56: 554–561. 10.1097/00007890-199309000-00012
Brazilian Geography and Statistics Institute – IBGE Available from: home http://www.ibge.gov.br/
Younossi Z, McCormick M, Boparai N, et al.: Impact of chronic liver disease on patients' utilities [Abstract]. Gastroenterology 1999, 116: A1292.
Brazilian Ministry of Health: Sistema de Gerenciamento da Tabela de Procedimentos, Medicamentos e OPM do SUS - SIGTAP. Available from sigtap.datasus.gov.br/. Accessed Nov 2009
Brazilian Official Gazette Section 3. n 129, July 7th 2011
Brazilian Official Gazette Section 3. n 121, June 27th 2011
Brazilian Official Gazette Section 3. n 122, June 28th 2011
Ascione A, De Luca M, Tartaglione MT, et al.: Peginterferon alfa-2a plus ribavirin is more effective than peginterferon alfa-2b plus ribavirin for treating chronic hepatitis C virus infection. Gastroenterology 2010, 138(no. 1):116–22. Epub 2009 Oct 20 10.1053/j.gastro.2009.10.005
Rumi MG, Aghemo A, Prati GM, et al.: Randomized study of peginterferon-alpha2a plus ribavirin vs peginterferon-alpha2b plus ribavirin in chronic hepatitis C. Gastroenterology 2010, 138(no. 1):108–15. Epub 2009 Sep 18 10.1053/j.gastro.2009.08.071
McHutchison JG, Lawitz EJ, Shiffman ML, et al.: Peginterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infection. N Engl J Med 2009, 361(6):580–93. 10.1056/NEJMoa0808010
Yenice N, Mehtap O, Gumrah M, et al.: The efficacy of pegylated interferon alpha 2a or 2b plus ribavirin in chronic hepatitis C patients. Turk J Gastroenterol 2006, 17(2):94–98.
Scotto G, Fazio V, Fornabaio C, Tartaglia A, et al.: Peg-interferon alpha-2a versus Peg-interferon alpha-2b in nonresponders with HCV active chronic hepatitis: a pilot study. J Interferon Cytokine Res 2008, 28(10):623–9. 10.1089/jir.2007.0116
Berak H, Kolakowska-Rzadzka A, Wasilewski M, et al.: Randomized, open label trial comparing efficacy and safety of pegylated interferon alfa 2b vs alfa 2b treatment of patients with chronic hepatitis C infected with non 2/3 genotypes - final analysis. Journal of Hepatology 2007, 46: S217-S218.
Kolakowska A, Berak H, Wasilewski M, et al.: Relevance between fibrosis and response to treatment with peginterferon alfa2a vs alfa2b with ribavirin in chronic hepatitis C genotype 3 patients. Randomized open label study. Hepatology 2008, 48(no. 4):878A.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
We declare that the article was funded by Hoffmann-La Roche Ltd. All authors have received financial sponsorship by Roche Pharmaceuticals. FMRB has also received financial support by Merck Sharp & Dohme, Bristol-Myers Squibb, Bayer HealthCare Pharmaceuticals and Biolab Sanus. HC has also received financial support by Bristol-Myers Squibb, Novartis and Janssen-Cilag.
Authors’ contributions
Fabio Barros and Hugo Cheinquer provided all medical and scientific support. Carolina Tsuchiya was responsible for writing, analysis and revision. Eduardo Santos provided health economic expertise, designed the model structure and economic evaluations, and supervised the writing, analysis and revision. All authors read and approved the final manuscript.
Hugo Cheinquer contributed equally to this work.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Barros, F.M., Cheinquer, H., Tsuchiya, C.T. et al. Cost-effectiveness analysis of treatment with peginterferon-alfa-2a versus peginterferon-alfa-2b for patients with chronic hepatitis C under the public payer perspective in Brazil. Cost Eff Resour Alloc 11, 25 (2013). https://doi.org/10.1186/1478-7547-11-25
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1478-7547-11-25