Abstract
The implantation process involves complex and synchronized molecular and cellular events between the uterus and the implanting embryo. These events are regulated by paracrine and autocrine factors. Trophoblast invasion and migration through the uterine wall is mediated by molecular and cellular interactions, controlled by the trophoblast and the maternal microenvironment. This review is focused on the molecular constituents of the human trophoblast, their actions and interactions, including interrelations with the uterine endometrium.
Similar content being viewed by others
1. Introduction
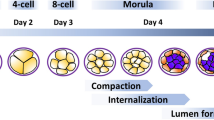
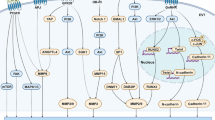
Successful implantation depends on synchronization between the developmental stages of the embryo itself and the complex series of molecular and cellular events that are induced in the pregnant uterus by paracrine and autocrine regulators [1]. Embryos prepare for implantation during their cleavage stage. Successive cleavages must produce sufficient cells by the time the blastocyst is formed to permit the full formation of inner cell mass and trophectodermal cells. The latter are the origin of the cytotrophoblastic cells which ensue to become either the villous cytotrophoblastic cells which will proliferate and differentiate by fusion to form the syncytiotrophoblast, or they will stream out of the syncytiotrophoblast to form mononuclear multilayered invasive extravillous cytotrophoblastic cells [2]. The process of implantation begins six to seven days following fertilization [3] and consists basically of three stages [4]. Apposition is the first stage denoting the initial, still unstable, adhesion of the blastocyst to the uterine wall. At this stage the pinopodes, which are micro protrusions from the apical uterine epithelium surface, inter-digitate with microvilli on the apical syncytiotrophoblast surface of the blastocyst[5] (Figure 1). The stable adhesion, which is the next step, reveals an increased physical contact between the blastocyst and the uterine epithelium, while the embryonic pole is oriented toward the epithelium. The last stage is the invasion process, which starts with the penetration of the syncytiotrophoblasts through the uterine epithelium and followed by infiltration of the mononuclear cytotrophoblasts invading the entire endometrium, the inner third of the myometrium and the uterine vasculature. Villous cytotrophoblast cells at the tip of the anchoring villi proliferate outwards from the underlying basement membrane to form cell columns from which cells migrate into the decidua (interstitial trophoblast cells) and invade the maternal spiral arteries and designate the endovascular trophoblast cells. The latter allows the trophoblasts to be in direct contact with the maternal blood establishing the uteroplacental circulation [4]. Trophoblast invasion and migration is probably controlled by components of the trophoblast itself and maternal microenvironment, through molecular and cellular interaction (Figure 2).
The human implantation process is unique thus no other mammals can provide a true model [2]. Ethical restrictions and limited availability of human placental tissue limits the possibilities of human implantation studies. Consequently most knowledge comes from in vitro experiments using cultured human trophoblasts or cell-lines, mainly obtained from choriocarcinoma. Although the differences in reproductive physiology between species limit the relevance, knowledge on the possible role of various factors in the implantation process comes from knockout-studies in mice. Primates provide a more physiological relevant model, therefore in vivo and in vitro studies in baboons have contributed to our knowledge [6]. All in all, human in vitro and mammal in vitro and in vivo studies have provided important information, which helped in understand at least part from the complex process of implantation. This review focuses on the molecular constituents of the human trophoblast, their actions and interactions, which seem to be crucial for successful implantation.
2. Cell adhesion molecules
2.1 Integrins
During the mid-luteal stage of the menstrual cycle, apparently in response to progesterone, epithelial apical membrane projections named pinopodes appear in the uterus [5]. The biological nature of the pinopodes has not been determined, but their short appearance during the implantation window, interconnecting with microvilli on the apical syncytiotrophoblast surface of the blastocyst, suggests an involvement in embryo implantation [7]. Adherence of the trophectoderm cells of the blastocyst to the pinopode membrane has been suggested to occur through adhesive molecules such as E-cadherin, present in the pinopode epithelial membrane [5]. In vitro studies showed that although no direct contact between trophectoderm and pinopode is seen, blastocysts tends to attach to areas of cultured endometrial epithelium containing pinopodes [5].
Integrins are heterodimeric membrane glycoproteins, composed of α and β subunits, capable of binding to various extracellular matrix (ECM) components and cell adhesion molecules, thereby influencing and mediating adhesion, migration, invasion, cytoskeleton reorganization and cellular signaling [8]. Endometrial integrins are hormone-dependent and vary throughout the menstrual cycle [9]. The integrin repertoire of the endometrium may play an important role for obtaining a successful implantation. According to a timed expression correlating with embryo attachment, the αVβ3 and the α4β1 integrins are considered markers of uterine receptivity [10–12]. αVβ3 has been shown to be highly expressed at the time of embryo attachment, and aberrant expression of αVβ3 is associated with infertility [13, 14]. Women with recurrent miscarriages were found to have a lower concentration of α4β1 and α5β1 integrins in the stroma during the implantation window, than women with unexplained infertility [15]. The relevance of integrins alteration, in glandular epithelium or stroma, but not in luminal epithelium is not well understood, since they are not likely to be involved in the early parts of implantation [16]. The trophectoderm also express several integrins, α3, α5, β1, β3, β4 and β5, supposed to be implicated in blastocyst attachment to the endothelial surface [17–19]. Trophoblasts modulate their integrin repertoire during invasion of the stroma and during differentiation, varying in invasive and non-invasive cells [2, 16]. Several factors known to be involved in the implantation process may act through mediation of integrins. For example, Insulin-like growth factor (IGF)-I-induced migration of extravillous trophoblast cells (EVT) probably involves internalization of the α5β1-integrin on EVT [19], as well as influencing the αVβ3-integrin pathway [18]. In female mice lacking a functional integrin β1 gene, embryos develop normally to the blastocyst stage but fail to implant properly and die [20]. No other integrins were found in knockout studies to be involved in implantation defects [21], but inactivation of αVβ3 by the disintegrin echistatin significantly reduced implantation sites in mice [22]. Apparently several integrins may have a redundant role in implantation [23].
2.2 MUC1
Uterine cell-surface glycoproteins such as MUC1 are thought to provide a barrier to trophoblast invasiveness, by controlling accessibility of integrin receptors to their ligands [8]. MUC1 increases from the proliferative to the secretory phase in endometrial tissue and then decreases in the late secretory phase [24]. Progesterone combined with estrogen up-regulates MUC1 at the receptive endometrium [24]. Blastocysts were shown to deplete MUC1 locally from the implantation site, whereas during the apposition phase, when the blastocyst apparently must be stationary in position, MUC1 was up-regulated [25]. MUC1 may thereby serve as an anti-adhesive molecule that hinders blastocyst attachment to the uterine epithelium until 3 days after entrance to the uterus [26].
3. Extracellular matrix proteins
The trophoblastic cells are confronted with various matrix proteins and basement membranes, when penetrating the uterine wall. These ECM components, including collagens (Col), fibronectin (FN), laminin (LN), vitronectin (VN), trophin and tastin, influence cell functions by binding to integrins, thereby effecting adhesion, migration, differentiation and spreading [2]. Trophin and Tastin for example can be found in endometrial epithelium as well as in trophoblasts, and may be involved in blastocyst attachment by forming a cell-adhesion molecular complex [26].
The components of the ECM are changed during the menstrual cycle [27]. The changes associated with decidualization, during the mid-secretory phase, are especially relevant for implantation. This includes an increase in hyaluronan, a decrease in collagen VI and a slower production of collagen III and I. During decidualization, stromal fibroblasts differentiate into decidual cells, and a basement membrane, containing laminin, entactin, collagen IV and heparan sulphate proteoglycan, appears around each cell [28].
ECM components affect the behavior and function of trophoblastic cells by affecting matrix metalloproteases (MMPs) and their tissue inhibitors (TIMPs) as shown by Xu et al, 2001 [29]. Trophoblasts, grown in the presence of VN, LN or FN, secreted more MMP-9 than in the presence of Col I, or IV, whereas MMP-2, TIMP-2 and MMP-14 were not affected. TIMP-3 on the contrary was inhibited by the presence of VN. Trophoblast adhesiveness was highest in the presence of Col I and IV compared with the other matrix proteins. Since cytotrophoblast cells also produce LN, FN and VN during the first trimester, these matrix proteins may be part of an autocrine mechanism, regulating MMP expression and cell invasiveness [29].
4. Growth factors
4.1 Epidermal Growth Factor
Epidermal Growth Factor (EGF) is expressed both in decidual and trophoblastic cells [30] and affects implantation in several ways. EGF induces trophoblast invasion [31, 32], trophoblast differentiation [33, 34] and trophoblast proliferation [35], and is therefore regarded a major regulator of the implantation process. EGF has been shown to increase MMP-2 and MMP-9 activity in trophoblastic cells [32, 36, 37], and also urokinase-type plasminogen activator (uPA) and plasminogen activator inhibitor-1 (PAI-1) activity [36] in trophoblastic cells, thereby inducing cell invasion. EGF stimulates secretion of human chorionic gonadotrophin (hCG) and human placental lactogen (hPL) from trophoblastic cells [38, 33], and has been shown to induce α2 integrin expression in a choriocarcinoma cell-line, an effect found to be related to increased invasiveness [38].
4.2 Heparin-binding EGF-like growth factor
Heparin-binding EGF-like growth factor (HB-EGF) shares a common receptor with EGF and transforming growth factor (TGF)-α. HB-EGF is expressed in stromal and epithelial cells of the uterus and is thought to regulate endometrial proliferation, secretion and decidualization [21]. The expression of HB-EGF is maximal in the mid-secretory phase in uterine epithelial cells; therefore HB-EGF may also be involved in the regulation of blastocyst implantation [21].
4.3 Transforming growth factor β
TGF-β is expressed both in endometrial and trophoblastic cells [2]. TGF-β was shown to inhibit trophoblast proliferation and invasion apparently by stimulating TIMP secretion and decreasing MMP activation through down-regulation of plasminogen activators [39]. In another study TGF-β was found to inhibit trophoblast invasion by reducing MMP-9 and uPA secretion, but did not affect TIMP levels or cell proliferation [40]. Elevated TGF-β activity has been reported in the plasma of pre-eclamptic mothers [41] and may be implicated in the impaired implantation associated with pre-eclampsia [42].
4.4 Insulin-like growth factor binding protein-1
Insulin-like growth factor binding protein-1 (IGFBP-1) is the main secretory product of the decidualized endometrium. IGFBP-1 modulates the metabolic effect of insulin-growth factor (IGF)-I and IGF-II and has been shown to increase the gelatinolytic activity of trophopblasts [43] and trophoblast invasiveness mainly by increasing cell migration [44, 45].
5. Cytokines
5.1 Leukaemia inhibitory factor
Leukaemia inhibitory factor (LIF) secreted from the uterus is regarded an important factor in embryo implantation. Female mice lacking a functional LIF gene are fertile, but their blastocysts fail to implant, even though they are viable and can implant when transferred to wild-type recipients [46]. Maximal expression of LIF in endometrial epithelium is during the implantation window [21]. LIF and its receptor are also expressed in pre-implantation embryos [47] and in cytotrophoblasts [48]. LIF inhibits gelatinase activity in cytotrophoblasts, thereby effecting cell invasiveness [49]. LIF may also be involved in immune tolerance through regulation of HLA-G, a class I MHC molecule specifically expressed by invasive cytotrophoblast cells [50].
5.2 Interleukin-1
Interleukin-1 (IL-1) is present at the feto-maternal interphase; trophoblastic cells and decidualized stromal cells produce IL-1, and the IL-1 receptor is present in endometrial epithelial cells as well as in trophoblasts [2]. IL-1 may be one of the first signals of the blastocyst acting upon the endometrium, since in vitro IL-1 increases endometrial secretion of prostaglandin E2, LIF and of integrin β3 subunit expression [51]. In mice IL-1 receptor antagonist given prior to implantation significantly reduces the number of implanted embryos, indicating a role for IL-1 in embryo implantation [52]. IL-1 can stimulate MMP-9 activity in trophoblasts [53] and expression in endometrial stroma cells [54], thereby inducing trophoblast invasion Among other cytokines also present at the maternal-feto interface are IL-6, which stimulates MMP-2 and MMP-9 activity [55] whereas IL-10 down-regulates MMP-9 and trophoblast invasion [56].
6. Hormones
6.1 Human Chorionic Gonadotropin
Syncytiotrophoblastic cells secrete hormones including progesterone and human chorionic gonadotropin (hCG), which play important roles in the implantation process. hCG affects several processes during pregnancy, besides the well-known maintenance of the corpus luteum, including cell growth and differentiation. The trophoblasts themselves express a truncated and inactive hCG receptor until ninth week of gestation, then switching to the full length receptor, allowing hCG autocrinic regulation of various functions including cell differentiation in the trophoblasts [57]. In trophoblastic neoplasms these receptors have been found to be over-expressed [2]. Positive correlation between hCG level and trophoblast invasion has been shown in ectopic pregnancies [58, 59], indicating a possible stimulatory effect on trophoblast invasiveness. hCG was found in vitro to increase invasiveness of a trophoblastic choriocarcinoma cell-line [60] however in vitro studies of primary trophoblast showed the opposite effect [61, 62]. Therefore, the influence of hCG on trophoblast in vivo invasion remains unclear. hCG stimulates the cAMP pathway, and forskolin, which directly activates adenylate cyclase and increases cAMP, also stimulates trophoblast invasiveness, both in a trophoblast choriocarcinoma cell-line and in primary trophoblastic cells [32]. This is in agreement with the finding that hCG increases MMP-9, an important key-factor in trophoblast invasion [57]. Lately hCG was shown in vitro to stimulate trophoblast migration through an IGF-II effect [63].
hCG influences several uterine factors, for example increases the expression of COX-2 gene, an enzyme involved in prostaglandin biosynthesis [21], LIF and vascular endothelial growth factor (VEGF) [57], suggesting a role in endometrial vascularization. In the baboon hCG was shown to cause physiological effects on the uterine endometrium in vivo, including an increase in glycodelin expression and secretion by the glandular epithelium, and differentiation of subepithelial stromal fibroblasts characterized by expression of the alpha smooth muscle actin, associated with the initiation of decidualization [64, 65]. This suggests that the primate blastocyst signal modulates the uterine environment prior to implantation [64].
Hyperglycosylated hCG (HhCG), also called invasive trophoblast antigen (ITA), is an hCG variant with extra-large O-linked oligosaccharides. HhCG is the predominant form of hCG in early pregnancy and in choriocarcinoma, where aggressive trophoblast invasion takes place [66]. It is produced by poorly differentiated or invasive trophoblast cells and decreases as pregnancy advances [67]. The fact that HhCG is dominant around the time of implantation and in the 3 weeks that follow [68] makes it an interesting molecule that deserves further exploration. Low maternal mid-trimester urine HhCG has been found to predict preeclampsia, which is associated with poor trophoblast invasion [69].
6.2 Progesterone
Progesterone has a crucial role in preparing the uterus for the developing embryo and for obtaining a successful pregnancy. Stromal cells differentiate into decidual cells in respond to progesterone during the decidualization process, characterized by morphological changes and secretion of prolactin [70]. Progesterone is also required for maintenance of the pregnancy by stimulating and maintaining uterine functions, necessary for early embryonic development, implantation, placentation and fetal development. Hormones such as hCG, produced by the trophoblast, maintain this progesterone production in early pregnancy [71]. Progesterone was suggested to affect trophoblast invasiveness through the down-regulation of MMP-9 [72]. Progesterone is known to restrain endometrial breakdown by inhibiting MMPs. This inhibitory effect implies that progesterone impedes the invasion of trophoblast cells into the endometrial tissue. In a recent report progesterone was found to decrease invasion and gelatinase expression in early first trimester trophoblast cells and to increase cell invasion and MMP-2 expression in late trophoblast cells [73]. A differential progesterone receptor (PR) profile was documented with the dominance of PRB in the early trophoblast and dominance of PRA in the late trophoblast [73]. This differential PR profile is compatible with the inverse temporal effect of the hormone on the trophoblast cells. In mice a similar dual role of progesterone in embryo implantation has been reported, when progesterone promoted attachment and invasion of primary trophoblasts, mainly through MMP-2 stimulation, but inhibited invasion of secondary trophoblasts [74].
7. Inflammatory factors
7.1 Cortico-releasing hormone
The implanting embryo suppresses the maternal immune process, thereby preventing rejection. Cortico-releasing hormone (CRH) is produced by both trophoblasts and placental deciduas [75]. In mice, implantation can be highly reduced by anti-CRH antibody, indicating a role in embryo implantation [76]. Fas and its ligand FasL, belongs to the tumor necrosis family (TNF). Fas and Fas-FasL interaction, plays an important role in the regulation of immune tolerance, mainly by inducing apoptosis in cells carrying Fas, including T and B lymphocytes [75]. FasL is expressed on cytotrophoblastc as well as on maternal decidual cells of the placenta [77]. CRH was found in vitro to stimulate FasL expression in extravillous human trophoblasts, thereby enabling them to induce apoptosis of the surrounding activated T lymphocytes [75]. Rats treated with a CRH receptor 1 (CRHR1) antagonist had diminished FasL endometrial expression and reduced number of implantation sites, suggesting that locally produced CRH promotes implantation and maintenance of early pregnancy mainly by killing activated T cells [75]. The CRH-Fas-FasL system is not the sole maternal immunetolerance mechanism preventing embryo rejection. Mice with an inactivating mutation of Fasl gene or CRH and CRHR1-deficient mice [78–80] can be fertile.
7.2 Tumor necrosis factor-α
The pleiotropic cytokine TNF-α and its two receptors are present in the endometrium, as well as in placental trophoblasts [81]. TNF-α was shown in vitro to increase uPA secretion from cytotrophoblasts, thereby properly enhances the degradation of fibronectin during trophoblast penetration of the endometrial ECM [84]. Although, TNF-α did not affect MMP-9 concentration, the up-regulation of uPA increases activation of MMP-9 through the plasminogen activation system, thereby enhancing trophoblast invasiveness [84]. This is consistent with another report describing TNF-α stimulation of MMP-9 gelatinolytic activity, without affecting MMP-9 concentration [53]. At the same time, TNF-α decreases MMP-2 concentration and activity as well as hCG secretion [53, 84]. It is therefore suggested, that high TNF-α levels presented during inflammatory responses [85], could be responsible in pathologic processes such as pregnancy loss, preterm delivery and preeclampsia, for abnormal trophoblast endocrine function [84, 86]. In a recent study, despite increased MMP-9 expression, TNF-α was found to inhibit in vitro trophoblast migration and invasion. However, the plasminogen activator inhibitor-1 (PAI-1) that blocks the plasminogen activator system, was found to be increased [86]. Thus, TNF-α seems to exert diverse in vitro effects, depending on individual trophoblast preparation, type of cell-line and cytokine concentration. This affects the value of conclusions which can be derived from in vitro studies.
A link between elevated PAI-1 levels detected in plasma and syncytium of preeclamptic women, and elevated TNF-α found in preeclamptic sera, villi and deciduas has been suggested [86]. The elevated TNF-α level could result from local hypoxic conditions developing under reduced maternal and fetal vascular perfusion [87].
7.3 Prostaglandins
Prostaglandins are synthesized from arachidonic acid by phospholipase A2, followed by cyclooxygenase (COX). Prostaglandin E2 (PGE2) is essential for mammalian female reproduction. PGE2 is involved in regulation of decidualization of endometrial stomal cells [88], apparently through stimulation of IL-11, since blocking of IL-11 in PGE2 treated cells reduces decidualization and COX inhibitor reduces IL-11 secretion from these cells [89]. In rats, expression of the PGE receptor EP2 is highly detected at implantation sites in luminal epithelium, peaking on day 6 of pregnancy [90]. Phospholipase A2 and PGE2 receptor were described to be up-regulated in human endometrium during the window of implantation [91]. Experiments in mice have shown that prostaglandins are essential for the correct timing of implantation [92]. Reduced levels of COX-2 or COX-2 ligands cause deferred implantation and reduced litter size, and prostaglandin treatment resumes on-time implantation [92]. COX-1, COX-2 and prostaglandin E synthase (PGES), which catalyses COX products to PGE2, are highly expressed in mice at the blastocyst stage [93].
8. Extracellular degrading matrix proteinases
8.1 MMPs
The matrix metalloproteinases (MMPs) are a family of zinc-containing endopeptidases capable of degrading all components of the ECM, both interstitial matrix and basement membrane. MMPs are thought to play an important role in tumor progression and metastasis [94]. Twenty-six mammalian and twenty-two human MMPs are known so far [28, 95]. The vertebrate MMPs each have distinct but often overlapping substrate specificities. Human MMPs fall into five classes according to primary structure and substrate specificity: collagenases, gelatinases, stromelysins, membrane-type and nonclassified MMPs [96]. MMPs regulate cell behavior in numerous ways, including cell-matrix and cell-cell interactions and the release, activation or inactivation of autocrine or paracrine signaling molecules and cell surface receptors. ECM-degradation permits cellular invasion to take place and influence processes such as cell shape, movement, cytoskeleton machinery and matrix-derived signals [95]. MMPs are regulated at several levels, including transcriptional, secretion, activation, inhibition and degradation. Transcriptional regulation is cell, tissue and MMP-specific and includes several cytokines and growth factors [97]. MMPs are produced as proenzymes, requiring removal of the propeptide domain for activation. The extracellular activation of most MMPs can be initiated by activated MMPs or by several serine proteinases, including uPA, plasminogen, thrombin and elastase. MMP-2 is activated at the cell surface through a multi-step pathway involving membrane type (MT) – MMPs and TIMP-2 [98]. MMPs are inhibited by α2-macroglobulin, in tissue fluids, and in tissue by TIMPs, which bind to MMPs in a 1:1 stoichiometric fashion [99].
In vitro studies suggest that successful implantation and placentation result from the balance between secretion of MMPs from the trophoblast and their inhibition by TIMPs [100, 101]. The gelatinases, MMP-2 and -9, degrade Collagen IV, the main component of the basement membrane, and are therefore regarded as key enzymes in the implantation process, enabling the invasion of the trophoblast cells through the decidua and into the maternal vasculature. Several in vitro studies have found the gelatinases to be required for trophoblast invasion [102–105, 32].
Gelatinases, mainly MMP-2, are secreted from the embryo already at the blastocyst stage [106–108]. Lately we and others have shown, that MMP-2 and MMP-9 have a differential expression throughout the first trimester, with MMP-2 being the main gelatinase in early first trimester (6–8 w) and MMP-9 being dominant in late first trimester (9–12 w) [32, 104]. MMPs may have other important actions in the implantation process, besides ECM degradation, including regulation of bioactivity of growth factors, cytokines and angiogenic factors [27]. This includes MMP-2 or -9 activation of TGFβ [109] or release of IGF by degradation of IGFBPs [110]. Another role may be modulation of angiogenic factors such as endothelin-1, a vasoconstrictor [111], or angiostatin, an angiogenic inhibitor [112]. Interestingly, knockout mice, deficient in MMP-2 or MMP-9, are fertile and only mild effects have been reported: MMP-2 deficient mice have a subtle delay in their growth [113] and MMP-9 deficient mice show a decreased litter size and an increase in percentage of infertile mice [114].
MT1-MMP deficient mice die before puberty; therefore no conclusions can be made on reproductive capacity [115]. In MMP-7 null-mice both MMP-3 and MMP-10 were up-regulated in the uterus, whereas in MMP-3 deficient mice MMP-7 and MMP-11 were up-regulated, thereby indicating the presence of a compensation mechanism [116].
The MMP gene promoter contains several cis-regulatory elements, often acting synergistically, with varying importance and effect, depending upon cell-type and inducer. AP-1 sites give several MMP genes in various cell types the ability to be induced by phorbol esthers and act in some cases synergistically with adjacent Ets-binding sites [117, 2, 118]. AP-1 was found to be necessary, but not sufficient for transactivation of the MMP-9 gene in human trophoblasts, and antisense against Jun and Fos transcription factors, belonging to the AP-1 complex, was found to inhibit MMP-9 gelatinolytic activity in trophoblasts [118]. The importance of the Ets site was shown by the study of knockout mice for ets2 transcription factor, which resulted in deficient MMP-9 expression and early embryonic lethality [119]. Lately, it has been shown that EGF induction of MMP-9 as well as TIMP-1 in an extravillous trophoblastic cell-line is through the MAPK and PI3K pathways [37].
Recently ADAM (a disintegrin and metalloproteinase, adamalysin)-19 has been detected during early pregnancy in the endometrium and the placenta of the rhesus monkey, and may therefore also be involved in trophoblast invasion and degradation of the ECM [120].
8.2 TIMPs
Tissue inhibitors of matrix metalloproteinases (TIMPs) are the main inhibitors of MMPs in tissue, physiologically controlling their activity. The four known TIMP proteins (TIMP 1–4) inhibit MMPs in a 1:1 stoichiometric fashion, by interaction of mainly the C-terminal with the MMP catalytic site. Individual TIMPs differ in their ability to inhibit various MMPs, and in their gene regulation and tissue-specific patterns of gene expression [121]. TIMP-1 and 2 exhibit inhibitory activity against the active forms of all MMPs, TIMP-1 preferentially binding MMP-9 in both active and latent form [122], and TIMP-2 preferentially binding active or latent MMP-2 [123]. TIMP-2 in addition has an important role in activation of MMP-2, together with MT1-MMP.
TIMPs (1–3) are produced by trophoblastic and decidual tissues throughout gestation [124, 125, 100]. TIMP-4 is secreted from mouse blastocyst, and the addition of specific TIMP-4 antibody increases the expression and activity of MMP-2 and MMP-9 [126]. In addition this group also found TIMP-4 to be expressed in a malignant choriocarcinoma human cell-line (JEG-3) [127]. Lately TIMP-1 and -3 and to a lesser extent TIMP-2 were detected in pre-implantation human embryos, indicating that MMP and TIMP genes are among the first genes to be expressed in the developing embryo, preparing for implantation [128]. Growth factors and cytokines known to effect trophoblast invasiveness may act by up or down-regulation of TIMPs. TGF-β for example inhibits trophoblast invasion by up-regulating TIMP-1 and PAI-1 and down-regulating uPA [129]. TIMPs may have additional roles besides MMP inhibition, including increasing cell proliferation [130, 131] and embryo development [132].
8.3 Serine proteases
The plasminogen activator system includes the urokinase-type plasminogen activator (uPA), the tissue-type plasminogen activator (tPA), the PA inhibitors PAI-1 and PAI-2 and the cell surface uPA receptor. The PA system converts plasminogen into the active serine protease plasmin, which can degrade ECM. The activity of the PA system is balanced by the inhibitors (PAI-1 and -2) [133]. Besides directly degrading ECM, the PA system has an indirect effect, through proteolytic activation of MMPs. Both uPA and plasmin are reported in the uterus [134] and in trophoblasts [135]. Studies in mice and rats suggest a role for the PA system in the implantation process [136]. A recent report found that Adrenomedullin (ADM), a polypeptide belonging to calcitonin gene-related peptide superfamily, enhances in vitro trophoblast proliferation and invasion [137]. Both ADM binding sites and ADM are present in the trophoblasts, the latter is most abundant in first trimester placenta [138]. The report showed that ADM decreased PAI-1 expression and increased MMP-2 activity, thereby enhancing the downstream reaction of cell invasion [137].
9. Endovascular invasion
The development of a placental vascular network is essential for the growth and maintenance of the developing embryo. Several factors are involved in this angiogenic process, including VEGF (vascular endothelial growth factor), PDGF (platelet-derived growth factor) and PAF (platelet-activating factor) [26].
VEGF induces angiogenesis and increases permeabilization of blood vessels. VEGF and its receptors are expressed in both the endometrium and in trophoblastic cells [1]. Mouse embryos with functional inactivation of one VEGF allele die on day 11–12 of pregnancy and show several malfunctions on the vascular system [139]. VEGF mRNA expression can be detected already in the blastocyst, enabling the implanting embryo to induce angiogenesis at the implantation site by binding to endometrial receptors [1]. VEGF expression is up-regulated in placental tissues by hypoxia, associated with early placental development, whereas another angiogenic factor PIGF (Placental growth factor) is down-regulated [140]. Surprisingly placental VEGF was reduced in pre-eclamptic pregnancies, despite the prolonged hypoxic condition associated with pre-eclampsia [141]. PIGF, on the other hand, was decreased in pre-eclampsia, as expected [142]. TGF-β and TNF-α, two pro-angiogenic factors present in the uterus, increased VEGF expression in a trophoblast cell-line [143]. This is especially interesting in light of the elevated TGF-β level, thought to be involved in pre-eclampsia [42], which may be an attempt to raise the low VEGF level.
ICAM (intercellular adhesion molecule), VCAM (vascular cell adhesion molecule) and PECAM (platelet endothelial cell adhesion molecule) are endothelial-cell adhesion molecules, playing an important role in endothelial activation and are elevated in the maternal circulation during pregnancy. Co-culture of endothelial cells and trophoblasts lead to an increased expression of ICAM, VCAM and E-selectin, indicating that factors released from the trophoblasts activate endothelial cells [108]. In pre-eclamptic pregnancies the expression of these adhesion molecules in the maternal circulation is further increased [108, 144]. Elevated expression of E-selectin, as found in pre-eclampsia and in placental tissues cultured under hypoxia-reoxygenation conditions, may be mediated by the cytokine tumor necrosis factor-alpha, since anti-TNFα antibody reduced this activation [145]. Elevated VCAM in maternal blood together with elevated hyperhomocyst(e)inemia, a preeclampsia risk factor, were found to be strongly associated with an increased risk of preeclampsia [146]. This is in contrast to the result of an immunocytochemical study of placental bed biopsies from normal and pre-eclamptic women, which found no difference in ICAM, PECAM, VCAM and E-selectin expression between the two groups, suggesting that these molecules are not implicated in the etiology of pre-eclampsia [147]. In another study MCAM (melanoma cell adhesion molecule) expression was reduced in trophoblasts in placentas from pre-eclamptic women compared to normal, as detected by immunohistochemistry [148]. VCAM is lower in term than in first trimester placenta, indicating importance in the developmental stage. The reduced VCAM expression found in pregnancies complicated with fetal growth restriction further supports this concept [149]. In a comparison study of first trimester serum between normal pregnant women and women with pregnancy-induced hypertension (PIH) ICAM and E-selectin, but not VCAM or P-selectin, were significant higher in women who developed PIH late in gestation, suggesting that these factors can serve as effective indicators of the onset of PIH [150].
10. Conclusion
Ethical restrictions and limited availability of human tissue confined our studies on human implantation. It must be appreciated that most knowledge comes from in vitro experiments using cultured human trophoblasts or cell-lines and in vivo studies from other species. All the same, it is clear today that the implantation process depends on appropriate timing and is regulated by various factors of both maternal and embryonic origin. The success of this process is a result of complex interactions between these factors and comprehension of the process demands further characterization of these interactions.
New technologies that allow the profiling of tissues at the genomic, transcriptomic and proteomic levels are becoming available will probably bring more information and hopefully will help to shed more light on the implantation process. Further understanding of the process will enable new strategies in treating implantation failure both in natural and in assisted reproduction.
References
Krüssel JS, Bielfeld P, Polan ML, Simón C: Regulation of embryonic Implantation. European Journal of Obstetrics and Gynecology and Reproductive Biology. 2003, 110: 2-9. 10.1016/S0301-2115(03)00167-2.
Bischof P, Campana A: Molecular mediators of implantation. Bailliere's Clinical Obstetrics and Gynaecology. 2000, 14 (5): 801-814. 10.1053/beog.2000.0120.
Vigano P, Mangioni S, Pompei F, Chiodo I: Maternal-conceptus Cross Talk – A Review. Placenta. 2003, 24: S56-S61. 10.1016/S0143-4004(03)00137-1.
Norwitz ER, Schust DJ, Fisher SJ: Implantation and the survival of early pregnancy. N Engl J Med. 2001, 345: 1400-1408. 10.1056/NEJMra000763.
Lopata A, Bentin-Ley U, Enders A: "Pinopodes" and Implantation. Reviews in Endocrine & metabolic Disorders. 2002, 3: 77-86. 10.1023/A:1015455709833.
Carver JC, Martin K, Spyropoulou I, Barlow D, Sargent I, Mardon H: An in-vitro model for stromal invasion during implantation of the human blastocyst. Human Reproduction. 2003, 18 (2): 283-290. 10.1093/humrep/deg072.
Nacas G: Pinopodes as markers of endometrial receptivity in clinical practice. Hum Reprod. 1999, 14 (Suppl 2): 99-106.
Burghardt RC, Johnson GA, Jarger LA, Ka H, Garlow JE, Spencer TE, Bazer FW: Integrins and Extracellular Matrix Proteins at the Maternal-Fetal Interface in Domestic Animals. Cell Tissues Organs. 2002, 172: 202-217. 10.1159/000066969.
Lessey BA: Endometrial integrins. Endocrinologist. 1995, 5: 214-221.
Lessey BA, Castelbaum AJ, Buck CA, Lei Y, Yowell CW, Sun J: Further characterization of endometrial integrins during the menstrual cycle and in pregnancy. Fertil Steril. 1994, 62 (3): 497-506.
Lessey BA: Endometrial integrins and the establishment of uterine receptivity. Hum Reprod. 1998, 13 (Suppl 3): 247-258. discussion 259–261
Nardo LG, Nikas G, Makrigiannakis A, Sinatra F, Nardo F: Synchronous expression of pinopodes and alpha v beta 3 and alpha 4 beta 1 integrins in the endometrial surface epithelium of normally menstruating women during the implantation window. J Reprod Med. 2003, 48 (5): 355-361.
Lessey BA, Castelbaum AJ, Sawin SW, Sun J: Integrin as markers of uterine receptivity in women with primary unexplained infertility. Fertil Steril. 1995, 63 (3): 535-542.
Lessey BA, Ilesanmi AO, Lessey MA, Riben M, Harris JF, Chwalisz K: Luminal and glandular endometrial epithelium express integrins differentially throughout the menstrual cycle: implications for implantation, contraception and infertility. Am J Reprod Immunol. 1996, 35: 195-204.
Skrzypczak J, Mikolajczyk M, Szymanowski K: Endomatrial receptivity: expression of alpha3beta1, alpha4beta1 and alphaVbeta1 endometrial integrins in women with impaired fertility. Reprod Biol. 2001, 1 (2): 85-94.
Bowen JA, Hunt JS: The role of Integrins in Reproduction. Proc Soc Exp Biol Med. 2000, 223 (4): 331-343. 10.1046/j.1525-1373.2000.22348.x.
Campbell S, Swann HR, Seif MW, Kimber SJ, Aplin JD: Cell adhesion molecules on the oocyte and preimplantation human embryo. Hum Reprod. 1995, 10 (6): 1571-1578.
Kabir-Salmani M, Shiokawa S, Akimoto Y, Sakai K, Nagamatsu S, Sakai S, Nakamura Y, Lofti A, Kawakami H, Iwashita M: Alphavbeta3 integrin signaling pathway is involved in insulin-like growth factor I-stimulated human extravillous trophoblast cell migration. Endocrinology. 2003, 144 (4): 1620-1630. 10.1210/en.2002-220886.
Kabir-Salmani M, Shiokawa S, Akimoto Y, Sakai K, Iwashita M: The role of alpha(5)beta(1)-integrin in the IGF-I-induced migration of extravillous trophoblast cells during the process of implantation. Mol Hum Reprod. 2004, 10 (2): 91-97. 10.1093/molehr/gah014.
Stephens LE, Sutherland AE, Klimanskaya IV, Andrieux A, Meneses J, Pedersen RA, Damsky CH: Deletion of beta 1 integrin in mice results in inner cell mass failure and periimplantation lethality. Genes Dev. 1995, 9 (15): 1883-1895.
Simón C, Martin JC, Pellicer A: Paracerine regulators of implantation. Bailliere's Clinical Obstetrics and Gynaecology. 2000, 14 (5): 815-826. 10.1053/beog.2000.0121.
Illera MJ, Cullinan E, Gui Y, Yuan L, Beyler SA, Lessey BA: Blockade of the α5β3 integrin adversely affects implantation in the mouse. Biol Reprod. 2000, 62: 1285-1290. 10.1095/biolreprod62.5.1285.
Aplin JD, Kimber SJ: Trophoblast-uterine interactions at implantation. Reproductive Biology and Endocrinology. 2004, 2: 48-10.1186/1477-7827-2-48.
Dominguez F, Pellicer A, Simon C: Paracrine dialogue in implantation. Molecular and Cellular Endocrinology. 2002, 186: 175-181. 10.1016/S0303-7207(01)00659-1.
Meseguer M, Aplin JD, Caballero-Campo P, O'Connor JE, Martin JC, Remohi J, Pellicer A, Simon C: Human endometrial mucin MUC1 is up-regulated by progesterone and down-regulated in vitro by the human blastocyst. Biol Reprod. 2001, 64: 590-601. 10.1095/biolreprod64.2.590.
Hill JA: Maternal-Embryonic Cross-Talk. Ann N Y Acad Sci. 2001, 943: 17-25.
Salamonsen LA, Shuster S, Stern R: Deposition of hyaluronan in human endometrium across the menstrual cycle: implications for implantation and menstruation. Cell Tissue Res. 2001, 306: 335-340. 10.1007/s004410100452.
Salamonsen LA, Nie G: Proteinases at the Endometrial-Trophoblast Interface: Their Role in Implantation. Reviews in Endocrine & Metabolic Disorders. 2002, 3: 133-143. 10.1023/A:1015407012559.
Xu P, Wang Y, Piao Y, Bai S, Xiao Z, Jia Y, Luo S, Zhuang L: Effects of Matrix Proteins on the Expression of Matrix Metalloproteinase-2, -9. and -14 and Tissue Inhibitors of Metalloproteinases in Human Cytotrophoblast Cells During the First Trimester. Biology of Reproduction. 2001, 65: 240-246. 10.1095/biolreprod65.1.240.
Hofmann GE, Scott RT, Bergh PA, Deligdisch L: Immunohistochemical localization of epidermal growth factor in human endometrium, decidua, and placenta. J Clin Endocrinol Metab. 1991, 73 (4): 882-887.
Bass KE, Morrish D, Roth I, Bhardwaj D, Taylor R, Zhou Y, Fisher SJ: Human Cytotrophoblast Invasion Is Up-regulated by Epidermal Growth Factor: Evidence That Paracrine Factors Modify This Process. Developmental Biology. 1994, 164: 550-561. 10.1006/dbio.1994.1223.
Staun-Ram E, Goldman S, Gabarin D, Shalev E: Expression and importance of matrix metalloproteinases 2 and 9 (MMP-2 and -9) in human trophoblast invasion. Reproductive Biology and Endocrinology. 2004, 2 (1): 59-10.1186/1477-7827-2-59.
Maruo T, Matsuo H, Otani T, Mochizuki M: Role of epidermal growth factor (EGF) and its receptor in the development of the human placenta. Reprod Fertil Dev. 1995, 7 (6): 1465-1470. 10.1071/RD9951465.
Dakour J, Li H, Chen H, Morrish DW: EGF promotes development of a differentiated trophoblast phenotype having c-myc and junB proto-oncogene activation. Placenta. 1999, 20 (1): 119-126. 10.1053/plac.1998.0336.
Li RH, Zhuang LZ: The effect of growth factors on human normal placental cytotrophoblast cell proliferation. Hum Reprod. 1997, 12 (4): 830-834. 10.1093/humrep/12.4.830.
Anteby EY, Greenfield C, Natanson-Yaron S, Goldman-Wohl D, Hamani Y, Khudyak V, Ariel I, Yagel S: Vascular endothelial growth factor, epidermal growth factor and fibroblast growth factor-4 and -10 stimulate trophoblast plasminogen activator system and metalloproteinase-9. Mol Hum Reprod. 2004, 10 (4): 229-235. 10.1093/molehr/gah031.
Qiu Q, Yang M, Tsang BK, Gruslin A: EGF-induced trophoblast secretion of MMP-9 and TIMP-1 involves activation of both PI3K and MAPK signaling pathways. Reproduction. 2004, 128 (3): 355-363. 10.1530/rep.1.00234.
Nakatsuji Y, Nishio Y, Tani N, Adachi K, Ohmichi M, Hisamoto K, Morishige K, Kurachi H, Tasaka K, Murata Y, Matsuura N: Epidermal growth factor enhances invasive activity of BeWo choriocarcinoma cells by inducing alpha2 integrin expression. Endocr J. 2003, 50 (6): 703-714. 10.1507/endocrj.50.703.
Graham CH, Connelly I, MacDougall JR, Kerbal RS, Stetler-Stevenson WG, Lala PK: Resistence of malignant trophoblast cells to both the anti-proliferative and anti-invasive effects of transforming growth factor beta. Experimental Cell Research. 1994, 103: 1641-1650.
Lash GE, Otun HA, Innes BA, Bulmer JN, Searle RF, Robson SC: Inhibition of Trophoblast Cell Invasion by TGFB1, 2, and 3 Is Associated with a Decrease in Active Proteases. Biol Reprod. 2005, Apr 27,
Djurovic S, Schjetlein R, Wisloff F, Haugen G, Husby H, Berg K: Plasma concentrations of Lp(a) lipoprotein and TGF-bera1 are altered in preeclampsia. Clin Genet. 1997, 52: 371-376.
Caniggia I, Winter J, Lye SJ, Post M: Oxygen and placental development during the first trimester: implications for the pathophysiology of pre-eclampsia. Placenta. 2000, 21 (Suppl A): S25-30. 10.1053/plac.1999.0522.
Bischof P, Meisser A, Campana A: Involvement of trophoblast in embryo implantation: regulation by paracrine factors. J Reprod Immunol. 1998, 39 (1–2): 167-177. 10.1016/S0165-0378(98)00020-5.
Hamilton GS, Lysiak JJ, Han VK, Lala PK: Autocrine-paracrine regulation of human trophoblast invasiveness by insulin-like growth factor (IGF)-II and IGF-binding protein (IGFBP)-1. Exp Cell Res. 1998, 244 (1): 147-156. 10.1006/excr.1998.4195.
Irving JA, Lala PK: Functional role of cell surface integrins on human trophoblast cell migration: regulation by TGF-beta, IGF-II and IGFBP-1. Exp Cell Res. 1995, 217 (2): 419-427. 10.1006/excr.1995.1105.
Stewart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, Kontgen F, Abbondanzo SJ: Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 1992, 359 (6390): 76-79. 10.1038/359076a0.
Chen HF, Shew JY, Ho HN, Hsu WL, Yang YS: Expression of leukemia inhibitory factor and its receptor in preimplantation embryos. Fertil Steril. 1999, 72 (4): 709-713. 10.1016/S0015-0282(99)00306-4.
Sharkey AM, King A, Clark DE, Burrows TD, Jokhi PP, Charnock-Jones DS, Loke YW, Smith SK: Localization of leukemia inhibitory factor and its receptor in human placenta throughout pregnancy. Biology of reproduction. 1999, 60: 355-364. 10.1095/biolreprod60.2.355.
Bischof P, Haenggeli L, Campana A: Effect of leukemia inhibitory factor on human cytotrophoblast differentiation along the invasive pathway. American Journal of Reproductive Immunology. 1995, 34: 225-230.
Bamberger AM, Jenatschke S, Schulte HM, Loning T, Bamberger MC: Leukemia inhibitory factor (LIF) stimulates the human HLA-G promoter in JEG3 chriocarcinoma cells. Endocrinol Metab. 2000, 85 (10): 3932-3936. 10.1210/jc.85.10.3932.
Vigano P, Mangioni S, Pompei F, Chiodo I: Maternal-conceptus Cross talk – A review. Placenta. 2003, 24: 56-61. 10.1016/S0143-4004(03)00137-1.
Simón C, Frances A, Piquette GN, el Danasouri I, Zurawski G, Dang W, Polan ML: Embryonic implantation in mice is blocked by interleukin-1 receptor antagonist. Endocrinology. 1994, 134: 521-528. 10.1210/en.134.2.521.
Meisser A, Chardonnens D, Campana A, Bischof P: Effects of tumour necrosis factor alpha, interleukin-I alpha, macrophage colony stimulating factor and transforming growth factor beta on trophoblastic matrix metalloproteinases. Molecular Human Reproduction. 1999, 5: 252-260. 10.1093/molehr/5.3.252.
Huang HY, Wen Y, Irvine JC, Kruessel JS, Soong YK, Polan ML: Cytokine mediated regulation of TIMP-1, TIMP-3 and 92-kDa Type IV collagenase messenger RNA expression in human endometrial stroma cells. J Clin Endiocrinology. 1998, 83: 1721-1729. 10.1210/jc.83.5.1721.
Meisser A, Cameo P, Islami D, Campana A, Bischof P: Effects of interleukin-6 (IL-6) on cytotrophoblastic cells. Molecular Human Reproduction. 1999, 5: 1055-1058. 10.1093/molehr/5.11.1055.
Roth I, Fisher SJ: IL-10 is an autocrine inhibitor of human placental cytotrophoblast MMP-9 production and invasion. Developmental Biology. 1999, 205 (1): 194-204. 10.1006/dbio.1998.9122.
Licht P, Russu V, Wildt L: On the role of human chorionic gonadotrophin (hCG) in the embryo-endometrial microenviroment: implications for differentiation and implantation. Semin Reprod Med. 2001, 19: 37-47. 10.1055/s-2001-13909.
Oktay K, Brzyski RG, Miller EB, Krugman D: Association of serum beta-hCG levels with myosalpingeal invasion and viable trophoblast mass in tubal pregnancy. Obstet Gynecol. 1994, 84 (5): 803-806.
Klein M, Graf A, Kiss H: The relation between depth of trophoblast invasion and beta-hCG levels in tubal pregnancies. Archives of Gynecology and Obstetrics. 1995, 256: 85-88.
Zygmunt M, Hahn D, Munstedt K, Bischof P, Lang U: Invasion of cytotrophoblastic JEG-3 cells is stimulated by hCG in vitro. Placenta. 1998, 19: 587-593. 10.1016/S0143-4004(98)90019-4.
Yagel S, Geva TE, Solomon H, Shimonovitz S, Reich R, Finci-Yeheskel Z, Mayer M, Milwidsky A: High levels of human chorionic gonadotropin retard first trimester trophoblast invasion in vitro by decreasing urokinase plasminogen activator and collagenase activities. J Clin Endocrinol Metab. 1993, 77 (6): 1506-1511. 10.1210/jc.77.6.1506.
Milwidsky A, Finci-Yeheskel Z, Yagel S, Mayer M: Gonadotropin-mediated inhibition of proteolytic enzymes produced by human trophoblast in culture. J Clin Endocrinol Metab. 1993, 76 (5): 1101-1105. 10.1210/jc.76.5.1101.
Zygmunt M, McKinnon T, Herr F, Lala PK, Han VK: HCG increases trophoblast migration in vitro via the insulin-like growth factor-II/mannose-6 phosphate receptor. Mol Hum Reprod. 2005, 11 (4): 261-267. 10.1093/molehr/gah160.
Fazleabas AT, Donnelly KM, Srinivasan S, Fortman JD, Miller JB: Modulation of the baboon (Papio anubis] uterine endometrium by chorionic gonadotrophin during the period of uterine receptivity. Proc Natl Acad Sci USA. 1999, 96 (5): 2543-2548. 10.1073/pnas.96.5.2543.
Srisuparp S, Strakova Z, Fazleabas AT: The role of chorionic gonadotrophin (CG) in blastocyst implantation. Arch Med Res. 2001, 32 (6): 627-634. 10.1016/S0188-4409(01)00330-7.
Cole LA, Khanlian SA, Sutton JM, Davies S, Stephens ND: Hyperglycosylated hCG (invasive trophoblast antigen, ITA) a key antigen for early pregnancy detection. Clin Biochem. 2003, 36 (8): 647-655. 10.1016/S0009-9120(03)00108-5.
Palomaki GE, Knight GJ, Roberson MM, Cunningham GC, Lee JE, Strom CM, Pandian R: Invasive trophoblast antigen (hyperglycosylated human chorionic gonadotropin) in second-trimester maternal urine as a marker for down syndrome: preliminary results of an observational study on fresh samples. Clin Chem. 2004, 50 (1): 182-189. 10.1373/clinchem.2003.023986.
Cole LA, Sutton JM, Higgins TN, Cembrowski GS: Between-method variation in human chorionic gonadotropin test results. Clin Chem. 2004, 50 (5): 874-882. 10.1373/clinchem.2003.026989.
Bahado-Singh RO, Oz AU, Kingston JM, Shahabi S, Hsu CD, Cole L: The role of hyperglycosylated hCG in trophoblast invasion and the prediction of subsequent pre-eclampsia. Prenat Diagn. 2002, 22 (6): 478-481. 10.1002/pd.329.
Dunn CL, Kelly RW, Critchley HO: Decidualization of the human endometrial stromal cell: an enigmatic transformation. Reprod Biomed Online. 2003, 7 (2): 151-161.
Spencer TE, Bazer FW: Conceptus signals for establishment and maintenance of pregnancy. Reproductive Biology and Endocrinology. 2004, 2: 49-10.1186/1477-7827-2-49.
Shimonovitz S, Hurwitz A, Hochner-Celnikier D, Dushnik M, Anteby E, Yagel S: Expression of gelatinase B by trophoblast cells: down-regulation by progesterone. American Journal of Obstetrics and Gynecology. 1998, 1788: 457-461. 10.1016/S0002-9378(98)70420-X.
Goldman S, Shalev E: Difference in Progesterone Receptor Isoforms Ratio, Between Early and Late First Trimester Human Trophoblast, Is Associated with Differential Cell Invasion and Matrix Metalloproteinase2 (MMP2) Expression. Biol Reprod. 2005 Aug 31,
Dai B, Cao Y, Liu W, Yang Y, Chen D, Duan E: Dual roles of progesterone in embryo implantation in mouse. Endocrine. 2003, 21 (2): 123-132. 10.1385/ENDO:21:2:123.
Makrigiannakis A, Zoumakis E, Kalantaridou S, Coutifaris C, Margioris AN, Coukos G, Rice KC, Gravanis A, Chrousos GP: Cortoco-releasing hormone promotes blastocyst implantation and early maternal tolerance. Nature Immunology. 2001, 2 (11): 1018-1023. 10.1038/ni719.
Athanassakis I, Farmakiotis V, Aifantis I, Gravanis A, Vassiliadis S: Expression of corticotrophin-releasing hormone in the mouse uterus: participation in embryo implantation. J Endocrino. 1999, 163 (2): 221-227. 10.1677/joe.0.1630221.
Bamberger AM, Shulte HM, Thuneke I, Erdmann I, Bamberger CM, Asa SL: Expression of the apoptosis-inducing Fas ligand (FasL) in human first and third trimester placenta and choriocarcinoma cells. J Clin Endocrinol Metab. 1997, 82 (9): 3173-3175. 10.1210/jc.82.9.3173.
Karray S, Kress C, Cuvellier S, Hue-Beauvais C, Damotte D, Babinet C, Levi-Strauss M: Complete loss of Fas ligand gene causes massive lymphoproliferation and early death, indicating a residual activity of gld allele. J Immunol. 2004, 172 (4): 2118-2125.
Muglia L, Jacobson L, Dikkes P, Majzoub JA: Corticotropin-releasing hormone deficiency reveals major fetal but not adult glucocorticoid need. Nature. 1995, 373 (6513): 427-432. 10.1038/373427a0.
Smith GW, Aubry JM, Dellu F, Contarino A, Bilezikjian LM, Gold LH, Chen R, Marchuk Y, Hauser C, Bently CA, Sawchenko PE, Koob GF, Vale W, Lee KF: Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron. 1998, 20 (6): 1093-1102. 10.1016/S0896-6273(00)80491-2.
Hunt JS: Expression and regulation of the tumour necrosis factor-alpha gene in the female reproductive tract. Reprod Fertil Dev. 1993, 5 (2): 141-153. 10.1071/RD9930141.
Tabibzadeh S, Zupi E, Babaknia A, Liu A, Marconi D, Romanini C: Site and menstrual cycle-dependent expression of proteins of the tumour necrosis factor (TNF) receptor family, and BCL-2 oncoprotein and phase-specific production. Hum Reprod. 1995, 10: 277-286.
Yang Y, Yelavarthi KK, Chen HL, Pace JL, Tettanova PF, Hunt JS: Molecular biochemical and functional characteristics of tumor necrosis factor alpha produced by human placental cytotrophoblastic cells. J Immuno. 1993, 150: 5614-5624.
Monzón-Bordonaba F, Vadillo-Ortega F, Feinberg RF: Modulation of trophoblast function by tumor necrosis factor-alpha: a role in pregnancy establishment and maintenance?. Am J Obstet Gyneco. 2002, 187 (6): 1574-1580. 10.1067/mob.2002.128028.
Hillier SL, Witkin SS, Khron MA, Watts DH, Kiviat NB, Eschenbach DA: The relationship of amniotic fluid cytokines and preterm delivery, amniotic fluid infection, histologic chorioamnionitis, and chorioamnion infection. Obstet Gyneco. 1993, 81 (6): 941-948.
Bauer S, Pollheimer J, Hartmann J, Husslein P, Aplin JD, Knofler M: Tumor necrosis factor-alpha inhibits trophoblast migration through elevation of plasminogen activator inhibitor-1 in first-trimester villous explant cultures. Clin Endocrinol Metab. 2004, 89 (2): 812-822. 10.1210/jc.2003-031351.
Benyo DF, Miles TM, Conrad KP: Hypoxia stimulates cytokine production by villous explants from the human placenta. J Clin Endocrinol Metab. 1997, 82 (5): 1582-1588. 10.1210/jc.82.5.1582.
Frank GR, Brar AK, Cedars MI, Handwerger S: Prostaglandin E2 enhances human endometrial stromal cell differentiation. Endocrinology. 1994, 134: 258-263. 10.1210/en.134.1.258.
Dimitriadis E, Stoikos C, Baca M, Fairlie WD, McCoubrie JE, Salamonsen LA: Relaxin and prostaglandin E(2) regulate interleukin 11 during human endometrial stromal cell decidualization. J Clin Endocrinol Metab. 2005, 90 (6): 3458-3465. 10.1210/jc.2004-1014.
Shi JJ, Ma XH, Diao HL, Ni H, Xu LB, Zhu H, Yang ZM: Differential expression of prostaglandin E receptor subtype EP2 in rat uterus during early pregnancy. Histol Histopathol. 2005, 20 (4): 1021-1028.
Kao LC, Tulac S, Lobo S, Imani B, Yang JP, Germeyer A, Osteen K, Taylor RN, Lessey BA, Giudice LC: Global gene profiling in human endometrium during the window of implantation. Endocrinology. 2002, 143 (6): 2119-2138. 10.1210/en.143.6.2119.
Dey SK: Fatty link to fertility. Nature. 2005, 435: 34-35. 10.1038/435034a.
Tan HN, Liu Y, Diao HL, Yang ZM: Cyclooxygenases and prostaglandin E synthases in preimplantation mouse embryos. Zygote. 2005, 13 (2): 103-108. 10.1017/S0967199405003187.
Westermarck J, Kahari VM: Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J. 1999, 13 (8): 781-972.
Sternlicht MD, Werb Z: How Matrix Metalloproteinases Regulate Cell Behavior. Annu Rev Cell Dev Biol. 2001, 17: 463-516. 10.1146/annurev.cellbio.17.1.463.
Hidalgo M, Eckhardt SG: Development of Matrix Metalloproteinase Inhibitors in Cancer Therapy. J Natl Cancer Inst. 2001, 93 (3): 178-193. 10.1093/jnci/93.3.178.
Fini ME, Cook JR, Mohan R, Brinckerhoff CE: Regulation of matrix metalloproteinase gene expression. Matrix Metalloproteinases. Edited by: Parks WC, Mecham RP. 1998, New York: Academic, 299-356.
Strongin AY, Collier I, Bannikov G, Marmer BL, Grant GA, Goldberg GI: Mechanism of cell surface activation of 72-kDa type IV collagenase. Isolation of the activated form of the membrane metalloproteinase. J Biol Chem. 1995, 270: 5331-5338. 10.1074/jbc.270.10.5331.
Goldman S, Shalev E: The role of matrix metalloproteinases in human endometrial and ovarien cycles. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2003, 111: 109-121. 10.1016/S0301-2115(03)00341-5.
Niu R, Okamoto T, Iwase K, Nomura S, Mizutani : Quantitative Analysis of Matrix Metalloproteinase-2 and -9 and their Tissue Inhibitors-1 and -2 in Human Placenta through gestation. Life Sciences. 2000, 66 (12): 1127-1137. 10.1016/S0024-3205(00)00416-1.
Dey SK, Lim H, Das SK, Reese J, Paria BC, Daikoku T, Wang H: Molecular Cues to Implantation. Endocrine Reviews. 2004, 25 (3): 341-373. 10.1210/er.2003-0020.
Librach CL, Werb Z, Fitzgerald ML, Chiu K, Corwin NM, Esteves RA, Grobelny D, Galardy R, Damsky CH: 92-kDa type IV collagenase mediates invasion of human cytotrophoblasts. J Cell Biol. 1991, 113: 437-449. 10.1083/jcb.113.2.437.
Bischof P, Matelli M, Campana A, Itoh Y, Ogata Y, Nagase H: Importance of metalloproteinases in human trophoblast invasion. Early Pregnancy Biology and Medicine. 1995, 1 (4): 263-269.
Xu P, Wang Y, Zhu S, Luo S, Piao Y, Zhuang L: Expression of Matrix Metalloproteinase-2, -9 and -14, Tissue Inhibitors of Metalloproteinse-1, and Matrix Proteins in Human Placenta During the First trimester. Biol Reprod. 2000, 62: 988-994. 10.1095/biolreprod62.4.988.
Isaka K, Usuda S, Ito H, Sagawa Y, Nakamura H, Nishi H, Suzuki Y, Li YF, Takayama M: Expression and Activity of Matrix Metalloproteinase 2 and 9 in Human Trophoblasts. Placenta. 2003, 24: 53-64. 10.1053/plac.2002.0867.
Puistola U, Ronnberg L, Martikainen H, Turpeenniemi-Hujanen T: The human embryo produces basement membrane collagen (type IV collagen)-degrading protease activity. Hum Reprod. 1989, 4: 309-311.
Turpeenniemi-Hujanen T, Feinberg RF, Kauppila A, Puistola U: Laminin in the human embryo implantation: analog to the invasion by malignant cells. Fertil Steril. 1992, 58: 105-113.
Wang Y, Zhang Y, Lewis DF, Gu Y, Li H, Granger DN, Alexander JS: Protease chymotrypsin mediates the endothelial expression of P- and E-selectin, but not ICAM and VCAM, induced by placental trophoblasts from pre-eclamptic pregnancies. Placenta. 2003, 24 (8–9): 851-861. 10.1016/S0143-4004(03)00132-2.
Yu Q, Stamenkovic I: Cell surface-localized matrix metalloproteinases-9 proteolytically activates THF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000, 14: 163-176.
Martin DC, Fowlkes JL, Babic B, Khokha R: Insulin-like growth factor II signaling in neoplastic proliferation is blocked by transgenic expression of the metalloproteinase inhibitor TIMP-1. J Cell Biol. 1999, 146: 881-892. 10.1083/jcb.146.4.881.
Fernandez-Patron C, Radomski MW, Davidge ST: Vascular matrix metalloproteinase-2 cleaves big endothelin-1 yielding a noval vasoconstrictor. Circ Res. 1999, 85: 906-911.
Patterson BC, sang QA: Angiostatin-converting enzyme activities of human matrilysin (MMP-7) and gelatinase B/type IV collagenase (MMP-9). J Biol Chem. 1997, 272: 28823-28825. 10.1074/jbc.272.46.28823.
Itoh T, Ikeda T, Gomi H, Nakao S, Suzuki T, Itohara S: Unaltered secretion of beta-amaloid precursor protein in gelatinase A (matrix metalloproteinase 2)-deficient mice. J Biol Chem. 1997, 272 (36): 22389-22392. 10.1074/jbc.272.36.22389.
Dubois B, Arnold B, Opdenakker G: Gelatinase B deficiency impairs reproduction. J Clin Invest. 2000, 106 (5): 627-628.
Holmbeck K, Bianco P, Caterina J, Yamada S, Kromer M, Kuznetsov SA, Mankani M, Robey PG, Poole AR, Pidoux I, Ward JM, Birkedal-Hansen H: MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen tuerover. Cell. 1999, 99 (1): 81-92. 10.1016/S0092-8674(00)80064-1.
Rudolph-Owen LA, Hulboy DL, Wilson CL, Mudgett J, Matrisian LM: Coordinate expression of matrix metalloproteinase family members in the uterus of normal, matrilysin-deficient, and stromelysin 1-deficient mice. Endocrinol. 1997, 138: 4902-4911. 10.1210/en.138.11.4902.
Pendas AM, Balbin M, Llana E, Jimenez MG, Lopez-Otin C: Structural analysis and promoter characterization of the human collagenase-3 gene (MMP13). Genomics. 1997, 40: 222-33. 10.1006/geno.1996.4554.
Bischof P, Truong K, Campana A: Regulation of Trophoblastic Gelatinases by Proto-oncogenes. Placenta. 2003, 24: 155-163. 10.1053/plac.2002.0890.
Yamamoto H, Flannery ML, Kupriyanov S, Pearce J, McKercher SR, Henkel GW, Maki RA, Werb Z, Oshima RG: Defective trophoblast function in mice with a targeted mutation of Ets2. Genes Dev. 1998, 12 (9): 1315-1326.
Wang HZ, Zhao YG, Wang HM, Yang Q, Lin HY, Sang QX, Zhu C: Expression of adamalysin 19/ADAM19 in the endometrium and placenta of rhesus monkey (Macaca mulatta) during early pregnancy. Mol Hum Reprod. 2005, 11 (6): 429-435. 10.1093/molehr/gah183.
Baker AH, Edwards DR, Murphy G: Metalloproteinase inhibitors: biological actions and therapeutic opportunities. J Cell Sci. 2002, 115 (Pt 19): 3719-3727. 10.1242/jcs.00063.
Goldberg GI, Strongin A, Collier IE, Genrich LT, Marmer BL: Interaction of 92 kDa type IV collagenase with the tissue inhibitor of metalloproteinases prevents dimerization, complex formation with interstitial collagenase, and activation of the pro-enzyme with stromelysin. J Biol Chem. 1992, 267: 4583-4591.
Stetler-Stevenson WG, Krutzsch HC, Liotta LA: Tissue inhibitor of metalloproteinase-2 (TIMP-2), a new member of metalloproteinase inhibitor family. J Biol Chem. 1989, 264: 17374-17378.
Hurskainen T, Hoyhtya M, Tuuttila A, Oikarinen A, Autio-Harmainen H: mRNA expression of TIMP-1, -2 and -3 and 92-kDa type IV collagenase in early human placenta and decidual membrane as studied by in situ hybridization. J Histochem Cytochem. 1996, 44: 1379-1388.
Ruck P, Marzusch K, Horny H-P, Diet J, Kaiserling E: The distribution of tissue inhibitor of metalloproteinases-2 (TIMP-2) in the human placenta. Placenta. 1996, 17: 263-266. 10.1016/S0143-4004(96)90047-8.
Zhang J, Zhao YG, Cao YJ, Sang QX, Duan EK: Expression and implications of tissue inhibitor of metalloproteinase-4 in mouse embryo. Mol Hum Reprod. 2003, 9 (3): 143-149. 10.1093/molehr/gag019.
Zhang J, Cao YJ, Zhao YG, Sang QX, Duan EK: Expression of matrix metalloproteinase-26 and tissue inhibitor of metalloproteinase-4 in human normal cytotrophoblast cells and a choriocarcinoma cell line, JEG-3. Mol Hum Reprod. 2002, 8 (7): 659-666. 10.1093/molehr/8.7.659.
Wang H, Wen Y, Mooney S, Li H, Behr B, Polan ML: Matrix metalloproteinase and tissue inhibitor of matrix metalloproteinase expression in human preimplantation embryos. Fertility and Sterility. 2003, 80 (suppl 2): 736-742. 10.1016/S0015-0282(03)00782-9.
Chakraborty C, Gleeson LM, McKinnon T, Lala PK: Regulation of human trophoblast migration and invasiveness. Can J Physiol Pharmacol. 2002, 80 (2): 116-124. 10.1139/y02-016.
Hayakawa T, Yamashita K, Uchijima M, Iwata K: Growth-promoting activity of tissue inhibitor of metalloproteinases (TIMP-1) for a wide range of cells. FEBS Letters. 1992, 298: 29-32. 10.1016/0014-5793(92)80015-9.
Hayakawa T, Yamashita K, Ohuchi E, Shinagawa A: Cell Growth promoting activity of tissue inhibitor of metalloproteinase-2 (TIMP-1). J Cell Sci. 1994, 107: 2372-2379.
Satoh T, Kobayashi K, Yamashita S, Kikuchi M, Sendai Y, Hoshi H: Tissue inhibitor of metalloproteinases (TIMP-1) produced by granolosa and oviduct cells enhances in vitro development of bovine embryos. Biol Reprod. 1994, 50: 835-844. 10.1095/biolreprod50.4.835.
Vassalli JD, Sappino AP, Berlin D: The plasminogen activator/plasmin system. J Clin Invest. 1991, 88: 1067-1072.
Nordengren J, Pilka R, Noskova V, Ehinger A, Domanski H, Andersson C, Hoyer-Hansen G, Hansson SR, Casslen B: Differential localization and expression of urokinase plasminogen activator (uPA), its receptor (uPAR), and its inhibitor (PAI-1) mRNA and protein in endometrial tissue during the menstrual cycle. Mol Hum Reprod. 2004, 10 (9): 655-663. 10.1093/molehr/gah081.
Floridon C, Nielsen O, Holund B, Sunde L, Westergaard JG, Thomsen SG, Teisner B: Localization and significance of urokinase plasminogen activator and its receptor in placental tissue from intrauterine, ectopic and molar pregnancies. Placenta. 1999, 20 (8): 711-721. 10.1053/plac.1999.0425.
Aflalo ED, Sod-Moriah UA, Potashnik G, Har-Vardi I: Differences in the implantation rates of rat embryos developed in vivo and in vitro: possible role for plasminogen activators. Fertil Steril. 2004, 81 (Suppl 1): 780-785. 10.1016/j.fertnstert.2003.10.014.
Zhang X, Green KE, Yallampalli C, Dong YL: Adrenomedullin Enhances Invasion by Trophoblast Cell Lines. Biol Reprod. 2005,
Moriyama T, Otani T, Matyo T: Expression of adrenomedullin by human placental cytotrophoblasts and choriocarcinoma JAr cells. J Clin Endocrinol Metab. 2001, 86 (8): 3958-3961. 10.1210/jc.86.8.3958.
Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A: Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996, 380 (6573): 435-439. 10.1038/380435a0.
Shore VH, Wang CL, Torry RJ, Caudle MR, Torry DS: Vascular endothelial growth factor, placenta growth factor and their receptors in isolated human trophoblast. Placenta. 1997, 18: 657-665. 10.1016/S0143-4004(97)90007-2.
Cooper JC, Sharkey AM, Charnock-Jones DS, Palmer CR, Smith SK: VEGF mRNA levels in placentae from pregnancies complicated by pre-eclampsia. Br J Obstet Gynaecol. 1996, 103 (12): 1191-1196.
Torry DS, Wang HS, Wang TH, Caudle MR, Torry RJ: Preeclampsia is associated with reduced serum levels of placenta growth factor. Am J Obstet Gynecol. 1998, 179 (6 Pt 1): 1539-1544. 10.1016/S0002-9378(98)70021-3.
Chung IB, Yelian FD, Zaher FM, Gonik B, Evans MI, Diamond MP, Svinarich DM: Expression and Regulation of Vascular Endothelial Growth Factor in a First Trimester Trophoblast Cell Line. Placenta. 2000, 21: 320-324. 10.1053/plac.1999.0481.
Llurba E, Gratacos E, Martin-Gallan P, Cabero L, Dominquez C: A comprehensive study of oxidative stress and antioxidant status in preeclampsia and normal pregnancy. Free Radic Biol Med. 2004, 37 (4): 557-570. 10.1016/j.freeradbiomed.2004.04.035.
Hung TH, Charnock-Jones DS, Skepper JN, Burton GJ: Secretion of tumor necrosis factor-alpha from human placental tissues induced by hypoxia-reoxygenation causes endothelial cell activation in vitro: a potential mediator of the inflammatory response in preeclampsia. Am J Pathol. 2004, 164 (3): 1049-1061.
Vadachkoria S, Sanchez SE, Qiu C, Muy-Rivera M, Malinow MR, Williams MA: Hyperhomocyst(e)inemia and elevated soluble vascular cell adhesion molecule-1 concentrations are associated with an increased risk of preeclampsia. Gynecol Obstet Invest. 2004, 58 (3): 133-139. 10.1159/000079067.
Tziotis J, Malamitsi-Puchner A, Vlaschos G, Creatsas G, Michalas S: Adhesion molecules expression in the placental bed of pregnancies with pre-eclampsia. BJOG. 2002, 109 (2): 197-201.
Liu Q, Yan X, Li Y, Zhang Y, Zhao X, Shen Y: Pre-eclampsia is associated with the failure of melanoma cell adhesion molecule (MCAM/CD146) expression by intermediate trophoblast. Lab Invest. 2004, 84 (2): 221-228. 10.1038/labinvest.3700033.
Rajashekhar G, Loganath A, Roy AC, Wong YC: Expression and secretion of the vascular cell adhesion molecule-1 in human placenta and its decrease in fetal growth restriction. J Soc Gynecol Investig. 2003, 10 (6): 352-360. 10.1016/S1071-5576(03)00121-7.
Matsubara K, Abe E, Ochi H, Kusanagi Y, Ito M: Changes in serum concentrations of tumor necrosis factor alpha and adhesive molecules in normal pregnant women and those with pregnancy-induced hypertension. J Obstet Gynaecol Res. 2003, 29 (6): 422-426. 10.1111/j.1341-8076.2003.00141.x.
Author information
Authors and Affiliations
Corresponding author
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Staun-Ram, E., Shalev, E. Human trophoblast function during the implantation process. Reprod Biol Endocrinol 3, 56 (2005). https://doi.org/10.1186/1477-7827-3-56
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1477-7827-3-56