Abstract
A zone of trophoblast specification is established when the embryo is amorula, presumably reflecting a unique combination of transcription factors in that zone of cells and the influence of various environmental cues and growth factors on them. A key first step in this process of specification is the down-regulation of Oct4, a transcription factor that acts as a negative regulator of trophoblast specification and of genes normally up-regulated as the trophectoderm first forms. The transcription factors believed to have a positive association with trophectoderm specification have been inferred primarily in two ways: by their expression patterns in embryos, ES cells and TS cells and by the consequences of gene disruption on embryonic development. Many of these transcription factors also control the expression of genes characteristically expressed in trophoblast but not in the epiblast, primitive endoderm and their derivatives. ES and TS cells from the mouse and other species are beginning to provide insights into the changes in gene expression that accompany lineage specification and the subsequent post-specification events that lead to functional trophoblast derivatives.
Similar content being viewed by others
Introduction
Trophectoderm is the progenitor tissue of the entire outer epithelial component of the placenta, known as trophoblast, and provides the functional bridge between the fetus and the mother. Trophoblast, which ultimately consists of a range of terminally differentiated cell types, performs the majority of the absorptive, immunoprotective and endocrinological functions of the placenta. Trophectoderm differentiates as a simple epithelium, enclosing the fluid-filled blastocoel cavity and the pluripotent inner cell mass (i.c.m.). In the mouse, the best studied of all species, the transition from the morula stage to the blastocyst occurs at about day 3.5 post fertilization, whereas in humans and farm species, the process begins a few days later. Shortly after the formation of the blastocoel, an additional cell layer, known as either primitive or extraembryonic endoderm, the precursor of the visceral and parietal yolk sac, grows out from the blastocoelic surface of the i.c.m. and along the inner surface of the trophectoderm. Although not discussed further in this review, there is recent evidence from the mouse that the precursors of primitive endoderm can be identified on blastocysts between day 3.5 and 4.5 embryos as a group of cells segregating from the rest of the i.c.m. that express the transcription factor GATA6 [1]. Some days after this simple placenta of two cell layers forms, derivatives of the i.c.m. provide extraembryonic mesoderm and the precursors of the allantoic and amniotic membranes.
At about the time that the primitive endoderm emerges as an identifiable lineage of cells, human and mouse blastocysts are beginning to expand to "hatch" from the enclosing zona pellucida. They then quickly attach and start to implant. In contrast, implantation in many other species is delayed. In pigs for example, the conceptuses first expand and then elongate end to end along the villous folds of the uterus. Even more surprising, the trophoblast never erodes the uterine epithelium of the mother and never gains direct access to the maternal blood stream [2]. The situation is only slightly different in cattle, sheep and other ruminant species, where the invasive component is restricted to a subpopulation of so-called binucleate cells that fuse with maternal uterine epithelial cells but do not breach the underlying basement membrane [3]. Not surprisingly, the "end" cells of trophoblast differentiation (Fig. 1) are functionally various and their relative homologies across species not entirely clear. For example, there is no obvious equivalent of the murine spongiotrophoblast in cattle, pigs and their relatives. This structure in the mouse may be equivalent to the supportive cytotrophoblast cell columns seen in the invading human trophoblast, but has no obvious homolog in species where the trophoblast does not invade [see [4]]. It is possible that the rodent trophoblast giant cell is the functional homolog of the invasive extravillous cytotrophoblast cells of humans and the invasive binucleate cells of ruminants and horses, but, since homologies are not altogether clear, this review concentrates on the specification of the early trophoblast. We focus largely on the transcription factors that play a part in these early events. Much of the genetic and developmental information has been derived from the mouse where most information exists (Fig. 1), but wherever possible we have ventured into comparative placentation, particularly where some obvious threads of connection exist across species.
A comparison of trophoblast lineage derivation in the mouse (upper) and human (lower). This diagram is based on that of Cross et al. [4]. Trophoblast lineage cell types are illustrated in black letters, with the direction of differentiation shown in black arrows; other lineages are shown in gray. Key transcription factors that either support or drive differentiation are shown adjacent to the arrows. Most of these are discussed in the text. Attempts have been made to illustrate possible homology between the two species. Oct4 is expressed in the ICM both species. Mouse trophectoderm is only specified when Oct4 becomes down-regulated. As discussed in the text, Cdx2 and Eomes are required early in the mouse trophectoderm development. Mouse TS cells can be derived from blastocysts and early postimplantation trophoblasts and grow in the presence of FGF4. Removal of FGF4 from the TS cell culture causes them to differentiate into trophoblast giant cells and other trophoblast subtypes. Human ES cells are derived from blastocyst and maintained in the presence of bFGF. They are able to give rise to all cell types of the embryo but can also differentiate into trophoblast cells either spontaneously or in a directed manner when provided with BMP4. Id2 is expressed in both mouse chorionic trophoblast and human villous cytotrophoblast [11, 79], while Mash2 is expressed in murine spongiotrophoblast and in the cytotrophoblast columns of the human placenta [77, 80]. Hand1, is necessary for the formation of mouse giant cells but not for the specification of spongiotrophoblast and syncytiotropphoblast. By contrast, Mash2 (or Hash2 in the human), has the opposite effect to Hand1. In the giant cell lineage, Hand1 must be down-regulated for giant cells to form. The gene mSNA, which represses the transcription of genes that promote the transition from mitotic to endoreplicative cell cycles in mouse trophoblast [74], becomes down regulated during giant cell differentiation, but has not been studied in other species. Human extravillous cytotrophoblast may be the functional homolog of the rodent trophoblast giant cells, although expression of Hand1 has not been detected in human placental villi [75]. On the other hand, expression of Gcm1 in mouse labyrinth [76] and human chorionic villi [77, 78] is consistent with structural homology of the tissues.
Specification of trophectoderm in the mouse
At the time the mouse blastocyst implants, it contains three types of cell: epiblast (or i.c.m.), primitive endoderm, and trophectoderm. The segregation of the lineages appears to occur according to the positions that the cells occupy in the compacted morula, so that by the time the blastocyst is ready to implant, the three lineages are no longer interconvertible [1], although they continue to exhibit interdependence during subsequent post-implantation development. In modern parlance, each lineage has its own population of stem cells and expresses its own characteristic set of genes by the time the embryo consists of 32 to 64 cells. This patterning is achieved by the action of transcription factors, whose combinatorial expression, most probably directed by growth factors and fine-tuned by environmental inputs such as oxygen and nutrient availability, establishes boundaries of cell lineage specification within the early embryo.
Transcription factors involved in trophoblast lineage specification
Knockout experiments in the mouse have revealed many genes that when deleted cause embryonic lethality. Sometimes these defects arise from failure within the epiblast lineage, but a significant number of such mutations cause the trophoblast to develop improperly and the conceptus to die before a fully functional placenta is formed [1, 4–8]. The majority of such mutations affecting the trophoblast have been discovered by chance when the investigators were in reality hoping to observe a phenotype in the adult animal, but were instead confronted by a failure to obtain homozygous mutants that died well before birth.
Other knockouts have been intentionally engineered after noting an association in expression of a particular gene with a specific placental cell type or after cloning out expressed cDNA from placental tissues. Examples of the latter have included knockouts for several genes encoding transcription factors that have been associated with some of the developmental transitions shown in Fig. 1. Thus, the basic helix-loop-helix (bHLH) gene, Hand1, is necessary for the formation of mouse giant cells but not for the specification of spongiotrophoblast and syncytiotrophoblast. By contrast, Mash2 (or Hash2 in the human) another bHLH gene, seems to have an opposing effect to Hand1 in the giant cell lineage and must be down-regulated for giant cells to form. Likewise, the gene, Gcm1, must be active for syncytiotrophoblast to develop from its precursors. This ability to limit a particular developmental transition in the trophoblast does not imply that these factors are expressed exclusively in that lineage of cells. For example, the Hand1 gene is expressed in heart and several other tissues derived from the epiblast [9]. Presumably, it is not the presence of either single transactivators or silencing factors that drive specification events, but a combination of such factors in the correct proportions within the context of the precursor cell (Fig. 1). For example, several additional positive and negative regulators have been implicated in the formation of trophoblast giant cells from their mitotic precursors. These include mSNA (a zinc finger transcription factor) [74], SOCS3 (a suppressor of cytokine signaling) [10], Id2, which is a dominant-negative antagonist of bHLH transcription factors [11], and the orphan nuclear receptor Errβ [12]. As the products identified in genetic screens for trophoblast-expressed genes increase and are further analyzed, the list of transcription factors involved in lineage specification will undoubtedly grow too [13].
Some transcription factors that are widely expressed in adult and fetal tissues have been demonstrated in knock out studies to be required for placental development. Good examples are Ets2 [14], AP-2γ [15, 16], and some of the subunit genes for AP-1 [17, 18]. In each case, blastocysts form and implant, but the trophoblast exhibits early abnormalities, and the conceptus soon dies. The role of these familiar transcription factors in lineage-specific events is of particular interest as they also regulate production of characteristic secretory products of trophoblast (discussed later).
Genes required for initial trophectoderm specification
Some murine genes, when deleted, cause developmental failure before or at implantation. A good example is the POU-domain transcription factor, Oct4 (encoded by Pou5f1, we refer here as Oct4). Normally, Oct4 is expressed in the nuclei of all cells of the cleavage stage embryo, but by the time the blastocyst forms, its expression is restricted to the i.c.m. [19]. When the gene is knocked out, however, no i.c.m. forms and all cells of the embryo default to trophectoderm [20]. Accordingly, trophectoderm is only specified in the mouse when Oct4 becomes down-regulated. This generality does not apply across all species, however. In bovine embryos, Oct4 can be detected in trophectoderm until day 10, two to three days after the blastocyst first forms [21, 22], although its expression there is clearly lower than in the i.c.m. (Fig. 2). Indeed it may be that the mouse is the exception rather than the rule, since Oct4 is also expressed in early human trophectoderm [23]. These observations indicate that Oct4 is not a binary off-on switch but that its dosage is critical.
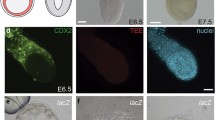
Oct-4 expression in an in vitro-derived d10 bovine embryo. Strong Oct-4 nuclear immunofluorescence (left panel; red) is detected in the i.c.m. at the left pole of the embryo, while weaker, more diffuse immunofluorescence is present over trophectoderm. DAPI nuclear staining (blue) is shown in the center panel. In the right panel, Oct-4 (red) and DAPI (blue) signals have been merged. The merged image indicates that, whereas all nuclei of the i.c.m. are strongly Oct-4 positive, Oct-4 signals are weaker and more variable over trophectoderm, with some cells apparently Oct-4 negative. Controls performed with a non-relevant IgG failed to show nuclear staining (not shown). Positive controls (also not shown) with the anti-Oct-4 IgG provided nuclear staining in undifferentiated F9 embryonic carcinoma cells but not in JAr choriocarcinoma cells (data not shown). The bovine embryos were fixed with 2% paraformaldehyde-PBS for 30 min at room temperature, permeabilized with 1% Triton X-100 for 30 min, and incubated overnight at 4 C with primary antibody (affinity purified rabbit anti-Oct-4 IgG in PBS; T.E., R.M.R. unpublished) at a concentration of 4 ng/μl. After washing, the blastocysts were exposed to secondary antibody (goat anti-rabbit IgG conjugated with Alexa Fluor 568; Molecular Probes, Eugene, OR) diluted 1:1000. Nuclear staining was performed with DAPI (Sigma, St. Louis, MO) at a concentration of 5 ng/μl. Bars represent 100 μm. Images were captured by using a Nikon Eclipse 800 microscope equipped with a CoolSnap HQ RTE/CCD 1217 digital camera operated by MetaMorph 4.6 software (Universal Imaging Corp., Downington, PA) and edited by Adobe Photoshop 6.0.
The Sox2 transcription factor exhibits a similar expression pattern to Oct4 during early development of the mouse except that it remains active in trophectoderm. Its deletion causes early post implantation failure, but does not prevent blastocyst formation and the differentiation of trophectoderm. Sox2 is, however, important in lineage specification because it and Oct4 together regulate the production of FGF4 by the epiblast, a necessary growth factor for trophectoderm proliferation [[1, 8, 24], and references therein]. Accordingly, Oct4 has a dual role. Its down-regulation is permissive for trophectoderm to be specified, but its expression with Sox2 in the i.c.m. leads to the production of a required trophoblast growth factor.
As pointed out by Rossant et al. [1], the formation of trophectoderm is not simply a default pathway initiated by the down-regulation of Oct4, but probably requires specific transcription factors. One of these is the homeodomain protein, Cdx2, and another is the T-box gene Eomes. Both show a reciprocal pattern of expression to Oct4 at the blastocyst stage [25, 26], i.e. they are absent from the i.c.m. but expressed in trophectoderm. Cdx2 [27] and Eomes [28] knockout embryos fail to implant, although they develop to the early blastocyst stage but generally advance no further, and do not form trophoblast outgrowths when cultured. Finally, Cdx2-/- embryos cannot be coaxed to produce trophectoderm stem cells [8]. It seems fair to conclude that Cdx2 and Eomes are required early in the development of trophectoderm, but possibly not in its earliest specification. At a minimum, murine trophectoderm requires the down-regulation of Oct4 followed by the up-regulation of Cdx2, Eomes. For the lineage to expand, the i.c.m. must produce the Sox2/Oct4-regulated FGF4 growth factor (Fig. 1). Whether these general rules apply universally across species remains unclear.
ES cells, trophoblast stem (TS) cells, and their interrelationship
Embryonic stem (ES) cells can now be routinely derived from the isolated i.c.m. of mouse blastocysts and maintained in a continuously dividing, undifferentiated mode as long as the cytokine leukemia inhibitory factor (LIF) is provided in the medium [[1, 8] and references therein]. These cell lines are generally pluripotent and able to give rise to a variety of differentiated cell types when LIF is withdrawn.
ES cell lines have been derived from two species of monkey [29] and from human blastocysts [30]. Paradoxically, the primate cell lines fail to show any dependence on LIF for continued proliferation in the undifferentiated state. Instead of LIF, these cells need basic FGF (bFGF; Fig. 1) and the support of additional factors that are best supplied by a "feeder" layer of embryonic fibroblasts [31]. ES cells from these primate species can be induced during in vitro culture to progress to a wide range of differentiated cell types and are considered, like those from mouse, to be pluripotent. This pluripotency endows ES cells with exciting potential for use in tissue repair and replacement. In the case of the mouse, pluripotency has been demonstrated conclusively by introducing the cells into blastocysts, where they colonize the i.c.m. rather than trophectoderm and ultimately contribute to the entire embryo, including the germ cells [see [1, 8]]. The failure of mouse ES cells to form trophoblast in such chimeras and not to differentiate into trophoblast cells in culture is puzzling since the ES cells derived from primate embryos spontaneously form trophoblast over time and can be induced to do so rapidly if supplied with the growth factor BMP4 [32]. Whether BMP4 is the signal for trophoblast differentiation in vivo remains to be determined. Such a determination will require either a genetic knock out approach, which can only ethically be performed on primates, or the use of RNAi or other silencing technologies whose outcome is often equivocal.
There are, however, circumstances when mouse ES cells can be directed towards trophoblast. The effects of mutating both copies of the Oct4 gene on embryo development has been noted earlier [20], and conditional knock out of the same gene in cultured ES cells causes cells expressing trophoblast markers to appear in culture [33]. On the other hand, removal of LIF, which effectively causes Oct4 to be silenced, does not lead to directed or widespread trophoblast differentiation of ES cell colonies. Instead, other differentiated cell types appear [34]. To explain this paradox, Hemburger et al. [35] have suggested that mouse ES cells contain some trophectoderm precursor cells at a low frequency. They have gone on to show that disruption of the gene for the enzyme poly(ADP-ribose) polymerase (Parp1) increases the likelihood of mouse ES cells differentiating spontaneously towards trophoblast. It seems conceivable that the difference between the primate ES cell, which tends to form trophoblast readily, and the mouse ES cell, which does not, may not be as profound as previously thought. Slight differences in responses to external signals, possibly the result of differences in receptor numbers or intracellular signaling pathways, and in the expression levels of a few key genes may be sufficient to favor one pathway of "spontaneous" differentiation over another. It should be recalled that mouse and human stem cells require different growth conditions to continue dividing and to remain pluripotent. Mouse ES cells need LIF, whereas LIF seems unimportant to human ES cells, which have a requirement for bFGF instead.
Cells with some features of ES cells have been derived from domestic species, such as sheep, cattle and swine [36–40], but there has been little follow-up since. It remains unclear whether or not these cells can transform into trophoblast, although they can form an array of differentiated derivatives suggestive of an epiblast origin.
Trophoblast stem (TS) cells, seemingly capable of forming only trophectoderm derivatives, i.e. restricted in their developmental potential, have been isolated for mouse but not yet from primate embryos (unless primate ES cells are regarded also as TS cells). Two approaches have been used to derive murine TS cells. The first has been to culture trypsin-dissociated cells from the ectoplacental cone of implanting day 6.5 mouse embryos [41]. The second has been to use primary outgrowths from cultured blastocysts [8]. In both, FGF4 and a growth medium conditioned by embryonic fibroblasts were critical requirements for the cells to proliferate. Removal of FGF4 led to spontaneous differentiation into trophoblast giant cells and the expression of a range of genes characteristic of end-stage trophoblast. A requirement for FGF4 was inferred from expression patterns in early embryos where it is expressed only by the epiblast and its precursor, the i.c.m. [1, 8, 42]. In contrast, its receptor first appears in outer blastomeres of morulae and then becomes localized on trophectoderm [43, 44]. Moreover, embryos lacking either a functional gene for FGF4 [45] or for FGFR2 [46], its receptor, fail to grow after implantation.
Various bovine, ovine, porcine and caprine trophoblast cell lines have been described [47–52], although, as discussed below, their status as continuously proliferating stem cells is unclear. One reasonably well characterized bovine cell line, CT-1, which was derived from a blastocyst outgrowth, has special growth requirements, best provided by a feeder layer of fibroblasts, and expresses genes, such as those for interferon (IFN)-τ [53], pregnancy-associated glycoproteins and trophoblast Kunitz-domain proteins characteristic of the bovine trophoblast lineage (J. MacLean, J. Green, & R.M. Roberts, unpublished results). Whether CT-1 or any of the other trophoblast lines are truly stem cells, representing the very earliest stages of lineage specification, is unclear, since they clearly express post-specification genes. They may be progenitor cells, one step along the committed lineage. They have not been well studied with regard to either gene expression or their capacity to differentiate in response to external cues.
There are, in addition, to the cell lines described above a number of trophoblast lines derived from tumors. These include the rat Rcho cell line [54], and an analogous human embryonal carcinoma line HT-H [55]. In both lines, dividing cells easily detach from substratum, but differentiate spontaneously under crowded culture conditions to larger, adherent cells with a more differentiated phenotype. Rcho cultures convert to giant cells with endoduplicated DNA in their nuclei and produce placental lactogen (PL)-I and -II, while HT-H cells form what resembles syncitial trophoblast that are active in steroid synthesis and hCG production. Such cell lines are proving to be valuable in following changes in gene expression that accompany differentiation. There are, in addition, many cell lines that have been derived from human choriocarcinomas, the best known of which are JAr, BeWo, and Jeg-3. Exactly where these cells fit in the in the human trophoblast lineage is unclear, but like Rcho and HT-H cells, they are probably transformed derivatives of early stage trophoblast progenitor cells. Human choriocarcinoma cell lines have been invaluable in determining the processes that control expression of trophoblast products such as hCG, placental lactogens, and steroid hormones [56].
Transcriptional profiling of differentiating TS cells
Attempts are being made to use various types of transcriptional profiling to define the phenotype of differentiating of TS cells, and the changes that occur over time as such cells differentiate [41, 57–59]. Oct4, for example, is not detectable in murine TS cells, while the transcription factors Errβ, Cdx2, and mEomes are strongly expressed when the cells are proliferating, but down-regulated as differentiation begins and when markers such as PL-I begin to appear.
Microarrays have been employed to follow the BMP4-induced transition of human ES cells to cells that actively produce hCG, HLA-G, estradiol and progesterone [32]. After 7 days of BMP4 treatment, the mRNA for two genes characteristic of pluripotent ES cells, Oct4 and telomerase reverse transcriptase (TERT), had significantly decreased. In contrast, BMP4 rapidly up-regulated some transcription factors, including Cdx2, GATA2, GATA3, and AP-2 genes (TFAP2), which had been previously implicated in either placental development or in regulating genes characteristically expressed in differentiating trophoblast. Curiously, the human orthologs of two other transcription factors, Errβ and Mash2, which have been discussed earlier in relation to their likely central role in specifying mouse trophoblast (Fig. 1), were expressed in the original ES cells and showed no significant change in response to BMP4. Attempts to make sense of this microarray information and to make valid comparisons across species are confounded by the sheer volume of the data and by the fact that the cultures undoubtedly contain a range of cell types. Resolving the inconsistencies will not be easy.
Transcription factors that control expression of trophoblast products
As trophectoderm emerges and then progresses to form the end-stage cells of mature trophoblast, a series of characteristic products, including several hormones, are released that play important roles in maintaining the pregnancy. For example, IFN-τ, the luteoprotective hormone of ruminant species, is first produced by the early bovine blastocyst and is massively up-regulated a few days later [60, 61]. A somewhat similar situation has been observed for hCG production in the human [62] and PL-I production by the mouse [63]. Analysis of the promoters regions of such genes has revealed that they are under the control of many of the same transcription factors, that, when knocked out in the mouse, cause trophoblast development to fail. A good example is Ets2, a gene essential for placenta formation in the mouse [14]. Ets2 is also a key transcriptional regulator of the IFN-τ genes (IFNT) in cattle and sheep [64], the hCGα and -β subunit genes [65, 66], and CYP11 (P-450 side-chain cleavage enzyme) genes in human [67], and the PL-II gene in the mouse [68]. Ets2 also regulates the urokinase-type plasminogen activator and several metalloproteinases, including MMP9, likely to be involved in invasive implantation [69, 70]. Cross et al. [4] discusses additional examples of transcription factors that regulate both trophoblast development and placental hormone production. It seems reasonable to conclude that many of the same genes that control cell fate in the trophoblast lineage also control the transcription of genes characteristically expressed in differentiated, end-stage trophoblast cells.
Several, possibly the majority, of the genes that are transcriptionally up-regulated as trophectoderm first forms and differentiates are silenced by Oct4 [see [71]]. We have suggested that one way that Oct4 acts in pluripotent ES cells and in epiblast is to silence the expression of genes that are lineage-specific for trophectoderm, such as those encoding the hCGα and -β subunits [72, 73], and IFNT [71]. Once Oct4 itself is down-regulated, this restraint is relieved and the genes can come under the control of transcriptional activators.
Conclusions
A zone of trophoblast specification is established when the embryo is a morula, presumably reflecting a unique combination of transcription factors in that zone of cells and the influence of various environmental cues and growth factors on them. A key first step in this process of specification is the down-regulation of Oct4, a transcription factor that acts as a negative regulator of trophoblast specification and of genes normally up-regulated as the trophectoderm first forms. The transcription factors believed to have a positive association with trophectoderm specification have been inferred primarily in two ways: by their expression patterns in embryos, ES cells and TS cells and by the consequences of gene disruption on embryonic development. Many of these transcription factors also control the expression of genes characteristically expressed in trophoblast but not in the epiblast, primitive endoderm and their derivatives. ES and TS cells from the mouse and other species are beginning to provide insights into the changes in gene expression that accompany lineage specification and the subsequent post-specification events that lead to functional trophoblast derivatives.
References
Rossant J, Chazaud C, Yamanaka Y: Lineage allocation and asymmetries in the early mouse embryo. Philos Trans R Soc Lond B Biol Sci. 2003, 358: 1341-1349.
Geisert RD, Yelich JV: Regulation of conceptus development and attachment in pigs. J Reprod Fertil Suppl. 1997, 52: 133-149.
Hoffman LH, Wooding FB: Giant and binucleate trophoblast cells of mammals. J Exp Zool. 1993, 266: 559-577.
Cross JC, Anson-Cartwright L, Scott IC: Transcription factors underlying the development and endocrine functions of the placenta. Recent Prog Horm Res. 2002, 57: 221-234. 10.1210/rp.57.1.221.
Rossant J, Cross JC: Placental development: lessons from mouse mutants. Nat Rev Genet. 2001, 2: 538-548. 10.1038/35080570.
Hemberger M, Cross JC: Genes governing placental development. Trends Endocrinol Metab. 2001, 12: 162-168. 10.1016/S1043-2760(01)00375-7.
Knofler M, Vasicek R, Schreiber M: Key regulatory transcription factors involved in placental trophoblast development-a review. Placenta. 2001, 22: S83-S92. 10.1053/plac.2001.0648.
Rossant J: Stem Cells from the mammalian blastocyst. Stem Cells. 2001, 19: 477-482.
Firulli AB: A HANDful of questions: the molecular biology of the heart and neural crest derivatives (HAND)-subclass of basic helix-loop-helix transcription factors. Gene. 2003, 312: 27-40. 10.1016/S0378-1119(03)00669-3.
Takahashi Y, Carpino N, Cross JC, Torres M, Parganas E, Ihle JN: SOCS3: an essential regulator of LIF receptor signaling in trophoblast giant cell differentiation. EMBO J. 2003, 22: 372-384. 10.1093/emboj/cdg057.
Janatpour MJ, McMaster MT, Genbacev O, Zhou Y, Dong J, Cross JC, Israel MA, Fisher SJ: Id-2 regulates critical aspects of human cytotrophoblast differentiation, invasion and migration. Development. 2000, 127: 549-558.
Luo J, Sladek R, Bader JA, Matthyssen A, Rossant J, Giguere V: Placental abnormalities in mouse embryos lacking the orphan nuclear receptor ERR-beta. Nature. 1997, 388: 778-782. 10.1038/42022.
Hemberger M, Cross JC, Ropers HH, Lehrach H, Fundele R, Himmelbauer H: UniGene cDNA array-based monitoring of transcriptome changes during mouse placental development. Proc Natl Acad Sci USA. 2001, 98: 13126-13131. 10.1073/pnas.231396598.
Yamamoto H, Flannery ML, Kupriyanov S, Pearce J, McKercher SR, Henkel GW, Maki RA, Werb Z, Oshima RG: Defective trophoblast function in mice with a targeted mutation of Ets2. Genes Dev. 1998, 12: 1315-1326.
Auman HJ, Nottoli T, Lakiza O, Winger Q, Donaldson S, Williams T: Transcription factor AP-2gamma is essential in the extra-embryonic lineages for early postimplantation development. Development. 2002, 129: 2733-2747.
Werling U, Schorle H: Transcription factor gene AP-2 gamma essential for early murine development. Mol Cell Biol. 2002, 22: 3149-3156. 10.1128/MCB.22.9.3149-3156.2002.
Schorpp-Kistner M, Wang ZQ, Angel P, Wagner EF: JunB is essential for mammalian placentation. EMBO J. 1999, 18: 934-948. 10.1093/emboj/18.4.934.
Schreiber M, Wang ZQ, Jochum W, Fetka I, Elliott C, Wagner EF: Placental vascularisation requires the AP-1 component Fra1. Development. 2000, 127: 4937-4948.
Palmieri SL, Peter W, Hess H, Scholer HR: Oct-4 transcription factor is differentially expressed in the mouse embryo during establishment of the first two extraembryonic cell lineages involved in implantation. Dev Biol. 1994, 166: 259-267. 10.1006/dbio.1994.1312.
Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, Scholer H, Smith A: Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998, 95: 379-391. 10.1016/S0092-8674(00)81769-9.
van Eijk MJ, van Rooijen MA, Modina S, Scesi L, Folkers G, van Tol HT, Bevers MM, Fisher SR, Lewin HA, Rakacolli D, Galli C, de Vaureix C, Trounson AO, Mummery CL, Gandolfi F: Molecular cloning, genetic mapping, and developmental expression of bovine POU5F1. Biol Reprod. 1999, 60: 1093-1103.
Kirchhof N, Carnwath JW, Lemme E, Anastassiadis K, Scholer H, Niemann H: Expression pattern of Oct-4 in preimplantation embryos of different species. Biol Reprod. 2000, 63: 1698-1705.
Hansis C, Grifo JA, Krey LC: Oct-4 expression in inner cell mass and trophectoderm of human blastocysts. Mol Hum Reprod. 2000, 6: 999-1004. 10.1093/molehr/6.11.999.
Avilion AA, Nicolis SK, Pevny LH, Perez L, Vivian N, Lovell-Badge R: Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 2003, 17: 126-140. 10.1101/gad.224503.
Beck F, Erler T, Russell A, James R: Expression of Cdx-2 in the mouse embryo and placenta: possible role in patterning of the extra-embryonic membranes. Dev Dyn. 1995, 204: 219-227.
Hancock SN, Agulnik SI, Silver LM, Papaioannou VE: Mapping and expression analysis of the mouse ortholog of Xenopus Eomesodermin. Mech Dev. 1999, 81: 205-208. 10.1016/S0925-4773(98)00244-5.
Chawengsaksophak K, James R, Hammond VE, Kontgen F, Beck F: Homeosis and intestinal tumours in Cdx2 mutant mice. Nature. 1997, 386: 84-87. 10.1038/386084a0.
Russ AP, Wattler S, Colledge WH, Aparicio SA, Carlton MB, Pearce JJ, Barton SC, Surani MA, Ryan K, Nehls MC, Wilson V, Evans MJ: Eomesodermin is required for mouse trophoblast development and mesoderm formation. Nature. 2000, 404: 95-99. 10.1038/35003601.
Thomson JA, Marshall VS: Primate embryonic stem cells. Curr Top Dev Biol. 1998, 38: 133-165.
Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM: Embryonic stem cell lines derived from human blastocysts. Science. 1998, 282: 1145-1147. 10.1126/science.282.5391.1145.
Jones JM, Thomson JA: Human embryonic stem cell technology. Semin Reprod Med. 2000, 18: 219-223. 10.1055/s-2000-12560.
Xu RH, Chen X, Li DS, Li R, Addicks GC, Glennon C, Zwaka TP, Thomson JA: BMP4 initiates human embryonic stem cell differentiation to trophoblast. Nat Biotechnol. 2002, 20: 1261-1264. 10.1038/nbt761.
Velkey JM, O'Shea KS: Oct4 RNA interference induces trophectoderm differentiation in mouse embryonic stem cells. Genesis . 2003, 37: 18-24. 10.1002/gene.10218.
Ishiwata I, Tokeida Y, Iguchi M, Ishiwata C, Kiguchi K, Yasumoto S, Sato K, Tachibana T, Hashimoto H, Ishikawa H: New approach for the establishment of mouse early embryonic stem cells and induction of their differentiation. Hum Cell. 2001, 14: 283-291.
Hemberger M, Nozaki T, Winterhager E, Yamamoto H, Nakagama H, Kamada N, Suzuki H, Ohta T, Ohki M, Masutani M, Cross JC: Parp1-deficiency induces differentiation of ES cells into trophoblast derivatives. Dev Biol. 2003, 257: 371-381. 10.1016/S0012-1606(03)00097-6.
Godkin JD, Bazer FW, Roberts RM: Protein production by cultures established from Day-14–16 sheep and pig conceptuses. J Reprod Fertil. 1985, 74: 377-382. 10.1530/jrf.0.0740377.
Notarianni E, Galli C, Laurie S, Moor RM, Evans MJ: Derivation of pluripotent, embryonic cell lines from the pig and sheep. J Reprod Fertil Suppl. 1991, 43: 255-260.
Wheeler MB: Development and validation of swine embryonic stem cells: a review. Reprod Fertil Dev. 1994, 6: 563-568.
Talbot NC, Powell AM, Rexroad CE: In vitro pluripotency of epiblasts derived from bovine blastocysts. Mol Reprod Dev. 1995, 42: 35-52.
Shim H, Gutierrez-Adan A, Chen LR, BonDurant RH, Behboodi E, Anderson GB: Isolation of pluripotent stem cells from cultured porcine primordial germ cells. Biol Reprod. 1997, 57: 1089-1095.
Tanaka S, Kunath T, Hadjantonakis AK, Nagy A, Rossant J: Promotion of trophoblast stem cell proliferation by FGF4. Science. 1998, 282: 2072-2075. 10.1126/science.282.5396.2072.
Goldin SN, Papaioannou VE: Paracrine action of FGF4 during periimplantation development maintains trophectoderm and primitive endoderm. Genesis. 2003, 36: 40-47. 10.1002/gene.10192.
Haffner-Krausz R, Gorivodsky M, Chen Y, Lonai P: Expression of Fgfr2 in the early mouse embryo indicates its involvement in preimplantation development. Mech Dev. 1999, 85: 167-172. 10.1016/S0925-4773(99)00082-9.
Rappolee DA, Patel Y, Jacobson K: Expression of fibroblast growth factor receptors in peri-implantation mouse embryos. Mol Reprod Dev. 1998, 51: 254-264. 10.1002/(SICI)1098-2795(199811)51:3<254::AID-MRD4>3.3.CO;2-8.
Feldman B, Poueymirou W, Papaioannou VE, DeChiara TM, Goldfarb M: Requirement of FGF-4 for postimplantation mouse development. Science. 1995, 267: 246-249.
Arman E, Haffner-Krausz R, Chen Y, Heath JK, Lonai P: Targeted disruption of fibroblast growth factor (FGF) receptor 2 suggests a role for FGF signaling in pregastrulation mammalian development. Proc Natl Acad Sci USA. 1998, 95: 5082-5087. 10.1073/pnas.95.9.5082.
Steven DH, Mallon KA, Nathanielsz PW: Sheep trophoblast in monolayer cell culture. Placenta. 1980, 1: 209-221.
Ramsoondar J, Christopherson RJ, Guilbert LJ, Wegmann TG: A porcine trophoblast cell line that secretes growth factors which stimulate porcine macrophages. Biol Reprod. 1993, 49: 681-694.
Flechon JE, Laurie S, Notarianni E: Isolation and characterization of a feeder-dependent, porcine trophectoderm cell line obtained from a 9-day blastocyst. Placenta. 1995, 16: 643-658. 10.1016/0143-4004(95)90033-0.
Shimada A, Nakano H, Takahashi T, Imai K, Hashizume K: Isolation and characterization of a bovine blastocyst-derived trophoblastic cell line, BT-1: development of a culture system in the absence of feeder cell. Placenta. 2001, 22: 652-662. 10.1053/plac.2001.0702.
Miyazaki H, Imai M, Hirayama T, Saburi S, Tanaka M, Maruyama M, Matsuo C, Meguro H, Nishibashi K, Inoue F, Djiane J, Gertler A, Tachi S, Imakawa K, Tachi C: Establishment of feeder-independent cloned caprine trophoblast cell line which expresses placental lactogen and interferon tau. Placenta. 2002, 23: 613-630. 10.1053/plac.2002.0846.
Marcelo P, Lefevre F: Induction of the IFN-gamma gene and protein is linked to the establishment of cell polarity in a porcine epithelial cell line. Exp Cell Res. 2002, 280: 33-44. 10.1006/excr.2002.5619.
Talbot NC, Caperna TJ, Edwards JL, Garrett W, Wells KD, Ealy AD: Bovine blastocyst-derived trophectoderm and endoderm cell cultures: interferon tau and transferrin expression as respective in vitro markers. Biol Reprod. 2000, 62: 235-247.
Faria TN, Soares MJ: Trophoblast cell differentiation: establishment, characterization, and modulation of a rat trophoblast cell line expressing members of the placental prolactin family. Endocrinology. 1991, 129: 2895-2906.
Izhar M, Siebert PD, Oshima RG, DeWolf WC, Fukuda MN: Trophoblastic differentiation of human teratocarcinoma cell line HT-H1. Dev Biol. 1986, 116: 510-518.
Ringler GE, Strauss JF: In vitro systems for the study of human placental endocrine function. Endocr Rev. 1990, 11: 105-123.
Yan J, Tanaka S, Oda M, Makino T, Ohgane J, Shiota K: Retinoic acid promotes differentiation of trophoblast stem cells to a giant cell fate. Dev Biol. 2001, 235: 422-432. 10.1006/dbio.2001.0300.
Tanaka TS, Kunath T, Kimber WL, Jaradat SA, Stagg CA, Usuda M, Yokota T, Niwa H, Rossant J, Ko MS: Gene expression profiling of embryo-derived stem cells reveals candidate genes associated with pluripotency and lineage specificity. Genome Res. 2002, 12: 1921-1928. 10.1101/gr.670002.
Tremblay GB, Kunath T, Bergeron D, Lapointe L, Champigny C, Bader JA, Rossant J, Giguere V: Diethylstilbestrol regulates trophoblast stem cell differentiation as a ligand of orphan nuclear receptor ERR beta. Genes Dev. 2001, 15: 833-838. 10.1101/gad.873401.
Roberts RM: A role for interferons in early pregnancy. Bioessays. 1991, 13: 121-126.
Larson MA, Kimura K, Kubisch HM, Roberts RM: Sexual dimorphism among bovine embryos in their ability to make the transition to expanded blastocyst and in the expression of the signaling molecule IFN-tau. Proc Natl Acad Sci USA. 2001, 98: 9677-9682. 10.1073/pnas.171305398.
Malassine A, Cronier L: Hormones and human trophoblast differentiation: a review. Endocrine. 2002, 19: 3-11. 10.1385/ENDO:19:1:3.
Yamaguchi M, Ogren L, Endo H, Thordarson G, Bigsby RM, Talamantes F: Production of mouse placental lactogen-I and placental lactogen-II by the same giant cell. Endocrinology. 1992, 131: 1595-1602. 10.1210/en.131.4.1595.
Ezashi T, Ealy AD, Ostrowski MC, Roberts RM: Control of interferon-tau gene expression by Ets-2. Proc Natl Acad Sci USA. 1998, 95: 7882-7887. 10.1073/pnas.95.14.7882.
Johnson W, Jameson JL: Role of Ets2 in cyclic AMP regulation of the human chorionic gonadotropin beta promoter. Mol Cell Endocrinol. 2000, 165: 17-24. 10.1016/S0303-7207(00)00269-0.
Ghosh D, Ezashi T, Ostrowski MC, Roberts RM: A central role for Ets-2 in the transcriptional regulation and cyclic adenosine 5'-monophosphate responsiveness of the human chorionic gonadotropin-beta subunit gene. Mol Endocrinol. 2003, 17: 11-26. 10.1210/me.2002-0223.
Pestell RG, Albanese C, Watanabe G, Lee RJ, Lastowiecki P, Zon L, Ostrowski M, Jameson JL: Stimulation of the P-450 side chain cleavage enzyme (CYP11A1) promoter through ras- and Ets-2-signaling pathways. Mol Endocrinol. 1996, 10: 1084-1094. 10.1210/me.10.9.1084.
Sun Y, Duckworth ML: Identification of a placental-specific enhancer in the rat placental lactogen II gene that contains binding sites for members of the Ets and AP-1 (activator protein 1) families of transcription factors. Mol Endocrinol. 1999, 13: 385-399. 10.1210/me.13.3.385.
Stacey KJ, Fowles LF, Colman MS, Ostrowski MC, Hume DA: Regulation of urokinase-type plasminogen activator gene transcription by macrophage colony-stimulating factor. Mol Cell Biol. 1995, 15: 3430-3441.
Watabe T, Yoshida K, Shindoh M, Kaya M, Fujikawa K, Sato H, Seiki M, Ishii S, Fujinaga K: The Ets-1 and Ets-2 transcription factors activate the promoters for invasion-associated urokinase and collagenase genes in response to epidermal growth factor. Int J Cancer. 1998, 77: 128-137. 10.1002/(SICI)1097-0215(19980703)77:1<128::AID-IJC20>3.0.CO;2-9.
Ezashi T, Ghosh D, Roberts RM: Repression of Ets-2 induced transactivation of the interferon-tau promoter by Oct-4. Mol Cell Biol. 2001, 21: 7883-7891. 10.1128/MCB.21.23.7883-7891.2001.
Liu L, Roberts RM: Silencing of the gene for the beta subunit of human chorionic gonadotropin by the embryonic transcription factor Oct-3/4. J Biol Chem. 1996, 271: 16683-16689. 10.1074/jbc.271.28.16683.
Liu L, Leaman D, Villalta M, Roberts RM: Silencing of the gene for the alpha-subunit of human chorionic gonadotropin by the embryonic transcription factor Oct-3/4. Mol Endocrinol. 1997, 11: 1651-1658. 10.1210/me.11.11.1651.
Nakayama H, Scott IC, Cross JC: The transition to endoreduplication in trophoblast giant cells is regulated by the mSNA zinc finger transcription factor. Dev Biol. 1998, 199: 150-163. 10.1006/dbio.1998.8914.
Knofler M, Meinhardt G, Bauer S, Loregger T, Vasicek R, Bloor DJ, Kimber SJ, Husslein P: Human Hand1 basic helix-loop-helix (bHLH) protein: extra-embryonic expression pattern, interaction partners and identification of its transcriptional repressor domains. Biochem J. 2002, 361: 641-651. 10.1042/0264-6021:3610641.
Anson-Cartwright L, Dawson K, Holmyard D, Fisher SJ, Lazzarini RA, Cross JC: The glial cells missing-1 protein is essential for branching morphogenesis in the chorioallantoic placenta. Nature Genet. 2000, 25: 311-314. 10.1038/77076.
Janatpour MJ, Utset MF, Cross JC, Rossant J, Dong J, Israel MA, Fisher SJ: A repertoire of differentially expressed transcription factors that offers insight into mechanisms of human cytotrophoblast differentiation. Dev Genet. 1999, 25: 146-157. 10.1002/(SICI)1520-6408(1999)25:2<146::AID-DVG9>3.0.CO;2-K.
Nait-Oumesmar B, Copperman AB, Lazzarini RA: Placental expression and chromosomal localization of the human Gcm 1 gene. J Histochem Cytochem. 2000, 48: 915-922.
Jen Y, Manova K, Benezra R: Each member of the Id family exhibits a unique expression pattern in mouse gastrulation and neurogenesis. Dev Dyn. 1997, 208: 92-106. 10.1002/(SICI)1097-0177(199701)208:1<92::AID-AJA9>3.0.CO;2-X.
Alders M, Hodges M, Hadjantonakis AK, Postmus J, van Wijk I, Bliek J, de Meulemeester M, Westerveld A, Guillemot F, Oudejans C, Little P, Mannens M: The human Achaete-Scute homologue 2 (ASCL2, HASH2) maps to chromosome 11p15.5, close to IGF2 and is expressed in extravillus trophoblasts. Hum Mol Genet. 1997, 6: 859-867. 10.1093/hmg/6.6.859.
Acknowledgements
The research from this laboratory was supported by NIH Grants HD 21896 and HD 42201.
Author information
Authors and Affiliations
Corresponding author
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Roberts, R.M., Ezashi, T. & Das, P. Trophoblast gene expression: Transcription factors in the specification of early trophoblast. Reprod Biol Endocrinol 2, 47 (2004). https://doi.org/10.1186/1477-7827-2-47
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1477-7827-2-47