Abstract
Embryonic stem cells (ES) can self-replicate and differentiate into all cell types including insulin-producing, beta-like cells and could, therefore, be used to treat diabetes mellitus. To date, results of stem cell differentiation into beta cells have been debated, largely due to difficulties in defining the identity of a beta cell. We have recently differentiated non-human primate (rhesus) embryonic stem (rES) cell lines into insulin producing, beta-like cells with the beta cell growth factor, Exendin-4 and using C-peptide as a phenotype marker. Cell development was characterized at each stage by gene and protein expression. Insulin, NKX6.1 and glucagon mRNA were expressed in stage 4 cells but not in early undifferentiated cells. We concluded that rES cells could be differentiated ex vivo to insulin producing cells. These differentiated rES cells could be used to develop a non-human primate model for evaluating cell therapy to treat diabetes. To facilitate the identification of beta-like cells and to track the cells post-transplantation, we have developed a marker gene construct: fusing the human insulin promoter (HIP) to the green fluorescent protein (GFP) gene. This construct was transfected into stage 3 rES derived cells and subsequent GFP expression was identified in C-peptide positive cells, thereby substantiating endogenous insulin production by rES derived cells. Using this GFP detection system, we will enrich our population of insulin producing rES derived cells and track these cells post-transplantation in the non-human primate model.
Similar content being viewed by others
Review
Diabetes Mellitus (DM) is a collection of heterogeneous disorders that result in glucose homeostasis abnormalities and produce metabolic complications that are frequently debilitating and life threatening. Currently, approximately 17 million Americans [1–6] are affected by DM; and this number is expected to increase by 165% in the USA in the next 30 years [7]. Identifying methods to treat or cure DM, along with efforts to prevent its development, will be a key in stemming this pandemic.
Central to the development of DM is the relative loss of insulin production from the pancreatic beta cells. Replacing these cells has been a therapeutic goal for decades and could prevent the morbidity and mortality associated with DM. Recently, islet transplantations were successful in restoring normal glycemic control [8]. This success provides proof that replacing functional β cell mass is an effective treatment for DM.
Although islet transplantation has shown significant promise, it remains an unlikely therapy for patients with DM primarily due to the lack of available human islet tissue [9, 10]. Furthermore, individual patients will require repeat islet transplantations to offset the slow but progressive loss of transplanted islet function [11]. Since β cells are the only sources of insulin in the body, an unlimited and renewable supply of β cells or islets will be needed to successfully treat DM by transplantation [12–14].
An ideal tissue source for transplantation would be β cell lines with glucose-mediated insulin release, that are not immunogenic, tumorogenic or at risk of transmitting infectious disease, and are able to replicate ex vivo without losing their differentiation potential [15]. While such a cell line does not yet exist, islet progenitor (adult stem cells) or embryonic stem (ES) cells are prime candidates [13]. Both adult and embryonic stem cells have the potential to proliferate ex vivo and differentiate into islet-like cells [16, 17]. If these techniques can be translated into the growth and isolation of islet cells, this would provide a source of replaceable islet tissue.
Embryonic stem cells
ES cells, present in the inner cell mass of the pre-implantation embryo, are immortal and pluripotent [18]. Clonal mouse ES cell lines differentiate into islet-like phenotypes, ex vivo [19] and in vivo [17]. This methodology has also been applied to human ES cells; however, the process produces a mixed population of cells containing only about 3% insulin positive cells [20]. Although ES cells have the potential to differentiate into islet like cells, early work was limited by the identification of the β cell phenotype using insulin immunocytochemistry. This identification method has recently been invalidated because insulin is a growth factor present in the conditioned media used to differentiate and grow the cells [21]. A recent publication demonstrates that insulin in the media is pinocytosed into apoptotic cells and thus, is indistinguishable to endogenous insulin when identified by immunocytochemical or radioimmuno assays. Therefore, the identification of insulin can falsely identify apoptotic cells as insulin producing cells [21]. Subsequent to this publication, mouse ES cells were differentiated into insulin producing cells in media containing no additional insulin demonstrating the capacity of ES cells to develop into insulin-producing, β-like cells [22]. These studies highlight the need for specific and irrefutable markers of the β cell phenotype.
Identifying beta like cells
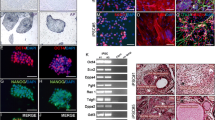
Gene expression can be used to identify cell lineage and is not adversely affected by compounds in the media. In addition to insulin production and release, lineage restricted gene expression can also be used to identify developing β cells [23, 24]. We utilized this information to identify β cell like phenotype development from differentiating rhesus ES cells and adult monkey islet cells. Total RNA was isolated from ES cells used in three separate differentiation experiments and RT-PCR performed using oligonucleotides based on the human gene sequence for genes of the pancreatic lineage. Genes associated with early β cell development such as NeuroD [25, 26] and Nestin were identified in stage two cells while PDX-1 and insulin gene expression was detectable only after stage two, consistent with the presence of cells with a differentiated phenotype (Figure 1). Gene markers of other cell lineages including amylase and enolase were detected in stage four cells. This suggested that cells have undergone some degree of differentiation into β-like cells as indicated by the expression of insulin and PDX-1.
Pancreatic Gene Expression RT-PCR was performed on cells from each stage of differentiation, from 3 independent differentiation experiments. Genes from the pancreatic cell lineage were identified and compared to control samples from rhesus adult islets. Reverse transcriptase negative samples confirmed the absence of genomic DNA (data not shown).
In addition to identifying β-cell lineage restricted genes, C-peptide, cleaved from proinsulin during insulin processing, can be used to identify an insulin producing, β-like cell. This methodology circumvents the problems associated with measuring mature insulin. Since C-peptide antibodies do not cross react with mature insulin or insulin in the growth media C-peptide is a direct measure of endogenous insulin production [27]. We were able to identify the production of insulin by rES cells grown in the presence of insulin using C-peptide immunocytochemistry (Figure 2). This data, along with the gene expression data, suggests that the rES cells are differentiating into insulin producing cells. At this time, however, further modifications to the culture conditions are needed to enhance cell differentiation and develop islet-like structures. Development of additional methods to identify the β cell phenotype that are sensitive and specific would greatly enhance these differentiation protocols.
C-peptide Expression Rhesus ES cells were grown in media containing 1 nM Exendin-4 to promote differentiation to β like cells. The cells were fixed, permeabilized and probed with anti-C-peptide antibody (1/500 dilution) from Linco. C-peptide staining is visualized with Texas-Red secondary anti-body and the nuclei are stained with DAPI.
Tagging ES cells for purification and identification post-transplantation
One approach to identifying the β cell phenotype is to tag insulin producing cells with a fluorescent marker. Marker genes such as the green fluorescent protein (GFP) can be used to purify cells populations by FACS and to track cells following transplantation [28]. We have generated a human insulin promoter (HIP)-GFP construct to be used specifically for these purposes. This construct will allow GFP expression to tag insulin producing cells and provide a means to purify insulin producing cells from the mixed cell population using fluorescent cell sorting (FACS).
We transfected rhesus ES derived cells at stage 3, prior to the addition of specific β cell growth factors. GFP expression was identified by fluorescent microscopy following 1 week of culture with the β cell growth factor, Exendin 4 [29–33]. Cells were co-stained for C-peptide to identify endogenous insulin production (Figure 3). We identified cells that co-expressed C-peptide and GFP supporting use of the HIP-GFP construct as a method to identify insulin producing ES derived cells. In addition to providing a means to identify and purify insulin producing cells, tagging the ES cells could allow their tracking post-transplantation.
GFP and C-peptide expression in HIP:GFP transfected rES cells Rhesus ES cells were grown in differentiation media through stage 3 (bFGF). These cells, growing predominately as a monolayer on a glass coverslip, were transfected with the HIP:GFP construct using ExGen 500. Cells were allowed to differentiate in stage 3 media (bFGF) for 3 weeks, followed by 2 weeks in stage 4 media containing 1 nM Exendin 4. The cells were fixed in 3.4% formaldehyde and permeabilized with acetone. Cells were co-stained with DAPI and c-peptide. GFP expression was identified using a specific GFP filter and C-peptide detected by Tx-Red secondary antibody. In this field, two cells show both GFP and C-peptide expression (large arrows) and one cell expressing only C-peptide (small arrow).
Transplantation of ES cells into diabetic animal models
Development of animal transplantation models will be necessary to study the safety and efficacy of ES derived cell transplants. For DM therapies, ES derived β cells transplanted into the streptozotocin induced diabetic SCID mouse should provide elementary insights. In the past, use of rhesus monkeys and other non-human primate models provided critical information resulting in changes to the islet transplant protocol [34, 35]. As with islet transplantation studies, the rhesus monkey has proved to be an invaluable model for other diabetes-related studies [36–38]. Therefore, an ideal primate model to study transplantation of ES cells is the chemically induced diabetic rhesus monkey. Tagging the ES derived cells prior to transplantation will provide an important tool in the investigation of ES cell transplantation.
Conclusion
The high cost and rising number of affected patients makes diabetes a major health crisis for the entire world. Current therapeutic options, with the exception of islet transplantation, have, at best, reduced the effects of diabetes. Stem cells (adult or embryonic) are currently the most promising candidates for islet cell replacement therapies. Recent advances differentiating embryonic stem cells into insulin producing cells have pointed out this potential, as well as, the pitfalls to the current approach. We have demonstrated insulin production from rhesus ES cells using C-peptide as a marker, thus avoiding the pitfall of insulin detection. In addition, we have transfected ES cells with a HIP-GFP construct to identify insulin producing, ES derived cells. This methodology will allow us to develop a pre-clinical model of cell transplantation in rhesus monkeys. Such a model is critical in the evaluation of the ES cell-based transplantation safety and efficacy.
References
Gabir MM, Hanson RL, Dabelea D, Imperatore G, Roumain J, Bennett PH, Knowler WC: The 1997 American Diabetes Association and 1999 World Health Organization criteria for hyperglycemia in the diagnosis and prediction of diabetes. Diabetes Care. 2000, 23: 1108-1112.
Harris MI: Diabetes in America: epidemiology and scope of the problem. Diabetes Care. 1998, 21: C11-14.
Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP: The continuing epidemics of obesity and diabetes in the United States. JAMA. 2001, 286: 1195-1200. 10.1001/jama.286.10.1195.
Mokdad AH, Ford ES, Bowman BA, Nelson DE, Engelgau MM, Vinicor F, Marks JS: Diabetes Trends in the US: 1990-1998. Diabetes Care. 2000, 23: 1278-1283.
Mokdad AH, Ford ES, Bowman BA, Nelson DE, Engelgau MM, Vinicor F, Marks JS: The continuing increase of diabetes in the US. Diabetes Care. 2001, 24: 412-
Narayan KM, Gregg EW, Fagot-Campagna A, Engelgau MM, Vinicor F: Diabetes-a common, growing, serious, costly, and potentially preventable public health problem. Diabetes Res Clin Pract. 2000, 50: S77-84. 10.1016/S0168-8227(00)00183-2.
Boyle JP, Honeycutt AA, Narayan KM, Hoerger TJ, Geiss LS, Chen H, Thompson TJ: Projection of Diabetes Burden Through 2050. Diabetes Care. 2001, 24: 1936-1940.
Shapiro AM, Lakey JRT, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV: Islet Transplantation in Seven Patients with Type 1 Diabetes Mellitus using A Glucocorticoid-Free Immunosuppressive Regimen. N Engl J Med. 2000, 343: 230-238. 10.1056/NEJM200007273430401.
Mathieu C: Current limitations of islet transplantation. Transplant Proc. 2001, 33: 1707-1708. 10.1016/S0041-1345(00)02650-6.
Zwillich T: Islet transplants not yet ready for prime time. Science. 2000, 289: 531-532. 10.1126/science.289.5479.531.
Ryan EA, Lakey JRT, Rajotte RV, Korbutt GS, Kin T, Imes S, Rabinovitch A, Elliot JF, Bigam D, Knetman NM, Warnock GL, Larsen I, Shapiro AM: Clinical outcomes and insulin secretion after islet transplantation with the Edmonton protocol. Diabetes. 2001, 50: 710-709.
Swenne I: Pancreatic Beta-cell growth and diabetes mellitus. Diabetologia. 1992, 35: 193-201.
Serup Palle, Madsen Ole D., Mandrup-Poulsen Thomas: Islet and stem cell transplantation for treating diabetes. BMJ. 2001, 322: 29-32. 10.1136/bmj.322.7277.29.
Oberholzer J, Toso C, Ris F, Bucher P, Triponez F, Demirag A, Lou J, Morel P: Beta Cell Replacement for the Treatment of Diabetes. Ann NY Acad Sci. 2001, 387: 373-384.
Beattie Gillian M., Cirulli Vincenzo, Lopez Ana D., Hayek Alberto: Ex Vivo Expansion of Human Pancreatic Endocrine Cells. Journal of Cinical Endocrinolgy Metab. 1997, 82: 1852-1856. 10.1210/jc.82.6.1852.
Bonner-Weir S, Taneja M, Weir GC, Tatarkiewicz K, Song KH, Sharma A, O'Neil JJ: In vitro cultivation of human islets from expanded ductal tissue. PNAS. 2000, 97: 7999-8004. 10.1073/pnas.97.14.7999.
Soria B: In-vitro differentiation of pancreatic beta-cells. Differentiation. 2001, 68: 205-219. 10.1046/j.1432-0436.2001.680408.x.
Thomson JA, Itskovitz-Eldor J, Shapiro SS: Embryonic stem cell lines derived from human blastocysts. Science. 1998, 282: 1145-1147. 10.1126/science.282.5391.1145.
Lumelsky N, Blondel O, Laeng P, Velasco I, Ravin R, McKay R: Differnetiation of Embryonic Stem Cells to Insulin-Secreting Structures Similar to Pancreatic Islets. Science. 2001, 292: 1389-1394. 10.1126/science.1058866.
Assady S, Maor G, Amit M, Itskovitz-Eldor J, Skorecki KL, Tzukerman M: Insulin Production by Human Embryonic Stem Cells. Diabetes. 2001, 50: 1691-1697.
Rajagopal J, Anderson WJ, Kume S, Martinez OI, Melton DA: Insulin Staining of ES Cell Progeny from Insulin Uptake. Science. 2003, 299: 363-
Kahan BW, Jacobson LM, Hullett DA, Ochoada JM, Oberley TD, Lang KM, Odorico JS: Panreatic Precursors and Differentiated Islet Cell Types From Murine Embryonic Stem Cells. An in Vitro Model to Study Islet Differentiation. Diabetes. 2003, 52: 2016-2024.
Edlund H: Pancreatic organogenesis: developmental mechanisms and implications for therapy. Nature Reviews Genetics. 2002, 3: 524-532. 10.1038/nrg841.
Wang H, Maechler P, Ritz-Laser B, Hagenfeldt KA, Ishihara H, Philippe J, Wolheim CB: Pdx1 Level Defines Pancreatic Gene Expression Pattern and Cell Lineage Differentiation. J Biol Chem. 2001, 276: 25279-25286. 10.1074/jbc.M101233200.
Lee JE, Hollenberg SM, Snider L, Turner DL, Lipnick N, Weintraub H: Conversion of Xenopus into neurons by NeuroD, a basic helix-loop-helix protein. Science. 1995, 268: 836-844.
Naya FJ, Huang HP, Qui Y, Mutoh H, DeMayo FJ, Leiter AB, Tsai MJ: Diabetes, defective pancreatic morphogenesis, and abnormal enteroendocrine differentiation in BETA2/neuroD-deficient mice. Genes and Development. 1997, 11: 2323-2334.
Hori Yuichi, Rulifson Ingrid, Tsal BC, Helt JJ, Cahoy JD, Kim Seung K.: Growth inhibitors promote differentiation of insulin-producing tissue from embryonic stem cells. Proc Natl Acad Sci USA. 2002, 99: 16105-16110. 10.1073/pnas.252618999.
Eiges R, Schuldiner M, Drukker M, Yanuka O, Itskovitz-Eldor J, Benveniste H: Establishment of human embryonic stem cell-transfected clones carrying a marker for undifferentiated cells. Current Biology. 2001, 11: 514-518. 10.1016/S0960-9822(01)00144-0.
Xu G, Stoffers DA, Habener JF, Bonner-Weier S: Exendin-4 stimulated both beta cell replication and neogenesis, resulting in increased beta cell mass and improved glucose tolerance in diabetic rats. Diabetes. 1999, 48: 2270-2276.
Tourrel Cecile, Bailbe Danielle, Lacorne Matthieu, Meile Marie-Jo, Kergoat Micheline, Portha Bernard: Persistent Improvement of Type 2 Diabetes in the Goto-Kakizaki Rat Model by Expansion of the {beta}-Cell Mass During the Prediabetic Period With Glucagon-Like Peptide-1 or Exendin-4 . Diabetes. 2002, 51: 1443-1452.
Movassat J, Beattie Gillian M., Lopez Ana D., Hayek Alberto: Exendin 4 up-regulates expression of PDX 1 and hastens differentiation and maturation of human fetal pancreatic cells. J Clin Endocrinol Metab. 2002, 87: 4775-4781. 10.1210/jc.2002-020137.
Rolin Bidda, Larsen Marianne O., Gotfredsen Carsten F., Deacon Carolyn F., Carr Richard D., Wilken Michael, Knudsen Lotte Bjerre: The long-acting GLP-1 derivative NN2211 ameliorates glycemia and increases beta -cell mass in diabetic mice. Am J Physiol Endocrinol Metab. 2002, 283: E745-752.
Tourrel Cecile, Bailbe Danielle, Meile Marie-Jo, Kergoat Micheline, Portha Bernard: Glucagon-Like Peptide-1 and Exendin-4 Stimulate {beta}-Cell Neogenesis in Streptozotocin-Treated Newborn Rats Resulting in Persistently Improved Glucose Homeostasis at Adult Age. Diabetes. 2001, 50: 1562-1570.
Hirshberg B, Mog Steven, Patterson Noelle, Leconte John, Harlan DM: Histopathological Study of Intrahepatic Islets Transplanted in the Nonhuman Primate Model Using Edmonton Protocol Immunosuppression. J Clin Endocrinol Metab. 2002, 87: 5424-5429. 10.1210/jc.2002-020684.
Hirshberg B, Montgomery S, Wysoki MG, Xu H, Tadaki D, Lee JE, Hines K, Gaglia J, Patterson Noelle, Leconte John, Hale Douglas, Chang Richard, Kirk AD, Harlan DM: Pancreatic Islet Transplantation Using the Nonhuman Primate (Rhesus) Model Predicts That the Portal Vein Is Superior to the Celiac Artery as the Islet Infusion Site. Diabetes. 2002, 51: 2135-2140.
Kenyon NS, Chatzipetrou M, Masetti M, Ranuncoli A, Oliviera M, Wagner JL, Kirk AD, Harlan DM, Burkly LC, Ricordi C: Long-term survival and function of intrahepatic islet allografts in rhesus monkey treated with humanized anti-CD154. Proc Natl Acad Sci USA. 1999, 96: 8132-8137. 10.1073/pnas.96.14.8132.
Theriault BR, Thistlethwaite JR, Levisetti MG, Wardrip CL, Szot G, Bruce DS, Rilo H, Li X, Gray GS, Bluestone JA, Padrid PA: Induction, maintenance, and reversal of streptozotocin-induced insulin-depdendent diabetes mellitus in the juvenile cynomolgus monkey (Macaca fascilularis). Transplantation. 1999, 68: 331-337. 10.1097/00007890-199908150-00003.
Thomas JM, Contreras JL, Smyth CA, Lobashevsky A, Jenkins S, Hubbard WJ, Eckhoff DE, Stavrou S, Neville DM, Thomas FT: Successful reversal of streptozotocin-induced diabetes with stable allogeneic islet function in a preclinical model of type 1 diabetes. Diabetes. 2001, 50: 1227-12236.
Acknowledgements
The Juvenile Diabetes Research Association (RS-2003 to LBL) and the NIH (RR 15199 to DPW) provided funding for these studies.
We are very grateful to Dr. C.V. Wright, Vanderbilt University, for the gift of the anti-PDX-1 antibody and Dr. Michael German, UCSF, for the gift of the human insulin promoter cDNA.
Author information
Authors and Affiliations
Corresponding author
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Lester, L.B., Kuo, HC., Andrews, L. et al. Directed differentiation of rhesus monkey ES cells into pancreatic cell phenotypes. Reprod Biol Endocrinol 2, 42 (2004). https://doi.org/10.1186/1477-7827-2-42
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1477-7827-2-42