Abstract
Ovarian cancer is the most common cause of death from gynecological cancers in the Western world. There are many genetic and environmental factors which can influence a woman's risk of getting ovarian cancer. A strong family history of breast or ovarian cancer is definitely one of the most important and best-defined epidemiological risk factors. This review evaluates current knowledge of hereditary ovarian cancer. Histologic, cytologic and molecular studies on the ovarian surface epithelium (OSE), which is the origin of ovarian epithelial carcinomas, from women with a strong family history for ovarian carcinomas or with a mutation in one of the two known cancer susceptibility genes – BRCA1 and BRCA2, provide a background to facilitate understanding of the early changes in ovarian carcinogenesis. This overview is followed by a discussion of recent hypotheses and research on two questions. First, is there a mutational hotspot of BRCA mutation for ovarian cancer? Second, why do mutations in BRCA1 and BRCA2, which are ubiquitously expressed genes that participate in general cellular activities, lead preferentially to breast and ovarian cancer?
Similar content being viewed by others
Introduction
Ovarian surface epithelium (OSE)-derived ovarian carcinoma is the most lethal gynecological malignancy in North America. 5–10% of epithelial ovarian cancer involves strong family histories. Thus, the familial component is one of the most important and best-defined risk factors for ovarian cancer. A woman's lifetime risk for ovarian cancer is 1.4% but is estimated to be 15–60% for women with a strong family history and/or those who inherited a germline mutation in certain cancer susceptibility genes [1, 2] (see below), suggesting that this increased risk has a genetic component. A strong family history refers to those having two or more first-degree relatives (parents, siblings and children) diagnosed with breast or ovarian cancer, and in some circumstances with features of a type of bowel cancer (hereditary non-polyposis colon cancer, HNPCC, also called Lynch Syndrome II), at age 45 or younger. There are at least three types of family history of ovarian cancer indicative of a putative autosomal dominantly inherited cancer susceptibility syndrome: hereditary site-specific ovarian cancer, Lynch syndrome II and hereditary breast/ovarian carcinoma. The discovery of DNA mismatch repair genes such as MSH2 and MLH1 for the Lynch Syndrome II [3–5], and the identification of BRCA1 and BRCA2 tumor suppressor proteins in hereditary breast/ovarian cancer syndrome [2, 6, 7], have advanced our knowledge on the etiology of familial ovarian cancer. Mutations in the BRCA1 and BRCA2 genes, in particular, account for as much as 90% of cancers in women with familial ovarian cancer histories and the lifetime risk for ovarian cancer in women carrying a BRCA1 or BRCA2 mutation is estimated to be as high as 60–70% [1]. The majority of BRCA1 or BRCA2 mutations are presumed to lead to premature protein truncations as a result of frameshift deletions/insertions or nonsense mutations and alter the functions of BRCA protein. Whereas the functions of the BRCA1 and BRCA2 proteins have yet to be fully elucidated, BRCA genes are believed to be tumor suppressor genes, where they inhibit the growth of cancer cells through their roles in the maintenance of genome integrity, DNA repair, cell cycle control and apoptosis [8].
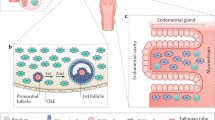
There is embryological and in vitro evidence that ovarian surface epithelium (OSE) is the origin of ovarian epithelial carcinomas [9]. OSE is a simple mesothelium that overlies the surface of the ovary. It is important to note that the adult OSE and the Mullerian epithelia arise from a common embryonic origin, the celomic epithelium. In early development, OSE cells form part of the celomic epithelium and the celomic epithelium adjacent to the presumptive gonads invaginates to give rise to the Mullerian ducts, i.e. the primordia for the epithelia of the oviduct, endometrium and endocervix. The relevance of this close developmental relationship between the OSE and the Mullerian epithelia could explain the frequent acquisition of architectural and functional characteristics of the Mullerian epithelia during neoplastic progression of OSE and the similarities between OSE-derived carcinomas and Mullerian epithelial malignancies. OSE cells from ovaries of women with strong familial history of ovarian cancer frequently undergo Mullerian metaplasia in adult life. This will become apparent later in this review.
Is there a premalignant lesion?
Histologic features
The question, "Is there a premalignant lesion that precedes the development of epithelial ovarian cancer", has been addressed through four approaches: (a) comparison of the concordance of ovarian aberrations between monozygotic twins where one had ovarian cancer; (b) identifying preneoplastic changes in normal ovaries contralateral to unilateral ovarian cancer; (c) evaluating architectural and cytologic changes of OSE adjacent to epithelial ovarian cancer; and (d) comparing the phenotype of overtly normal ovaries, prophylactically removed from cancer-prone women with an inherited predisposition for ovarian cancer, to normal ovaries from women of the general population. The first clue to the clincopathological evidence was provided by Gusberg and Deligdisch (1984), who examined the grossly normal ovaries that were prophylactically removed from identical twin sisters of patients with invasive carcinoma of the ovary [10]. Surface papillations, inclusion cysts, nuclear polymorphism or stratification in surface and invaginated epithelial lining are frequently found in these ovaries. All of these characteristics have been postulated by various investigators to be potentially premalignant histologic features. Other earlier studies using both light microscopy and image cytometry have reported similar cellular and nuclear atypia in the non-cancerous OSE or cyst epithelium adjacent to primary ovarian tumors [11, 12].
Ovaries from BRCA mutation carriers provide an excellent opportunity to identify candidate lesions for the study of early molecular changes in ovarian carcinogenesis, because these women are at a significant risk for developing ovarian cancer compared to women without such a family history (Table 1). Salazar et al. (1996), in a non-blinded study of 20 high-risk individuals and 20 women with no family history of ovarian cancer, revealed a higher rate of potentially preneoplastic features such as surface papillomatosis, surface epithelial stratification, epithelial inclusion cysts, invaginations and ovarian stromal hyperplasia in prophylactically removed ovaries from high-risk individuals [13]. Although only 9 of the 20 women studied by Salazar et al. (1996) have documented germline BRCA1 mutations, the study provides us with valuable information on phenotypic alterations in ovarian tissues due to hereditary influences. The positive family history of breast/ovarian cancer strongly suggests that most if not all of these women carry mutations in the BRCA1 or BRCA2 genes, even though they are not tested. The study also identified two unanticipated microscopic ovarian neoplasms in these high-risk ovaries. Consistent with these data, Werness et al. (1999) reported an increased frequency of inclusion cysts in a blinded study of 64 prophylactic oophorectomy specimens removed from women with a strong family history of ovarian cancer compared to 30 controls [14]. In contrast, 3 blinded studies on prophylactically removed ovaries from BRCA1 and BRCA2 mutation carriers found no significant difference in these early morphologic features related to ovarian carcinogenesis [15–17]. On the other hand, the blinded histopathologic analysis of Werness et al. (1999) revealed an important finding: although no microscopic (pre)malignant features such as papillomatosis and increased stromal activities were found, image analysis identified changes in the nuclei, where those from the ovaries of high-risk individuals were larger, of irregular contours, and contained more heterogeneously dense chromatin than nuclei of control ovaries [14]. Similarly, precancerous changes in the nuclei of high-risk ovaries were demonstrated by a novel computational analysis in the study of Deligdisch et al. (1999), which focused on BRCA founder mutations in Jewish patients [18]. 77.6% of ovaries removed from women of Ashkenazi Jewish descent who are at high risk for ovarian cancer because of the prevalence of BRCA1 mutations showed significantly larger nuclei and non-homogenous chromatin distribution than in control ovaries. Similar nuclear atypia was also identified in the non-cancerous surface epithelium adjacent to primary ovarian tumors compared to OSE from control ovaries [12]. However, there is no evidence of premalignant alterations in tumor suppressor proteins and oncogenes, such as the p53 tumor suppressor protein and the c-erbB2 oncoprotein, which are most frequently implicated in ovarian carcinogenesis, between OSE or cyst epithelium of ovaries from women with inherited BRCA1 mutations and controls. Neither was there a difference in cell proliferation and apoptosis [15, 19].
It is noteworthy that surface invaginations and inclusion cysts are more frequent in high-risk ovaries than in control ovaries. This is an exciting prospect because the ovarian surface epithelium (OSE) is normally separated from the ovarian stroma by the tunica albuginea, and the entrapment of the OSE cells within the stroma to form inclusion cysts generates a microenvironment in which the OSE is in close proximity to the paracrine influence of adjacent ovarian cortical stroma. Such epithelial-stromal interaction is important for epithelial differentiation, and may account for the Mullerian metaplasia commonly seen in the inclusion cysts. This hypothesis is supported by the observation that tubal epithelial metaplasia was frequently present in inclusion cysts of ovaries contralateral to ovaries containing unilateral carcinomas compared to control ovaries [20, 21]. It has been postulated that metaplastic and dysplastic changes of cyst-lining OSE cells could also be promoted by increased levels of estrogen, growth factors and bioactive cytokines in cyst fluid.
In culture
It is interesting that OSE of women with strong family histories of breast and ovarian cancer differs not only genetically but also phenotypically from OSE of women with no such family history (Table 2). Normal OSE is a mesothelium of an uncommitted phenotype. It is a simple squamous-to-cuboidal epithelium in vivo. In tissue culture, normal OSE cells are highly responsive to environmental signals and have a tendency to undergo epithelio-mesenchymal conversion over time. In contrast to OSE cells from women in the general population (NFH-OSE) that become mesenchymal after a few passages, OSE cells from women with strong family history of breast/ovarian cancer (FH-OSE) retain predominantly epithelial morphologies and growth patterns. Furthermore, FH-OSE cells are unable to contract three-dimensional sponge matrices, suggesting that these cells are more committed to epithelial differentiation and less responsive to wound-healing stimuli such as those associated with ovulation [22]. The ovarian carcinoma cell lines show an even more committed epithelial phenotype. Another (pre)neoplastic characteristic of FH-OSE in culture is to form colonies with whorls of elongated, irregularly shaped overlapping cells in primary culture, which appear to be metaplastic [23].
These altered morphologic features are concomitant with inappropriate expression of epithelial differentiation markers. FH-OSE cultures contained more cytokeratin and less mesenchymal collagen type III than NFH-OSE [22]. Two of the more important indications to suggest an increased commitment to an epithelial phenotype in overtly normal OSE cells from women with family histories of ovarian cancer (FH-OSE) is the enhanced expression of CA125 [24] and E-cadherin [25]. CA125 and E-cadherin are epithelial differentiation markers, which have little if any expression in normal human OSE, but are prominent in ovarian epithelial-lined clefts and inclusion cysts, Mullerian epithelia, for example oviductal and endometrial epithelia, and in ovarian carcinomas. The high intensity and number of cells expressing both markers also relate to the embryonic proximity of OSE to Mullerian duct epithelia. The malignant tumors often have histologic features that are reminiscent of the architecture and function of epithelia of the oviduct, endometrium and endocerix. It is interesting that the distributions of E-cadherin and CA125 in culture were similar, although there is no evidence that the functions of these two cellular components are related.
While the presence of hormone and growth factor receptors of OSE and their ability to secrete cytokines and growth factors is an integral part in normal OSE physiology, the cells appear to become less responsive to environmental signals and engaged into dysregulated autocrine loops with malignant progression. For example, macrophage-colony stimulating factor (M-CSF) is secreted by normal OSE cells and acts in a paracrine manner but become an autocrine regulatory factor in ovarian cancer cells, which also express its receptor [26, 27]. Importantly, FH-OSE cells produce hepatocyte growth factor (HGF) which acts as an autocrine growth regulator in other ovarian carcinoma cell lines, but rarely by normal OSE (9%). 61% of FH-OSE cultures express HGF and Met receptor concomitantly [28]. Several signaling molecules, including those of the phosphoinositol-3-kinase pathway are already activated in FH-OSE cultures independent of exogenous growth factor stimulation, which could be attributed to the autocrine HGF-Met regulation in these cells or other cytokines and growth factors. It is also possible that the PI3KCA gene which could lead to increased phosphoinositol-3-kinase activity is already amplified in FH-OSE cells [29], similar to the amplification of this gene in many ovarian cancers [30].
Perhaps one of the most interesting observations in these studies is that FH-OSE has a more limited growth potential and tends to senescence earlier than NFH-OSE in culture. This appears at a first glance to be different from the increased cell growth that confers tumorigenesis, but is particularly relevant to the paradoxical acquisition of Mullerian epithelial differentiation in early stages of ovarian neoplastic progression. As discussed earlier, most FH-OSE cultures have a propensity to undergo metaplasia to a Mullerian phenotype under physiological conditions, however routine culture condition does not support expansion of these transformed characteristics. Thus, the differentiated, metaplastic surface epithelium tends to senescence earlier in culture. Alternatively, the metaplastic phenotype may be reversible and is lost in culture, because causative factors, present in vivo, are missing. A shorter telomeric length of FH-OSE than NFH-OSE cells may provide an alternative explanation to the reduced growth potential in these cells [31]. Loss of telomere protection which represents a greater proximity to cell senescence and a decrease in genomic stability could contribute to the earlier age of onset of ovarian cancer in women with familial ovarian cancer syndromes.
Although the populations chosen for comparative studies are likely to represent cancer-prone and non cancer-prone groups have some limitations. First, it is difficult to accurately define the risk of ovarian cancer. Women in the general population who are carriers of BRCA mutations may be at a greater risk for ovarian cancer due to genetic reasons, however this does not account for other known non-genetic factors such as reproductive history and use of oral contraceptives. Similarly, the control population may not be a pure low-risk population, since in most studies neither extensive family history analysis nor BRCA1 testing has been performed on these women. Second, it is difficult to control statistically for age differences between the high-risk cases and controls. Because familial ovarian cancer is diagnosed at a younger age than sporadic ovarian cancer, women who decide to have their ovaries removed prophylactically to prevent the development of ovarian cancer are typically young, usually before age 45 as soon as childbearing is completed. Ovaries are very rarely removed from women at this young age under any other circumstances.
BRCA-associated ovarian tumors
Many studies have sought to determine potential serum biomarkers by comparing expression patterns of ovarian carcinomas and normal human OSE cells using high-throughput genomic and proteomic technologies [32, 33]. Important to this review, Jazaeri et al. (2002) used microarray technology to examine the role of mutations in the BRCA1 and BRCA2 genes in ovarian carcinogenesis by comparing gene expression patterns in ovarian cancers that are associated with germline BRCA1 and BRCA2 mutations and sporadic ovarian cancers [34]. Interestingly, BRCA1 and BRCA2-associated ovarian tumors display distinct gene expression profiles. 110 genes showed statistically significant different expression levels. This result suggests that BRCA1 and BRCA2 have different functions in ovarian carcinogenesis even though both proteins have been implicated in DNA damage repair, chromatin remodeling and transcriptional regulation. These data are in agreement with a previous study illustrating differences in gene expression profiles in BRCA1 and BRCA2-linked breast cancer [35]. The second important finding is that the molecular profiles of hereditary and sporadic ovarian cancers are not significantly different; sporadic ovarian tumors shared gene expression features of either the BRCA1 or BRCA2-associated tumors. The parallels in hereditary and sporadic ovarian tumor phenotypes suggest that sporadic tumors may also result from epigenetic loss of BRCA functions through inactivation of the BRCA1 or BRCA2 genes. Although mutations of BRCA genes are rare in sporadic ovarian tumors, loss of BRCA function through high rates of loss of heterozygosity, hypermethylation of the BRCA promoter or other mechanisms is a frequent event in sporadic ovarian tumors [36]. These alternate pathways may play a role in the loss of function of BRCA1 or BRCA2 which is required for the disease phenotype to develop. Consistently, decreased expression of BRCA1 in the nucleus is observed in sporadic ovarian tumors, suggesting defects in normal nuclear function of this protein [37, 38]. It is also possible that the molecular mechanisms in sporadic tumors are altered in a similar way as in BRCA-associated ovarian tumors. It has been suggested that there are other overriding key pathways driving ovarian cancers, which are as yet undescribed [39]. Although the clinical and pathological features of ovarian cancers in women with inherited germline BRCA1 and BRCA2 mutations compared to ovarian cancers that arise sporadically have been less consistent [40], ovarian cancers arising from BRCA1 or BRCA2 mutation-positive families are more likely to be invasive, high grade and of serous histologic type than cancers arising in women without BRCA mutations [40, 41].
Is the relative risk for ovarian cancer associated with the location of BRCA mutations?
Although there is little evidence for mutational hotspots or clustering on BRCA1 and BRCA2 genes except in certain populations, e.g. Ashkenazi Jewish, a defined repertoire of mutations have been detected, it has been suggested that the location of BRCA mutations is associated with different ovarian cancer risk. Mutations at the C-terminal of BRCA1 protein appear to be associated with breast cancer, whereas mutations at the N-terminal of the protein are more strongly associated with ovarian cancer [42]. The RING-finger domain of the N-terminus of BRCA1 protein appears to play a role in the anti-apoptotic function in OSE cells [43]. Among BRCA2 mutation carriers, the risk of ovarian cancer is greatest for women with mutations clustered in a region of 3.3 kb in exon 11 [44]. However, other genetic and environmental factors are also important. For example, rare alleles of HRAS1 have been shown to increase the risk for ovarian cancer at least two times in women with the same BRCA1 mutation [45]. Rare HRAS1 alleles also contribute to a greater risk of ovarian cancer in the general population [46]. Preliminary data suggest that the 17β-hydroxysteroid dehydrogenase-2 gene may function as a linked modifier of ovarian cancer risk in BRCA1 mutation carriers [47].
The nature of germline BRCA1 and BRCA2 mutations are to some extent dependent on the ethnicity of the population [see review in [48]]. The most notable example of mutations in these genes is the Ashkenazi Jewish women with early-onset ovarian cancer (and breast cancer), where two specific mutations in BRCA1 (185del AG and 538insC) and one mutation in BRCA2 (6174delT) appear to be particularly common. While another BRCA2 999del5 mutation is more common to the Icelandic population and a unique BRCA1 mutation (3452delA) was identified in two women diagnosed with ovarian cancer from Mongolia, a geographically isolated population.
Gender and tissue-specific properties
Although germline BRCA1 or BRCA2 mutations are present in all tissues and BRCA proteins exhibit fundamental cellular functions in maintaining genomic integrity, mutations in BRCA strongly predispose for breast and ovarian cancers in women. There are two possible hypotheses to explain why mutations in BRCA1 and BRCA2 lead specifically to breast and ovarian cancer: first, the loss of a second allele of BRCA preferentially occurs in women, in whom breast and ovarian tissues are preferred sites, but what causes this preferential loss is not known. Second, the putative tissue-specific activities of BRCA, such as its effect on hormone functions, may favor transformation in hormone-responsive epithelial tissues. Beginning in puberty, both the breast and ovarian epithelium proliferate rapidly in response to changes in levels of estrogen. Reproductive factors linked to estrogen, such as oral contraceptive use, are associated with breast and ovarian cancer risk.
A recent study by Ganesan et al. (2002) may provide some insights to the question of why BRCA1 mutation is a predisposing factor for hereditary cancer syndromes more frequently in women than in men. BRCA1 is found to contribute to the X-chromosome inactivation, which is a process specific to female cells [49]. Moreover, breast and ovarian carcinoma cells lacking BRCA1 show evidence of defects in X-chromatin structure. Such defects could be reversed by the expression of wild-type BRCA1 [49]. In support of this model, a preliminary study suggests that X-inactivation may not be a random process in women with BRCA1 mutations. Interestingly, the process is in some ways favorably occurring on the alternative X-chromosome carrying the wild-type BRCA1 allele [50]. This suggests that the combination of a germline mutation of the BRCA1 gene as well as nonrandom X-chromosome inactivation could eliminate wild-type activity of this gene and thus contributes to the increased incidence of cancer in these females. However, further experiments will be required to verify this issue. It is also intriguing to ask whether such properties could be related to the abnormal nuclear structure seen in high-risk ovaries described earlier in this review [14, 18].
BRCA1 has been reported to interact with the estrogen receptor (ER) and inhibit both ligand-dependent and -independent ER activation [51, 52]. Estrogen is a principal determinant in the epithelial cell proliferation, differentiation and normal functional status of breast and ovary, which are both estrogen-responsive organs. Therefore, BRCA1 mutations might possibly promote the growth and differentiation of ovarian and mammary epithelial cells through regulation of estrogen receptor activity, and by implication, contribute to the initiation of ovarian and breast cancer. However, it seems confusing that BRCA1 mutations have never been linked to tumors of other estrogen-responsive tissues, including the endometrium. Alternatively, not mutually exclusive with the model described above, certain oxidative forms of estrogen have been reported to be genotoxic [53], and BRCA1 has been proposed to play a role in protecting breast and ovarian tissue from estrogen-induced DNA damage. BRCA1 has also been documented to enhance androgen-dependent transactivation by androgen receptor [54], allelic variants of which could modify breast or ovarian cancer penetrance in BRCA1 mutation carriers [47, 55].
BRCA1-/- mouse embryos exhibit early embryonic lethality, which hinders the study of the tumor suppression activity of BRCA1 [56]. Mice heterozygous for mutations in either the BRCA1 or BRCA2 gene have been generated. Although these mice are not predisposed to mammary or ovarian tumor development, they display defects in mammary duct branching and atrophic ovaries with significantly arrested follicular development in response to estrogen [57]. The incidence of breast tumors increases when the mice were crossed into a p53+/- background [58], consistent with the idea that loss of BRCA gene alone is not sufficient to confer tumor formation and requires the accumulation of addition mutations in genes for checkpoint controls, including the inactivation of p53.
Conclusion
One reason for the high mortality of ovarian cancer is that almost 70% of the disease is diagnosed at a late stage when disease has spread beyond pelvis. However, if a woman is diagnosed with an early stage (stage I) ovarian cancer, the survival rate is close to 90% without altering current therapeutic approaches. Data presented in this review suggest that ovaries removed prophylactically from women with familial ovarian cancer syndromes may appear macroscopically normal; however, a careful histopathological examination may reveal a number of cancer-prone phenotypes and perhaps even unanticipated malignant neoplasms. The identification of early molecular changes in OSE cells of ovaries from women with familial ovarian cancer is encouraging, as this contributes to our understanding about the biology of ovarian tissue in women at increased risk of developing ovarian cancer, which to date is still largely unknown. Such knowledge may help in the development of possible screening tools for high-risk women to better define their risk of developing ovarian carcinomas and the management of the disease.
References
Antoniou A, Pharoah PD, Narod S, Risch HA, Eyfjord JE, Hopper JL, Loman N, Olsson H, Johannsson O, Borg A, et al.: Average Risks of Breast and Ovarian Cancer Associated with BRCA1 or BRCA2 Mutations Detected in Case Series Unselected for Family History: A Combined Analysis of 22 Studies. Am J Hum Genet. 2003, 72: 1117-1130. 10.1086/375033.
Boyd J: Hereditary ovarian cancer: What we know. Gynecol Oncol. 2003, 88: S8-S10. 10.1006/gyno.2002.6674.
Lindblom A, Tannergard P, Werelius B, Nordenskjold M: Genetic mapping of a second locus predisposing to human nonpolyposis colorectal cancer. Nat Genet. 1993, 5: 279-282.
Lynch HT, Lynch JF: Hereditary cancer: family history, diagnosis, molecular genetics, ecogenetics, and management strategies. Biochimie. 2002, 84: 3-17. 10.1016/S0300-9084(01)01363-3.
Peltomaki P, Aaltonen L, Sistonen P, Pylkkanen L, Mecklin JP, Jarvinen H, Green JS, Jass JR, Weber JL, Weber FS, et al.: Genetic mapping of a locus predisposing to human colorectal cancer. Science. 1993, 260: 810-812.
Miki Y, Swensen J, Shattuck-Eidens D, Futreal PA, Harshman K, Tavtigian S, Liu Q, Cochran C, Bennett LM, Ding W, et al.: A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994, 266: 66-71.
Tavtigian SV, Simard J, Rommens J, Couch F, Shattuck-Eidens D, Neuhausen S, Merajver S, Thorlacius S, Offit K, Stoppa-Lyonnet D, et al.: The complete BRCA2 gene and mutations in chromosome 13q-linked kindreds. Nat Genet. 1996, 12: 333-337.
Welcsh PL, King MC: BRCA1 and BRCA2 and the genetics of breast and ovarian cancer. Hum Mol Genet. 2001, 10: 705-713. 10.1093/hmg/10.7.705.
Auersperg N, Wong AS, Choi KC, Kang SK, Leung PC: Ovarian surface epithelium: biology, endocrinology, and pathology. Endocr Rev. 2001, 22: 255-88. 10.1210/er.22.2.255.
Gusberg SB, Deligdisch L: Ovarian dysplasia. A study of identical twins. Cancer. 1984, 54: 1-4.
Deligdisch L, Gil J: Characterization of ovarian dysplasia by interactive morphometry. Cancer. 1989, 63: 748-755.
Plaxe SC, Deligdisch L, Dottino PR, Cohen CJ: Ovarian intraepithelial neoplasia demonstrated in patients with stage I ovarian carcinoma. Gynecol Oncol. 1990, 38: 367-372.
Salazar H, Godwin AK, Daly MB, Laub PB, Hogan WM, Rosenblum N, Boente MP, Lynch HT, Hamilton TC: Microscopic benign and invasive malignant neoplasms and a cancer-prone phenotype in prophylactic oophorectomies. J Natl Cancer Inst. 1996, 88: 1810-20.
Werness BA, Afify AM, Bielat KL, Eltabbakh GH, Piver MS, Paterson JM: Altered surface and cyst epithelium of ovaries removed prophylactically from women with a family history of ovarian cancer. Hum Pathol. 1999, 30: 151-157.
Barakat RR, Federici MG, Saigo PE, Robson ME, Offit K, Boyd J: Absence of premalignant histologic, molecular, or cell biologic alterations in prophylactic oophorectomy specimens from BRCA1 heterozygotes. Cancer. 2000, 89: 383-390. 10.1002/1097-0142(20000715)89:2<383::AID-CNCR25>3.0.CO;2-T.
Sherman ME, Lee JS, Burks RT, Struewing JP, Kurman RJ, Hartge P: Histopathologic features of ovaries at increased risk for carcinoma. A case-control analysis. Int J Gynecol Pathol. 1999, 18: 151-7.
Stratton JF, Buckley CH, Lowe D, Ponder BA: Comparison of prophylactic oophorectomy specimens from carriers and noncarriers of a BRCA1 or BRCA2 gene mutation. United Kingdom Coordinating Committee on Cancer Research (UKCCCR) Familial Ovarian Cancer Study Group. J Natl Cancer Inst. 1999, 91: 626-628. 10.1093/jnci/91.7.626.
Deligdisch L, Gil J, Kerner H, Wu HS, Beck D, Gershoni-Baruch R: Ovarian dysplasia in prophylactic oophorectomy specimens: cytogenetic and morphometric correlations. Cancer. 1999, 86: 1544-1550. 10.1002/(SICI)1097-0142(19991015)86:8<1544::AID-CNCR22>3.3.CO;2-9.
Werness BA, Afify AM, Eltabbakh GH, Huelsman K, Piver MS, Paterson JM: p53, c-erbB, and Ki-67 expression in ovaries removed prophylactically from women with a family history of ovarian cancer. Int J Gynecol Pathol. 1999, 18: 338-343.
Mittal KR, Zeleniuch-Jacquotte A, Cooper JL, Demopoulos RI: Contralateral ovary in unilateral ovarian carcinoma: a search for preneoplastic lesions. Int J Gynecol Pathol. 1993, 12: 59-63.
Resta L, Russo S, Colucci GA, Prat J: Morphologic precursors of ovarian epithelial tumors. Obstet Gynecol. 1993, 82: 181-186.
Dyck HG, Hamilton TC, Godwin AK, Lynch HT, Maines-Bandiera S, Auersperg N: Autonomy of the epithelial phenotype in human ovarian surface epithelium: changes with neoplastic progression and with a family history of ovarian cancer. Int J Cancer. 1996, 69: 429-436. 10.1002/(SICI)1097-0215(19961220)69:6<429::AID-IJC1>3.0.CO;2-6.
Wong AST, Leung PC, Maines-Bandiera SL, Auersperg N: Metaplastic changes in cultured human ovarian surface epithelium. In Vitro Cell Dev Biol Anim. 1998, 34: 668-70.
Auersperg N, Maines-Bandiera S, Booth JH, Lynch HT, Godwin AK, Hamilton TC: Expression of two mucin antigens in cultured human ovarian surface epithelium: influence of a family history of ovarian cancer. Am J Obstet Gynecol. 1995, 173: 558-565.
Wong AST, Maines-Bandiera SL, Rosen B, Wheelock MJ, Johnson KR, Leung PC, Roskelley CD, Auersperg N: Constitutive and conditional cadherin expression in cultured human ovarian surface epithelium: influence of family history of ovarian cancer. Int J Cancer. 1999, 81: 180-8. 10.1002/(SICI)1097-0215(19990412)81:2<180::AID-IJC3>3.3.CO;2-Z.
Berchuck A, Kohler MF, Boente MP, Rodriguez GC, Whitaker RS, Bast RC: Growth regulation and transformation of ovarian epithelium. Cancer. 1993, 71: 545-551.
Ziltener HJ, Maines-Bandiera S, Schrader JW, Auersperg N: Secretion of bioactive IL-1, IL-6 and colony stimulating factors by human ovarian surface epithelium. Biol Reprod. 1993, 49: 635-641.
Wong AST, Pelech SL, Woo MM, Yim G, Rosen B, Ehlen T, Leung PC, Auersperg N: Coexpression of hepatocyte growth factor-Met: an early step in ovarian carcinogenesis?. Oncogene. 2001, 20: 1318-1328. 10.1038/sj.onc.1204253.
Wong AST, Kim SO, Leung PC, Auersperg N, Pelech SL: Profiling of protein kinases in the neoplastic transformation of human ovarian surface epithelium. Gynecol Oncol. 2001, 82: 305-311. 10.1006/gyno.2001.6280.
Shayesteh L, Lu Y, Kuo WL, Baldocchi R, Godfrey T, Collins C, Pinkel D, Powell B, Mills GB, Gray JW: PIK3CA is implicated as an oncogene in ovarian cancer. Nat Genet. 1999, 21: 99-102. 10.1038/5042.
Kruk PA, Godwin AK, Hamilton TC, Auersperg N: Telomeric instability and reduced proliferative potential in ovarian surface epithelial cells from women with a family history of ovarian cancer. Gynecol Oncol. 1999, 73: 229-36. 10.1006/gyno.1999.5348.
Mok SC, Chao J, Skates S, Wong K, Yiu GK, Muto MG, Berkowitz RS, Cramer DW: Prostasin, a potential serum marker for ovarian cancer: identification through microarray technology. J Natl Cancer Inst. 2001, 93: 1458-1464. 10.1093/jnci/93.19.1458.
Petricoin EF, Ardekani AM, Hitt BA, Levine PJ, Fusaro VA, Steinberg SM, Mills GB, Simone C, Fishman DA, Kohn EC, Liotta LA: Use of proteomic patterns in serum to identify ovarian cancer. Lancet. 2002, 359: 572-577. 10.1016/S0140-6736(02)07746-2.
Jazaeri AA, Yee CJ, Sotiriou C, Brantley KR, Boyd J, Liu ET: Gene expression profiles of BRCA1-linked, BRCA2-linked, and sporadic ovarian cancers. J Natl Cancer Inst. 2002, 94: 990-1000. 10.1093/jnci/94.13.990.
Hedenfalk IA: Gene expression profiling of hereditary and sporadic ovarian cancer reveals unique BRCA1 and BRCA2 signatures. J Natl Cancer Inst. 2002, 94: 960-961. 10.1093/jnci/94.13.960.
Hilton J, Geisler J, Rathe J, Hattermann-Zogg M, DeYoung B, Buller R: Inactivation of BRCA1 and BRCA2 in ovarian cancer. J Natl Cancer Inst. 2002, 94: 1396-1406. 10.1093/jnci/94.18.1396.
Wilson CA, Ramos L, Villasenor MR, Anders KH, Press MF, Clarke K, Karlan B, Chen JJ, Scully R, Livingston D, et al.: Localization of human BRCA1 and its loss in high-grade, non-inherited breast carcinomas. Nat Genet. 1999, 21: 236-240. 10.1038/6029.
Zheng W, Luo F, Lu JJ, Baltayan A, Press MF, Zhang ZF, Pike MC: Reduction of BRCA1 expression in sporadic ovarian cancer. Gynecol Oncol. 2000, 76: 294-300. 10.1006/gyno.1999.5664.
Hedenfalk I, Duggan D, Chen Y, Radmacher M, Bittner M, Simon R, Meltzer P, Gusterson B, Esteller M, Kallioniemi OP, et al.: Gene-expression profiles in hereditrary breast cancer. N Engl J Med. 2001, 344: 539-548. 10.1056/NEJM200102223440801.
Werness BA, Eltabbakh GH: Familial ovarian cancer and early ovarian cancer: biologic, pathologic, and clinical features. Int J Gynecol Pathol. 2001, 20: 48-63. 10.1097/00004347-200101000-00005.
Shaw PA, McLaughlin JR, Zweemer RP, Narod SA, Risch H, Verheijen RH, Ryan A, Menko FH, Kenemans P, Jacobs IJ: Histopathologic features of genetically determined ovarian cancer. Int J Gynecol Pathol. 2002, 21: 407-411. 10.1097/00004347-200210000-00011.
Gayther SA, Warren W, Mazoyer S, Russell PA, Harrington PA, Chiano M, Seal S, Hamoudi R, van Rensburg EJ, Dunning AM, et al.: Germline mutations of the BRCA1 gene in breast and ovarian cancer families provide evidence for a genotype-phenotype correlation. Nat Genet. 1995, 11: 428-433.
Johnson NC, Kruk PA: BRCA1 Zinc RING Finger Domain Disruption Alters Caspase Response in Ovarian Surface Epithelial Cells. Cancer Cell Int. 2002, 2: 7-10.1186/1475-2867-2-7.
Gayther SA, Mangion J, Russell P, Seal S, Barfoot R, Ponder BA, Stratton MR, Easton D: Variation of risks of breast and ovarian cancer associated with different germline mutations of the BRCA2 gene. Nat Genet. 1997, 15: 103-5.
Phelan CM, Rebbeck TR, Weber BL, Devilee P, Ruttledge MH, Lynch HT, Lenoir GM, Stratton MR, Easton DF, Ponder BA, et al.: Ovarian cancer risk in BRCA1 carriers is modified by the HRAS1 variable number of tandem repeat (VNTR) locus. Nat Genet. 1996, 12: 309-11.
Weitzel JN, Ding S, Larson GP, Nelson RA, Goodman A, Grendys EC, Ball HG, Krontiris TG: The HRAS1 minisatellite locus and risk of ovarian cancer. Cancer Res. 2000, 60: 259-261.
Narod SA: Modifiers of risk of hereditary breast and ovarian cancer. Nat Rev Cancer. 2002, 2: 113-123. 10.1038/nrc726.
Liede A, Narod SA: Hereditary breast and ovarian cancer in Asia: genetic epidemiology of BRCA1 and BRCA2. Hum Mutat. 2002, 20: 413-424. 10.1002/humu.10154.
Ganesan S, Silver DP, Greenberg RA, Avni D, Drapkin R, Miron A, Mok SC, Randrianarison V, Brodie S, Salstrom J, et al.: BRCA1 supports XIST RNA concentration on the inactive X chromosome. Cell. 2002, 111: 393-405.
Buller RE, Sood AK, Lallas T, Buekers T, Skilling JS: Association between nonrandom X-chromosome inactivation and BRCA1 mutation in germline DNA of patients with ovarian cancer. J Natl Cancer Inst. 1999, 91: 339-346. 10.1093/jnci/91.4.339.
Fan S, Ma YX, Wang C, Yuan RQ, Meng Q, Wang JA, Erdos M, Goldberg ID, Webb P, Kushner PJ, et al.: Role of direct interaction in BRCA1 inhibition of estrogen receptor activity. Oncogene. 2001, 20: 77-87. 10.1038/sj.onc.1204073.
Zheng L, Annab LA, Afshari CA, Lee WH, Boyer TG: BRCA1 mediates ligand-independent transcriptional repression of the estrogen receptor. Proc Natl Acad Sci U S A. 2001, 98: 9587-92. 10.1073/pnas.171174298.
Liehr JG: Genotoxicity of the steroidal oestrogens oestrone and oestradiol: possible mechanism of uterine and mammary cancer development. Hum Reprod Update. 2001, 7: 273-81. 10.1093/humupd/7.3.273.
Park JJ, Irvine RA, Buchanan G, Koh SS, Park JM, Tilley WD, Stallcup MR, Press MF, Coetzee GA: Breast cancer susceptibility gene 1 (BRCAI) is a coactivator of the androgen receptor. Cancer Res. 2000, 60: 5946-5949.
Levine DA, Boyd J: The androgen receptor and genetic susceptibility to ovarian cancer: results from a case series. Cancer Res. 2001, 61: 908-911.
Brodie SG, Deng CX: BRCA1-associated tumorigenesis: what have we learned from knockout mice?. Trends Genet. 2001, 17: S18-22. 10.1016/S0168-9525(01)02451-9.
Bennett LM, McAllister KA, Malphurs J, Ward T, Collins NK, Seely JC, Gowen LC, Koller BH, Davis BJ, Wiseman RW: Mice heterozygous for a Brca1 or Brca2 mutation display distinct mammary gland and ovarian phenotypes in response to diethylstilbestrol. Cancer Res. 2000, 60: 3461-3469.
Xu X, Wagner KU, Larson D, Weaver Z, Li C, Ried T, Hennighausen L, Wynshaw-Boris A, Deng CX: Conditional mutation of BRCA1 in mammary epithelial cells results in blunted ductal morphogenesis and tumor formation. Nat Genet. 2001, 22: 37-43.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Wong, A.S., Auersperg, N. Ovarian surface epithelium: family history and early events in ovarian cancer. Reprod Biol Endocrinol 1, 70 (2003). https://doi.org/10.1186/1477-7827-1-70
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1477-7827-1-70