Abstract
In most mammals oogonia proliferate by mitosis and begin meiotic development during fetal life. Previous studies indicated that there is a delay in the progression to the first stage of meiotic arrest in germ cells of female fetuses of undernourished ewes. We report that underfeeding (50% NRC requirement beginning on Day 28 of pregnancy) provokes an increase in oxidative base lesions within DNA of mid-gestational (Day 78) fetal oogonia; this condition was associated with up-regulation of the tumor suppressor/cell-cycle arrest modulator p53, antiapoptotic factor Bcl-2, and base-excision repair polymerase β. Fetal ovarian weights and germ cell concentrations were not altered by nutrient deprivation. Ovaries of ewes on control diets (100% NRC) contained more tertiary follicles than their restricted counterparts; however, peripheral venous estradiol-17β was not different between groups. There was no effect of treatment on p53 accumulation in maternal oocytes. Luteal structure-function was not perturbed by undernutrition. No fetal losses were attributed to the dietary restriction. It is proposed that DNA of interphase fetal oogonia is vulnerable to oxidative insults perpetrated by a nutritional stress to the dam, and that multiple/integrated adaptive molecular response mechanisms of cell-cycle inhibition (providing the time required for base repairs) and survival hence sustain the genomic integrity and population stability of the germline.
Similar content being viewed by others
Background
That maternal undernutrition can affect fetal ovarian development has been documented [1]. Experiments using the sheep as a paradigm indicate that meiotic maturation of germ cells is delayed by feed restriction [2, 3]; putative molecular mechanisms which mediate this effect are unknown. We hypothesized that fetal oogonia distressed as a result of nutrient deprivation express the tumor suppressor protein p53. Cells respond to p53 by cycle arrest at the G1/S or G2/M genomic checkpoints. Outcome is dictated by the intensity of the affliction. The apoptotic pathway is invoked in cells subjected to a severe trauma that causes irreparable damages. In the presence of sublethal disturbances to DNA, p53 affords the time necessary for enzymatic repairs and proof-reading. Thus, p53, the so-called "guardian of the genome," serves to maintain genetic fidelity [4–7].
There is a lack of fundamental information on potential influences of undernutrition on the functional morphology of ovaries of pregnant animals. The corpus luteum provides steroid hormonal support essential for the establishment and maintenance of early pregnancy [8, 9]. Follicles undergo cycles of antral development and atresia throughout gestation [10–12].
The primary objective of this investigation was to compare p53 responses of germ cells in fetal ovaries of ewes receiving adequate or restricted diets. Alterations in p53 were related to accretions of apoptotic/internucleosomal DNA cleavage sites [13], the oxidative DNA stress marker 8-oxoguanine [14], the survival protein Bcl-2 [15], and base-excision repair polymerase β [16]. Corpora lutea were evaluated for progesterone and steroidogenic cell and vascular compositions. Tertiary follicles were quantified and maternal oocytes were assessed for p53.
Materials and Methods
This project was conducted with the approval of the University of Wyoming Animal Care and Use Committee. Reagents were purchased from Sigma Chemical Co. (St. Louis, MO) unless indicated otherwise.
Animals and diets
Multiparous western-range ewes were synchronized to estrus (= Day 0) with prostaglandin F2α (dinoprost tromethamine i.m.; Pharmacia & Upjohn, Kalamazoo, MI) and bred to fertile rams. Diets consisted of a pelleted beet pulp (79.68% total digestible nutrients, 93.48% dry matter, 9.99% crude protein) supplemented with a mineral-vitamin mix (51.43% sodium triphosphate, 47.62% potassium chloride, 0.39% zinc oxide, 0.06% cobalt acetate; 8,000,000 IU vitamin A, 800,000 IU vitamin D3, 400,000 IU vitamin E per pound). Animals which did not return to estrus (N = 13) were weighed on Day 20 and dietary intakes calculated on a dry matter basis for total digestible nutrients recommended for early gestation (NRC). Feeding in individual pens commenced on Day 21. Ewes were assigned on Day 28 to a control (100% NRC; n = 7) or nutrient-restricted group (50% NRC; n = 6). Diets were adjusted for weight gain/loss (to maintain a constant level of energy) at seven-day intervals until slaughter (Day 78 ± 0.9). Pregnancies were confirmed by ultrasonography on Day 45. Four-of-seven control and 5-of-6 restricted ewes were pregnant with female fetuses; twin females were present in two control animals and in one restricted animal.
Processing of samples
Maternal blood samples were collected by jugular venipuncture on the day of slaughter, placed into heparinized tubes, and mixed by inverting. Plasma was harvested from cells after centrifugation and stored at -20 C.
Parameters recorded after slaughter included: weights of ewes, fetuses, maternal and fetal ovaries, and isolated corpora lutea; fetal sex; corpora lutea per ewe; and numbers of follicles ≥3 mm diameter visible at the surface of maternal ovaries. A small portion of luteal tissue was excised from each gland and frozen in liquid nitrogen.
Fetal ovaries, corpora lutea, and maternal interstitial/follicular (residual) tissues were fixed by immersion in 10% buffered formalin, washed in phosphate-buffered saline (PBS), dehydrated in a graded series of ethanol, cleared in xylene, infiltrated with and embedded in paraffin wax, and sectioned at 6 μm thickness. Sections were floated on deionized water, transferred onto microscope slides treated with subbing solution (0.025% chromium potassium sulfate, 0.25% gelatin), air-dried, deparaffinized, rehydrated, and stained with hematoxylin and eosin (H & E) or processed for flourescence microscopy (Olympus BH-2, Tokyo, Japan).
General morphology and cellular composition of fetal ovaries
Fetal ovarian sections stained with H & E were examined for surface epithelium and arrangements of pregranulosa and germ cells. Germ cells were counted (two fields from three different mid-ovarian sections per fetus at × 400 magnification) and relative manifestations of apoptosis (nuclear pyknosis and cytoplasmic condensation) were noted.
p53, 8-oxoguanine, Bcl-2, and polymerase β immunohistochemistry
Purified antibodies to p53 (mouse monoclonal KAM-CC002) and Bcl-2 (rabbit polyclonal AAP-070) were obtained from StressGen Biotechnologies (Victoria BC, Canada). Mouse monoclonal anti-8-oxoguanine (4355-MC-100) was purchased from Trevigen (Gaithersburg, MD). Antipeptide polymerase β antibodies were affinity-purified from rabbit serum [17].
Sections of fetal and residual maternal ovarian tissues were incubated for 30 min with 10% normal goat serum and for 1 h with primary antibodies (1 μg/ml), washed in two changes of PBS, incubated for 30 min with secondary goat antirabbit (F0382) or antimouse (F0257) immunoglobulin G-fluorescein isothiocyanate (FITC) (1:40), and washed in two changes of PBS. Serum and antibodies were diluted in freshly-prepared PBS containing 0.5% bovine serum albumin. Negative controls were carried out without primary antibodies and with primary antibodies preabsorbed (100-fold molar excess, 2 h, 25 C) with an 8-oxoguanine oligonucleotide (3850-100-01; Trevigen), human recombinant p53 (Santa Cruz Biotechnology, Santa Cruz, CA), Bcl-2 peptide (amino acids 41–54; StressGen), or recombinant polymerase β (Trevigen).
DNA fragmentation analysis of germ cells
End-labeling of fragmented DNA was used to monitor progressive (nuclear) apoptosis [18, 19]. Briefly, 3'-OH ends of DNA were linked with digoxigenin-11-d uridine triphosphate by terminal deoxynucleotidyl transferase (TdT) catalysis. Incorporated nucleotide heteropolymers were localized with antidigoxigenin Fab-FITC (ApopTag Kit S7110; Intergen Co., Purchase, NY). Conjugate or TdT were omitted in negative control reactions.
Fluorescence measurements of immunostained oogonia/oocytes
Images of cells sectioned through the nucleus were captured (× 400 magnification) by computer-interfaced digital photography (1.2 million pixel resolution; Pixera, Los Gatos, CA) and assessed for luminance intensities (continuous inverted gray-scale = 0 [black]-255 [white]; Optimas Software, Bothell, WA). Measures were made on twenty germ cells (within a respective section) per fetus for each analytical procedure: p53, apoptotic DNA fragmentation, 8-oxoguanine, Bcl-2, and polymerase β. Ten oocytes per ewe (primary-antral follicles; n = 4–6 ovarian sections) were evaluated for p53 immunoreactions.
Radioimmunoassays
Maternal plasma were assayed for progesterone [20] and estradiol-17β [21] and luteal tissues (wet basis) for progesterone [22] using validated procedures. All samples were analyzed in the same assays; intra-assay coefficients of variation were < 10%.
Luteal morphometry
Percentage areas occupied by large steroidogenic cells, small cells, and blood vessels (luminal space) were determined (Optimas) within images of H & E-stained tissues (× 400 magnification; two fields within each of three different sections per gland). Small luteal cells (12–22 μm diameter) were defined as spindle-shaped with dark-staining cytoplasm. Large luteal cells (> 30 μm diameter) were distinguished as polyhedral with light-staining cytoplasm [23].
Statistics
Assignments of animals to treatments and selections of fields/cells for microscopic examination were made at random. Subsample values were averaged. Fetal twin and luteal data were averaged within-ewe. Treatment (control vs. restricted) mean comparisons were made by Student's t-test. Contrasts were considered significantly different at P < 0.05. Data are presented as means ± standard errors.
Results
Ewes in the restricted-diet group weighed less at slaughter than controls. Fetal weights also were reduced by nutritional deprivation (Table 1).
There were no salient differences observed in the histoarchitecture of ovaries recovered from fetal lambs of control and restricted ewes. Surface epithelia of ovaries consisted of cuboidal cells; a definitive basal lamina (which eventually supports and separates surface cells from the ovarian cortical interstitium) was not readily discernible. Germ cells were typically spherical in shape, contained a conspicuous centrally-located nucleus, were larger than other cell-types, and were organized into clusters or cords sometimes associated with progenitor granulosa cells. Evidence of apoptotic oogonial degeneration was apparent (approximately 10% of cells) within ovaries of both nutritional groups (Figure 1).
Light microscopic morphology of fetal ovaries. A low-power view of the ovarian surface epithelium (OSE) and cortex are shown in the upper left panel. High-power views of healthy oogonia (O), an apoptotic oogonium (AO), and pregranulosa cells (GC) are illustrated in the lower left panel. A mid-power view of oogonial clusters/cords is depicted in the right panel. Scale bars = 5 μm.
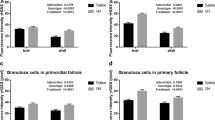
There were no significant influences of nutrition on weights of fetal ovaries or tissue concentrations of germ cells (Table 2). Increases above controls in immunoreactive 8-oxoguanine, p53, Bcl-2, and polymerase β were detected in germ cells of fetal ovaries from diet-limited ewes. No group differences in rates of apoptosis as indicated by immunoreactive DNA fragments were detected. Levels of p53 in maternal oocytes were not altered overall (irrespective of follicular classification) by undernutrition (Figure 2); and therefore, we did not monitor the inductive damage (8-oxoguanine) or response survival (Bcl-2)/repair (polymerase β) markers.
Fluorescence intensity scores of oogonia/oocytes: A, fetal 8-oxoguanine; B, fetal p53; C, fetal Bcl-2; D, fetal polymerase β; E, fetal apoptosis (DNA fragmentation); F, maternal p53 (fetal control, n = 4; fetal restricted, n = 5; maternal control, n = 7; maternal restricted, n = 6). Asterisks indicate pairwise increases (P < 0.01). Values for negative controls (not subtracted from the data shown) were < 45. Representative photomicrographs of p53-immunostained oogonia of control (left panel) and restricted (center panel) fetuses are shown in the inset (note the contrast in magnitudes of nuclear fluorescence); a negative preabsorption control cell (restricted fetus) is shown in the right panel.
Additional data relative to maternal ovaries and jugular plasma sex steroid hormone determinations are compiled in Table 3. Weights of maternal ovaries and (dissected) corpora lutea were not influenced by nutritional status. Numbers of corpora lutea and fetuses diagnosed by ultrasound matched the number of fetuses retrieved at slaughter (i.e., no embryonic/fetal losses occurred during gestation or due to treatments). Ovaries of control ewes contained more follicles 3 mm or greater in diameter than animals receiving restricted diets; albeit, there was no significant difference in jugular venous concentrations of estradiol-17β. Likewise, there was no differential effect of treatments on peripheral progesterone. Luteal progesterone and tissue areas occupied by blood vessels and large and small steroidogenic cells were similar between groups.
Discussion
Results of this study indicate that in underfed ewes p53, Bcl-2, and polymerase β are up-regulated in fetal oogonia containing elevated contents of 8-oxoguanine. Oxoguanine has become the benchmark for oxidative DNA modifications; it is arguably the most important mutagenic lesion in DNA (mispairing with adenine during chromosomal replications causes GC → TA transversions) [24]. It is suggested that untoward oxidative stresses to DNA prompt cell-cycle delay, antiapoptotic, and repair responses. Indeed, p53 controls the low-level damage-dependent premeiotic checkpoint which facilitates DNA repair during spermatogenesis [25]. Gametogenesis is inherently error-prone [26] and subject to mutations [27]. Nevertheless, it is imperative to avoid genotoxic complications that could become integrated into the germline.
Bcl-2 belongs to a family of cellular proteins which arbitrate decisions in life-or-death situations. In the presence of Bcl-2, irregularities within the DNA sequence are corrected before the p53 suicidal program is executed. Bcl-2 impedes the subcellular trafficking of p53, inhibits downstream adapters necessary for stimulation of the apoptotic caspases, and can act as an antioxidant [15, 28].
Polymerase β is a penultimate mediator of mammalian DNA base-excision repair. The base-excision cascade is characteristically limited to the repair of small lesions in DNA (e.g., single nucleotide modifications). Short-patch reconstruction is initiated by a proof-reading glycosylase that hydrolyzes the N-glycosylic bond linking an improper base to deoxyribose. The abasic sugar-phosphate backbone is then cleaved by an apurinic/apyrimidinic endonuclease or lyase. Polymerase β fills the nucleotide gap created in DNA with the deoxyribonucleoside triphosphate complementary to the template. Finally, the nick is sealed by a DNA ligase [16]. The base-excision pathway is a principal contributor to the amendment of 8-oxoguanine corruptions in DNA [29].
Unlike males, which continue to generate sperm cells by mitosis throughout their reproductive lives, mammalian females are generally born with their full complement of (meiotic) gametes [30]. The normal chronology of ovarian development follows a definitive pattern. Critical windows of activity in the sheep include: somatic ovarian tissue differentiation (from the gonadal ridges of the mesonephros) and growth (up to Day 50 of gestation); germ (from yolk sac endoderm) and pregranulosa (derived from the ovarian surface epithelium) cellular migration into and colonization of the ovaries (Days 30–65); clonal expansion of oogonia (Days 35–75); onset of meiosis and arrest (by Day 80) at the dictyate stage of prophase (the first division of meiosis is not completed until ovulation); and ovigerous cord regression and primordial follicle emergence (Days 65–110) [1, 31–33]. It seems apparent that germ cells are particularly vulnerable to a metabolic insult when in the phasic transition between mitosis and meiosis I.
Relatively innocuous base damages to DNA caused by oxidations are an inevitable by-product of physiological metabolism (e.g., leakage of radicals associated with the reduction of oxygen to water during mitochondrial respiration) [34]. The etiological basis for the formation of abnormally high levels of 8-oxoguanine adducts in oogonia of lambs of undernourished ewes is unknown. Altered placental vascular dynamics and shifts in fetal organ blood flow precipitated by nutrient deficiencies are of possible significance [35]. Ischemia-reperfusion is a well known stimulus of free radical formation [36]. Micronutrient imbalances or insufficiencies also can negatively impact the functional capacities of antioxidant vitamin (C, E) and enzymatic (superoxide dismutase, glutathione peroxidase, catalase) defense systems [37].
While cellular proliferation is a generic feature of normal germline development, so is programmed physiological death. In fact, the apoptotic demise (yielding double-stranded DNA breaks) of female germ cells throughout gestations of most vertebrates will encompass more than one-half of precursor populations [38, 39]. Day 75 is the approximate time-point when a maximum number of oogonia is achieved in lambs; a nearly five-fold loss then occurs between Days 75 and 90 [40]. In some cases of nutritional deprivation cellular losses are accelerated [38] and reproductive performance of the female offspring during adulthood is attenuated [1]. Prenatal exposure to famine (1944–45 Dutch cohort) did not affect subsequent fertility of women [41]. Numbers of oogonia/oocytes and weights of mid-gestational fetal ovaries of sheep were increased [2, 42] or not influenced by undernutrition [[3], present study]. It has been suggested that oogonia which have matured to oocytes are less susceptible to degeneration as pregnancy advances [2]. A prominent role for the aromatic hydrocarbon receptor (e.g., which is responsive to polycyclic ligands present in environmental toxicants) in promoting apoptosis during fetal oogenesis has recently been identified in mice [43, 44]. Prospective endocrine/paracrine modifiers (which are altered by nutritional regimen) of the fates of fetal germ cells include glucocorticoids, thyroid hormones, insulin, growth hormone, insulin-like growth factors, and leptin [1, 45].
The physiological relevance, if any, of the diminution in numbers of surface antral ovarian follicles in dams of the restricted nutrition group is unclear – this was not reflected by a perturbation in circulatory estradiol-17β. Structural and functional properties of ovine corpora lutea of pregnancy were not altered by plane of nutrition. Moreover, maternal nutrient restriction during the middle third of gestation in pigs had no effect on ovarian progesterone production or fetal survival [46]. Morphometric data for sheep corpora lutea are consistent with previous reports summarized by Sawyer [23]. It therefore appears that maternal ovaries are comparatively resilient to repercussions of undernutrition.
In conclusion, we surmise that oxidative base damages to the DNA of fetal oogonia of pregnant animals faced with a nutritional adversity are a potential threat to the genetic character and reproductive capacity of their progeny; the predicament can evidently be reconciled by response mechanisms of cell-cycle arrest, survival, and repair. Evolutionary pressures to resist or adapt to the stresses of caloric constraints have almost certainly served to assure the successes of mammalian reproduction (natural selection) for millions of years.
References
Rhind SM, Rae MT, Brooks AN: Effects of nutrition and environmental factors on the fetal programming of the reproductive axis. Reproduction. 2001, 122: 205-214.
Borwick SC, Rhind SM, McMillen SR, Racey PA: Effect of undernutrition of ewes from the time of mating on fetal ovarian development in mid gestation. Reprod Fertil Dev. 1997, 9: 711-715. 10.1071/R97011.
Rae MT, Palassio S, Kyle CE, Brooks AN, Lea RG, Miller DW, Rhind SM: Effect of maternal undernutrition during pregnancy on early ovarian development and subsequent follicular development in sheep fetuses. Reproduction. 2001, 122: 915-922.
Amundson SA, Myers TG, Fornace AJ: Roles for p53 in growth arrest and apoptosis: putting on the breaks after genotoxic stress. Oncogene. 1998, 17: 3287-3299. 10.1038/sj.onc.1202576.
Evan G, Littlewood T: A matter of life and death. Science. 1998, 281: 1317-1322. 10.1126/science.281.5381.1317.
Eastman A, Rigas JR: Modulation of apoptosis signaling pathways and cell cycle regulation. Semin Oncol. 1999, 26: 7-16.
Sionov RV, Haupt Y: The cellular response to p53: the decision between life and death. Oncogene. 1999, 18: 6145-6157. 10.1038/sj.onc.1203130.
Wathes DC: Embryonic mortality and the uterine environment. J Endocrinol. 1992, 134: 321-325.
Niswender GD, Juengel JL, Silva PJ, Rollyson MK, McIntush EW: Mechanisms controlling the function and life span of the corpus luteum. Physiol Rev. 2000, 80: 1-29.
Rexroad CE, Casida LE: Ovarian follicular development in cows, sows and ewes in different stages of pregnancy as affected by number of corpora lutea in the same ovary. J Anim Sci. 1975, 41: 1090-1097.
Taylor C, Rajamahendran R: Follicular dynamics and corpus luteum growth and function in pregnant versus nonpregnant cows. J Dairy Sci. 1991, 74: 115-123.
Bartlewski PM, Beard AP, Rawlings NC: Ultrasonographic study of ovarian function during early pregnancy and after parturition in the ewe. Theriogenology. 2000, 53: 673-689. 10.1016/S0093-691X(99)00266-6.
Compton MM: A biochemical hallmark of apoptosis: internucleosomal degradation of the genome. Cancer Metast Rev. 1992, 11: 105-119.
de Zwart LL: Biomarkers of free radical damage: applications in experimental animals and in humans. Free Rad Biol Med. 1999, 26: 202-226. 10.1016/S0891-5849(98)00196-8.
Adams JM, Cory S: The Bcl-2 protein family: arbiters of cell survival. Science. 1998, 281: 1322-1326. 10.1126/science.281.5381.1322.
Wilson SH: Mammalian base excision repair and DNA polymerase β. Mutat Res. 1998, 407: 203-215. 10.1016/S0921-8777(98)00002-0.
Murdoch WJ, Townsend RS, McDonnel AC: Ovulation-induced DNA damage in ovarian surface epithelial cells of ewes: prospective regulatory mechanisms of repair/survival and apoptosis. Biol Reprod. 2001, 65: 1417-1424.
Murdoch WJ: Programmed cell death in preovulatory ovine follicles. Biol Reprod. 1995, 53: 8-12.
Allen RT, Hunter WJ, Agrawal DK: Morphological and biochemical characterization and analysis of apoptosis. J Pharmacol Toxicol Meth. 1997, 37: 215-228. 10.1016/S1056-8719(97)00033-6.
Eggleston DL, Wilken C, Van Kirk EA, Slaughter RG, Ji TH, Murdoch WJ: Progesterone induces expression of endometrial messenger RNA encoding for cyclooxygenase. Prostaglandins. 1990, 39: 675-683. 10.1016/0090-6980(90)90027-S.
Field RA, Maiorano G, Hinds FC, Murdoch WJ, Riley ML: Bone ossification and carcass characteristics of wethers given silastic implants containing estradiol. J Anim Sci. 1990, 68: 3663-3668.
McPherson LA, Van Kirk EA, Murdoch WJ: Localization of stress protein-70 in ovine corpora lutea during prostaglandin-induced luteolysis. Prostaglandins. 1993, 46: 433-440. 10.1016/0090-6980(93)90079-M.
Sawyer HR: Structural and functional properties of the corpus luteum of pregnancy. J Reprod Fertil Suppl. 1995, 49: 97-110.
Grollman AP, Moriya M: Mutagenesis by 8-oxoguanine: an enemy within. Trends Genet. 1993, 9: 246-249. 10.1016/0168-9525(93)90089-Z.
Schwartz D, Goldfinger N, Kam Z, Rotter V: p53 controls low DNA damage-dependent premeiotic checkpoint and facilitates DNA repair during spermatogenesis. Cell Growth Diff. 1999, 10: 665-675.
Hunt PA, Hassold TJ: Sex matters in meiosis. Science. 2002, 296: 2181-2183. 10.1126/science.1071907.
Baarends WM, van der Laan R, Grootegoed JA: DNA repair mechanisms and gametogenesis. Reproduction. 2001, 121: 31-39.
Tsujimoto Y, Shimizu S: Bcl-2 family: life-or-death switch. FEBS Lett. 2000, 466: 6-10. 10.1016/S0014-5793(99)01761-5.
Dianov G, Bischoff C, Piotrowski J, Bohr VA: Repair pathways for processing of 8-oxoguanine in DNA by mammalian cell extracts. J Biol Chem. 1998, 273: 33811-33816. 10.1074/jbc.273.50.33811.
Albertini DF, Carabatsos MJ: Comparative aspects of meiotic cell cycle control in mammals. J Mol Med. 1998, 76: 795-799. 10.1007/s001090050283.
McNatty KP, Smith P, Hudson NL, Heath DA, Tisdall DJ, O WS, Braw-Tal R: Development of the sheep ovary during fetal and early neonatal life and the effect of fecundity genes. J Reprod Fertil Suppl. 1995, 49: 123-135.
Picton H, Briggs D, Gosden R: The molecular basis of oocyte growth and development. Mol Cell Endocrinol. 1998, 145: 27-37. 10.1016/S0303-7207(98)00166-X.
Sawyer HR, Smith P, Heath DA, Juengel JL, Wakefield SJ, McNatty KP: Formation of ovarian follicles during fetal development in sheep. Biol Reprod. 2002, 66: 1134-1150.
Collins AR: Oxidative DNA damage, antioxidants, and cancer. Bioessays. 1999, 21: 238-246. 10.1002/(SICI)1521-1878(199903)21:3<238::AID-BIES8>3.0.CO;2-3.
Bauer MK, Harding JE, Bassett NS, Brier BH, Oliver MH, Gallaher BH, Evans PC, Woodall SM, Gluckman PD: Fetal growth and placental function. Mol Cell Endocrinol. 1998, 140: 115-129. 10.1016/S0303-7207(98)00039-2.
Li C, Jackson RM: Reactive species mechanisms of cellular hypoxia-reoxygenation injury. Am J Physiol Cell Physiol. 2002, 282: C227-C241.
Ashworth CJ, Antipatis C: Micronutrient programming of development throughout gestation. Reproduction. 2001, 122: 527-535.
Matova N, Cooley L: Comparative aspects of animal oogenesis. Dev Biol. 2001, 231: 291-320. 10.1006/dbio.2000.0120.
Tilly JL: Commuting the death sentence: how oocytes strive to survive. Nat Rev Mol Cell Biol. 2001, 2: 838-848. 10.1038/35099086.
Smith P, O WS, Hudson NL, Shaw L, Heath DA, Condell L, Phillips DJ, McNatty KP: Effects of the Booroola gene (FecB) on body weight, ovarian development and hormone concentrations during fetal life. J Reprod Fertil. 1993, 98: 41-54.
Lumey LH, Stein AD: In utero exposure to famine and subsequent fertility: the Dutch famine birth cohort study. Am J Public Health. 1997, 87: 1962-1966.
Osgerby JC, Wathes DC, Howard D, Gadd TS: The effect of maternal undernutrition on ovine fetal growth. J Endocrinol. 2002, 173: 131-141.
Robles R, Morita Y, Mann KK, Perez GI, Yang S, Matikainen T, Sherr DH, Tilly JL: The aryl hydrocarbon receptor, a basic helix-loop-helix transcription factor of the PAS gene family, is required for normal ovarian germ cell dynamics in the mouse. Endocrinology. 2000, 141: 450-453.
Matikainen TM, Moriyama T, Morita Y, Perez GI, Korsmeyer SJ, Sherr DH, Tilly JL: Ligand activation of the aromatic hydrocarbon receptor transcription factor drives Bax-dependent apoptosis in developing fetal ovarian germ cells. Endocrinology. 2002, 143: 615-620.
Symonds ME, Budge H, Stephenson T, McMillen IC: Fetal endocrinology and development – manipulation and adaptation to long-term nutritional and environmental challenges. Reproduction. 2001, 121: 853-862.
Hard DL, Anderson LL: Maternal starvation and progesterone secretion, litter size, and growth in the pig. Am J Physiol. 1979, 237: E273-E278.
Author information
Authors and Affiliations
Corresponding author
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Murdoch, W.J., Van Kirk, E.A., Vonnahme, K.A. et al. Ovarian responses to undernutrition in pregnant ewes, USA. Reprod Biol Endocrinol 1, 6 (2003). https://doi.org/10.1186/1477-7827-1-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1477-7827-1-6