Abstract
Background
A growing body of evidences suggests that the ovary is a site of inflammatory reactions, and thus, ovarian cells could represent sources and targets of the interleukin-1 (IL-1) system. The purpose of this study was to examine the IL-1 system gene expressions in equine granulosa cells, and to study the IL-1β content in follicular fluid during the follicle maturation. For this purpose, granulosa cells and follicular fluids were collected from the largest follicle at the early dominance stage (diameter 24 ± 3 mm) or during the preovulatory maturation phase, at T0 h, T6 h, T12 h, T24 h and T34 h after induction of ovulation. Cells were analysed by RT-PCR and follicular fluids were studied by gel electrophoresis and immunoblotting.
Results
We demonstrated that interleukin-1β (IL-1β), interleukin-1 receptor 2 (IL-1R2) and interleukin-1 receptor antagonist (IL-1RA) genes are expressed in equine granulosa cells. We observed that the IL-1β and IL-1RA mRNA content changed in granulosa cells during the terminal follicular maturation whereas IL-1R2 mRNA did not vary. In follicular fluid, IL-1β content fluctuated few hours after induction of ovulation.
Conclusions
The expression of IL-1β gene in granulosa cells and the follicular fluid IL-1β content seem to be regulated by gonadotropins suggesting that IL-1β could be an intermediate paracrine factor involved in ovulation.
Similar content being viewed by others
Background
Interleukins are polypeptide cytokine components of the immune system originally defined by their role in cellular communication among leucocytes. The IL-1 system is composed mainly of 2 bioactive ligands IL-1α and IL-1β [1], 2 types of receptors IL-1R1 and IL-1R2 [2, 3], and a natural receptor antagonist IL-1RA which regulates IL-1 ligand biological activity by a competitive interaction at receptors [4]. The ovary is a site of cyclic ovulation process which is now widely assumed to an inflammatory reaction [5]. Interleukins and mainly interleukin-1 family could be key mediators of this process. Interleukins are expressed and have effects on a large range of tissues [6]. Studies in the mouse, rat, and human have shown that the ovary could be a site of IL-1 system member expression [7–10]. IL-1 activity was measured in porcine [11] and human [12] follicular fluids. At the ovary level IL-1β effects were mainly investigated in vitro. It was shown that IL-1β regulates cellular activities of granulosa and theca cells such as the synthesis of proteases [13], plasminogen activator [14, 15], and prostaglandins [16, 17]. IL-1β also regulates steroidogenesis [18, 19]. On the other hand, it has been shown that IL-1β promotes the ovulation process in rat [20] and rabbit [21] models. Moreover, IL-1β seems to act at the oocyte level since it promotes the ex vivo oocyte maturation in the rabbit [21]. In contrast, we demonstrated recently that IL-1β inhibits the eLH-induced in vitro meiosis resumption in equine oocytes [22].
Taken together, these observations lead to hypothesize that, in mammalian species, IL-1 could be a factor involved in the cascade of some events leading to ovulation [23] and oocyte maturation. This factor could be regulated by gonadotropins and be locally produced. In the mare, the relations between the endocrine system and the ovarian activity are well known [24–26]. Nevertheless, the conditions for in vivo follicular and oocyte maturation differ somewhat from those observed in other domestic mammals and in humans [27, 28]. The ovulatory LH surge occurs as a progressive rise that lasts several days, with a maximum one day after ovulation. Moreover the maturation rate of the equine oocyte remains low in culture conditions adapted from those used in the bovine species [29–31] and in vitro fertilization gives only limited success [32]. A better knowledge of the local mechanisms involved in equine follicle maturation could allow to encompass some of these problems. IL-1β could be involved in some of these species-specific mechanisms.
In this context, our purpose was first to study the IL-1 system gene expression in granulosa cells from the dominant follicle at different physiological stages of its growth and maturation. Second, we determined the pattern of follicular fluid content of IL-1β in the dominant follicle during the preovulatory maturation. The transvaginal ultrasound-guided puncture was used to collect granulosa cells, follicular fluid and cumulus oocyte complexes. Granulosa cells and follicular fluids were analyzed by RT-PCR and immunoblotting, respectively.
Results
Recovery of cumulus oocyte complexes, cumulus morphology and oocyte nuclear stage
Four out of the 72 punctures performed failed because ovulation occurred before puncture. Twenty nine cumulus-oocyte complexes were collected (43%; 29/68). The collection rate did not differ significantly (p > 0.05) between the 6 groups.
Morphology of COCs was analyzed at collection (Figure 1). Follicles from group 1 (early dominance: 'ED') contained compact cumulus only. In contrast, cumulus from group 5 (T24 h after CEG injection) and 6 (T34 h after CEG injection) were expanded cumulus. In groups 2 (T0 h), 3 (T6 h after CEG injection) and 4 (T12 h after CEG injection) both cumulus morphologies were observed. These profiles differed significantly (p < 0.01). Groups 5 and 6 were significantly different from groups 1, 2 and 3(p < 0.05). Group 4 differed from group 1(p < 0.05).
Morphology of cumulus recovered at different stages of the follicular maturation in the mare. The number of compact and expanded cumulus were illustrated by histograms in each of the 6 follicular maturation stages (early dominance [ED], end of growth [T0 h], 6 h [T6 h], 12 h [T12 h], 24 h [T24 h] and 34 h [T34 h] after injection of crude equine gonadotropin). Cumulus morphology in each group was compared with the non parametric Kruskall Wallis test. ** p < 0.05 at least.
Follicles from group 6 (T34 h after CEG injection) contained oocytes in metaphase II only (Figure 2). In contrast, all oocytes were immature (germinal vesicle stage: GV) in groups 1 (early dominance: 'ED') and 3 (T6 h after CEG injection). Groups 2 (T0 h), and 5 (T24 h after CEG injection) showed immature (GV) and metaphase I oocytes, whereas group 4 (T12 h after CEG injection) showed the 3 nuclear stages (GV, MI and MII). These profiles differed significantly (p < 0.01). Group 6 oocytes differed significantly from all other groups (p < 0.05). Group 5 was significantly different from group 3(p < 0.05), tended to be different from groups 1, 2 and 4 but the differences were not significant (p > 0.05).
Nuclear stage of oocyte recovered at different stages of the follicular maturation. The number of oocytes at the germinal vesicle (GV), MI and MII stages were illustrated by histograms in each of the 6 follicular maturation stages (early dominance [ED], end of growth [T0 h], 6 h [T6 h], 12 h [T12 h], 24 h [T24 h] and 34 h [T34 h] after injection of crude equine gonadotropin). Nuclear stage in each group were compared with the non parametric Kruskall Wallis test. *: p < 0.1; **: p < 0.05 at least.
IL-1 System Gene Expression In Granulosa Cells During Follicular Maturation
PCR amplification of gapdh, IL-1β, IL-1RA, and IL-1R2 mRNA in granulosa cells is illustrated in Figure 3. The amplified fragments were of the expected sizes (255 bp, 266 bp, 425 bp, and 311 bp, respectively; Table 1). IL-1α and IL-1R1 mRNA were not detected in any granulosa cell pellets analyzed, in spite of their amplification in the positive controls (cDNA from LPS stimulated equine macrophages, data not shown). The PCR signals for IL-1R2 were very faint and did not significantly vary during final follicular maturation. The semi-quantificative analysis of IL-1β and IL-1RA signals displayed differences between groups, as illustrated in Figure 4A and 4B, respectively. IL-1β mRNA content progressively increased in granulosa cells from the early dominance stage (group 1) to the beginning of maturation 6 hours after CEG injection (T6 h; group 3). Expression of IL-1β sharply decreased (p < 0.05) at T12 h (group 4) and increased again from T12 h onwards, with a significant difference between T12 h and T24 h (group 4 vs group 5; p < 0.05). A progressive decrease in IL-1RA mRNA content in granulosa cells was observed (Figure 4B) from the early dominance stage to T6 h (p < 0.05). The IL-1RA mRNA content was low at T6 h and T12 h and progressively increased from T12 h onwards (p < 0.05).
Gapdh, IL-1β, IL-1RA and IL-1R2 gene expression in equine granulosa cells at different stages. Lane M, molecular weight markers (100-bp DNA ladder, Promega); lane P, positive control of PCR (cDNA from lipopolysaccharide-stimulated equine macrophages); lane N, negative control of PCR (no cDNA); lane 1, early dominance [ED]; lane 2, end of growth [T0 h]; lanes 3, 4, 5 and 6, 6 h [T6 h], 12 h [T12 h], 24 h [T24 h], and 34 h [T34 h] after injection of crude equine gonadotropin, respectively. The expected sizes of amplified fragments are indicated.
Granulosa cell expression of A) IL-1β and B) IL-1RA mRNA at different stages: early dominance [ED], end of growth [T0 h], 6 h [T6 h], 12 h [T12 h], 24 h [T24 h] and 34 h [T34 h] after injection of crude equine gonadotropin. The relative amount of each PCR product isolated by electrophoresis was obtained by scanning each band. Gene expression intensity (arbitrary unit) was measured by ImageQuant software and is expressed as an intensity value normalized to the gapdh intensity value. The results are expressed in arbitrary units and represent means ± SEM of seven to ten samples. a and b are significantly different (p < 0.05 at least), a and c tended to be different (p < 0.06), b and c are not different according to the non parametric Wilcoxon Mann Whitney test. The data presented are representative of two experiments (samples collection, RNA extraction and PCR).
IL-1β Quantity In Follicular Fluid During Maturation Of The Dominant Follicle
Follicular fluids were submitted to SDS-PAGE and immunoblotting in order to evaluate their IL-1β content. One band at 28 kDa was visualized in most samples of equine follicular fluid (Figure 5A). It was slightly lower than the band visualized in ovine follicular fluids (29 kDa). The quantitative analysis of blots (Figure 5B) showed a steady-state level of IL-1β in follicular fluid from early dominance to T6 h after CEG injection. The IL-1β intrafollicular content significantly decreased from T6 h to T24 h after CEG injection (p < 0.05). It significantly increased between T24 h and T34 h after CEG injection (p < 0.05).
Content of IL-1β in follicular fluid at different stages: early dominance [ED], end of growth [T0 h], 6 h [T6 h], 12 h [T12 h], 24 h [T24 h] and 34 h [T34 h] after injection of crude equine gonadotropin A) Western immunoblots using a mouse anti-ovine IL-1β. Lane 1, early dominance [ED]; lane 2, end of growth [T0 h]; lanes 3, 4, 5 and 6, 6 h [T6 h], 12 h [T12 h], 24 h [T24 h], and 34 h [T34 h] after CEG injection respectively. Lanes P1 and P2, positive controls (ovine follicular fluids). B) Quantification of IL-1β signals visualized in follicular fluid at different stages as described above. IL-1β signal in each lane was measured by ImageQuant software and is expressed per follicle. Results are expressed in arbitrary units and represent means ± SEM of four to seven samples. a, b, and c are significantly different (p < 0.05 at least) according to the non parametric Wilcoxon Mann Whitney test. The data presented are representative of four experiments (electrophoresis and immunoblotting).
Discussion
In the present study, COC data were measured as a physiological control (COC maturation) of the kinetics of the follicle maturation. The cumulus morphology and the nuclear stage of the oocytes displayed a progressive pattern from the early dominance stage to the preovulatory one (T34 h after i.v. injection of CEG). We observed that cumulus expansion took place between 0 h and 6 h after induction of ovulation by CEG injection. In oocytes, resumption of meiosis occurred from 12 h after CEG injection onwards, and meiosis was completed at the time of ovulation (i.e. 34 h after CEG injection). These results are consistent with previous observation of the in vivo equine COCs maturation [33, 34].
Expression of IL-1β, IL-1RA and IL-1R2 genes is described in the present work for the first time in equine granulosa cells, at various physiological stages of the dominant follicle progression. IL-1β gene expression has been previously observed in rat [9] and human [35–37] granulosa cells at different physiological stages. By contrast in the mouse, IL-1β was not observed in granulosa cells but only in the luteal tissue after ovulation [7]. This could be attributed to some species-specific differences concerning ovarian IL-1β expression. To date, only two studies described the expression of IL-1R2 gene in the ovary. These studies were made on rat granulosa cells. In contrast with our results, they demonstrated the absence of IL-1R2 gene expression in rat granulosa cells [9, 38]. Our work suggests that equine granulosa cells could be some potential target of IL-1 system as it was shown in rat [15, 39], rabbit [40] and human [41] cultured granulosa cells, in which IL-1β seems to regulate plasminogen activator activity and steroidogenesis. Concerning IL-1RA, its expression was shown in granulosa cells of rat [8, 10] and human [35]. To our knowledge, no other study was performed on IL-1RA expression in granulosa cells of either species. In the present study, IL-1α mRNA was never detected in granulosa cells. This is in accordance with our previous study in which we failed to amplify some IL-1α cDNA from equine cumulus cells and oocytes [22]. The type 1 IL-1 receptor was not detected in equine granulosa cells whereas it has been shown to be expressed in granulosa cells of the rat [8, 9, 38], mouse [7] and human [13] species, as well as in equine cumulus cells [22] suggesting that equine cumulus cells are potential targets of IL-1 system in spite of absence of IL-1β effect on cumulus expansion in vitro [22]. The absence of IL-1α and IL-1R1 mRNA argues in favor of the absence of immune cells in granulosa cell preparations. Taken together, these data indicate the existence in mammals of a granulosa cell IL-1 system consisting of ligands (IL-1β rather than IL-1α), receptors (IL-1R1 and/or IL-1R2), and receptor antagonist. Although the IL-1 mechanism of action is still unclear, the expression of the IL-1 system members in equine granulosa cells suggests its involvement in equine ovarian physiology. Thus, one can hypothesize that in equine granulosa cells, IL-1 could regulate some cell functions, as observed in other species [13–17, 19]. It supposes that the transduction of IL-1 action occurs via IL-1R2, which is mainly considered to date by immunologists as an IL-1 binding protein and a 'decoy' receptor [2]. Therefore, the effect of IL-1β on the equine granulosa cell function needs to be demonstrated.
In the present study, expression of IL-1β and IL-1RA genes in the dominant follicle varied mainly after induction of ovulation by CEG injection. Gonadotropin injection induced a rapid and transient (T6 h) increase in IL-1β expression in granulosa cells, followed by a marked decrease (T12 h). The high variability of IL-1β mRNA content at T6 h could be explained by the fact that the response delay to exogenous gonadotropins fluctuates by a few hours between mares, or that the endogenous LH increase preceded the injection in some of the mares. The peak of IL-1β expression in granulosa cells at T6 h after gonadotropins injection could suggest that, in the equine preovulatory follicle, IL-1β gene expression is gonadotropin dependent. This was suggested for human granulosa cells [35] and demonstrated in rat theca cells IL-1β [42].
We observed that the lowest level of IL-1RA expression in equine granulosa cells was detected after the induction of ovulation with gonadotropins (T6 h and T12 h). This observation led us to hypothesize that IL-1RA expression by equine granulosa cells is gonadotropin dependent. Then, an increase in IL-1RA expression was observed until the expected ovulation time, as it was previously demonstrated in rat whole ovarian cell preparations [10]. At the end of follicular maturation (T24 h and T34 h), the increase in IL-1RA expression could be explained by an upregulation of the IL-1RA gene expression by IL-1, as it was demonstrated for IL-1RA gene in rat granulosa cells [10], and for IL-1β and IL-1R1 genes in human luteal cells [41].
Equine follicular fluid has been shown in the present work to contain IL-1β protein. This was demonstrated by western blot using an anti ovine-IL-1β antibody. The IL-1β presence in equine follicular fluid confirms previous results obtained in the human [12, 43] and porcine [11] species. The signal we obtained in western blot was at about 28 kDa in reducing and denaturing conditions. Mature equine IL-1β was recently produced in vitro and its molecular weight was calculated at 21 kDa size [44]. The discrepancy between these two molecular weights could be explained by the glycosylation rate since IL-1β is well known to be a glycosylated protein [1]. The difference we observed between ovine and equine IL-1β molecular weight can be explained by some species-specific feature of the proteins. Of note is the fact that the present work demonstrates for the first time, the IL-1β content in follicular fluid during maturation of the dominant follicle. No significant peak was detected but a decrease in IL-1β content was observed 12 h after induction of ovulation. This decrease could either be a consequence of a decrease in IL-1β transcription in ovarian cells, or a decrease in protein biosynthesis, or an increase in degradation of the protein. The profile of IL-1β mRNA in equine granulosa cells seems to argue in favor of the decrease in mRNA transcription. This result again suggests an intermediary and early role of IL-1β in follicle maturation. Moreover, the decrease in IL-1β in follicular fluid could represent a positive signal to oocyte maturation, since it was demonstrated in vitro that IL-1β inhibits the eLH-induced maturation of oocyte [22]. By contrast, just before the decrease, the high content of IL-1β could activate cumulus expansion by inducing hyaluronic acid biosynthesis. Indeed, it has been demonstrated that IL-1β induced accumulation of hyaluronic acid in the medium of rat ovarian dispersates [45].
Conclusions
This study demonstrates for the first time the expression of IL-1β, IL-1RA and IL-1R2 genes in equine granulosa cells. We showed that IL-1β and IL-1RA expression in granulosa cells vary during follicular maturation, probably under the influence of gonadotropins. Moreover, the presence of IL-1β was clearly shown in follicular fluid. Although the mechanism by which IL-1 regulates follicle maturation is unclear, our observations demonstrate that equine ovarian cells are a site of IL-1 system member production and suggest that IL-1 system could play a key role in the follicular preovulatory maturation and in the ovulatory process.
Methods
Animals and Ovarian Monitoring
Adult cyclic pony mares in good body condition, aged 2–21 years, kept indoors and fed with concentrates were used from March to May. Mares received a luteolytic dose of a prostaglandin F2α analogue (Cloprostenol [Estrumate], 125 μg/mare i.m.; Scherring-Plough, Levallois-Peret, France) during the mid-luteal phase (5–6 days after ovulation). Follicle size was then assessed by routine rectal ultrasound scanning until the day of experimental follicular puncture.
Experimental Follicular Punctures
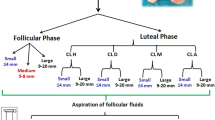
A total of 72 mares were used. Samples were collected using an in vivo transvaginal ultrasound-guided follicular aspiration technique with a 7.5-MHz sectorial probe (Kretz; Soframed, Truchtersheim, France) as previously described [46]. In group 1 (n = 12 mares), punctures were performed on the largest growing follicle, the day it reached a diameter of 22 to 25 mm (early dominance: 'ED'; 24 ± 3 mm). In groups 2, 3, 4, 5 and 6, (n = 12 mares, each) the dominant follicle was punctured 0, 6, 12, 24 or 34 h, respectively after induction of ovulation by an i.v. injection of 20 mg of crude equine gonadotropin (CEG) [47] the day when the dominant follicle reached a diameter of 33.5 ± 1 mm. This treatment is known to induce ovulation between 34 and 40 h after injection [48].
Before follicle puncture, mares were sedated with detomidine (0.6 mg/100 kg body weight (BW) i.v. Domosedan; Pfizer, Amboise, France), and relaxation of the rectum was induced by an injection (i.v.) of propantheline bromide (60 mg/100 kg BW i.v., Sigma, Saint Quentin Fallavier, France). After puncture sessions, mares were injected with an antibiotic (Intramicine: 1600000 IU penicillin/100 kg BW and 1.3 g dihydrostreptomycin/100 kg BW i.m.; Sanofi, Libourne, France).
Collection of Follicular Fluid, Granulosa Cells, and Cumulus-Oocyte Complex (COC)
During puncture, the follicle wall was scraped with a needle as previously described [22]. Follicular fluid and rinsings were individually examined under a stereomicroscope for cumulus-oocyte complexes. Follicular fluids devoid of blood were centrifuged for 10 min at 2700 g to pellet cells and pure follicular fluids were individually stored at -80°C.
COCs Morphology And Oocyte Nuclear Stage Examinations
Recovered COCs were classified morphologically as compact or expanded. They were then rinsed twice and stripped of their cumulus cells with a small glass pipette in PBS at 37°C. Totally denuded oocytes were rinsed in PBS, stained with 1 μg/mL bis-benzimide solution (Hoechst 33342; Sigma), and observed in a drop on a slide under a light and fluorescence microscope in order to determine their nuclear stage. Oocytes showing a polar body (light microscopy) and 2 distinct spots of chromosomes stained by Hoechst (fluorescence microscopy) were considered in metaphase II (MII), as described previously [29].
Granulosa Cells Preparation
Granulosa cells were prepared as previously described [49]. Briefly, the pellets of follicular cells devoid of blood cells were resuspended in 2 mL of PBS, then layered onto 3 mL of Ficoll-paque (research grade; Pharmacia Biotech, Uppsala, Sweden), and centrifuged (10 min, 1400 g, 4°C) in order to get rid of blood cells. Granulosa cells were recovered by aspiration at the interface layer and were rinsed several time with sterile PBS. They were frozen in liquid nitrogen and stored at -80°C until mRNA analysis. Aliquots of cells were analyzed by immunohistochemical staining with an equine antibody specific for a marker of immune cells (CD 44, Serotec, Oxford, UK). In any case, less than 0.5% of immune cells was detected.
RNA Extraction Of Granulosa Cells And DNAse 1 Treatment
The RNable Kit (Eurobio, Les Ulis, France) was used as recommended by the manufacturer to extract total RNA from granulosa cells. RNA pellets were dissolved in 70 μL of H2O and treated with DNAse 1 (Gibco, Cergy Pontoise, France) at 37°C for 60 min to eliminate residual DNA. DNase digestion products were then added to 100 μL of phenol and 100 μL of mix chloroform / isoamylic alcohol (49 v/1v), centrifuged and prepared as described above in order to eliminate DNAse 1. RNA pellets were dissolved in 13 μL H2O.
Reverse Transcription And PCR
RNA pellets were reverse transcribed (RT) in a final volume of 30 μL containing 2 μM of oligo(dT)12–18 (Amersham Pharmacia, Orsay, France), 1.25 mM of each dNTP, 2.5 mM of MgCl2, 40 IU of recombinant ribonuclease inhibitor (RNasin) and 200 IU of Moloney murine leukemia virus reverse transcriptase (Promega, Charbonnière, France). The reaction was performed for 60 min at 37°C, then reverse transcriptase was heat-inactivated at 95°C for 5 min. PCR amplification of IL-1α, IL-1β, IL-1RA, IL-1R1 and IL-1R2 cDNAs was performed with specific oligo-nucleotide pairs (Eurogentec, Nantes, France) whose sequences and references are shown in Table 1. Moreover, PCR amplification of glyceraldehyde-3-phosphate dehydrogenase (gapdh), a constitutively expressed gene, was used to verify the quality of extracted RNA from granulosa cells and to normalize the IL-1 member data. The PCR reaction mixture contained 2 μL RT products from granulosa cell mRNA, 0.5 μM of each sense and antisense primer, 1.5 mM of MgCl2, 0.2 μM of each dNTP, 1 μCi [α-33P]dATP (Amersham) and 1IU of Taq DNA polymerase, in 25 μL of the appropriate Taq buffer (Promega). For each pair of primers, a negative control of PCR was made, by adding ultra-pure water instead of mRNA. PCR reactions were performed in a thermal cycler (geneamp PCR system 9700, Perkin Elmer, USA) and were initiated by 3 min of denaturation at 94°C, followed by 35 cycles each consisting of 30 sec at 94°C, 30 sec at primer hybridization temperature (Table 1) and 45 sec at 72°C. PCR was ended by a final elongation step of 3 min at 72°C. PCR efficiency was checked by using a positive control. This corresponds to a cDNA from alveolar equine macrophages which were recovered from horse lungs by washing with PBS, and cultured in RPMI 1640 (Gibco) supplemented with 10% fetal calf serum (Gibco), 5.10-5M 2-mercaptoethanol (Sigma), 2 mM L-glutamine (Gibco), and 1 mM sodium pyruvate (Gibco) [50] before to be stimulated for 48 h with 10 μg/mL of lipopolysaccharides (LPS). PCR products were electrophoresed through a 2% agarose gel, stained with 0.2 μg/mL ethidium bromide and visualized by ultraviolet illumination. Gels were then dried and exposed to a radiosensitive screen for 10 h in order to detect incorporated radioactivity using a phosphoimager (STORM, Molecular Dynamics, USA). Signals were quantified using image analysis software (ImagQuant). The relative amount of IL-1α, IL-1β, IL-1RA, IL-1R1 and IL-1R2 mRNA was normalized to a constant amount of gapdh mRNA.
Follicular Fluid Analysis By Gel Electrophoresis And Immunoblotting
Follicular fluids were submitted to one-dimensional SDS-PAGE (15%) under reducing conditions [51]. Acrylamide-bisacrylamide solution was purchased from Serva GmbH & Co. (Heidelberg, Germany). Prestained standards (range 6.9–202 kDa; Bio-Rad, Hercules, CA) were run simultaneously to the samples at 25 mA per gel. Ovine follicular fluids were run simultaneously as positive controls. 5 μL of each sample was diluted in 5 μL of electrophoresis buffer (Tris-HCl 160 mM pH 6.8, EDTA 10 mM, SDS 10%, β-mercaptoethanol 10%, glycerol 20% and Bromophenol blue) and boiled for 4 min. After electrophoresis, gels were electroblotted at 200 mA onto hydrophobic PolyVinylidene DiFluoride membranes (PVDF, Amersham Pharmacia, Orsay, France) overnight at 4°C. For immunological detection, membranes were washed in TBS-Tween 20 and incubated for 1 h in a saturating buffer (TBS containing 5% dry milk, 0.2% Igepal, pH 7.4). Membranes were then incubated for 3 h with the mouse anti ovine-IL-1β antibody (Serotec, Oxford, UK) diluted in saturating solution (1/100), and rinsed twice in TBS-Tween 20. A 30 min incubation in saturating buffer was made before an incubation of the membrane for 1 h with peroxidase-conjugated secondary antibody. This peroxydase-conjugated second antiserum was a goat anti mouse-IgG (Tebu, Le Perray en Yvelines, France) diluted in saturating solution (1/500). The ECLplus western blotting detection system (Amersham Pharmacia, Orsay, France) was used to detect and quantify immunoreactive polypeptide with a phosphoimager (Image master Amersham). A control without either the first or the second antibody was made and no signal was detected.
After quantification of IL-1β signals, IL-1β content per follicle was calculated, considering that each signal was determined for 5 μl of follicular fluid and that the total volume of each punctured follicle was known.
Statistical Analysis
The distribution of cumulus morphologies and of oocyte maturation stages were globally analyzed during follicular maturation with the non parametric Jonckerheere-Terpstra test for independent ordered data. In the two cases, differences were significant (p < 0.05). Thus, groups were compared between them with the non parametric Kruskal-Wallis test. Immunoblotting and densitometry results were analyzed with the non parametric Jonckerheere-Terpstra test. Data were expressed as mean ± SEM. In order to compare results between the different follicular stages analyzed, the non parametric Wilcoxon rank sum Mann Whitney test was used. Tests were all performed using StatXact 5.0 software (CYTEL, Cambridge, MA, USA, http://www.cytel.com).
References
Dinarello CA: The interleukin-1 family: 10 years of discovery. FASEB J. 1994, 8: 1314-1325.
Colotta F, Dower SK, Sims JE, Mantovani A: The type II "decoy" receptor: a novel regulatory pathway for interleukin-1. Immunol Today. 1991, 15: 562-566. 10.1016/0167-5699(94)90217-8.
Sims JE, Dower SK: Interleukin-1 receptors. Eur Cytokine Network. 1994, 5: 539-546.
Arend WP: Interleukin 1 receptor antagonist: A new member of the interleukin 1 family. J Clin Invest. 1991, 88: 1445-1451.
Espey LL: Ovulation as an inflammatory reaction – a hypothesis. Biol Reprod. 1980, 22: 73-106.
Terranova P, Montgomery Rice V: Cytokine involvement in ovarian processes. Am J Reprod Immunol. 1997, 37: 50-63.
Simon C, Frances A, Piquette G, Polan ML: Immunohistochemical localization of the interleukin-1 system in the mouse ovary during follicular growth, ovulation, and luteinization. Biol Reprod. 1994, 50: 449-57.
Wang LJ, Brannstrom M, Cui KH, Simula AP, Hart RP, Maddocks S, Norman RJ: Localisation of mRNA for interleukin-1 receptor and interleukin-1 receptor antagonist in the rat ovary. J Endocrinol. 1997, 152: 11-17.
Kol S, Ruutiainen-Altman K, Scherzer WJ, Ben-Shlomo I, Ando M, Rohan RM, Adashi EY: The rat intraovarian interleukin (IL)-1 system: cellular localization, cyclic variation and hormonal regulation of IL-1beta and of the type I and type II IL-1 receptors. Mol Cell Endocrinol. 1999, 149: 115-28. 10.1016/S0303-7207(98)00260-3.
Kol S, Donesky BW, Ruutiainen-Altman K, Ben-Shlomo I, Irahara M, Ando M, Rohan RM, Adashi EY: Ovarian interleukin-1 receptor antagonist in rats: gene expression, cellular localization, cyclic variation, and hormonal regulation of a potential determinant of interleukin-1 action. Biol Reprod. 1999, 61: 274-82.
Takakura K, Taii S, Fukuoka M, Yasuda K, Tagaya Y, Yodoi J, Mori T: Interleukin-2 receptor/p55(Tac)-inducing activity in porcine follicular fluids. Endocrinology. 1989, 125: 618-623.
Khan SA, Schmidt K, Hallin P, Di Pauli R, De Geyter C, Nieschlag E: Human testis cytosol and ovarian follicular fluid contain high amounts of interleukin-1-like factor(s). Mol Cell Endocrinol. 1988, 58: 221-230. 10.1016/0303-7207(88)90158-X.
Hurwitz A, Dushnik M, Solomon H, Ben-Chetrit A, Finci-Yeheskel Z, Milwidsky A, Mayer MD, Adashi EY, Yagel S: Cytokine-mediated regulation of rat ovarian function: interleukin-1 stimulates the accumulation of a 92-kilodalton gelatinase. Endocrinology. 1993, 132: 2709-2714.
Bonello NP, Norman RJ, Brännström M: Interleukin-1β inhibits luteinizing hormone-induced plasminogen activator activity in rat preovulatory follicles in vitro. Endocrine. 1995, 3: 49-54.
Karakji EG, Tsang BK: Regulation of rat granulosa cell plasminogen activator system: influence of interleukin-1β and ovarian follicular development. Biol Reprod. 1995, 53: 1302-1310.
Kokia E, Hurwitz A, Ricciarelli E, Tedeschi C, Resnick CE, Mitchell MD, Adashi EY: Interleukin-1 stimulates ovarian prostaglandin biosynthesis: evidence for heterologous contact-independent cell-cell interaction. Endocrinology. 1992, 130: 3095-3097.
Brännström M, Wang L, Norman RJ: Effects of cytokines on prostaglandin production and steroidogenesis of incubated preovulatory follicles of the rat. Biol Reprod. 1993, 48: 165-171.
Nakamura Y, Kato H, Terranova PF: Interleukin-1 alpha increases thecal progesterone production of preovulatory follicles in cyclic hamsters. Biol Reprod. 1990, 43: 169-173.
Best CL, Hill JA: Interleukin-1α and -β modulation of luteinized human granulosa cell oestrogen and progesterone biosynthesis. Human Reproduction. 1995, 10: 3206-3210.
Brännström M, Wang L, Norman RJ: Ovulatory effect of interleukin-1 beta on the perfused rat ovary. Endocrinology. 1993, 132: 399-404.
Takehara Y, Dharmarajan AM, Kaufman G, Wallach EE: Effect of interleukin-1 beta on ovulation in the in vitro perfused rabbit ovary. Endocrinology. 1994, 134: 1788-1793.
Martoriati A, Lalmanach AC, Goudet G, Gerard N: Expression of Interleukin-1 (IL-1) System Genes in Equine Cumulus-Oocyte Complexes and Influence of IL-1beta During In Vitro Maturation. Biol Reprod. 2002, 67: 630-636.
Ben-Shlomo I, Adashi EY: Interleukin-1 as a mediator in the ovulatory sequence: evidence for a meaningful role of cytokines in ovarian physiology. Curr Sci Series. 1994, 1: 187-192.
Palmer E: New results on follicular growth and ovulation in the mare. In Follicular growth and ovulation rate in farm animals. Edited by: Roche JF, O'Callaghan D. 1987, Dordecht: Martinus Nijhoff Publishers, 237-255.
Ginther OJ, Bergfelt DR: Growth of small follicles and concentrations of FSH during the equine oestrous cycle. J Reprod Fertil. 1993, 99: 105-111.
Daels PF, Hughes JP: The normal estrous cycle. In Equine Reproduction. Edited by: Kinnon AO & Voss JL. 1993, Lea & Febiger Publ, Philadelphia, Pennsylvani a: Mc, 121-132.
Whitmore HL, Wentworth BC, Ginther OJ: Circulating concentrations of luteinizing hormone during estrous cycle of mares as determined by radioimmunoassay. Am J Vet Res. 1973, 34: 631-636.
Irvine CHG, Alexander SL: The dynamics of gonadotrophin-releasing hormone, LH and FSH secretion during the spontaneous ovulatory surge of the mare as revealed by intensive sampling of pituitary venous blood. J Endocrinol. 1994, 140: 283-295.
Goudet G, Bézard J, Duchamp G, Gérard N, Palmer E: Equine oocyte competence for nuclear and cytoplasmic in vitro maturation: effect of follicle size and hormonal environment. Biol Reprod. 1997, 57: 232-245.
Goudet G, Belin F, Bezard J, Gerard N: Maturation-promoting factor (MPF) and mitogen activated protein kinase (MAPK) expression in relation to oocyte competence for in vitro maturation in the mare. Mol Hum Reprod. 1998, 4: 563-570. 10.1093/molehr/4.6.563.
Hinrichs K, Williams KA: Relationships among oocyte-cumulus morphology, follicular atresia, initial chromatin configuration, and oocyte meiotic competence in the horse. Biol Reprod. 1997, 57: 377-384.
Palmer E, Bezard J, Magistrini M, Duchamp G: In vitro fertilization in the horse. A retrospective study. J Reprod Fertil Suppl. 1991, 44: 75-84.
King WA, Bézard J, Bousquet D, Palmer E, Betteridge KJ: The meiotic stage of preovulatory oocytes in mares. Genome. 1987, 29: 679-682.
Bezard J, Mekarska A, Goudet G, Duchamp G, Palmer E: Timing of in vivo maturation of equine preovulatory oocytes and competence for in vitro maturation of immature oocytes collected simultaneously. Equine Vet J. 1997, Suppl 25: 33-37.
Hurwitz A, Loukides J, Ricciarelli E, Botero L, Katz E, McAllister JM, Garcia JE, Rohan R, Adashi EY, Hernandez ER: Human intraovarian interleukin-1 (IL-1) system: highly compartmentalized and hormonally dependent regulation of the genes encoding IL-1, its receptor, and its receptor antagonist. J Clin Invest. 1992, 89: 1746-1754.
Machelon V, Nome F, Durand-Gasselin I, Emilie D: Macrophage and granulosa interleukin-1 beta mRNA in human ovulatory follicles. Hum Reprod. 1995, 10: 2198-2203.
Chen HF, Shew JY, Chao KH, Chang LJ, Ho HN, Yang YS: Luteinizing hormone up-regulates the expression of interleukin-1 beta mRNA in human granulosa-luteal cells. Am J Reprod Immunol. 2000, 43: 125-133.
Scherzer WJ, Ruutiainen-Altman KS, Putowski LT, Kol S, Adashi EY, Rohan RM: Detection and in vivo hormonal regulation of rat ovarian type I and type II interleukin-I receptor mRNAs: increased expression during the periovulatory period. J Soc Gynecol Investig. 1996, 3: 131-139. 10.1016/1071-5576(96)00010-X.
Ghersevich S, Isomaa V, Vihko P: Cytokine regulation of the expression of estrogenic biosynthetic enzymes in cultured rat granulosa cells. Mol Cell Endocrinol. 2001, 172: 21-30. 10.1016/S0303-7207(00)00396-8.
Breard E, Delarue B, Benhaim A, Feral C, Leymarie P: Inhibition by gonadotropins of interleukin-1 production by rabbit granulosa and theca cells: effects on gonadotropin-induced progesterone production. Eur J Endocrinol. 1998, 138: 328-336.
Piquette GN, Simon C, el Danasouri I, Frances A, Polan ML: Gene regulation of interleukin-1 beta, interleukin-1 receptor type I, and plasminogen activator inhibitor-1 and -2 in human granulosa-luteal cells. Fertil Steril. 1994, 62: 760-770.
Hurwitz A, Ricciarelli E, Botero L, Rohan RM, Hernandez ER, Adashi EY: Endocrine- and autocrine-mediated regulation of rat ovarian (theca-interstitial) interleukin-1 beta gene expression: gonadotropin-dependent preovulatory acquisition. Endocrinology. 1991, 129: 3427-3429.
Wang LJ, Norman RJ: Concentrations of immunoreactive interleukin-1 and interleukin-2 in human preovulatory follicular fluid. Human Reproduction. 1992, 7: 147-150.
Tung JT, Fenton JI, Arnold C, Alexander L, Yuzbasiyan-Gurkan V, Venta PJ, Peters TL, Orth MW, Richardson DW, Caron JP: Recombinant equine interleukin-1beta induces putative mediators of articular cartilage degradation in equine chondrocytes. Can J Vet Res. 2002, 66: 19-25.
Kokia E, Hurwitz A, Ben-Shlomo I, Adashi EY, Yanagishita M: Receptor-mediated stimulatory effect of IL-1 beta on hyaluronic acid and proteoglycan biosynthesis by cultured rat ovarian cells: role for heterologous cell-cell interactions. Endocrinology. 1993, 133: 2391-2394.
Duchamp G, Bézard J, Palmer E: Oocyte yield and the consequences of punture of all follicles larger than 8 millimeters in mares. In Equine Reproduction VI. Edited by: Sharp DC, Bazer FW. 1995, Madison, WI: society for the study of reproduction, 233-241.
Duchamp G, Bour B, Combarnous Y, Palmer E: Alternative solutions to hCG induction of ovulation in the mare. J Reprod Fertil. 1987, Suppl 35: 221-228.
Bézard J, Magistrini M, Duchamp G, Palmer E: Chronology of equine fertilization and embryonic development in vivo and in vitro. Equine Vet J. 1989, suppl 8: 105-110.
Belin F, Goudet G, Duchamp G, Gerard N: Intrafollicular concentrations of steroids and steroidogenic enzymes in relation to follicular development in the mare. Biol Reprod. 2000, 62: 1335-1343.
Pépin M, Cannella D, Fontaine JJ, Pittet JC, Le Pape A: Ovine mononuclear phagocytes in situ: identification by monoclonal antibodies and involvement in experimental pyogranulomas. J Leukoc Biol. 1992, 60: 188-198.
Laemmli UK: Cleavage of structural proteins during the assemblyof the head of bacteriophage T4. Nature. 1970, 227: 680-685.
Acknowledgements
This work was supported by grants from the Haras Nationaux, France. Alain Martoriati was supported by a fellowship from the Institut National de la Recherche Agronomique, France, and the Région Centre, France. The authors wish to thank Anne-Christine Lalmanach, Ghylène Goudet, and the staff of the experimental stud for technical assistance. We are grateful to Annick Lacombe for proof reading and correction of the English.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors' contribution
AM carried out the immunoblotting, granulosa cells preparation, RNA extraction and RT-PCR, participated to the collection of biological samples, analyzed results and drafted the manuscript. NG conceived of the study, and participated in its design, coordination and in the collection of biological samples and analysis of cumulus oocyte complexes. NG participated to the final analysis of results and corrected the manuscript. The two authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Martoriati, A., Gérard, N. Interleukin-1 (IL-1) system gene expression in granulosa cells: kinetics during terminal preovulatory follicle maturation in the mare. Reprod Biol Endocrinol 1, 42 (2003). https://doi.org/10.1186/1477-7827-1-42
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1477-7827-1-42